Abstract
Objectives
To determine 4-decade temporal trends in the prevalence of diabetes and cardiovascular risk factors among patients undergoing coronary artery bypass grafting (CABG) and to compare in-hospital outcomes, resource utilization, and long-term survival after CABG in diabetics versus nondiabetics.
Methods
From January 1972 to January 2011, 10,362 pharmacologically treated diabetics and 45,139 nondiabetics underwent first-time CABG. Median follow-up was 12 years. Direct technical cost data were available from 2003 onward (n = 4679). Propensity matching by diabetes status was used for outcome comparisons. Endpoints were in-hospital adverse events, resource utilization, and long-term survival.
Results
Diabetics undergoing CABG increased from 7%in the 1970s to 37%in the 2000s. Their outcomes were worse, with more (P < .05) in-hospital deaths (2.0% vs 1.3%), deep sternal wound infections (2.3% vs 1.2%), strokes (2.2% vs 1.4%), renal failure (4.0% vs 1.3%), and prolonged postoperative hospital stay (9.6% vs 6.0%); and their hospital costs were 9% greater (95% confidence interval 7%–11%). Survival after CABG among diabetics versus nondiabetics at 1, 5, 10, and 20 years was also worse: 94% versus 94%, 80% versus 84%, 56% versus 66%, and 20% versus 32%, respectively. Propensity-matched patients incurred similar costs, but the prevalence of postoperative deep sternal wound infections and stroke, as well as long-term survival, remained worse in diabetics.
Conclusions
Diabetes is both a marker for high-risk, resource-intensive, and expensive care after CABG and an independent risk factor for reduced long-term survival. These issues, coupled with the increasing proportion of patients needing CABG who have diabetes, are a growing challenge in reining in health care costs.
Keywords: Coronary artery bypass grafting, diabetes, health care costs
Graphical abstract
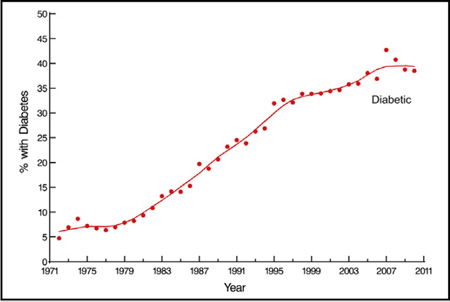
Four-decade trend in prevalence of diabetes among patients undergoing primary isolated coronary artery bypass grafting. Each circle represents a yearly percentage, and the solid line is the locally estimated scatterplot smoother (loess) estimate.
Diabetes is an emerging worldwide epidemic that affects 382 million people,1 including 25.8 million in the United States alone.2 In 2013, global health expenditures resulting from diabetes were estimated at $548 billion and are expected to exceed $627 billion by 2035.1 In the United States, the total economic burden of diabetes was $245 billion in 2012.3 Because coronary artery disease is common in diabetics,4 it is an important driver of diabetes-related health care cost.3
As the prevalence of diabetes has risen, cardiovascular disease associated with it has increased as well.5 Today, diabetics represent an important subset of patients undergoing coronary artery bypass grafting (CABG), an expensive procedure. Therefore, we sought to determine 4-decade temporal trends in the prevalence of diabetes and cardiovascular risk factors for patients undergoing CABG, compare overall in-hospital adverse outcomes, hospital resource utilization and costs, and long-term survival after CABG in diabetics versus nondiabetics, then compare these same factors in diabetics versus nondiabetics who have similar high-risk profiles using propensity matching.
METHODS
Patients
From January 1, 1972, to January 1, 2011, 57,278 patients underwent first-time isolated CABG at Cleveland Clinic. Data on the presence or absence of pharmacologically treated diabetes mellitus (insulin or oral hypoglycemic agent) were available for 55,501 (97%) of these patients: 45,139 nondiabetics and 10,362 diabetics.
Patients were identified, and preoperative, operative, and postoperative variables (Appendix E1) were retrieved from the prospective Cleveland Clinic Cardiovascular Information Registry. This database is populated concurrently with patient care and has been approved for use in research by the institutional review board, with the requirement for patient consent waived.
Variables and Definitions
A coronary artery system was considered meaningfully stenotic if it contained a ≥50%-diameter obstruction. Incomplete revascularization was defined as failure to graft any coronary system containing ≥50% stenosis, or both left anterior descending and circumflex coronary systems for ≥50% left main trunk stenosis. Left ventricular function was echocardiographically graded as normal (ejection fraction ≥60%), mild dysfunction (ejection fraction 40%–59%), moderate dysfunction (ejection fraction 25%–39%), or severe dysfunction (ejection fraction <25%).
Endpoints
Endpoints were: (1) in-hospital adverse outcomes defined as in the Society of Thoracic Surgeons national database (www.ctsnet.org/file/rptDataSpecifications252_1_ForVendorsPGS.pdf); (2) in-hospital direct technical costs (the sum of direct preoperative, operative, and postoperative costs), both total and broken down according to resource utilization areas; and (3) time-related mortality. Actual direct technical cost data (not charge data), exclusive of physician professional salaries, were obtained from Decision Support Services of Cleveland Clinic. Data were available for patients from only 2003 onward (n = 4679: 1776 diabetics and 2903 nondiabetics). Costs were corrected to constant 2011 dollars.6 Indirect costs, including institutional costs and capital equipment costs used for all operations, could not be estimated on a per patient basis and are not included, but they were assumed to be distributed uniformly across groups.
Vital status after hospital discharge was obtained by routine anniversary follow-up questionnaires supplemented with data from the Social Security Death Master File,7 accessed on October 27, 2011, with a closing date of April 27, 2011. A total of 714,709 patient-years of follow-up data were available for analyses. Median follow-up was 11.8 years, with 25% of survivors followed for >21 years, and 10% for >30 years.
