Abstract
Background
In spite of significant improvement after multi-modality treatment, prognosis of most patients with glioblastoma remains poor. Standard clinical prognostic factors (age, gender, extent of surgery and performance status) do not clearly predict long-term survival. The aim of this case-control study was to evaluate immuno-histochemical and genetic characteristics of the tumour as additional prognostic factors in glioblastoma.
Patients and methods
Long-term survivor group were 40 patients with glioblastoma with survival longer than 30 months. Control group were 40 patients with shorter survival and matched to the long-term survivor group according to the clinical prognostic factors. All patients underwent multimodality treatment with surgery, postoperative conformal radiotherapy and temozolomide during and after radiotherapy. Biopsy samples were tested for the methylation of MGMT promoter (with methylation specific polymerase chain reaction), IDH1 (with immunohistochemistry), IDH2, CDKN2A and CDKN2B (with multiplex ligation-dependent probe amplification), and 1p and 19q mutations (with fluorescent in situ hybridization).
Results
Methylation of MGMT promoter was found in 95% and in 36% in the long-term survivor and control groups, respectively (p < 0.001). IDH1 R132H mutated patients had a non-significant lower risk of dying from glioblastoma (p = 0.437), in comparison to patients without this mutation. Other mutations were rare, with no significant difference between the two groups.
Conclusions
Molecular and genetic testing offers additional prognostic and predictive information for patients with glioblastoma. The most important finding of our analysis is that in the absence of MGMT promoter methylation, longterm survival is very rare. For patients without this mutation, alternative treatments should be explored.
Key words: glioblastoma, long-term survival, methyl guanine methyl transferase, prognostic factor
Introduction
Treatment with surgery, conformal radiotherapy and chemotherapy led to modest improvement in the prognosis of patients with glioblastoma (GBM). In the past, surgery alone or combined with less precise techniques of radiotherapy led to median survival of 12 months and only about 20% of patients survived beyond 2 years.1,2 After introduction of modern tri-modality treatment, median survival extended to 14 months.3,4 For the majority of patients, however, the prognosis remains poor and long-term survival beyond 3 years after diagnosis is still rare.5
Several clinical characteristics have been confirmed as independent prognostic factors. Worse survival has been associated with advanced age, poor performance status and incomplete surgical resection of the tumour.6-8 Regarding post-surgical treatment, over 8 weeks of delay with postoperative radiotherapy and omission of postoperative chemotherapy had negative impact upon survival, as well.9-12
Study of the prognostic importance of immunohistochemical and genetic characteristics of brain tumours is a relatively recent approach. In low-grade gliomas, mutation of enzyme izocitratedehidrogenaze1 (IDH1) has been found in more than 60% of cases.13 Patients with mutated IDH1 fare better than those who have IDH1 wild type.14,15 In anaplastic oligodendroglioma, co-deletion of 1p and 19q chromosomes conferred a significant benefit when compared to patients without this mutation.16 In GBM, mutation of IDH1 is far rarer than in lower grade gliomas and the prognostic importance of this mutation has not been confirmed.17-19 However, GBMs often gain methylation of the promoter of methyl guanine methyl transferase (MGMT), an enzyme otherwise un-methylated and as such has been found to diminished its influence on temozolomide therapeutic function and ultimately improve patient survival repair alkylation caused by temozolomide. Some studies have used MGMT methylation status as a stratification tool.20-23
The genes cyclin-dependent kinase inhibitor 2A (CDKN2A) and CDKN2B, are sometimes used as a poor prognosis marker in various neoplasms, and the decreased expression of these genes, has been shown to correlate with the poor prognosis.24 It has been proposed, that as CDKN2A is present in paediatric low grade gliomas, recurring as a high grade one of prognostic factors is CDKN2A deletion and that probably patients expressing could be treated differently at the first presentation.25
This study focused on some genetic characteristics of the GBMs and used to test the hypothesis that long-term survival is in association with these genetic alterations. We compare GBM patients with survival beyond 2.5 years with a control group matched by age, extent of surgery, performance status and therapy, but with standard median survival of GBM.
