Abstract
Due to the need to balance the requirement for efficient respiration in the face of tremendous levels of exposure to endogenous and environmental challenges, it is crucial for the lungs to maintain sustainable defense that minimizes damage caused by exposures and the detrimental effects of inflammation to delicate gas exchange surfaces. Accordingly, epithelial and macrophage defenses constitute essential 1st and 2nd lines of protection that prevent the accumulation of potentially harmful agents in the lungs, and under homeostatic conditions do so effectively without inducing inflammation. Though seemingly distinct, recent data show that epithelial and macrophage mediated defenses are linked through their shared reliance on airway mucins, in particular the polymeric mucin MUC5B. This review highlights our understanding of novel mechanisms that link mucus and macrophage defenses. The roles of phagocytosis and the effects of factors that are contained within mucus on phagocytosis, as well as newly identified roles for mucin glycoproteins in the direct regulation of leukocyte functions are discussed. The emergence of this nascent field of glycoimmunobiology sets forth a new paradigm for considering how homeostasis is maintained under healthy conditions and how it is restored in disease.
Introduction
The principal function of the lungs is gas exchange. To this end, under normal tidal breathing, 8,000–12,000 liters of air pass through lungs each day. Gas flows through multiple generations of conducting airways, which ultimately terminate in the alveoli. Alveoli are bounded by type I epithelial cells that cover over 95% of the lung surface, and to allow for efficient exchange of O2 and CO2, type I epithelia are extremely thin and together with alveolar capillaries create a diffusion distance of <1 µm. Consequently, these thin surfaces are protected by elaborate defense mechanisms that must trap and eliminate particulates and pathogens before they reach the alveolar walls, while simultaneously preventing and/or suppressing potentially inflammatory responses that could injure delicate gas exchange structures. This review concentrates on the mucociliary escalator and alveolar macrophages (AMs) as crucial first and second lines of host defense in the lungs.
Airway tissues are exposed to ~100 billion inhaled particles daily (1). Airborne particles can arise from natural and manmade sources, can vary in size and chemical composition, can differ in concentrations based on geography and local environments, and can thus result in heterogeneous pathological responses (2–8). Most inspired materials are large enough to impact upon nasopharyngeal and tracheal mucosae where they are transported proximally by mucociliary clearance (MCC) and are ultimately eliminated by expectoration or swallowing. The remainder deposit in the lung periphery where they are ingested by AMs. Under healthy conditions, particulate deposition in the periphery is primarily limited to small particles (<1 µm diameter). However, under conditions where particulate concentrations are high or in pathological settings where MCC is impaired, larger particles can also accumulate in the lung periphery. Together, the coordinated functions of MCC and AMs eliminate inhaled particulates from the alveoli and airways, and hence comprise robust mechanisms for exogenous clearance. At the same time, clearance also removes endogenous materials that are generated during normal cell turnover or as a consequence of disease. Critically, although AM and MCC functions are ordinarily considered distinct, emerging data show that their functions are tightly linked through physiological and biochemical mechanisms. Below we describe mucus and macrophages separately, and this is followed by a discussion of emerging knowledge of interactions between them.
The mucus barrier and MCC
MCC involves the coordinated activities of secretory cells that release polymeric mucin glycoproteins, and multi-ciliated cells whose apically localized motile cilia provide a means for transport and elimination. Cilia are molecular machines whose structural and motile components are highly regulated; their complex assembly, function, and dysfunction in diseases are reviewed elsewhere (9, 10). For the purposes of this review, we consider physiological roles of motile cilia, and we highlight key aspects of mucociliary interactions that are essential in the airways. MCC requires the coordinated regulation of airway surface liquid to control the osmolarity, viscoelasticity, and resultant transportability of secreted mucus (11, 12). This control is driven by electrolyte transport machinery intracellularly as well as the presence of osmolytes in the extracellular space. Although ciliated and mucous layers have been considered as separate entities (‘sol’ and ‘gel’ phases), this distinction is challenged by recent studies demonstrating it as a more continuous glycoprotein hydrogel. Membrane mucins (MUC1, MUC4, and MUC16) that are present along cilia surfaces form a hydrated brush that allows for the free movement of cilia. The overlying, viscoelastic mucus layer is positioned atop this grafted brush of cilia. As a result, airway surface hydration regulates the balance between cilia and mucus structures maintained in a ‘gel-on-brush’ conformation that promotes effective motility and MCC (13).
Loss of MCC is a significant cause of respiratory infections. For instance, impaired MCC is a primary pathophysiological feature of infection-related diseases such as primary ciliary dyskinesia (PCD) where cilia motility is impaired or absent, and cystic fibrosis (CF) where airway surface dehydration causes mucus adhesion to airway surfaces and hyperosmotic collapse of underlying cilia. Less appreciated perhaps are findings in COPD and asthma, which also show significant MCC impairment (14–21). Unlike the primary roles of altered mucus and ciliary structures in CF and PCD, COPD and asthma-related changes are secondary to inflammatory or injurious stimuli that cause impairments in ciliary motility and the dysregulated production of the two major secreted mucins, MUC5AC and MUC5B (22–25).
Expression of the airway mucins MUC5AC and MUC5B
Under healthy conditions, MUC5AC and MUC5B are both produced in the lungs. MUC5AC is found predominantly in surface epithelia throughout the central conducting airways, whereas MUC5B is found mainly in submucosal glands of central airways (trachea and bronchi) and in non-ciliated surface epithelial cells of peripheral airways. MUC5AC levels increase in both airway surface and glandular epithelia in asthma (22, 23) and COPD (24, 26, 27). By contrast, MUC5B levels are more variable. For example, in patients with established CF and COPD, MUC5B levels are increased in sputum (28, 29), which is predominated by central airway secretions that are supplied by tracheobronchial submucosal glands. However, in patients with early or pre-clinical COPD or with strong allergic asthma MUC5B levels actually decrease, especially within epithelial cells that line central and peripheral airway surfaces where MUC5B transcript levels are reduced by 90% or more (22–24, 27). It is thus plausible that differential repression of MUC5B could affect MCC and contribute to lung pathologies. Indeed, recent studies in mice provide mechanistic support for this.
In mice, deletion of the Muc5b gene caused severe upper and lower airway MCC impairments and led to the development of lethal spontaneous infections (30). Interestingly, although chronic infection and inflammation were prominent outcomes in Muc5b knockout mice, their pathobiological impacts were stronger than those observed in models of PCD. In cilia-defective Dnaic, Pcdip1, Spef2, and Cby knockout mice, although MCC is severely impaired, upper airway pathologies were not reported to be lethal, and they did not carry over to the lower respiratory tract (31–33). Thus, among MCC components in the lungs, Muc5b is a dominant regulator of homeostatic microbial elimination. In addition, during chronic spontaneous and acute experimental infections, Muc5ac production increased in Muc5b knockout mice. Although not entirely protective itself, Muc5ac could have played a role in delaying the effects of infections (30). Possible explanations for the mucin functions in airway defense (as well as differences between Muc5ac and Muc5b) may reflect differences in their polymeric structures, glycosylation, and interactions with microbes or anti-microbial molecules. Determination of the specific and overlapping roles of Muc5ac and Muc5b remains an area of urgent investigation.
Mucin Expression
MUC5AC/Muc5ac and MUC5B/Muc5b gene expression levels are regulated by endogenous and environmental factors. For human MUC5B, single nucleotide polymorphisms have been shown to regulate expression via control of promoter activity (34–36). These genetic controls likely impact (or are impacted upon) by numerous innate and adaptive immune cytokine signaling pathways, as well as growth factor regulated mechanisms that are associated with responses to inflammation, injury, and tissue repair. These are reviewed extensively elsewhere (37–42). Lastly, endogenous factors include developmental (43–46) and epigenetic (47–49) regulatory mechanisms, which may play roles in the expression of mucins in cancers.
Mucin polymerization
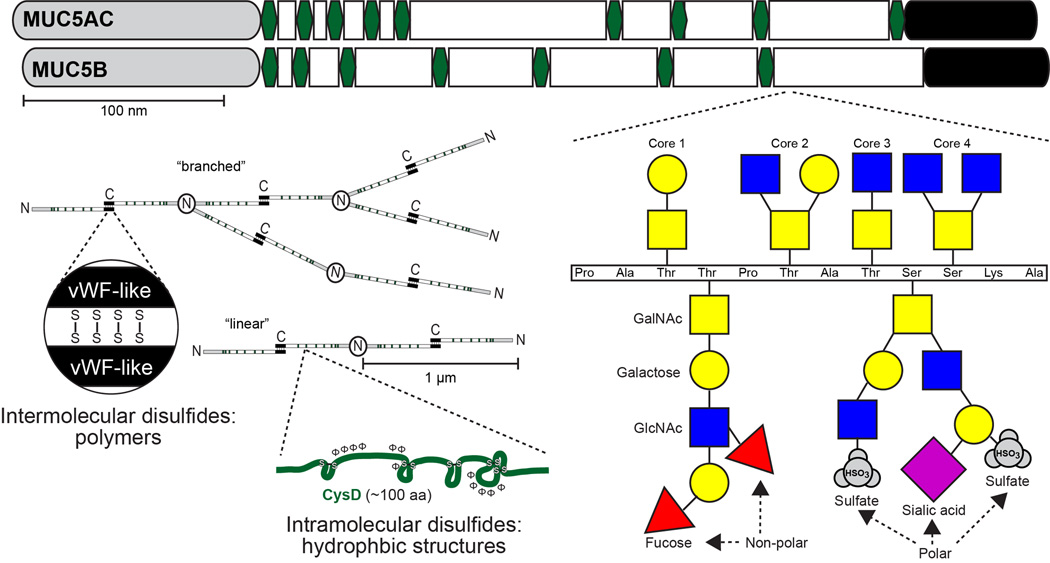
The abilities of secreted mucins to regulate MCC are largely dependent on their polymer structures formed through disulfide bonds (Figure 1). Like other members of the secreted polymeric mucin family, Muc5ac and Muc5b are composed of ~5–6% cysteines (~250–300 per molecule). They have cysteine-rich N- and C- terminal von Willebrand factor (vWF) type D-like and C- terminal cysteine knot disulfide bonding domains that are critical for intermolecular mucin assembly (50–52). Additional highly conserved cysteine-rich CysD domains are interspersed in varying numbers in polymeric mucin carbohydrate-rich repeats (53–55). Through intramolecular disulfide linkages, CysD domains are proposed to form hydrophobic loop structures that facilitate mucin alignment and regulate mucus mesh spacing (56). Furthermore, in each mucin at least 100 cysteines exist that are not found in defined “domains”. In all cases, the majority of disulfide bonds are thought to form intracellularly during assembly. In the extracellular environment, free cysteines that do exist may become oxidized and form additional cross-links that increase the elastic moduli of mucus gels (57). Disruption of N- and C-terminal bonds or CysD’s may be sufficient to “loosen” obstructive mucus. Accordingly, current mucolytic therapies such as N-acetylcysteine, as well as investigative therapies, target these by reducing disulfides and decreasing mucus viscoelasticity, thereby enhancing mucus transport (58–60). A current challenge is to determine which therapies can be given at doses that are well-tolerated and still maintain the benefits of efficient defense.