For diabetic patients, 86,153 patient-years of follow-up data were available for analyses, with a median follow-up period of 7.5 years; 25% of survivors were followed for >12 years, and 10% for >18 years. For nondiabetic patients, 628,556 patient-years of follow-up data were available for analyses, with a median follow-up period of 13 years; 25% of survivors were followed for >24 years, and 10% for >31 years.
Statistical Analysis
Temporal trends
Four-decade temporal trends in the prevalence of diabetes and cardiovascular risk factors among patients undergoing CABG were assessed using plots of yearly percentages or averages over time. A nonparametric locally estimated scatterplot smoother, PROC LOESS (SAS Institute, Cary, NC), was used to smooth these temporal trends.
In-hospital adverse outcomes
Comparisons of outcomes after CABG between diabetics and nondiabetics, unmatched and propensity matched, were made using the χ2 test for categoric endpoints.
Resource utilization and total direct technical cost
Hours in the intensive care unit and postoperative and total hospital lengths of stay had right-skewed distributions, so the Wilcoxon rank-sum test, and the median score test for continuous endpoints, were used for comparisons between diabetics and nondiabetics. To identify the relative difference in direct technical costs, we estimated the ratio of the median cost between the 2 groups. The percentile bootstrap confidence method8 was used to estimate 95%confidence intervals. This procedure was applied to the overall and matched cohorts.
Long-term survival
Survival was assessed nonparametrically, using the Kaplan-Meier method, and parametrically, using a multiphase hazard model.9 The latter involved resolving the number of hazard phases for instantaneous risk of death (hazard function), and estimating shaping parameters. (For details, see www.lerner.ccf.org/qhs/software/hazard.) Because the shape of time-varying risk of death may differ for diabetics versus nondiabetics, we constructed separate hazard models for each group.
Propensity-score matching
Although overall assessment of outcomes in diabetics compared with nondiabetics represents the realities of the real world, diabetics as a group present with a higher-than-average risk profile. We therefore treated diabetes as a “natural experiment,”10 comparing outcomes of propensity-matched diabetics and nondiabetics.11–13 This comparison was accomplished in 2 steps. First, a parsimonious multivariable logistic regression was used to identify differences in preoperative characteristics of diabetic versus nondiabetic patients, to obtain insight into these differences (see Appendix E1 for a list of variables analyzed). Bootstrap bagging for variable selection with automated analysis of 500 resampled datasets was used to accomplish this, followed by tabulation of the frequency of both single factors and closely related clusters of factors.14 We retained factors that occurred in ≥50% of the bootstrap models (Table E1). The C-statistic for this parsimonious model was .83.
Second, the parsimonious model was augmented into a saturated propensity model by including patient characteristics that were not statistically significantly different between groups. These characteristics were demographic, cardiac, and noncardiac comorbidities not represented (see Appendix E1). The C-statistic for this model was .84.
A propensity score representing the probability of diabetes—group membership given the variables included in the propensity model, regardless of whether the patient had diabetes—was then calculated for each patient. A greedy matching strategy based on the propensity scores alone was used to match diabetic with nondiabetic patients, yielding 8926 well-matched pairs (86% of possible matches; Figure E1). Diabetes cases with propensity scores that deviated >0.10 from those of nondiabetes cases were considered unmatched. Standardized differences demonstrated that covariable balance was achieved across nearly all variables (Figure E2).
Using a similar approach, separate propensity matching was done between the subset of diabetics and nondiabetics undergoing an operation in the period from 2003 to 2011, during which cost data were available. This yielded 1368 well-matched pairs (77% of possible matches).
Missing values
Several variables examined in multivariable analyses had missing values. We used fivefold multiple imputation15 with a Markov chain Monte Carlo technique to impute missing values (SAS PROC MI; SAS Institute, Cary, NC). In multivariable modeling, for each imputed complete dataset, we estimated regression coefficients and their variance–covariance matrix. After this step, following Rubin,15 we combined estimates from the 5 models (SAS PROC MIANALYZE; SAS Institute, Cary, NC) to yield final regression coefficient estimates, the variance–covariance matrix, and P values.
Presentation
Continuous variables are summarized by mean ± SD, or equivalent 15th, 50th (median), and 85th percentiles when the distribution of values is skewed. Analyses were performed using SAS statistical software, version 9.1 (SAS Institute, Cary, NC) and R (version 3.0.2). Uncertainty is expressed by confidence limits (CLs) equivalent to ±1 SE (68%).
RESULTS
Compared with nondiabetics, diabetic patients undergoing CABG were older and more likely to be overweight, and more likely to be women. In addition, they were more likely to have a history of heart failure, peripheral arterial disease, carotid disease, hypertension, renal failure, stroke, and advanced coronary artery disease (Table 1).
TABLE 1.