Patients and methods
Selection of patients
In Slovenia, surgery for brain tumours is done in Departments for Neurosurgery of the University Clinical Centre in Ljubljana and in Maribor. In case of indication for radiotherapy and/or chemotherapy, patients are referred to the Institute of Oncology Ljubljana.
The pool of patients covered by this analysis comprises all cases with biopsy-proven GBM and treated at the Institute of Oncology in Ljubljana between 1997 and 2011. Initial analysis included data on demographics, extent of surgery, performance status, post-surgical treatment, time to progression and survival. Follow-up for progression and update on survival were completed on September 2014.
From this series of patients, two groups of patients were selected for detailed immunohistochemical and molecular genetic analysis. All tumours were initially classified and graded according to current WHO 2007 classification of the tumours of the central nervous system.26 Long-term survival (LTS) group were patients with survival beyond 30 months. Control group were patients matched to the LTS group according to age, extent of surgery, performance status, radio/chemo therapy and with survival shorter than 30 months.
Immunohistochemical and genetic tissue analysis
From selected paraffin blocks of GBMs with LTS and control groups the samples were taken and tissue microarray (TMA) was made in which the sample of each tissue was 2 mm thick in diameter. After sampling the tissue for TAM 10 sections of 5-μm thickness from each block we additionally cut for molecular epigenetic and genetic analysis for MGMT promoter methylation, IDH1 and IDH2 mutations, presence of 1p/19q co-deletion and CDKN2A, CDKN2Bmutations.
Immunohistochemistry was applied on 4-μm thick sections of TAM to detect IDH1 R132H mutation using mouse MAb clone H09, diluted 1:50 (Dianova, Hamburg, Germany) on a Bench Mark XT immunostainer (Ventana Medical Systems, Tuscon, AZ, USA).
DNA isolation
Tissue samples were cut at 10 μm from formalin-fixed paraffin-embedded tissue blocks and for the isolation procedure, six to eight 10 μm sections were used. Total DNA isolation was performed using QIAamp DNA FFPE Tissue Kit (Qiagen) according to the manufacturer’s protocol. The DNA was eluted in 60 μl of nuclease-free water. The yield was measured fluorescently using Quant-It (Life Technologies) according to manufacturer instruction and Rotor Gene Q (Qiagen).27-29
MGMT methylation detection
For MGMT methylation detection, methyl-specific polymerase chain reaction (MSP) was used in a two-step approach with primers previously described.28 Briefly, prior to MSP, 500 ng of DNA was used for bisulfite conversion using innuCONVERT Bisulfite Basic Kit according to manufacturer instruction (Analytik Jena) and stored at -20° for subsequent MSP. For MSP, 15 ng of bisulfite converted DNA was used with 0.2 μM of each primer for methylated form and 0.3 μM of primer for unmethylated form, 2 mM of dNTP and 0.25 U of Hot Master Polymerase (5 Prime) in 10 μl reaction. Amplification was performed according to manufacturer instruction using 59 °C for primer annealing. In each run, fully methylated (EpiTect Control DNA, methylated, Qiagen) as well as fully unmethylated controls (EpiTect Control DNA, unmethlyated, Qiagen) were used as assay controls. Results were analysed using 2% agarose gel. The investigator who analysed the glioma samples were blinded to all clinical information.
Multiplex ligation-dependent probe amplification (MLPA) analysis was used to detect copy number changes of multiple loci simultaneously (http://www.mlpa.com) and all assays used were prepared by MRC-Holland (Amsterdam, the Netherlands). MLPA assay P088-C1 was used to detect complete or partial losses involving chromosome 1p (19 probes), 19q (11 probes) and genes CDKN2A (3 probes), CDKN2B (2 probes) and identification of the most common IDH1 (R132H, R132C) and IDH2 (R172K, R172M) mutations. MLPA was performed as described by the manufacturer and data analysis was performed with Coffalyser software. Detection thresholds were set at 1.2 and 0.8 for the detection of low-level gains and hemizygous losses, respectively. For chromosome 1pand 19q losses, a distinction was made between complete and partial losses, the latter were defined as a ratio < 0.8 for at least 3 adjacent probes but not of all probes for these chromosome arms.30
SPSS 20 statistical package was used for statistical analysis. The prognostic importance of individual parameters for the LPS and control groups were compared with chi-square test and then with non-parametric test package. The difference of corticosteroid dose between groups at different time intervals was calculated using T-test. Cox regression was used for survival analysis.