Figure 1. Polymeric and macromolecular structures of the major secreted mucins in the airways - MUC5AC and MUC5B.
MUC5AC and MUC5B (and their orthologs) have amino (N) and carboxyl (C) termini that are evolutionarily conserved in polymeric mucins and von Willebrand factor (vWF, grey and black regions). The vWF-like domains are involved in covalent intermolecular disulfide assembly of C-terminal linked dimers and N-terminal linked multimers. Multimers may exist as linear or branched structures with sizes in the 1 to >10 MDa range. Between vWF-like domains are additional cysteine rich regions (CysD domains, green hexagons) that are rich in hydrophobic amino acids and intramolecular disulfide bonds. CysD’s are suggested to mediate the distribution of mucin strands and gel-pore size after secretion in healthy mucus, but may become oxidized and increase in polymer size and stiffness in disease (57). Lastly, the majority of the remaining mucin apoprotein backbone is rich in proline, serine, and threonine. This ‘PTS’ domain (white) is an imperfect repeat region and is the primary site of O-linked glycosylation. O-linkages on serine and threonine residues form Core1–4 structures, which are defined the presence of N-acteylgalactosamine (GalNAc, yellow squares) linkages on the hydroxyl groups of serine and threonine followed by single or paired attachments of galactose (yellow circles) and/or N-acetylglucosamine (GlcNAc, blue squares). Lastly, galactose and GlcNAc glycans can be further substituted with fucose, sialic acid, and sulfates that impart diverse charges that may affect mucus gel hydration and also form 3-D structural confirmations that are critical for interactions with both pathogens and host-cell lectins. Glycan structures shown are examples of possible linkages and do not necessarily represent those found on specific mucins. Polar and non-polar glycans can be found on sugars from each core type, and may be found along the same or different branches.
Mucin glycosylation
While disulfide polymerization is an important but underappreciated aspect of secreted mucins, their glycosylation is perhaps more eminent. Mucins are defined by their heavy glycosylation, especially within variable-sized glycan-rich domains (see Figure 1). In MUC5AC and MUC5B, these regions are called ‘PTS’ domains due to their enrichment in prolines, threonines, and serines. PTS-rich repeats are sites of O-glycosylation, starting with N-acetylgalactosamine on serine and threonine residues. Galactose and N-acetylglucosamine are then attached and elaborated linearly or in branches, and the sugars can be modified by sulfation or by the addition of terminal sialic acid and fucose glycans. Two chief purposes of mucin glycans are to adsorb water and to participate in host defense. For water adsorption, glycan variations can greatly affect the osmotic pressures imparted by mucus gels. For example, sialylated and sulfated termini are strongly charged, and their large polar surface areas promote both hydration and electronegative repulsion (11, 13). On the other hand, fucose has a lower charge and an approximately 50% lower polar surface area, which hypothetically promotes mucus aggregation, increases viscoelasticity, and thereby inhibits MCC. For host defense, mucin glycans are known to interact with sugar binding molecules on a variety of bacteria that colonize or infect the lungs (61–67) and gastrointestinal tract (68–74), fungi such as Aspergillus fumigatus (75) and respiratory viruses such as respiratory syncytial virus and influenza (76, 77). Whether these interactions are beneficial to the host or the microbe vary widely. Nonetheless, as the result of host genetics and environmental exposures (such as infectious or allergic states) protection is limited. Impaired defense may be affected by changes in the properties of mucus (e.g., through variations in MUC5AC/Muc5ac vs MUC5B/Muc5b expression levels or glycosylation) that are often coupled with ciliary dysfunction (e.g., through loss/absence of ciliated cells or components of motile cilia) (78–91). Taken together, the roles of mucins in the formation and maintenance of a mucus gel, and their abilities to bind microorganisms demonstrate the coordinated function and dysfunction of mucus binding and clearance dynamics in host defense.
In summary, this conventional view of the mucociliary barrier as a defense system regulated by mucus and ciliary functions has been refined by the identification of key factors such as Muc5b and by the dissection of complex biophysical regulation of mucociliary interactions. An immediate challenge is to relate these to specific and required molecular components that regulate their intrinsic biophysical functions. Furthermore, new findings have introduced a novel set of interactions through which mucins regulate defense and inflammation in the lungs via resident and recruited pulmonary leukocyte populations. In particular, dendritic cell, eosinophil, and macrophage functions in various tissues have been demonstrated to be regulated specifically by mucin terminal glycans. Below we focus on macrophage and eosinophil functions that are regulated by extracellular oligosaccharides, including the airway mucin Muc5b.
Macrophage ontogeny and clearance mechanisms
Particulates and microbes that evade the first line of defense--epithelial mucus--reach the distal lung where they must be cleared rapidly and efficiently by the second line of defense--phagocytes. AMs are the dominant phagocytic cell in the lungs, and during health account for up to 90% of the leukocytes in airspaces (92–95) They reside in the alveolar lumen, and perhaps also in the airways. In addition to clearing inhaled particulates, they are critical for removing dying cells and maintaining alveolar homeostasis. Recent evidence suggests that AMs arise from progenitors that occupy the fetal liver and yolk sac during embryogenesis (96–98). At birth, these cells populate the airspaces where they quickly mature into resident AMs. Importantly, AMs self-renew throughout life, and in the absence of disease, they are not replaced by monocytes from the circulation (99–101). During inflammation, resident AMs proliferate locally (102). At the same time monocytes from the circulation migrate to inflamed regions where they mature into macrophages, termed monocyte-derived AMs (MDAMs) (103). Hence, the inflammatory AM pool contains cells of both embryonic and post-natal origin. Although both macrophage subsets demonstrate phagocytic capacity, their respective contributions to the clearance of exogenous particulates and pathogens and to the removal of endogenous debris and cells remain unknown. Intriguingly, as inflammation resolves MDAMs undergo programmed cell death and are removed from the lungs, leaving behind the embryonically derived resident AMs to maintain alveolar homeostasis (103).
During health, resident AMs function as sentinels, constantly surveying the luminal environment for pathogens and inhaled particulates. Under most circumstances, such agents are cleared silently and quickly - without inducing systemic inflammatory responses that could injure alveolar gas exchange structures. Indeed, experimental depletion of AMs results in exaggerated inflammatory responses (104–112), yet at the same time AM absence impairs the ability to control infection (107, 110, 113) demonstrating that restrained responses are more efficacious and beneficial. As discussed below, the alveolar environment plays an essential role in regulating AM endocytic and inflammatory responses, and it also contains a diverse array of molecules that recognize pathogens and facilitates clearance by non-inflammatory phagocytic defense.
Phagocytic Mechanisms
AMs employ a number of mechanisms to ingest particulates and pathogens, all of which involve endocytosis, a process in which the plasma membrane surrounds a target, invaginates, and then pinches off to form a membrane bound vesicle (reviewed in (114, 115)). Phagocytosis is the primary endocytic process by which AMs clear exogenous materials and is driven by cytoskeletal rearrangements that lead to rapid internalization of pathogens such as bacteria or fungi in a membrane bound phagosome. The phagosome becomes acidified after sequential fusion with endosomes and lysosomes, which contain hydrolytic enzymes and reactive oxygen species that digest and destroy the target. An initial interface that AM’s have with particles and pathogens occurs through a phagocytic synapse formed by a diverse array of plasma membrane proteins that recognize phagocytic targets through specific moieties on them, including microbial and host cell glycoconjugates. These AM receptors initiate and/or modulate phagocytosis.
Phagocytic Receptors
AMs are equipped with a vast repertoire of phagocytic receptors. Importantly, during microbial contact many different receptor families are often simultaneously activated. Some receptors directly recognize specific molecules on phagocytic targets (e.g., phosphatidyl serine or inflammasome molecules), whereas others bind to targets coated with opsonins (e.g., immunoglobulins, complement, and surfactant materials). In addition, whereas some (e.g. Fc receptors) lead directly to pathogen engulfment, others (e.g. Toll-like receptors (TLRs)) promote phagocytosis indirectly by upregulating the expression of phagocytic receptors and their downstream signaling molecules (116–118). Here we discuss main classes of receptors on AMs in the context of opsonins and signals present in airway mucus (119–125).
Immunoglobulin (Ig) signaling is an important adaptive immune process that mediates AM phagocytosis. AMs express high levels of Fcγ-receptors I (CD64), II (CD32) and III (CD16) that recognize the Fc region of IgG. Biologically relevant concentrations IgG can be found in the alveolar lining fluid of healthy humans (126). To trigger phagocytosis, Fcγ-receptors bind multiple IgG molecules within an immune complex. FcγRI is a high affinity receptor that in addition to respiratory burst and microbial killing also leads to phagocytosis. In comparison, FcγRII and FcγRIII may also promote phagocytosis but have low binding affinity. Respiratory epithelial cells secrete IgA by transcytosis, and IgA can easily be detected in the lumens of both the proximal airways and alveoli (126, 127). AMs express low levels of both FcαRI (CD89) and Fcα/µR that bind IgA and drive phagocytosis (128). Adaptive immune Ig functions are linked to glycan structures through the recognition of carbohydrate antigens, N- and O-glycosylation of their Fc domains, and physical association with secreted mucins that have specific Ig binding domains (129–132).
The complement system aids in innate host defense by opsonizing immune complexes and pathogens, enhancing their killing and removal. Alveolar lavage fluid of healthy humans contains components of the classical (C1q, C2, C3, C4) and alternative (C3, Factor B) pathways (133–135). The classical pathway is primarily activated by the interaction of C1q with antigen-antibody complexes, but it can also be activated by direct binding of C1q to bacterial, fungal and virus membrane components (136, 137). Opsonization of targets by either means can stimulate phagocytosis. AMs express three complement receptors (CRs), CR1, CR3 and CR4. CR1 is incapable of internalizing opsonized particles on its own, but can enhance Fc-mediated phagocytosis. CR3 and CR4 are heterodimers that share a common β2 integrin chain (CD18) paired with specific α chains. CR4 contains the αX subunit (CD11c) and binds to particles opsonized with C3b and iC3b fragments. CR3 contains an αM chain (also known as CD11b) with a carbohydrate-binding lectin site. Accordingly, in addition to binding particles opsonized with C3b and iC3b fragments, CR3 binds microbial cell wall glycan-containing components including LPS, mannan, β-glucan, and others (138, 139). While CR3 appears to be capable of internalizing opsonized bacteria independently (140, 141) it also functions cooperatively with other receptors including CR1, CD14, FcγR and FcαRI (138, 142–144) to enhance particle clearance. Not surprisingly, mice deficient in CR3 have impaired host defense to gram-negative bacteria, gram-positive bacteria and yeast (145, 146). Importantly, studies from rodents demonstrate that cell surface expression of complement receptors varies markedly on resident AMs versus recruited MDAMs (103): Resident AMs express high levels of CD11c/CR4 but not CD11b/CR3, whereas recruited MDAMs have high CD11b/CR3 but low CD11c/CR4. This raises the intriguing hypothesis that AM subpopulations have complementary functions to control infectious and inflammatory host defense. Like Ig’s, complement components are found in airway mucus, and their levels are upregulated in inflammation (147, 148). Furthermore, complement also increases the expression of Muc5ac in airway epithelial cells (149).