Patient characteristics and revascularization details of nondiabetics and diabetics undergoing coronary artery bypass grafting
| Nondiabetics (n = 45,139) | Diabetics (n = 10,362) | ||||
|---|---|---|---|---|---|
| Characteristic | n | No. (% or mean ± SD | n | No. (%) or mean ± SD | P value |
| Demographics | |||||
| Age (y) | 45,139 | 60 ± 10 | 10,362 | 63 ± 9.7 | <.001 |
| Male | 45,139 | 37,626 (83) | 10,362 | 7249 (70) | <.001 |
| Body mass index (kg/m2) | 27,739 | 28 ± 4.7 | 8883 | 30 ± 5.8 | <.001 |
| Symptoms and surgical priorities | |||||
| NYHA functional class | 44,775 | 10,317 | <.001 | ||
| I | 7645 (17) | 1800 (17) | |||
| II | 16,533 (37) | 3982 (39) | |||
| III | 4702 (11) | 1468 (14) | |||
| IV | 15,895 (35) | 3067 (30) | |||
| Emergency operation | 45,137 | 1242 (2.8) | 10,362 | 248 (2.4) | .04 |
| Cardiac comorbidity | |||||
| Myocardial infarction | 45,139 | 22,916 (51) | 10,362 | 5932 (57) | <.001 |
| Left ventricular dysfunction | 42,404 | 8996 | <.001 | ||
| None | 37,013 (87) | 6300 (70) | |||
| Mild | 2492 (5.9) | 948 (11) | |||
| Mild to moderate | 514 (1.2) | 238 (2.6) | |||
| Moderate | 1315 (3.1) | 688 (7.6) | |||
| Moderate to severe | 492 (1.2) | 384 (4.3) | |||
| Severe | 578 (1.4) | 438 (4.9) | |||
| Preoperative AF or flutter | 36,357 | 411 (1.1) | 8967 | 163 (1.8) | <.001 |
| Heart failure | 45,139 | 2219 (4.9) | 10,362 | 1750 (17) | <.001 |
| Coronary artery disease | 35,015 | 20,687 (59) | 7042 | 3446 (49) | <.001 |
| No. of coronary systems diseased (stenosis ≥50%) | 43,113 | 9908 | <.001 | ||
| 0 | 366 (0.85) | 69 (0.70) | |||
| 1 | 5310 (12) | 615 (6.2) | |||
| 2 | 13,807 (32) | 2332 (24) | |||
| 3 | 23,630 (55) | 6892 (70) | |||
| Left main disease (stenosis ≥50%) | 41,909 | 6704 (16) | 9277 | 1749 (19) | <.001 |
| Noncardiac comorbidity | |||||
| Peripheral arterial disease | 45,139 | 4551 (10) | 10,362 | 1840 (18) | <.001 |
| Carotid disease | 45,139 | 3434 (7.6) | 10,362 | 2278 (22) | <.001 |
| Stroke | 45,139 | 1515 (3.4) | 10,362 | 880 (8.5) | <.001 |
| Hypertension | 29,346 | 17,615 (60) | 9077 | 6966 (77) | <.001 |
| COPD | 14,360 | 1079 (7.5) | 6639 | 584 (8.8) | <.001 |
| Smoking | 44,244 | 24,133 (55) | 10,208 | 5319 (52) | <.001 |
| Creatinine (mg/dL) | 13,969 | 1.2 ± 0.83 | 6464 | 1.3 ± 1.2 | .07 |
| Blood urea nitrogen (mg/dL) | 13,954 | 18 ± 9.0 | 6460 | 22 ± 12 | <.001 |
| Renal dialysis | 7375 | 59 (0.80) | 4060 | 98 (2.4) | <.001 |
| Total cholesterol (mg/dL) | 35,236 | 235 ± 56 | 7422 | 208 ± 60 | <.001 |
| HDL cholesterol (mg/dL) | 14,734 | 40 ± 12 | 5283 | 39 ± 13 | <.001 |
| LDL cholesterol (mg/dL) | 9616 | 128 ± 46 | 4309 | 113 ± 45 | <.001 |
| Triglycerides (mg/dL) | 29,248 | 185 ± 116 | 6672 | 203 ± 173 | .002 |
| Bilirubin (mg/dL) | 12,683 | 0.65 ± 0.50 | 5803 | 0.59 ± 0.51 | <.001 |
| Hematocrit (%) | 13,037 | 40 ± 5.1 | 5993 | 38 ± 5.5 | <.001 |
| Experience | |||||
| January 1, 1972 to index operation | 45,139 | 14 ± 9.5 | 10,362 | 21 ± 9.6 | <.001 |
| Revascularization details | |||||
| ITA grafts at index operation | 45,139 | 10,362 | <.001 | ||
| None | 14,888 (33) | 2018 (19) | |||
| Single | 25,160 (56) | 7570 (73) | |||
| Bilateral | 5091 (11) | 774 (7.5) | |||
| Complete revascularization* | 45,139 | 40,405 (90) | 10,362 | 9432 (91) | <.001 |
| Cardiopulmonary bypass | 45,139 | 43,761 (97) | 10,362 | 9817 (95) | <.001 |
Values in “n” columns are numbers of patients with data available. SD, Standard deviation; NYHA, New York Heart Association; AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ITA, internal thoracic artery.
Incomplete revascularization was defined as failure to graft any system containing 50% stenosis or both the left anterior descending and circumflex coronary artery systems for 50% left main trunk stenosis.