The study was approved by Ethics and Study Protocol Assessment Committee at the Institute of Oncology Ljubljana and by the Slovenian Ethics Committee for Research in Medicine (approval ref. no. 12/07/2011) and was carried out according to the Helsinki Declaration.
Results
During the period covered by this study, 862 patients (501 male, 361 female) with GBM were treated at the Institute of Oncology in Ljubljana. Median age was 60 years (range: 18 to 86 years). From this series, 40 patients with survival beyond 30 months (LTS group), and 40 patients with shorter survival and matched according to age, extent of surgery, performance status, and therapy (Control group) were selected for further analysis.
Data on demographics and basic prognostic factors for the LTS and control groups are presented in Table 1. Regarding standard prognostic factors and treatment, no difference is seen between LTS group and control group.
Table 1.
Demographics, standard prognostic factors and treatment for long-term survivor (LTS) group and control group
| Variable | LTS group | Control group | |
|---|---|---|---|
| Mean age (range) | 47,5 (21–74) | 49, 6 (23–74) | |
| Gender | Male | 24 | 31 |
| Female | 16 | 9 | |
| Surgery | Gross total | 22 | 23 |
| Reduction | 15 | 15 | |
| Biopsy | 3 | 2 | |
| WHO PS | 0 | 9 | 5 |
| 1 | 25 | 26 | |
| 2 | 4 | 9 | |
| 3 | 2 | 0 | |
| Mean RT dose (Gy) | 58 | 57,9 | |
| RT technique | 1D | 1 | 0 |
| 2D | 3 | 3 | |
| 3D | 36 | 37 | |
| No. of fractions | 25–33 | 25–33 | |
| Chemotherapy | Yes | 37 | 39 |
| Adjuvant only | 3 | 1 |
PS = performance status; RT = radiotherapy
Table 2.
Response to primary treatment
| LTS group | Control group | P value | |
|---|---|---|---|
| Complete response* | 24 | 4 | < 0.001 |
| Partial response | 5 | 2 | n.s. |
| Stable disease | 4 | 13 | 0.013 |
| Progression | 7 | 20 | 0.002 |
| Overall response | 31/40 | 19/40 | < 0.001 |
| Non evaluable | 0 | 1 |
Includes patients reported by surgeon as gross total resection; LTS = long term survivor
Median overall survival for all 863 patients was 10 months. Median survival for the LTS group and for control group was 58 and 12 months, respectively (Figure 1).
Figure 1.