Other classes of carbohydrate lectins, the C-type lectins, are calcium-dependent carbohydrate binding proteins that contain a conserved glycan recognition domain and are involved in pathogen recognition and phagocytosis (150). In the context of lung host defense, two groups of C-type lectins are well recognized: the pulmonary collectins (surfactant proteins A and D), and pathogen-binding receptors (namely the mannose receptor (CD206) and dectin-1). Surfactant proteins A and D (SP-A, SP-D) are comprised of highly oligomerized monomers that are formed by N-terminal collagen-like domains linked to a C-terminal carbohydrate recognition domain (CRD) by a central hinge region. Through their CRDs, SP-A and SP-D recognize sugar residues on microbial pathogens. Consequently, they opsonize gram-negative and gram-positive bacteria, mycobacteria, fungi, and viruses such as influenza A and respiratory syncytial virus. A number of candidate receptors for collectin-opsonized particles exist on AMs, including C1qRp, SP-R210, CD14, and the calreticulin-CD91 complex (reviewed extensively in (151)). In addition to enhancing phagocytosis through their opsonizing effects, collectins may also promote phagocytosis indirectly. For example, SP-A enhances expression of scavenger receptor A (SR-A) and may augment Fc-receptor and CR-mediated phagocytosis (152–154). In addition, both SP-A and SP-D appear to increase cell surface localization and hence the phagocytic function of the mannose receptor (155–157). The mannose receptor (CD206) is highly expressed on AMs, and contains an extracellular domain that recognizes mannose, N-acetylglucosamine, and fucose glycans. Accordingly, CD206 promotes phagocytosis of pulmonary pathogens with diverse extracellular carbohydrate signatures including Streptococcus pneumoniae, Klebsiella pneumoniae, Mycobacterium tuberculosis, Pneumocystis jerovecii, and fungi such as candida and aspergillus (158). Precise mechanisms by which CD206 participates in phagocytosis are unclear, and it is likely that interactions with co-receptors are required (159). Dectin-1 was originally identified as a dendritic cell-specific receptor, but it is also expressed on AMs (160). Dectin-1 recognizes β-glucans found in fungal cell walls (161, 162) and also particles opsonized with pentraxin-3, a protein rapidly synthesized and secreted by mononuclear phagocytes in response to pro-inflammatory signals (163). Together these classes of receptors highlight a group of surface molecules that interact with exogenous and endogenous constituents of airway surface liquid and mucus to mediate AM phagocytic defense.
In immunocompetent individuals, defensive components such as IgG increase in the lungs during infection, promoting pathogen clearance through recognition of numerous antigen types, including carbohydrate epitopes. Indeed bacterial targets such as surface polysacharrides are exploited for use in developing effective pneumococcal vaccines (164). Conversely, recurrent sinopulmonary infections and impaired pathogen clearance are common in patients with Ig deficiencies (165–171). In addition, in common chronic airway diseases including asthma, COPD and cystic fibrosis, impaired clearance of microbial pathogens by AMs has been extensively documented (172–175). AM dysfunction correlates with disease severity and exacerbation frequency (176–178). While etiologies vary among diseases, common features include altered expression of phagocytic receptors, reduced lysosomal killing, and enhanced production of mediators that can worsen inflammation by inducing collateral damage to surrounding tissues. These defects in AMs are either absent or reduced in mononuclear phagocytes isolated from other sites (e.g. blood). Therefore, perturbations in the local environment appear to play a dominant role in altering AM function in these diseases.
Emerging links between airway mucins and AM function
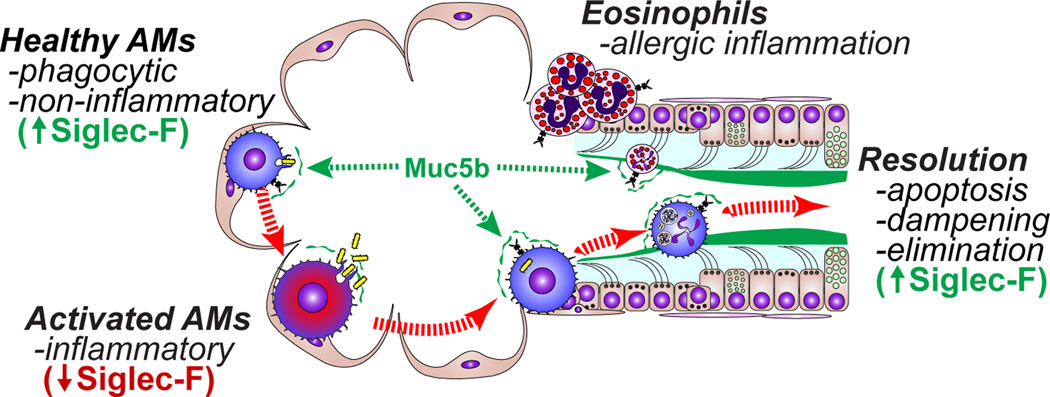
Based on the distinct anatomical localization and the highly dedicated cellular mechanisms involved in the specification of mucin-producing goblet cells in the airways and phagocytic macrophages in the alveoli, there is an outward appearance of discrete compartmentalization of their functions. However, the limiting the localization of resident AM’s to the alveolar space is not entirely warranted, as intraluminal macrophages in conducting airways account for 2–8% of the total resident macrophage population in rat lungs (179–185). Even within the alveolar compartment recent evidence demonstrates that a subpopulation of AMs, termed sessile AMs, can communicate across great distances through via a calcium-dependent signaling AM:alveolar epithelial circuit that ultimately suppresses immune function (186). Recent studies show that there are indeed functional links between airway mucus and macrophage function, and that these links are crucial for host defense. At one level, secreted factors such as Ig’s and complement are abundant in secreted mucus, suggesting that mucus is an important carrier of these defensive molecules. In addition, there are also direct links between secreted mucins and resident innate immune cells through their coordinated activities during resolving inflammation and physical interactions between glycans on mucins and carbohydrate-binding lectin receptors on leukocytes such as the sialic acid binding immunoglobulin-like lectins (siglec’s). We propose that mucin-leukocyte interactions regulate homeostatic, inflammatory, and resolving immune functions through signaling and physical clearance mechanisms (Figure 2).
Figure 2. Mucin:leukocyte interactions during homeostasis and inflammation.
In healthy lungs, resident resting alveolar macrophages (AMs) are defensive and non-inflammatory. MUC5B from bronchioles mixes with alveolar fluids, providing a route for MUC5B to contact alveolar AMs. Homeostatic or low dose stimuli elicit defensive functions such as phagocytosis. During inflammation resident AMs can become activated, and this is associated with a decrease in their Siglec-F surface expression. In addition, leukocytes, such as monocyte-derived macrophages (which lack Siglec-F) or eosinophils (which express Siglec-F) are recruited and persist for brief periods of time. These transient populations are eliminated as inflammation resolves. In mice, resolution involves Siglec-F-mediated reductions in leukocyte activation and survival. Dampened and apoptotic cells are subsequently eliminated by MUC5B-mediated MCC.
In the mouse, the intestinal mucin, Muc2, interacts with glycan-selective immuno-regulatory receptors on dendritic cells that mediate the development of inflammatory and regulatory lymphocyte subsets. In this setting, Muc2 glycans bind to two lectins (Dectin-1 and Galectin-3) that function cooperatively with the inhibitory IgG receptor FcγR3 to suppress inflammatory signals and promote tolerance (187). In a similar vein, goblet cells have also been shown to be an important mechanism for the delivery of antigens to resident monocyte-derived dendritic cells in the small intestine (188). The result of these activities is the development of tolerance to foreign antigens introduced by ingested food particles.
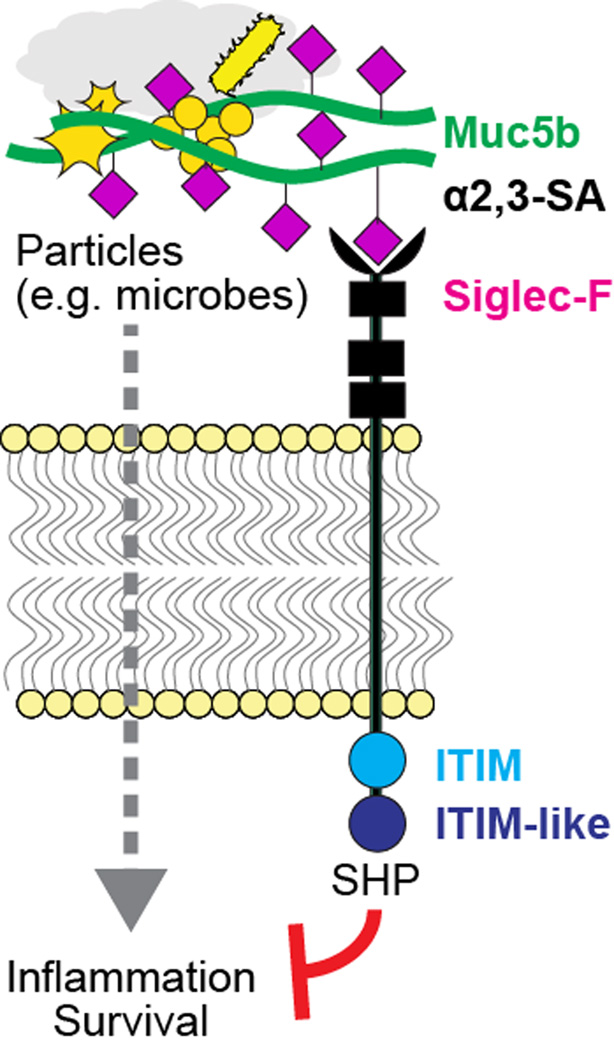
In the lungs, inhibitory regulation of leukocyte functions appears to be mediated by acute control of leukocyte activation states. In mice, Muc5b, through its α2,3-linked sialoside glycans binds to Siglec-F, an inhibitory SHP-phosphatase signaling immunoreceptor on eosinophils and AMs (189) (Figure 3). On eosinophils, Siglec-F mediates apoptosis (190–193), thereby functioning as a significant mechanism for resolving allergic inflammation. Indeed, mice lacking Siglec-F or one particular enzyme needed for this Muc5b sialylation step, ST3Gal-III, fail to make airway ligands for Siglec-F, and these mice display exaggerated and selective lung eosinophilia in a type 2 allergic inflammation lung model (194–198). In this context, Muc5b presumably contributes to the physical removal of cells by MCC while simultaneously preventing continued activation and mediator release into airspaces during elimination from the mouse lung. In humans, the Siglec-F paralog Siglec-8 also reduces eosinophil survival via sialylated and sulfated ligands, but the specificity observed between Muc5b and Siglec-F in mice is not as well conserved between MUC5B and Siglec-8 in humans (199–201). Rather, Siglec-9 is an isoform that is bound by MUC5B sialosides, and it is expressed on neutrophils, natural killer cells, dendritic cells, and monocytes/macrophages (199). Indeed, resident alveolar macrophages in healthy mouse lungs also express Siglec-F, but its role beyond that of a cell surface marker is not yet clear. Given the associations of mucus and macrophage dysfunction in numerous lung pathologies, determining the nature of their interactions will be of tremendous interest as the field advances. With the emergence of mucins as important mediators of defense, and the recognition of the crucial significance of the glycobiology of innate and adaptive immunity, efforts to interrogate these will involve both challenging and exciting experimental approaches.