Temporal Trends
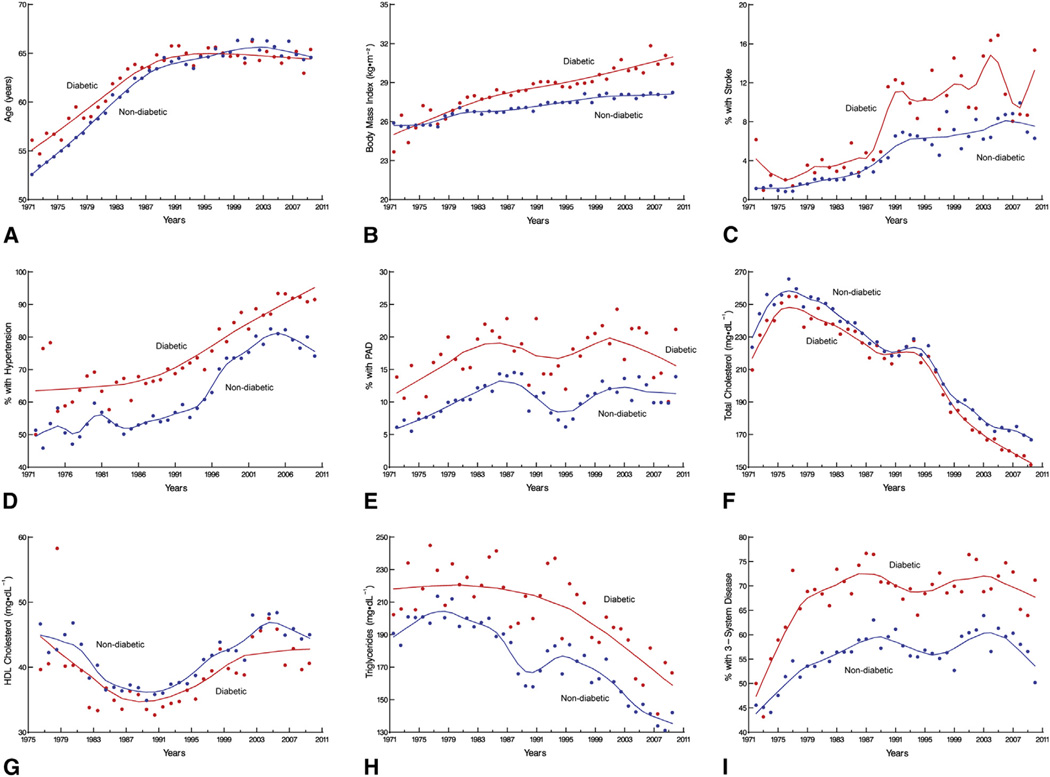
The proportion of patients presenting for CABG who have diabetes increased from 7% per year in the 1970s to 37% in the 2000s (Central Image). The cardiovascular risk factor profile also changed during this time, more so for diabetics than nondiabetics. Today, patients are likely to be older (Figure 1, A) and obese (Figure 1, B); to have had a stroke (Figure 1, C); and to have hypertension (Figure 1, D), peripheral arterial disease (Figure 1, E), lower total cholesterol (Figure 1, F), higher high-density lipoprotein cholesterol (Figure 1, G), lower triglycerides (Figure 1, H), and more-advanced coronary artery disease (Figure 1, I) (see Table E1).
FIGURE 1.
Four-decade trends in prevalence of diabetes and cardiovascular risk factors among patients undergoing primary isolated coronary artery bypass grafting. Each circle is a yearly percentage or mean value, and solid lines are loess estimates. The factors shown are: (A) age; (B) body mass index; (C) stroke; (D) hypertension; (E) PAD; (F) total cholesterol; (G) HDL cholesterol; (H) triglycerides; and (I) percentage with 3-system disease. PAD, Peripheral arterial disease; HDL, high-density lipoprotein.
Overall Outcomes
In-hospital adverse outcomes, and resource utilization and direct technical costs
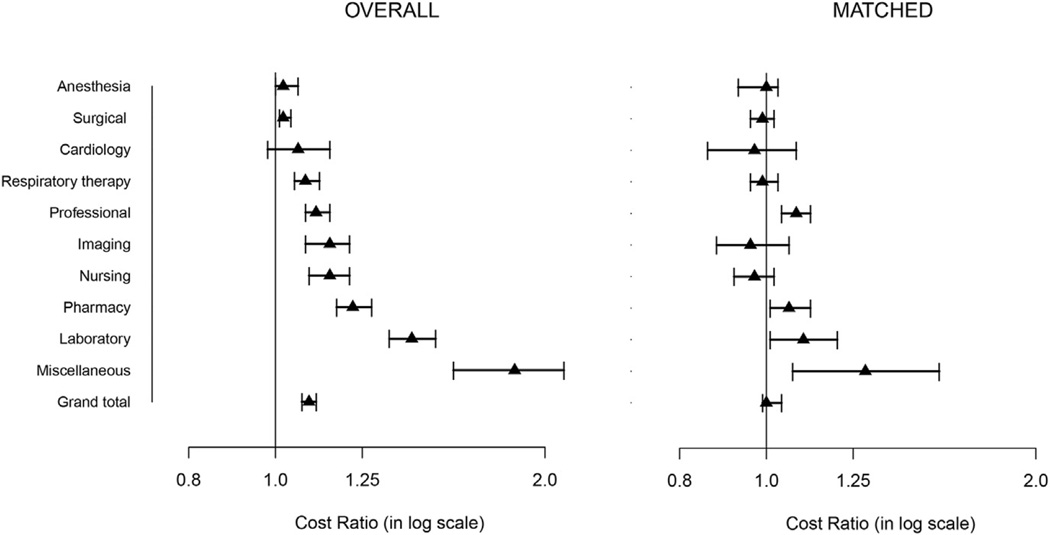
Diabetics had higher in-hospital mortality and greater occurrence of deep sternal wound infection, stroke, atrial fibrillation, renal failure, and respiratory failure (Table 2). Hours spent in the intensive care unit and of length of stay > 14 days were higher in diabetics than nondiabetics (Table 2). As a result, the total cost of CABG was 9% greater (95% CI, 7%–11%) in diabetics. Most of this difference was due to higher costs of clinical and laboratory testing, diagnostic imaging, pharmacy services, and nursing care (Figure 2).
TABLE 2.