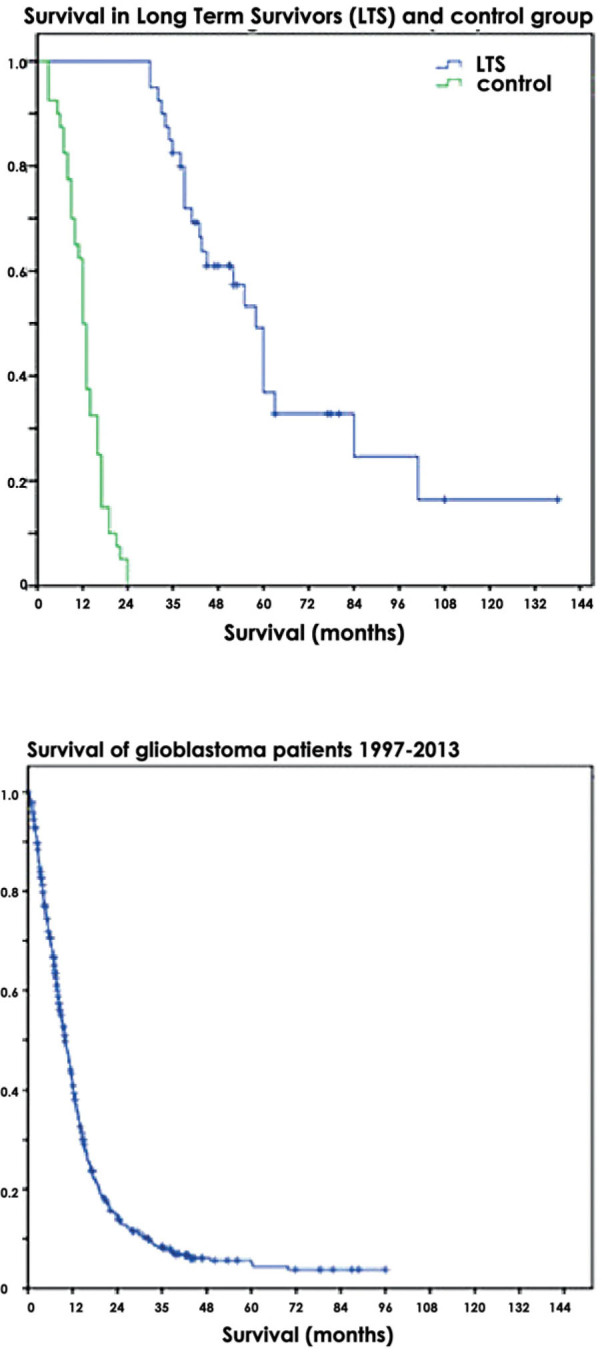
Survival of glioblastoma patients.
Results of genetic analysis
MGMT promoter methylation
Using MSP, 48 of 74 (65%) samples from patients with GBM have methylated analysed CpG islands of MGMT. One sample failed to amplify, therefore analysis was not possible, and one sample gave non-conclusive results. Kaplan-Meyer analysis revealed that overall survival was significantly longer in patients with methylated MGMT compared to those with unmethylated MGMT (43 vs. 16 months respectively, p < 0.001). Similarly, time to progression was significantly longer in patients with methylated MGMT compared to those with unmethylated MGMT (36 vs. 11 months respectively, p < 0.001).
In 2 cases in the LTS group and 7 cases in the control group, the bioptic material was not sufficient for MGMT promoter methylation analysis.
Methylation of MGMT promoter was confirmed in 36/38 patients in the LTS group and in 12 of 33 patients in the control group (chi square test: p < 0.0001) (Figure 2).
Figure 2.

Methyl guanine methyl transferase (MGMT) promoter methylation in long term survivors and control group.
IDH1 R132H mutation
Immunohistochemically IDH1 R132H mutation was identified in 6 cases in the LTS group and in 1 case in the control group (p = 0.043). The same results were achieved with genetic analysis of IDH1 R132H mutation (Figure 3).
Figure 3.
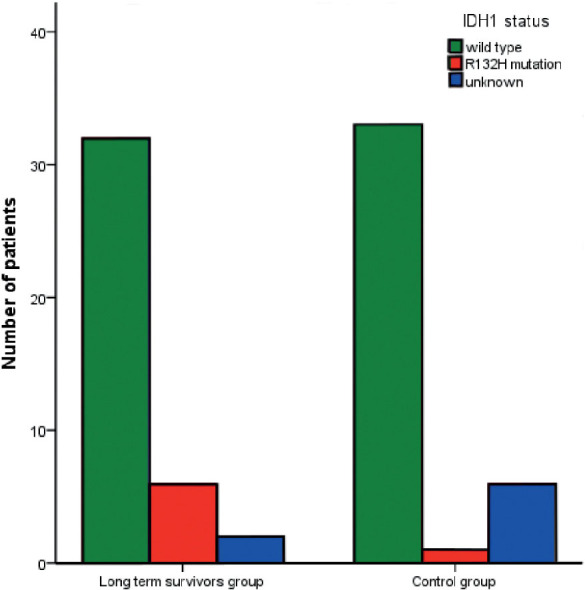
Izocitratedehidrogenaze1 (IDH1) mutations in long term survivors and control group.