Figure 3. Putative Muc5b:Siglec-F signal transduction mechanism.
Muc5b, via its display of multivalent α2,3-sialic acid (SA) linkages on galactose residues, binds to the N-terminal lectin domain of Siglec-F, thereby driving immunoreceptor tyrosine-based inhibitory motif (ITIM) and ITIM-like domain activation. ITIM signals putatively activate SH2 domain-containing phosphatase (SHP) enzymes that suppress kinase-activated inflammatory signals and can also promote apoptosis.
Conclusion
Innate defenses in the lungs are essential for maintaining efficient gas exchange. As first and second lines of host defense, mucins and macrophages play critical roles that are integrated by their physical and physiological interactions. The emergence of these links presents a convergence of new challenges that connect epithelial and innate immune programs.
Acknowledgments
Grant Support: WJJ: NIH HL109517, NIH HL114381. BSB: NIH AI72265, NIH HL107151; CME: NIH HL080396, NIH ES023384, AHA 14GRNT19990040.
References
- 1.O'Connell S, Au-Yeung HK, Gregory CJ, Matthews IP. Outdoor and indoor respirable air particulate concentrations in differing urban traffic microenvironments. Journal of toxicology and environmental health Part A. 2008;71:1069–1072. doi: 10.1080/15287390802112000. [DOI] [PubMed] [Google Scholar]
- 2.Fromme H. particulate matter in indoor environments--exposure situation in residences, schools, pubs, and related recreational spaces. Gesundheitswesen. 2006;68:714–723. doi: 10.1055/s-2006-927248. [DOI] [PubMed] [Google Scholar]
- 3.Kliucininkas L, Martuzevicius D, Krugly E, Prasauskas T, Kauneliene V, Molnar P, Strandberg B. Indoor and outdoor concentrations of fine particles, particle-bound pahs and volatile organic compounds in kaunas, lithuania. Journal of environmental monitoring : JEM. 2011;13:182–191. doi: 10.1039/c0em00260g. [DOI] [PubMed] [Google Scholar]
- 4.Guo H, Morawska L, He C, Zhang YL, Ayoko G, Cao M. Characterization of particle number concentrations and pm2.5 in a school: Influence of outdoor air pollution on indoor air. Environmental science and pollution research international. 2010;17:1268–1278. doi: 10.1007/s11356-010-0306-2. [DOI] [PubMed] [Google Scholar]
- 5.Gordon T. Linking health effects to pm components, size, and sources. Inhalation toxicology. 2007;19(Suppl 1):3–6. doi: 10.1080/08958370701490312. [DOI] [PubMed] [Google Scholar]
- 6.Kappos AD, Bruckmann P, Eikmann T, Englert N, Heinrich U, Hoppe P, Koch E, Krause GH, Kreyling WG, Rauchfuss K, Rombout P, Schulz-Klemp V, Thiel WR, Wichmann HE. Health effects of particles in ambient air. International journal of hygiene and environmental health. 2004;207:399–407. doi: 10.1078/1438-4639-00306. [DOI] [PubMed] [Google Scholar]
- 7.Health effects of outdoor air pollution. Committee of the environmental and occupational health assembly of the american thoracic society. Am J Respir Crit Care Med. 1996;153:3–50. doi: 10.1164/ajrccm.153.1.8542133. [DOI] [PubMed] [Google Scholar]
- 8.Kelly FJ, Kelly J. London air quality: A real world experiment in progress. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2009;14(Suppl 1):5–11. doi: 10.1080/13547500902965252. [DOI] [PubMed] [Google Scholar]
- 9.Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013;188:913–922. doi: 10.1164/rccm.201301-0059CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Button B, Okada SF, Frederick CB, Thelin WR, Boucher RC. Mechanosensitive atp release maintains proper mucus hydration of airways. Science signaling. 2013;6:ra46. doi: 10.1126/scisignal.2003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Button B, Picher M, Boucher RC. Differential effects of cyclic and constant stress on atp release and mucociliary transport by human airway epithelia. J Physiol. 2007;580:577–592. doi: 10.1113/jphysiol.2006.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Riordan TG, Zwang J, Smaldone GC. Mucociliary clearance in adult asthma. The American review of respiratory disease. 1992;146:598–603. doi: 10.1164/ajrccm/146.3.598. [DOI] [PubMed] [Google Scholar]
- 15.Messina MS, O'Riordan TG, Smaldone GC. Changes in mucociliary clearance during acute exacerbations of asthma. The American review of respiratory disease. 1991;143:993–997. doi: 10.1164/ajrccm/143.5_Pt_1.993. [DOI] [PubMed] [Google Scholar]
- 16.Foster WM, Langenback EG, Bergofsky EH. Lung mucociliary function in man: Interdependence of bronchial and tracheal mucus transport velocities with lung clearance in bronchial asthma and healthy subjects. The Annals of occupational hygiene. 1982;26:227–244. [PubMed] [Google Scholar]
- 17.Thomas B, Rutman A, Hirst RA, Haldar P, Wardlaw AJ, Bankart J, Brightling CE, O'Callaghan C. Ciliary dysfunction and ultrastructural abnormalities are features of severe asthma. The Journal of allergy and clinical immunology. 2010;126:722–729. e722. doi: 10.1016/j.jaci.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 18.Moller W, Felten K, Sommerer K, Scheuch G, Meyer G, Meyer P, Haussinger K, Kreyling WG. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am J Respir Crit Care Med. 2008;177:426–432. doi: 10.1164/rccm.200602-301OC. [DOI] [PubMed] [Google Scholar]
- 19.Morgan L, Pearson M, de Iongh R, Mackey D, van der Wall H, Peters M, Rutland J. Scintigraphic measurement of tracheal mucus velocity in vivo. Eur Respir J. 2004;23:518–522. doi: 10.1183/09031936.04.00061404. [DOI] [PubMed] [Google Scholar]
- 20.Smaldone GC, Foster WM, O'Riordan TG, Messina MS, Perry RJ, Langenback EG. Regional impairment of mucociliary clearance in chronic obstructive pulmonary disease. Chest. 1993;103:1390–1396. doi: 10.1378/chest.103.5.1390. [DOI] [PubMed] [Google Scholar]
- 21.Hasani A, Pavia D, Rotondetto S, Clarke SW, Spiteri MA, Agnew JE. Effect of oral antibiotics on lung mucociliary clearance during exacerbation of chronic obstructive pulmonary disease. Respir Med. 1998;92:442–447. doi: 10.1016/s0954-6111(98)90289-x. [DOI] [PubMed] [Google Scholar]
- 22.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, Fahy JV. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med. 2001;163:517–523. doi: 10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]
- 23.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Innes AL, Woodruff PG, Ferrando RE, Donnelly S, Dolganov GM, Lazarus SC, Fahy JV. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest. 2006;130:1102–1108. doi: 10.1378/chest.130.4.1102. [DOI] [PubMed] [Google Scholar]
- 25.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caramori G, Casolari P, Di Gregorio C, Saetta M, Baraldo S, Boschetto P, Ito K, Fabbri LM, Barnes PJ, Adcock IM, Cavallesco G, Chung KF, Papi A. Muc5ac expression is increased in bronchial submucosal glands of stable copd patients. Histopathology. 2009;55:321–331. doi: 10.1111/j.1365-2559.2009.03377.x. [DOI] [PubMed] [Google Scholar]
- 27.Caramori G, Di Gregorio C, Carlstedt I, Casolari P, Guzzinati I, Adcock IM, Barnes PJ, Ciaccia A, Cavallesco G, Chung KF, Papi A. Mucin expression in peripheral airways of patients with chronic obstructive pulmonary disease. Histopathology. 2004;45:477–484. doi: 10.1111/j.1365-2559.2004.01952.x. [DOI] [PubMed] [Google Scholar]
- 28.Kirkham S, Kolsum U, Rousseau K, Singh D, Vestbo J, Thornton DJ. Muc5b is the major mucin in the gel phase of sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:1033–1039. doi: 10.1164/rccm.200803-391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: Variations in the amounts and glycoforms of the major oligomeric mucins muc5ac and muc5b. Biochem J. 2002;361:537–546. doi: 10.1042/0264-6021:3610537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, Tuvim MJ, Adachi R, Romo I, Bordt AS, Bowden MG, Sisson JH, Woodruff PG, Thornton DJ, Rousseau K, De la Garza MM, Moghaddam SJ, Karmouty-Quintana H, Blackburn MR, Drouin SM, Davis CW, Terrell KA, Grubb BR, O'Neal WK, Flores SC, Cota-Gomez A, Lozupone CA, Donnelly JM, Watson AM, Hennessy CE, Keith RC, Yang IV, Barthel L, Henson PM, Janssen WJ, Schwartz DA, Boucher RC, Dickey BF, Evans CM. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostrowski LE, Yin W, Rogers TD, Busalacchi KB, Chua M, O'Neal WK, Grubb BR. Conditional deletion of dnaic1 in a murine model of primary ciliary dyskinesia causes chronic rhinosinusitis. Am J Respir Cell Mol Biol. 2010;43:55–63. doi: 10.1165/rcmb.2009-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voronina VA, Takemaru K, Treuting P, Love D, Grubb BR, Hajjar AM, Adams A, Li FQ, Moon RT. Inactivation of chibby affects function of motile airway cilia. The Journal of cell biology. 2009;185:225–233. doi: 10.1083/jcb.200809144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKenzie CW, Klonoski JM, Maier T, Trujillo G, Vitiello PF, Huber VC, Lee L. Enhanced response to pulmonary streptococcus pneumoniae infection is associated with primary ciliary dyskinesia in mice lacking pcdp1 and spef2. Cilia. 2013;2:18. doi: 10.1186/2046-2530-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakano Y, Yang IV, Walts AD, Watson AM, Helling BA, Fletcher AA, Lara AR, Schwarz MI, Evans CM, Schwartz DA. Muc5b promoter variant rs35705950 affects muc5b expression in the distal airways in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193:464–466. doi: 10.1164/rccm.201509-1872LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamio K, Matsushita I, Hijikata M, Kobashi Y, Tanaka G, Nakata K, Ishida T, Tokunaga K, Taguchi Y, Homma S, Azuma A, Kudoh S, Keicho N. Promoter analysis and aberrant expression of the muc5b gene in diffuse panbronchiolitis. Am J Respir Crit Care Med. 2005;171:949–957. doi: 10.1164/rccm.200409-1168OC. [DOI] [PubMed] [Google Scholar]
- 36.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, Evans CM, Garantziotis S, Adler KB, Dickey BF, du Bois RM, Yang IV, Herron A, Kervitsky D, Talbert JL, Markin C, Park J, Crews AL, Slifer SH, Auerbach S, Roy MG, Lin J, Hennessy CE, Schwarz MI, Schwartz DA. A common muc5b promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. 2015;16:27–35. doi: 10.1038/ni.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boucherat O, Boczkowski J, Jeannotte L, Delacourt C. Cellular and molecular mechanisms of goblet cell metaplasia in the respiratory airways. Exp Lung Res. 2013;39:207–216. doi: 10.3109/01902148.2013.791733. [DOI] [PubMed] [Google Scholar]