In-hospital outcomes after coronary artery bypass grafting: overall and propensity matched
| Overall | Propensity matched | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nondiabetic (total n = 45,139) |
Diabetic (total n = 10,362) |
Nondiabetic (total n = 8926) |
Diabetic (total n = 8926) |
|||||||
| Outcome | n | No. (%) | n | No. (%) | P value | n | No. (%) | n | No. (%) | P value |
| Hospital death | 45,139 | 566 (1.3) | 10,362 | 211 (2.0) | <.001 | 8926 | 152 (1.7) | 8926 | 174 (1.9) | .2 |
| Deep sternal wound infection | 45,139 | 526 (1.2) | 10,362 | 239 (2.3) | <.001 | 8926 | 116 (1.3) | 8926 | 197 (2.2) | <.001 |
| Septicemia | 14,298 | 226 (1.6) | 6633 | 151 (2.3) | .004 | 6393 | 139 (2.2) | 5352 | 113 (2.1) | .8 |
| Stroke | 45,139 | 640 (1.4) | 10,362 | 233 (2.2) | <.001 | 8926 | 134 (1.5) | 8926 | 200 (2.2) | <.001 |
| Perioperative MI | 45,139 | 1023 (2.3) | 10,362 | 135 (1.3) | <.001 | 8926 | 118 (1.3) | 8926 | 123 (1.4) | .8 |
| Bleeding or tamponade | 45,139 | 1791 (4.0) | 10,362 | 348 (3.4) | .004 | 8926 | 271 (3.0) | 8926 | 314 (3.5) | .07 |
| Atrial fibrillation | 45,139 | 5148 (12) | 10,362 | 1979 (19) | <.001 | 8926 | 1914 (21) | 8926 | 1672 (19) | <.001 |
| Renal failure | 45,139 | 569 (1.3) | 10,362 | 418 (4.0) | <.001 | 8926 | 258 (2.9) | 8926 | 285 (3.2) | .2 |
| Renal failure requiring dialysis | 14,298 | 104 (0.73) | 6633 | 86 (1.3) | <.001 | 6393 | 79 (1.2) | 5352 | 60 (1.1) | .6 |
| Prolonged ventilation (>24 h) | 3492 | 320 (9.2) | 2100 | 249 (12) | .001 | 1838 | 212 (12) | 1563 | 160 (10) | .2 |
| Length of stay* | ||||||||||
| ICU (h) | 14,296 | 24/24/72 | 631 | 24/26/95 | <.001 | 6391 | 24/24/75 | 5351 | 24/24/76 | <.001 |
| Postoperative (d) | 44,014 | 6.1/8.0/11 | 10,263 | 5.9/7.9/12 | <.001 | 8864 | 5.2/7.0/11 | 8829 | 5.9/7.9/11 | <.001 |
| Hospital (d) | 44,014 | 7/11/17 | 10,263 | 6.3/10/18 | <.001 | 8864 | 6.3/9.3/16 | 8829 | 6.3/10/17 | <.001 |
| Prolonged (>14 d) | 45,139 | 2688 (6.0) | 10,362 | 995 (9.6) | <.001 | 8926 | 679 (7.6) | 8926 | 785 (8.8) | .004 |
Values in “n” columns are numbers of patients with data available. P values are given for the median score test. MI, Myocardial infarction; ICU, intensive care unit.
15th/50th/85th percentiles. Median score test was used to compare medians and Wilcoxon rank-sum test to compare tails of distributions.
FIGURE 2.
Median (triangles) ratio of total direct technical costs (overall and propensity matched) in diabetics versus nondiabetics. Error bars are 95% confidence intervals.
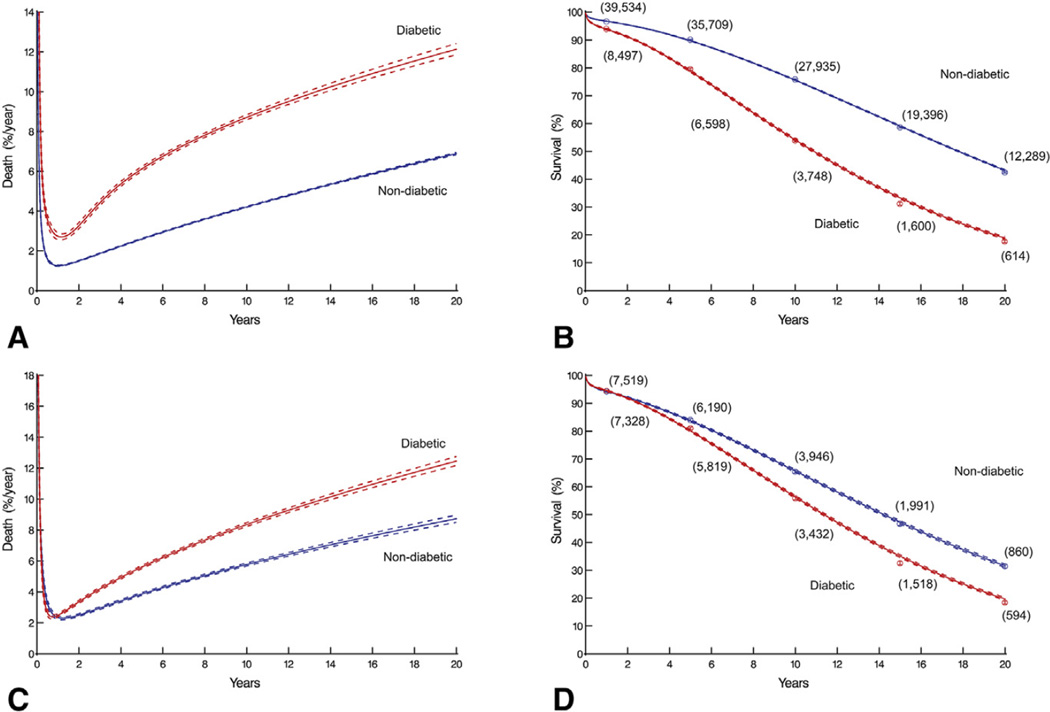
Long-term survival
The instantaneous risk of death was high immediately after CABG, decreased over the ensuing 6 months, and then gradually increased for both diabetics and nondiabetics (Figure 3, A), resulting in early divergence of their survival curves (Figure 3, B). In addition, late hazard was elevated in diabetics; thus, ever-increasing divergence of survival was observed out to at least 20 years. Among diabetics, overall survival at 6 months, 1, 5, 10, 15, and 20 years after CABG was 95%, 94%, 80%, 54%, 31%, and 18%, respectively. In contrast, for nondiabetics, it was 97%, 97%, 90%, 76%, 59%, and 42%, respectively (P < .0001).
FIGURE 3.
Time-related death after primary isolated coronary artery bypass grafting in diabetics and nondiabetics. Solid lines are parametric estimates enclosed within dashed 68% confidence bands equivalent to ±1 SE. The panels show: (A) instantaneous risk of death (overall); (B) survival (overall); (C) instantaneous risk of death (propensity-matched cohort); and (D) survival (propensity-matched cohort). Each symbol represents a death; vertical bars are confidence limits equivalent to ±1 SE; and values in parentheses are numbers of patients remaining at risk.
Comparison of Diabetics with Similar High-Risk Nondiabetics
In-hospital adverse outcomes, and resource utilization and direct technical costs
After matching, the incidence of deep sternal wound infection and stroke remained significantly higher among diabetics (Table 2). After propensity matching, no significant difference remained in total cost of CABG between diabetics and nondiabetics (Figure 2). Hours spent in the intensive care unit were similar, but length of stay > 14 days remained higher for diabetics (Table 2).
Long-term survival
Among propensity-matched patients, instantaneous risk of death (hazard function) was similar for both diabetics and nondiabetics until 1 year after surgery, after which risk of death was greater for diabetics (Figure 3, C). Early survival was similar between the 2 groups, but late survival was worse for diabetics (Figure 3, D). Late survival continued to diverge as long as patients were followed, because of the substantial difference in late hazard for at least 20 years after CABG. Survival of diabetics at 6 months, 1, 5, 10, 15, and 20 years after operation was 96%, 94%, 80%, 56%, 35%, and 20%, respectively, versus 96%, 94%, 84%, 66%, 47%, and 32% for nondiabetics.
DISCUSSION
Principal Findings
This study shows that the proportion of patients presenting for CABG who have diabetes increased each year during the past 4 decades, as did the proportion with cardiovascular risk factors. Thus, compared with diabetics undergoing operation in the 1970s, 1980s, and 1990s, those operated on more recently were more likely to be obese and have more comorbidities and advanced coronary artery disease. For diabetics, CABG was more resource intensive and expensive, and in-hospital adverse events and long-term survival were worse. However, the increase in in-hospital resource utilization was not specific to diabetics, but rather commensurate with that of patients coming to surgery with a similar extent of comorbidities, but without diabetes. Unadjusted in-hospital and early mortality (1-year) were higher in diabetics than in nondiabetics, but similar for propensity-matched patients with a similar comorbidity profile. Long-term survival was worse in diabetics than in either nondiabetic patients or matched, nondiabetic, high-risk patients. Thus, diabetes is both a marker for highrisk, resource-intensive, and expensive care after CABG, and an independent risk factor for reduced long-term survival.
Trends
In 2010, nearly 40% of those undergoing CABG at our institution were diabetic, paralleling the rising prevalence of diabetes in the general population. However, the increasing use of percutaneous coronary intervention for revascularization, and the choice of CABG as the preferred revascularization strategy for diabetics, could also have been contributing factors.16 Although cardiovascular disease–related morbidity and mortality has clearly been reduced in the United States over the past 50 years, the cardiovascular disease burden attributable to diabetes has increased.5 Current estimates are that 18.8 million people in the United States have been diagnosed with diabetes, and 7 million remain undiagnosed.2 In addition, nearly 79 million people aged ≥20 years have prediabetes,2 a condition that places them at increased risk of developing diabetes and cardiovascular disease.17
We also observed a change in the cardiovascular risk-factor profile over time. Diabetics undergoing CABG in recent years are more likely to be obese and to have more-advanced coronary artery disease than those operated on in the 1970s, 1980s, and 1990s. In addition, they are more likely to have hypertension, one component of metabolic syndrome, together with diabetes, hyperlipidemia, and obesity—all risk factors for coronary artery disease.18 Obesity is now considered a national epidemic and is of particular importance as a well-recognized contributor to diabetes. More than one third of US adults are obese,19 and in the next 20 years, obesity may play a contributing role in an estimated 6 million cases of diabetes.20 On the other hand, diabetics undergoing CABG in recent years had lower total cholesterol, higher high-density lipoprotein cholesterol, and lower triglycerides than did patients operated on in earlier years; this difference may be attributable to better control of lipids in the statin era.
In-Hospital Adverse Outcomes
In-hospital adverse outcomes after CABG were more common in diabetics than nondiabetics. In part, this difference is attributable to diabetic patients being sicker and having more comorbidities than nondiabetics, because some of the differences, including hospital death, septicemia, renal failure, and respiratory failure, became statistically insignificant after comparison with similar high-risk nondiabetic patients through propensity matching. However, occurrence of deep sternal wound infection and stroke remained significantly higher in diabetics even after matching. Deep sternal wound infection results in prolonged postoperative length of stay and thus increases hospital resource utilization. Strokes may cause permanent disability, resulting in unemployment and thus increasing the indirect cost of diabetes through loss of productivity.