- 39.Evans CM, Koo JS. Airway mucus: The good, the bad, the sticky. Pharmacology and Therapeutics. 2009;121:332–348. doi: 10.1016/j.pharmthera.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thai P, Loukoianov A, Wachi S, Wu R. Regulation of airway mucin gene expression. Annu Rev Physiol. 2008;70:405–429. doi: 10.1146/annurev.physiol.70.113006.100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voynow JA, Gendler SJ, Rose MC. Regulation of mucin genes in chronic inflammatory airway diseases. Am J Respir Cell Mol Biol. 2006;34:661–665. doi: 10.1165/rcmb.2006-0035SF. [DOI] [PubMed] [Google Scholar]
- 42.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 43.Tompkins DH, Besnard V, Lange AW, Wert SE, Keiser AR, Smith AN, Lang R, Whitsett JA. Sox2 is required for maintenance and differentiation of bronchiolar clara, ciliated, and goblet cells. PloS one. 2009;4:e8248. doi: 10.1371/journal.pone.0008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reid CJ, Gould S, Harris A. Developmental expression of mucin genes in the human respiratory tract. Am J RespirCell Mol Biol. 1997;17:592–598. doi: 10.1165/ajrcmb.17.5.2798. [DOI] [PubMed] [Google Scholar]
- 45.Buisine MP, Devisme L, Copin MC, Durand-Reville M, Gosselin B, Aubert JP, Porchet N. Developmental mucin gene expression in the human respiratory tract. Am J Respir Cell Mol Biol. 1999;20:209–218. doi: 10.1165/ajrcmb.20.2.3259. [DOI] [PubMed] [Google Scholar]
- 46.Roy MG, Rahmani M, Hernandez JR, Alexander SN, Ehre C, Ho SB, Evans CM. Mucin production during prenatal and postnatal murine lung development. Am J Respir Cell Mol Biol. 2011;44:755–760. doi: 10.1165/rcmb.2010-0020OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vincent A, Perrais M, Desseyn JL, Aubert JP, Pigny P, Van SI. Epigenetic regulation (DNA methylation, histone modifications) of the 11p15 mucin genes (muc2, muc5ac, muc5b, muc6) in epithelial cancer cells. Oncogene. 2007;26:6566–6576. doi: 10.1038/sj.onc.1210479. [DOI] [PubMed] [Google Scholar]
- 48.Perrais M, Pigny P, Buisine MP, Porchet N, Aubert JP, Van Seuningen-Lempire I. Aberrant expression of human mucin gene muc5b in gastric carcinoma and cancer cells. Identification and regulation of a distal promoter. J Biol Chem. 2001;276:15386–15396. doi: 10.1074/jbc.M010534200. [DOI] [PubMed] [Google Scholar]
- 49.Ho JJ, Han SW, Pan PL, Deng G, Kuan SF, Kim YS. Methylation status of promoters and expression of muc2 and muc5ac mucins in pancreatic cancer cells. International journal of oncology. 2003;22:273–279. [PubMed] [Google Scholar]
- 50.Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16209–16214. doi: 10.1073/pnas.0705984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park SW, Zhen G, Verhaeghe C, Nakagami Y, Nguyenvu LT, Barczak AJ, Killeen N, Erle DJ. The protein disulfide isomerase agr2 is essential for production of intestinal mucus. Proc Natl Acad Sci U S A. 2009;106:6950–6955. doi: 10.1073/pnas.0808722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schroeder BW, Verhaeghe C, Park SW, Nguyenvu LT, Huang X, Zhen G, Erle DJ. Agr2 is induced in asthma and promotes allergen-induced mucin overproduction. Am J Respir Cell Mol Biol. 2012;47:178–185. doi: 10.1165/rcmb.2011-0421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo X, Zheng S, Dang H, Pace RG, Stonebraker JR, Jones CD, Boellmann F, Yuan G, Haridass P, Fedrigo O, Corcoran DL, Seibold MA, Ranade SS, Knowles MR, O'Neal WK, Voynow JA. Genome reference and sequence variation in the large repetitive central exon of human muc5ac. Am J Respir Cell Mol Biol. 2014;50:223–232. doi: 10.1165/rcmb.2013-0235OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johansson ME, Sjovall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nature reviews Gastroenterology & hepatology. 2013;10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ambort D, van der Post S, Johansson ME, Mackenzie J, Thomsson E, Krengel U, Hansson GC. Function of the CysD domain of the gel-forming muc2 mucin. The Biochemical journal. 2011;436:61–70. doi: 10.1042/BJ20102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan S, Hollinger M, Lachowicz-Scroggins ME, Kerr SC, Dunican EM, Daniel BM, Ghosh S, Erzurum SC, Willard B, Hazen SL, Huang X, Carrington SD, Oscarson S, Fahy JV. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Science translational medicine. 2015;7:276ra227. doi: 10.1126/scitranslmed.3010525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nair GB, Ilowite JS. Pharmacologic agents for mucus clearance in bronchiectasis. Clinics in chest medicine. 2012;33:363–370. doi: 10.1016/j.ccm.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 59.Decramer M, Janssens W. Mucoactive therapy in copd. European respiratory review : an official journal of the European Respiratory Society. 2010;19:134–140. doi: 10.1183/09059180.00003610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balsamo R, Lanata L, Egan CG. Mucoactive drugs. European respiratory review : an official journal of the European Respiratory Society. 2010;19:127–133. doi: 10.1183/09059180.00003510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shuter J, Hatcher VB, Lowy FD. Staphylococcus aureus binding to human nasal mucin. Infect Immun. 1996;64:310–318. doi: 10.1128/iai.64.1.310-318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scharfman A, Van Brussel E, Houdret N, Lamblin G, Roussel P. Interactions between glycoconjugates from human respiratory airways and pseudomonas aeruginosa. Am J Respir Crit Care Med. 1996;154:S163–S169. doi: 10.1164/ajrccm/154.4_Pt_2.S163. [DOI] [PubMed] [Google Scholar]
- 63.Juge N. Microbial adhesins to gastrointestinal mucus. Trends in microbiology. 2012;20:30–39. doi: 10.1016/j.tim.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 64.Davies J, Carlstedt I, Nilsson AK, Hakansson A, Sabharwal H, van Alphen L, van Ham M, Svanborg C. Binding of haemophilus influenzae to purified mucins from the human respiratory tract. Infect Immun. 1995;63:2485–2492. doi: 10.1128/iai.63.7.2485-2492.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trivier D, Houdret N, Courcol RJ, Lamblin G, Roussel P, Davril M. The binding of surface proteins from staphylococcus aureus to human bronchial mucins. Eur Respir J. 1997;10:804–810. [PubMed] [Google Scholar]
- 66.Linden S, Nordman H, Hedenbro J, Hurtig M, Boren T, Carlstedt I. Strain- and blood group-dependent binding of helicobacter pylori to human gastric muc5ac glycoforms. Gastroenterology. 2002;123:1923–1930. doi: 10.1053/gast.2002.37076. [DOI] [PubMed] [Google Scholar]
- 67.Linden SK, Wickstrom C, Lindell G, Gilshenan K, Carlstedt I. Four modes of adhesion are used during helicobacter pylori binding to human mucins in the oral and gastric niches. Helicobacter. 2008;13:81–93. doi: 10.1111/j.1523-5378.2008.00587.x. [DOI] [PubMed] [Google Scholar]
- 68.Marionneau S, Airaud F, Bovin NV, Le Pendu J, Ruvoen-Clouet N. Influence of the combined abo, fut2, and fut3 polymorphism on susceptibility to norwalk virus attachment. The Journal of infectious diseases. 2005;192:1071–1077. doi: 10.1086/432546. [DOI] [PubMed] [Google Scholar]
- 69.Marionneau S, Ruvoen N, Le Moullac-Vaidye B, Clement M, Cailleau-Thomas A, Ruiz-Palacois G, Huang P, Jiang X, Le Pendu J. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology. 2002;122:1967–1977. doi: 10.1053/gast.2002.33661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang P, Farkas T, Marionneau S, Zhong W, Ruvoen-Clouet N, Morrow AL, Altaye M, Pickering LK, Newburg DS, LePendu J, Jiang X. Noroviruses bind to human abo, lewis, and secretor histo-blood group antigens: Identification of 4 distinct strain-specific patterns. The Journal of infectious diseases. 2003;188:19–31. doi: 10.1086/375742. [DOI] [PubMed] [Google Scholar]
- 71.Linden S, Semino-Mora C, Liu H, Rick J, Dubois A. Role of mucin lewis status in resistance to helicobacter pylori infection in pediatric patients. Helicobacter. 2010;15:251–258. doi: 10.1111/j.1523-5378.2010.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Celli JP, Turner BS, Afdhal NH, Keates S, Ghiran I, Kelly CP, Ewoldt RH, McKinley GH, So P, Erramilli S, Bansil R. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc Natl Acad Sci U S A. 2009;106:14321–14326. doi: 10.1073/pnas.0903438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, Fukuda MN, Fukuda M, Katsuyama T, Nakayama J. Natural antibiotic function of a human gastric mucin against helicobacter pylori infection. Science. 2004;305:1003–1006. doi: 10.1126/science.1099250. [DOI] [PubMed] [Google Scholar]
- 74.Pacheco AR, Curtis MM, Ritchie JM, Munera D, Waldor MK, Moreira CG, Sperandio V. Fucose sensing regulates bacterial intestinal colonization. Nature. 2012;492:113–117. doi: 10.1038/nature11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kerr SC, Fischer GJ, Sinha M, McCabe O, Palmer JM, Choera T, Yun Lim F, Wimmerova M, Carrington SD, Yuan S, Lowell CA, Oscarson S, Keller NP, Fahy JV. Flea expression in aspergillus fumigatus is recognized by fucosylated structures on mucins and macrophages to prevent lung infection. PLoS pathogens. 2016;12:e1005555. doi: 10.1371/journal.ppat.1005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okada SF, Zhang L, Kreda SM, Abdullah LH, Davis CW, Pickles RJ, Lazarowski ER, Boucher RC. Coupled nucleotide and mucin hypersecretion from goblet-cell metaplastic human airway epithelium. Am J Respir Cell Mol Biol. 2011;45:253–260. doi: 10.1165/rcmb.2010-0253OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ehre C, Worthington EN, Liesman RM, Grubb BR, Barbier D, O'Neal WK, Sallenave JM, Pickles RJ, Boucher RC. Overexpressing mouse model demonstrates the protective role of muc5ac in the lungs. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16528–16533. doi: 10.1073/pnas.1206552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Corfield AP, Berry M. Glycan variation and evolution in the eukaryotes. Trends Biochem Sci. 2015;40:351–359. doi: 10.1016/j.tibs.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 79.Corfield AP. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochimica et biophysica acta. 2015;1850:236–252. doi: 10.1016/j.bbagen.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 80.Hanisch FG, Bonar D, Schloerer N, Schroten H. Human trefoil factor 2 is a lectin that binds alpha-glcnac-capped mucin glycans with antibiotic activity against helicobacter pylori. The Journal of biological chemistry. 2014;289:27363–27375. doi: 10.1074/jbc.M114.597757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tan L, Psaltis A, Baker LM, McGuckin M, Rousseau K, Wormald PJ. Aberrant mucin glycoprotein patterns of chronic rhinosinusitis patients with bacterial biofilms. American journal of rhinology & allergy. 2010;24:319–324. doi: 10.2500/ajra.2010.24.3504. [DOI] [PubMed] [Google Scholar]