Other studies have also revealed worse hospital and long-term outcomes of CABG in diabetics.21,22 The SYNTAX trial showed that at 3 years, diabetes had little effect on outcomes of CABG, and diabetes control (as indicated by baseline hemoglobin A1c levels) was not predictive of major adverse cardiac and cerebrovascular events. In our study, overall postoperative prevalence of stroke and in-hospital death was higher in diabetics, and occurrence of myocardial infarction was higher in nondiabetics. However, after comparison with similar, high-risk, nondiabetic patients, occurrence of death and myocardial infarction was similar in the 2 groups, as was true in the SYNTAX trial, but stroke remained higher in diabetics.23
Health Care Costs
Our study additionally shows that resource utilization and the actual direct technical cost of CABG were greater in diabetics, mainly because of longer intensive care unit and postoperative stays, and higher costs of clinical and laboratory testing, diagnostic imaging, pharmacy services, and nursing care. Greater severity of disease among diabetics necessitates preoperative admission and more-extensive laboratory and diagnostic workup. Furthermore, managing postoperative adverse events and the resulting increase in postoperative length of stay both lead to higher in-hospital costs.
After using propensity matching to compare similar high-risk, nondiabetic patients, no significant difference between the total cost of CABG in diabetics versus nondiabetics with a similar high-risk profile was observed, demonstrating that most of the increased cost was due to diabetics being sicker and having more comorbidities. In 2012, $176 billion was spent on direct health care for diabetics in the United States, with in-hospital care representing 43% of that amount.3 Because heart disease is one of the leading causes of hospitalization in diabetics, a large share of in-hospital costs can be attributed to CABG and its postoperative complications.
Others have also demonstrated the association of diabetes with increased cost of CABG.24,25 A study of 114 diabetics and 198 nondiabetics showed that insulin-treated diabetics have longer hospital stays and higher hospital charges than non–insulin-treated diabetics and nondiabetics.24 A recently published study from China also showed that CABG was more costly, with worse long-term results, in diabetics than in nondiabetics.26
Survival
Diabetic patients as a group had a higher early (1-year) risk of death after CABG than nondiabetics, as has been documented by others.21 However, an interesting finding of our study is that among propensity-matched patients, early risk was similar to that of nondiabetic high-risk patients with a similar comorbidity profile. Long-term survival, however, was worse in diabetics than in both nondiabetic patients and nondiabetic high-risk patients. Other studies have also demonstrated diabetes to be an independent risk factor for reduced long-term survival after CABG.21,26 Although long-term survival after CABG is worse in diabetics and high-risk nondiabetics, in general, high-risk patients reap the greatest survival benefit from CABG.27 Moreover, using surgical techniques that are associated with better long-term survival after CABG in diabetics could further enhance this survival benefit.28
Diabetes: An Avoidable Economic Burden
Diabetes is a growing threat to the US economy. Diabetic patients’ medical expenses are nearly 2.3 times higher than those of nondiabetics,3 and this economic burden is expected to increase as the number of diabetes cases rises. Fortunately, however, this situation is largely preventable. In people with prediabetes, cost-effective lifestyle interventions have been shown to have a positive effect on preventing development of the disease.29–31 In people with diagnosed diabetes, controlling blood sugar and cardiovascular disease risk factors, such as hypertension and hypercholesterolemia, has been shown to help reduce cardiovascular events.32,33 With appropriate use of these approaches, we can help prevent development of diabetes and its complications, reducing the diabetes-related economic burden.
Strengths and Limitations
This study includes 4 decades of patients who underwent CABG at a single, high-volume academic medical center. An advantage of a long observation period is having a long follow-up period, but generalizing these late results to a contemporary patient population may not accurately reflect the changing patient case mix and advances in managing these patients over time. For adjusted comparison of outcomes, propensity-score matching was performed. Although the patient pairs were well matched, any patient factors that significantly affect outcomes after CABG but were not included in the propensity analysis might bias our adjusted results. In addition, circumstances of each death, which may differ among diabetic and nondiabetic patients, were not reliably captured during follow-up inquiries.
CONCLUSIONS
Diabetes is both a marker for high-risk, resource-intensive, and expensive care after CABG and an independent risk factor for reduced long-term survival. These issues, coupled with the annually increasing number of patients needing CABG who are diabetic, present a growing challenge to reining in health care costs, both in the United States and internationally. Diabetic patients, and those with a similar high-risk profile, set to undergo CABG should be made aware that their risks of postoperative complications are higher than average, and measures should be taken to reduce their postoperative complications. Moreover, trying to reverse the diabetes epidemic is important; if left uncontrolled, it will increase the prevalence of cardiovascular disease and add to the ever-increasing economic health care burden. Research and policies focused on reducing diabetes, and programs that raise awareness about preventive strategies, should be developed and implemented to check rising health care costs.
Supplementary Material
Central Message.
Increasingly, patients needing CABG have diabetes, a marker for high-risk, resource-intensive, expensive care after CABG, and an independent risk factor for reduced long-term survival, creating a growing challenge to health care cost reduction.
Perspective.
The proportion of patients needing CABG who have diabetes has increased to nearly 40% of all patients undergoing CABG at our institution. The procedure is more resource intensive and expensive for diabetics than for nondiabetics, partly because of postoperative complications. However, when surgeons present the risks and benefits of CABG to diabetic patients, they should explain that CABG offers the best chance for long-term survival.
Acknowledgments
This study was funded in part by the Sheikh Hamdan bin Rashid Al Maktoum Distinguished Chair in Thoracic and Cardiovascular Surgery (held by JFS), and the Kenneth Gee and Paula Shaw, PhD, Chair in Heart Research (held by EHB). These philanthropists played no role in the design of the study, collection of data, analysis and interpretation of the data, or writing of the report, and did not approve or disapprove publication of the article.
Abbreviation and Acronym
- CABG
coronary artery bypass grafting
Footnotes
Supplemental material is available online.
Conflict of Interest Statement
Dr Sabik is the North American principal investigator for the Abbott Laboratories–sponsored left main coronary disease randomized trial (EXCEL), is on the Society of Thoracic Surgeons Board of Directors, and is on the scientific advisory board of Medtronic. All other authors have nothing to disclose with regard to commercial support.