- 82.Magalhaes A, Gomes J, Ismail MN, Haslam SM, Mendes N, Osorio H, David L, Le PJ, Haas R, Dell A, Boren T, Reis CA. Fut2-null mice display an altered glycosylation profile and impaired baba-mediated helicobacter pylori adhesion to gastric mucosa. Glycobiology. 2009;19:1525–1536. doi: 10.1093/glycob/cwp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mata M, Sarrion I, Armengot M, Carda C, Martinez I, Melero JA, Cortijo J. Respiratory syncytial virus inhibits ciliagenesis in differentiated normal human bronchial epithelial cells: Effectiveness of n-acetylcysteine. PloS one. 2012;7:e48037. doi: 10.1371/journal.pone.0048037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oshansky CM, Pickens JA, Bradley KC, Jones LP, Saavedra-Ebner GM, Barber JP, Crabtree JM, Steinhauer DA, Tompkins SM, Tripp RA. Avian influenza viruses infect primary human bronchial epithelial cells unconstrained by sialic acid alpha2,3 residues. PloS one. 2011;6:e21183. doi: 10.1371/journal.pone.0021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lachowicz-Scroggins ME, Boushey HA, Finkbeiner WE, Widdicombe JH. Interleukin-13-induced mucous metaplasia increases susceptibility of human airway epithelium to rhinovirus infection. Am J Respir Cell Mol Biol. 2010;43:652–661. doi: 10.1165/rcmb.2009-0244OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pittet LA, Hall-Stoodley L, Rutkowski MR, Harmsen AG. Influenza virus infection decreases tracheal mucociliary velocity and clearance of streptococcus pneumoniae. Am J Respir Cell Mol Biol. 2010;42:450–460. doi: 10.1165/rcmb.2007-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Winter C, Herrler G, Neumann U. Infection of the tracheal epithelium by infectious bronchitis virus is sialic acid dependent. Microbes and infection/Institut Pasteur. 2008;10:367–373. doi: 10.1016/j.micinf.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu CA, Peluso JJ, Shanley JD, Puddington L, Thrall RS. Murine cytomegalovirus influences foxj1 expression, ciliogenesis, and mucus plugging in mice with allergic airway disease. The American journal of pathology. 2008;172:714–724. doi: 10.2353/ajpath.2008.070462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thompson CI, Barclay WS, Zambon MC, Pickles RJ. Infection of human airway epithelium by human and avian strains of influenza a virus. Journal of virology. 2006;80:8060–8068. doi: 10.1128/JVI.00384-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang T, You Y, Spoor MS, Richer EJ, Kudva VV, Paige RC, Seiler MP, Liebler JM, Zabner J, Plopper CG, Brody SL. Foxj1 is required for apical localization of ezrin in airway epithelial cells. J Cell Sci. 2003;116:4935–4945. doi: 10.1242/jcs.00830. [DOI] [PubMed] [Google Scholar]
- 91.Look DC, Walter MJ, Williamson MR, Pang L, You Y, Sreshta JN, Johnson JE, Zander DS, Brody SL. Effects of paramyxoviral infection on airway epithelial cell foxj1 expression, ciliogenesis, and mucociliary function. The American journal of pathology. 2001;159:2055–2069. doi: 10.1016/S0002-9440(10)63057-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zaynagetdinov R, Sherrill TP, Kendall PL, Segal BH, Weller KP, Tighe RM, Blackwell TS. Identification of myeloid cell subsets in murine lungs using flow cytometry. Am J Respir Cell Mol Biol. 2013;49:180–189. doi: 10.1165/rcmb.2012-0366MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Desch AN, Gibbings SL, Goyal R, Kolde R, Bednarek J, Bruno T, Slansky JE, Jacobelli J, Mason R, Ito Y, Messier E, Randolph GJ, Prabagar M, Atif SM, Segura E, Xavier RJ, Bratton DL, Janssen WJ, Henson PM, Jakubzick CV. Flow cytometric analysis of mononuclear phagocytes in non-diseased human lung and lung-draining lymph nodes. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201507-1376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heron M, Grutters JC, ten Dam-Molenkamp KM, Hijdra D, van Heugten-Roeling A, Claessen AM, Ruven HJ, van den Bosch JM, van Velzen-Blad H. Bronchoalveolar lavage cell pattern from healthy human lung. Clinical and experimental immunology. 2012;167:523–531. doi: 10.1111/j.1365-2249.2011.04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lensmar C, Elmberger G, Sandgren P, Skold CM, Eklund A. Leukocyte counts and macrophage phenotypes in induced sputum and bronchoalveolar lavage fluid from normal subjects. Eur Respir J. 1998;12:595–600. doi: 10.1183/09031936.98.12030595. [DOI] [PubMed] [Google Scholar]
- 96.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via gm-csf. The Journal of experimental medicine. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coggle JE, Tarling JD. The proliferation kinetics of pulmonary alveolar macrophages. Journal of leukocyte biology. 1984;35:317–327. doi: 10.1002/jlb.35.3.317. [DOI] [PubMed] [Google Scholar]
- 100.Tarling JD, Coggle JE. Evidence for the pulmonary origin of alveolar macrophages. Cell and tissue kinetics. 1982;15:577–584. doi: 10.1111/j.1365-2184.1982.tb01064.x. [DOI] [PubMed] [Google Scholar]
- 101.Sawyer RT. The significance of local resident pulmonary alveolar macrophage proliferation to population renewal. Journal of leukocyte biology. 1986;39:77–87. doi: 10.1002/jlb.39.1.77. [DOI] [PubMed] [Google Scholar]
- 102.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of th2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med. 2011;184:547–560. doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. The Journal of experimental medicine. 1989;170:499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thepen T, Hoeben K, Breve J, Kraal G. Alveolar macrophages down-regulate local pulmonary immune responses against intratracheally administered t-cell-dependent, but not t-cell-independent antigens. Immunology. 1992;76:60–64. [PMC free article] [PubMed] [Google Scholar]
- 106.Bang BR, Chun E, Shim EJ, Lee HS, Lee SY, Cho SH, Min KU, Kim YY, Park HW. Alveolar macrophages modulate allergic inflammation in a murine model of asthma. Experimental & molecular medicine. 2011;43:275–280. doi: 10.3858/emm.2011.43.5.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kooguchi K, Hashimoto S, Kobayashi A, Kitamura Y, Kudoh I, Wiener-Kronish J, Sawa T. Role of alveolar macrophages in initiation and regulation of inflammation in pseudomonas aeruginosa pneumonia. Infect Immun. 1998;66:3164–3169. doi: 10.1128/iai.66.7.3164-3169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Holt PG. Down-regulation of immune responses in the lower respiratory tract: The role of alveolar macrophages. Clinical and experimental immunology. 1986;63:261–270. [PMC free article] [PubMed] [Google Scholar]
- 109.Zaslona Z, Przybranowski S, Wilke C, van Rooijen N, Teitz-Tennenbaum S, Osterholzer JJ, Wilkinson JE, Moore BB, Peters-Golden M. Resident alveolar macrophages suppress, whereas recruited monocytes promote, allergic lung inflammation in murine models of asthma. J Immunol. 2014;193:4245–4253. doi: 10.4049/jimmunol.1400580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Broug-Holub E, Toews GB, van Iwaarden JF, Strieter RM, Kunkel SL, Paine R, 3rd, Standiford TJ. Alveolar macrophages are required for protective pulmonary defenses in murine klebsiella pneumonia: Elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997;65:1139–1146. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Machado-Aranda D, M VS, Yu B, Dolgachev V, Hemmila MR, Raghavendran K. Alveolar macrophage depletion increases the severity of acute inflammation following nonlethal unilateral lung contusion in mice. The journal of trauma and acute care surgery. 2014;76:982–990. doi: 10.1097/TA.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Elder A, Johnston C, Gelein R, Finkelstein J, Wang Z, Notter R, Oberdorster G. Lung inflammation induced by endotoxin is enhanced in rats depleted of alveolar macrophages with aerosolized clodronate. Exp Lung Res. 2005;31:527–546. doi: 10.1080/019021490944223. [DOI] [PubMed] [Google Scholar]
- 113.Qiu H, KuoLee R, Harris G, Van Rooijen N, Patel GB, Chen W. Role of macrophages in early host resistance to respiratory acinetobacter baumannii infection. PloS one. 2012;7:e40019. doi: 10.1371/journal.pone.0040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 115.Kumari S, Mg S, Mayor S. Endocytosis unplugged: Multiple ways to enter the cell. Cell research. 2010;20:256–275. doi: 10.1038/cr.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Doyle SE, O'Connell RM, Miranda GA, Vaidya SA, Chow EK, Liu PT, Suzuki S, Suzuki N, Modlin RL, Yeh WC, Lane TF, Cheng G. Toll-like receptors induce a phagocytic gene program through p38. The Journal of experimental medicine. 2004;199:81–90. doi: 10.1084/jem.20031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 118.Kong L, Ge BX. Myd88-independent activation of a novel actin-cdc42/rac pathway is required for toll-like receptor-stimulated phagocytosis. Cell research. 2008;18:745–755. doi: 10.1038/cr.2008.65. [DOI] [PubMed] [Google Scholar]
- 119.Kotlar HK, Harbitz O, Jenssen AO, Smidsrod O. Quantitation of proteins in sputum from patients with chronic obstructive lung disease. Ii. Determination of albumin, transferrin, alpha1-acid glycoprotein, igg, igm, lysozyme and c3-complement factor. European journal of respiratory diseases. 1980;61:233–239. [PubMed] [Google Scholar]
- 120.Hill SL, Mitchell JL, Burnett D, Stockley RA. Igg subclasses in the serum and sputum from patients with bronchiectasis. Thorax. 1998;53:463–468. doi: 10.1136/thx.53.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sloane AJ, Lindner RA, Prasad SS, Sebastian LT, Pedersen SK, Robinson M, Bye PT, Nielson DW, Harry JL. Proteomic analysis of sputum from adults and children with cystic fibrosis and from control subjects. Am J Respir Crit Care Med. 2005;172:1416–1426. doi: 10.1164/rccm.200409-1215OC. [DOI] [PubMed] [Google Scholar]
- 122.Aitken ML, Greene KE, Tonelli MR, Burns JL, Emerson JC, Goss CH, Gibson RL. Analysis of sequential aliquots of hypertonic saline solution-induced sputum from clinically stable patients with cystic fibrosis. Chest. 2003;123:792–799. doi: 10.1378/chest.123.3.792. [DOI] [PubMed] [Google Scholar]
- 123.Wright SM, Hockey PM, Enhorning G, Strong P, Reid KB, Holgate ST, Djukanovic R, Postle AD. Altered airway surfactant phospholipid composition and reduced lung function in asthma. J Appl Physiol (1985) 2000;89:1283–1292. doi: 10.1152/jappl.2000.89.4.1283. [DOI] [PubMed] [Google Scholar]
- 124.Shimura S, Masuda T, Takishima T, Shirato K. Surfactant apoprotein-a concentration in airway secretions for the detection of pulmonary oedema. Eur Respir J. 1996;9:2525–2530. doi: 10.1183/09031936.96.09122525. [DOI] [PubMed] [Google Scholar]
- 125.Masuda T, Shimura S, Sasaki H, Takishima T. Surfactant apoprotein-a concentration in sputum for diagnosis of pulmonary alveolar proteinosis. Lancet. 1991;337:580–582. doi: 10.1016/0140-6736(91)91640-g. [DOI] [PubMed] [Google Scholar]
- 126.Peebles RS, Jr, Liu MC, Lichtenstein LM, Hamilton RG. Iga, igg and igm quantification in bronchoalveolar lavage fluids from allergic rhinitics, allergic asthmatics, and normal subjects by monoclonal antibody-based immunoenzymetric assays. Journal of immunological methods. 1995;179:77–86. doi: 10.1016/0022-1759(94)00275-2. [DOI] [PubMed] [Google Scholar]
- 127.Sato T, Kitajima A, Ohmoto S, Chikuma M, Kado M, Nagai S, Izumi T, Takeyama M, Masada M. Determination of human immunoglobulin a and secretory immunoglobulin a in bronchoalveolar lavage fluids by solid phase enzyme immunoassay. Clinica chimica acta; international journal of clinical chemistry. 1993;220:145–156. doi: 10.1016/0009-8981(93)90043-4. [DOI] [PubMed] [Google Scholar]
- 128.Bakema JE, van Egmond M. The human immunoglobulin a fc receptor fcalphari: A multifaceted regulator of mucosal immunity. Mucosal immunology. 2011;4:612–624. doi: 10.1038/mi.2011.36. [DOI] [PubMed] [Google Scholar]
- 129.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 130.Wolfert MA, Boons G-J. Adaptive immune activation: Glycosylation does matter. Nat Chem Biol. 2013;9:776–784. doi: 10.1038/nchembio.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Crook CK, Lipsitt LP. Neonatal nutritive sucking: Effects of taste stimulation upon sucking rhythm and heart rate. Child development. 1976;47:518–522. [PubMed] [Google Scholar]
- 132.Pelaseyed T, Bergstrom JH, Gustafsson JK, Ermund A, Birchenough GM, Schutte A, van der Post S, Svensson F, Rodriguez-Pineiro AM, Nystrom EE, Wising C, Johansson ME, Hansson GC. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunological reviews. 2014;260:8–20. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Watford WT, Ghio AJ, Wright JR. Complement-mediated host defense in the lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L790–L798. doi: 10.1152/ajplung.2000.279.5.L790. [DOI] [PubMed] [Google Scholar]
- 134.Reynolds HY, Newball HH. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. The Journal of laboratory and clinical medicine. 1974;84:559–573. [PubMed] [Google Scholar]
- 135.Robertson J, Caldwell JR, Castle JR, Waldman RH. Evidence for the presence of components of the alternative (properdin) pathway of complement activation in respiratory secretions. J Immunol. 1976;117:900–903. [PubMed] [Google Scholar]
- 136.Nayak A, Ferluga J, Tsolaki AG, Kishore U. The non-classical functions of the classical complement pathway recognition subcomponent c1q. Immunology letters. 2010;131:139–150. doi: 10.1016/j.imlet.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 137.Nayak A, Pednekar L, Reid KB, Kishore U. Complement and non-complement activating functions of c1q: A prototypical innate immune molecule. Innate immunity. 2012;18:350–363. doi: 10.1177/1753425910396252. [DOI] [PubMed] [Google Scholar]
- 138.Ehlers MR. Cr3: A general purpose adhesion-recognition receptor essential for innate immunity. Microbes and infection/Institut Pasteur. 2000;2:289–294. doi: 10.1016/s1286-4579(00)00299-9. [DOI] [PubMed] [Google Scholar]
- 139.Gasque P. Complement: A unique innate immune sensor for danger signals. Molecular immunology. 2004;41:1089–1098. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 140.Ross GD, Cain JA, Lachmann PJ. Membrane complement receptor type three (cr3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for ic3b. J Immunol. 1985;134:3307–3315. [PubMed] [Google Scholar]
- 141.Thornton BP, Vetvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (cd11b/cd18) J Immunol. 1996;156:1235–1246. [PubMed] [Google Scholar]
- 142.Troelstra A, de Graaf-Miltenburg LA, van Bommel T, Verhoef J, Van Kessel KP, Van Strijp JA. Lipopolysaccharide-coated erythrocytes activate human neutrophils via cd14 while subsequent binding is through cd11b/cd18. J Immunol. 1999;162:4220–4225. [PubMed] [Google Scholar]
- 143.Sutterwala FS, Rosenthal LA, Mosser DM. Cooperation between cr1 (cd35) and cr3 (cd 11b/cd18) in the binding of complement-opsonized particles. Journal of leukocyte biology. 1996;59:883–890. doi: 10.1002/jlb.59.6.883. [DOI] [PubMed] [Google Scholar]
- 144.van Egmond M, van Vuuren AJ, Morton HC, van Spriel AB, Shen L, Hofhuis FM, Saito T, Mayadas TN, Verbeek JS, van de Winkel JG. Human immunoglobulin a receptor (fcalphari, cd89) function in transgenic mice requires both fcr gamma chain and cr3 (cd11b/cd18) Blood. 1999;93:4387–4394. [PubMed] [Google Scholar]
- 145.Rijneveld AW, de Vos AF, Florquin S, Verbeek JS, van der Poll T. Cd11b limits bacterial outgrowth and dissemination during murine pneumococcal pneumonia. The Journal of infectious diseases. 2005;191:1755–1760. doi: 10.1086/429633. [DOI] [PubMed] [Google Scholar]
- 146.Agramonte-Hevia J, Gonzalez-Arenas A, Barrera D, Velasco-Velazquez M. Gram-negative bacteria and phagocytic cell interaction mediated by complement receptor 3. FEMS immunology and medical microbiology. 2002;34:255–266. doi: 10.1111/j.1574-695X.2002.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 147.Humbles AA, Lu B, Nilsson CA, Lilly C, Israel E, Fujiwara Y, Gerard NP, Gerard C. A role for the c3a anaphylatoxin receptor in the effector phase of asthma. Nature. 2000;406:998–1001. doi: 10.1038/35023175. [DOI] [PubMed] [Google Scholar]
- 148.Wu J, Kobayashi M, Sousa EA, Liu W, Cai J, Goldman SJ, Dorner AJ, Projan SJ, Kavuru MS, Qiu Y, Thomassen MJ. Differential proteomic analysis of bronchoalveolar lavage fluid in asthmatics following segmental antigen challenge. Molecular & cellular proteomics : MCP. 2005;4:1251–1264. doi: 10.1074/mcp.M500041-MCP200. [DOI] [PubMed] [Google Scholar]
- 149.Dillard P, Wetsel RA, Drouin SM. Complement c3a regulates muc5ac expression by airway clara cells independently of th2 responses. Am J Respir Crit Care Med. 2007;175:1250–1258. doi: 10.1164/rccm.200701-049OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zelensky AN, Gready JE. The c-type lectin-like domain superfamily. The FEBS journal. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 151.Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T, Chakraborty T. Surfactant proteins sp-a and sp-d: Structure, function and receptors. Molecular immunology. 2006;43:1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 152.Kuronuma K, Sano H, Kato K, Kudo K, Hyakushima N, Yokota S, Takahashi H, Fujii N, Suzuki H, Kodama T, Abe S, Kuroki Y. Pulmonary surfactant protein a augments the phagocytosis of streptococcus pneumoniae by alveolar macrophages through a casein kinase 2-dependent increase of cell surface localization of scavenger receptor a. The Journal of biological chemistry. 2004;279:21421–21430. doi: 10.1074/jbc.M312490200. [DOI] [PubMed] [Google Scholar]
- 153.Watford WT, Smithers MB, Frank MM, Wright JR. Surfactant protein a enhances the phagocytosis of c1q-coated particles by alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1011–L1022. doi: 10.1152/ajplung.00366.2001. [DOI] [PubMed] [Google Scholar]
- 154.Gil M, McCormack FX, Levine AM. Surfactant protein a modulates cell surface expression of cr3 on alveolar macrophages and enhances cr3-mediated phagocytosis. The Journal of biological chemistry. 2009;284:7495–7504. doi: 10.1074/jbc.M808643200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Beharka AA, Crowther JE, McCormack FX, Denning GM, Lees J, Tibesar E, Schlesinger LS. Pulmonary surfactant protein a activates a phosphatidylinositol 3-kinase/calcium signal transduction pathway in human macrophages: Participation in the up-regulation of mannose receptor activity. J Immunol. 2005;175:2227–2236. doi: 10.4049/jimmunol.175.4.2227. [DOI] [PubMed] [Google Scholar]
- 156.Beharka AA, Gaynor CD, Kang BK, Voelker DR, McCormack FX, Schlesinger LS. Pulmonary surfactant protein a up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J Immunol. 2002;169:3565–3573. doi: 10.4049/jimmunol.169.7.3565. [DOI] [PubMed] [Google Scholar]
- 157.Kudo K, Sano H, Takahashi H, Kuronuma K, Yokota S, Fujii N, Shimada K, Yano I, Kumazawa Y, Voelker DR, Abe S, Kuroki Y. Pulmonary collectins enhance phagocytosis of mycobacterium avium through increased activity of mannose receptor. J Immunol. 2004;172:7592–7602. doi: 10.4049/jimmunol.172.12.7592. [DOI] [PubMed] [Google Scholar]
- 158.Kerrigan AM, Brown GD. C-type lectins and phagocytosis. Immunobiology. 2009;214:562–575. doi: 10.1016/j.imbio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Le Cabec V, Emorine LJ, Toesca I, Cougoule C, Maridonneau-Parini I. The human macrophage mannose receptor is not a professional phagocytic receptor. Journal of leukocyte biology. 2005;77:934–943. doi: 10.1189/jlb.1204705. [DOI] [PubMed] [Google Scholar]
- 160.Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. Dectin-1 is a major beta-glucan receptor on macrophages. The Journal of experimental medicine. 2002;196:407–412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. The Journal of experimental medicine. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 163.Diniz SN, Nomizo R, Cisalpino PS, Teixeira MM, Brown GD, Mantovani A, Gordon S, Reis LF, Dias AA. Ptx3 function as an opsonin for the dectin-1-dependent internalization of zymosan by macrophages. Journal of leukocyte biology. 2004;75:649–656. doi: 10.1189/jlb.0803371. [DOI] [PubMed] [Google Scholar]