References
- 1.International Diabetes Federation. IDF diabetes atlas. Available at: http://www.idf.org/diabetesatlas. [Google Scholar]
- 2.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Atlanta: US Department of Health and Human Services; 2011. [Google Scholar]
- 3.Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 5.Fox CS, Coady S, Sorlie PD, D’Agostino RB, Sr, Pencina MJ, Vasan RS, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115:1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 6.US Department of Labor, Bureau of Labor Statistics. CPI inflation calculator. Available at: http://www.bls.gov/data/inflation_calculator.htm.
- 7.Boyle CA, Decoufle P. National sources of vital status information: extent of coverage and possible selectivity in reporting. Am J Epidemiol. 1990;131:160–168. doi: 10.1093/oxfordjournals.aje.a115470. [DOI] [PubMed] [Google Scholar]
- 8.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman and Hall/CRC; 1998. [Google Scholar]
- 9.Blackstone EH, Naftel DC, Turner ME., Jr The decomposition of time-varying hazard into phases, each incorporating a separate stream of concomitant information. J Am Stat Assoc. 1986;81:615–624. [Google Scholar]
- 10.Pattakos G, Koch CG, Brizzio ME, Batizy LH, Sabik JF, III, Blackstone EH, et al. Outcome of patients who refuse transfusion after cardiac surgery: a natural experiment with severe blood conservation. Arch Intern Med. 2012;172:1154–1160. doi: 10.1001/archinternmed.2012.2449. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 12.Rubin DB. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med. 2007;26:20–36. doi: 10.1002/sim.2739. [DOI] [PubMed] [Google Scholar]
- 13.Sauerbrei W, Schumacher M. A bootstrap resampling procedure for model building: application to the Cox regression model. Stat Med. 1992;11:2093–2109. doi: 10.1002/sim.4780111607. [DOI] [PubMed] [Google Scholar]
- 14.Breiman L. Bagging predictors. Machine Learning. 1996;24:123–140. [Google Scholar]
- 15.Rubin DB. Multiple Imputation for Non-Response in Surveys. New York: Wiley; 1987. [Google Scholar]
- 16.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635–643. doi: 10.1016/j.jacc.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 18.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 19.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief No 82. 2012 Jan; Available at: http://www.cdc.gov/nchs/data/databriefs/db82.pdf. [PubMed]
- 20.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 21.Thourani VH, Weintraub WS, Stein B, Gebhart SS, Craver JM, Jones EL, et al. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg. 1999;67:1045–1052. doi: 10.1016/s0003-4975(99)00143-5. [DOI] [PubMed] [Google Scholar]
- 22.Barzilay JI, Kronmal RA, Bittner V, Eaker E, Evans C, Foster ED. Coronary artery disease and coronary artery bypass grafting in diabetic patients aged > or = 65 years (report from the Coronary Artery Surgery Study [CASS] Registry) Am J Cardiol. 1994;74:334–339. doi: 10.1016/0002-9149(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 23.Mack MJ, Banning AP, Serruys PW, Morice MC, Taeymans Y, Van Nooten G, et al. Bypass versus drug-eluting stents at three years in SYNTAX patients with diabetes mellitus or metabolic syndrome. Ann Thorac Surg. 2011;92:2140–2146. doi: 10.1016/j.athoracsur.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Stewart RD, Lahey SJ, Levitsky S, Sanchez C, Campos CT. Clinical and economic impact of diabetes following coronary artery bypass. J Surg Res. 1998;76:124–130. doi: 10.1006/jsre.1998.5306. [DOI] [PubMed] [Google Scholar]
- 25.Smith LR, Milano CA, Molter BS, Elbeery JR, Sabiston DC, Jr, Smith PK. Preoperative determinants of postoperative costs associated with coronary artery bypass graft surgery. Circulation. 1994;90:II124–II128. [PubMed] [Google Scholar]
- 26.Zhang H, Yuan X, Osnabrugge RL, Meng D, Gao H, Zhang S, et al. Influence of diabetes mellitus on long-term clinical and economic outcomes after coronary artery bypass grafting. Ann Thorac Surg. 2014;97:2073–2079. doi: 10.1016/j.athoracsur.2014.02.047. [DOI] [PubMed] [Google Scholar]
- 27.Hlatky MA, Boothroyd DB, Baker L, Kazi DS, Solomon MD, Chang TI, et al. Comparative effectiveness of multivessel coronary bypass surgery and multivessel percutaneous coronary intervention: a cohort study. Ann Intern Med. 2013;158:727–734. doi: 10.7326/0003-4819-158-10-201305210-00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raza S, Sabik JF, Masabni K, Ainkaran P, Lytle BW, Blackstone EH. Surgical revascularization techniques that minimize surgical risk and maximize late survival after coronary artery bypass grafting in diabetics. J Thorac Cardiovasc Surg. 2014;148:1257.e9–1266.e9. doi: 10.1016/j.jtcvs.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 29.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 31.Herman WH, Hoerger TJ, Brandle M, Hicks K, Sorensen S, Zhang P, et al. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med. 2005;142:323–332. doi: 10.7326/0003-4819-142-5-200503010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang ES, Meigs JB, Singer DE. The effect of interventions to prevent cardiovascular disease in patients with type 2 diabetes mellitus. Am J Med. 2001;111:633–642. doi: 10.1016/s0002-9343(01)00978-0. [DOI] [PubMed] [Google Scholar]
- 33.Mannucci E, Dicembrini I, Lauria A, Pozzilli P. Is glucose control important for prevention of cardiovascular disease in diabetes? Diabetes Care. 2013;36(Suppl 2):S259–S263. doi: 10.2337/dcS13-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.