- 164.Pilishvili T, Bennett NM. Pneumococcal disease prevention among adults: Strategies for the use of pneumococcal vaccines. Vaccine. 2015;33(Suppl 4):D60–D65. doi: 10.1016/j.vaccine.2015.05.102. [DOI] [PubMed] [Google Scholar]
- 165.Schwitzguebel AJ, Jandus P, Lacroix JS, Seebach JD, Harr T. Immunoglobulin deficiency in patients with chronic rhinosinusitis: Systematic review of the literature and meta-analysis. The Journal of allergy and clinical immunology. 2015;136:1523–1531. doi: 10.1016/j.jaci.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 166.Gathmann B, Mahlaoui N, Gerard L, Oksenhendler E, Warnatz K, Schulze I, Kindle G, Kuijpers TW, van Beem RT, Guzman D, Workman S, Soler-Palacin P, De Gracia J, Witte T, Schmidt RE, Litzman J, Hlavackova E, Thon V, Borte M, Borte S, Kumararatne D, Feighery C, Longhurst H, Helbert M, Szaflarska A, Sediva A, Belohradsky BH, Jones A, Baumann U, Meyts I, Kutukculer N, Wagstrom P, Galal NM, Roesler J, Farmaki E, Zinovieva N, Ciznar P, Papadopoulou-Alataki E, Bienemann K, Velbri S, Panahloo Z, Grimbacher B. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. The Journal of allergy and clinical immunology. 2014;134:116–126. doi: 10.1016/j.jaci.2013.12.1077. [DOI] [PubMed] [Google Scholar]
- 167.Oksenhendler E, Gerard L, Fieschi C, Malphettes M, Mouillot G, Jaussaud R, Viallard JF, Gardembas M, Galicier L, Schleinitz N, Suarez F, Soulas-Sprauel P, Hachulla E, Jaccard A, Gardeur A, Theodorou I, Rabian C, Debre P. Infections in 252 patients with common variable immunodeficiency. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46:1547–1554. doi: 10.1086/587669. [DOI] [PubMed] [Google Scholar]
- 168.Kainulainen L, Suonpaa J, Nikoskelainen J, Svedstrom E, Vuorinen T, Meurman O, Ruuskanen O. Bacteria and viruses in maxillary sinuses of patients with primary hypogammaglobulinemia. Archives of otolaryngology--head & neck surgery. 2007;133:597–602. doi: 10.1001/archotol.133.6.597. [DOI] [PubMed] [Google Scholar]
- 169.Busse PJ, Farzan S, Cunningham-Rundles C. Pulmonary complications of common variable immunodeficiency. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2007;98:1–8. doi: 10.1016/S1081-1206(10)60853-8. quiz 8–11, 43. [DOI] [PubMed] [Google Scholar]
- 170.Kainulainen L, Varpula M, Liippo K, Svedstrom E, Nikoskelainen J, Ruuskanen O. Pulmonary abnormalities in patients with primary hypogammaglobulinemia. The Journal of allergy and clinical immunology. 1999;104:1031–1036. doi: 10.1016/s0091-6749(99)70085-0. [DOI] [PubMed] [Google Scholar]
- 171.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: Clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 172.Fitzpatrick AM, Holguin F, Teague WG, Brown LA. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. The Journal of allergy and clinical immunology. 2008;121:1372–1378. 1378, e1371–e1373. doi: 10.1016/j.jaci.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Berenson CS, Garlipp MA, Grove LJ, Maloney J, Sethi S. Impaired phagocytosis of nontypeable haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. The Journal of infectious diseases. 2006;194:1375–1384. doi: 10.1086/508428. [DOI] [PubMed] [Google Scholar]
- 174.Taylor AE, Finney-Hayward TK, Quint JK, Thomas CM, Tudhope SJ, Wedzicha JA, Barnes PJ, Donnelly LE. Defective macrophage phagocytosis of bacteria in copd. Eur Respir J. 2010;35:1039–1047. doi: 10.1183/09031936.00036709. [DOI] [PubMed] [Google Scholar]
- 175.Marti-Lliteras P, Regueiro V, Morey P, Hood DW, Saus C, Sauleda J, Agusti AG, Bengoechea JA, Garmendia J. Nontypeable haemophilus influenzae clearance by alveolar macrophages is impaired by exposure to cigarette smoke. Infect Immun. 2009;77:4232–4242. doi: 10.1128/IAI.00305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Berenson CS, Kruzel RL, Eberhardt E, Sethi S. Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. The Journal of infectious diseases. 2013;208:2036–2045. doi: 10.1093/infdis/jit400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Alexis NE, Soukup J, Nierkens S, Becker S. Association between airway hyperreactivity and bronchial macrophage dysfunction in individuals with mild asthma. Am J Physiol Lung Cell Mol Physiol. 2001;280:L369–L375. doi: 10.1152/ajplung.2001.280.2.L369. [DOI] [PubMed] [Google Scholar]
- 178.Donnelly LE, Barnes PJ. Defective phagocytosis in airways disease. Chest. 2012;141:1055–1062. doi: 10.1378/chest.11-2348. [DOI] [PubMed] [Google Scholar]
- 179.Lehnert BE. Pulmonary and thoracic macrophage subpopulations and clearance of particles from the lung. Environmental health perspectives. 1992;97:17–46. doi: 10.1289/ehp.929717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lehnert BE, Valdez YE, Sebring RJ, Lehnert NM, Saunders GC, Steinkamp JA. Airway intra-luminal macrophages: Evidence of origin and comparisons to alveolar macrophages. Am J Respir Cell Mol Biol. 1990;3:377–391. doi: 10.1165/ajrcmb/3.4.377. [DOI] [PubMed] [Google Scholar]
- 181.Lehnert BE, Morrow PE. Association of 59iron oxide with alveolar macrophages during alveolar clearance. Exp Lung Res. 1985;9:1–16. doi: 10.3109/01902148509061524. [DOI] [PubMed] [Google Scholar]
- 182.Lehnert BE, Valdez YE, Holland LM. Pulmonary macrophages: Alveolar and interstitial populations. Exp Lung Res. 1985;9:177–190. doi: 10.3109/01902148509057522. [DOI] [PubMed] [Google Scholar]
- 183.Warheit DB, Hill LH, Brody AR. Surface morphology and correlated phagocytic capacity of pulmonary macrophages lavaged from the lungs of rats. Exp Lung Res. 1984;6:71–82. doi: 10.3109/01902148409087896. [DOI] [PubMed] [Google Scholar]
- 184.Yeh HC, Schum GM, Duggan MT. Anatomic models of the tracheobronchial and pulmonary regions of the rat. The Anatomical record. 1979;195:483–492. doi: 10.1002/ar.1091950308. [DOI] [PubMed] [Google Scholar]
- 185.Sorokin SP, Brain JD. Pathways of clearance in mouse lungs exposed to iron oxide aerosols. The Anatomical record. 1975;181:581–625. doi: 10.1002/ar.1091810304. [DOI] [PubMed] [Google Scholar]
- 186.Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506:503–506. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, He B, Cassis L, Bigas A, Cols M, Comerma L, Huang B, Blander JM, Xiong H, Mayer L, Berin C, Augenlicht LH, Velcich A, Cerutti A. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to cd103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Patnode ML, Cheng CW, Chou CC, Singer MS, Elin MS, Uchimura K, Crocker PR, Khoo KH, Rosen SD. Galactose-6-o-sulfotransferases are not required for the generation of siglec-f ligands in leukocytes or lung tissue. The Journal of biological chemistry. 2013 doi: 10.1074/jbc.M113.485409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kiwamoto T, Katoh T, Evans CM, Janssen WJ, Brummet ME, Hudson SA, Zhu Z, Tiemeyer M, Bochner BS. Endogenous airway mucins carry glycans that bind siglec-f and induce eosinophil apoptosis. The Journal of allergy and clinical immunology. 2015;135:1329–1340. e1321–e1329. doi: 10.1016/j.jaci.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mao H, Kano G, Hudson SA, Brummet M, Zimmermann N, Zhu Z, Bochner BS. Mechanisms of siglec-f-induced eosinophil apoptosis: A role for caspases but not for shp-1, src kinases, nadph oxidase or reactive oxygen. PloS one. 2013;8:e68143. doi: 10.1371/journal.pone.0068143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, Crocker PR, Rothenberg ME, Bochner BS. Siglec-f antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63:1156–1163. doi: 10.1111/j.1398-9995.2008.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Song DJ, Cho JY, Lee SY, Miller M, Rosenthal P, Soroosh P, Croft M, Zhang M, Varki A, Broide DH. Anti-siglec-f antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol. 2009;183:5333–5341. doi: 10.4049/jimmunol.0801421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guo JP, Myers AC, Choi O, Lee HS, Zhu Z, Hudson SA, Brummet M, Bovin NV, Crocker PR, Bochner BS. Ligands for siglec-8 and siglec-f: Binding characteristics and tissue distribution. Journal of Allergy and Clinical Immunology. 119:S299. [Google Scholar]
- 195.Kiwamoto T, Brummet ME, Wu F, Motari MG, Smith DF, Schnaar RL, Zhu Z, Bochner BS. Mice deficient in the st3gal3 gene product alpha2,3 sialyltransferase (st3gal-iii) exhibit enhanced allergic eosinophilic airway inflammation. The Journal of allergy and clinical immunology. 2014;133:240–247. e241–e243. doi: 10.1016/j.jaci.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Suzukawa M, Miller M, Rosenthal P, Cho JY, Doherty TA, Varki A, Broide D. Sialyltransferase st3gal-iii regulates siglec-f ligand formation and eosinophilic lung inflammation in mice. J Immunol. 2013;190:5939–5948. doi: 10.4049/jimmunol.1203455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Defining the in vivo function of siglec-f, a cd33-related siglec expressed on mouse eosinophils. Blood. 2007;109:4280–4287. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cho JY, Song DJ, Pham A, Rosenthal P, Miller M, Dayan S, Doherty TA, Varki A, Broide DH. Chronic ova allergen challenged siglec-f deficient mice have increased mucus, remodeling, and epithelial siglec-f ligands which are up-regulated by il-4 and il-13. Respiratory research. 2010;11:154. doi: 10.1186/1465-9921-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jia Y, Yu H, Fernandes SM, Wei Y, Gonzalez-Gil A, Motari MG, Vajn K, Stevens WW, Peters AT, Bochner BS, Kern RC, Schleimer RP, Schnaar RL. Expression of ligands for siglec-8 and siglec-9 in human airways and airway cells. The Journal of allergy and clinical immunology. 2015;135:799–810. e797. doi: 10.1016/j.jaci.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kano G, Almanan M, Bochner BS, Zimmermann N. Mechanism of siglec-8-mediated cell death in il-5-activated eosinophils: Role for reactive oxygen species-enhanced mek/erk activation. The Journal of allergy and clinical immunology. 2013;132:437–445. doi: 10.1016/j.jaci.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of siglec-8: A selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–5020. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]