All six IDH1 R132H mutations were present in MGMT methylated GBMs of LTS patients.
We calculated the risk ratio using Cox regression analysis, and in the patients with the IDH1 R132H mutation, the risk of dying of glioblastoma in the observed period was found to be 0.7 of the risk in IDH1 wild type patients. Due to the small number of patients, this was not statistically significant (p = 0.437).
IDH2 mutations
No IDH2 mutations were found.
1p/19q co-deletion
Chromosomal deletions in 1p and 19q were found in 40% of the patients in the LTS group and in 23.5% of patients in control group (p = 0.167), deletions or partial deletions on 1p or 19q were equally distributed in both groups. No significant differences were found when looking for specific deletions and duplications in single or both chromosomes. Only one patient had a 1p/19q co-deletion, thus suggesting GBM arising from previously undiagnosed oligodendroglial tumour.
CDKN2A and CDKN2B
There were no differences between the two groups in the expression of CDKN2A and CDKN2B
The frequencies of the assessed markers are summarized in the Table 3.
Table 3.
Molecular and genetic markers
| LTS group | Control group | P value | |
|---|---|---|---|
|
IDH1 (immunohistochemistry) |
6/40 | 1/34 | 0.043 |
| IDH1 (genetic) | 6/38 | 1/33 | 0.043 |
| IDH2 (genetic) | 0/38 | 0/33 | n.s. |
| 1p/19q | 0/38 | 1/34 | n.s. |
| 1p | 3/38 | 1/34 | n.s. |
| 19q | 10/38 | 6/34 | n.s. |
| MGMT methylation | 36/38 | 12/33 | < 0.001 |
| CDKN2A (deletion) | 29/39 | 25/34 | n.s. |
| CDKN2B (deletion) | 27/39 | 24/34 | n.s. |
IDH = izocitratedehidrogenaze; LTS = long term survivor; MGMT = methyl guanine methyl transferase; n.s. = non-significant
Discussion
For any malignancy, prognostic factors are essential when comparing different treatments within a randomised clinical trial or among several separate reports. In addition, predictive factors are helpful in assessing susceptibility to a particular treatment. In combination, identification of prognostic and predictive factors may define categories of patients for whom alternative treatments should be explored.
Regarding glioblastoma, the widely recognized favourable prognostic factors for longer survival are younger age, gross total surgical resection of the tumour, good performance status and initiation of postoperative radiotherapy within 2 months after surgery. Patients over 70 years of age have a median survival of just half a year regardless of the treatment, and the majority of those over 75 don’t even reach the end of initial radiotherapy. On the other hand, average survival of patients under 50 exceeds 2 years, as recently reported by our group4 and by several other authors.31-33 Type of surgery is also important: median survival is three months shorter for patients after biopsy, when compared with those after maximal safe resection.34,35 Performance status after surgery plays a major role in a patient’s survival: patients unable to perform daily chores have a median survival of around 3 months.36-38 Finally, shorter survival has been reported for patients who start radiotherapy after a delay of more than 6 to 8 weeks after surgery.9
To ad those known factors into the frame, which can help clinician to decide on the optimal treatment of the glioblastoma patients, recursive partitioning analysis (RPA) of those factors has been performed. Patients are then stratified to RPA classes which closely correspond to median survival.39-41
Still, in spite of significant improvement of median survival after tri-modality treatment, the prognosis for most patients remains grim.42,43
Our study is an attempt to assess an epigenetic and several genetic characteristics of the tumour as prognostic and predictive factors in glioblastoma. While long-term survivors with this disease are indeed a minority, some patients do survive beyond three years. A group of patients with LTS was therefore compared with a control group of patients with shorter survival. Since the two groups were balanced according to classical prognostic factors, novel prognostic factors would emerge.
Among molecular prognostic factors, MGMT methylation has been the most widely studied.44-46 MGMT has been proposed as a major factor determining prognosis and also predicting response to temozolomide-based chemotherapy. An overall survival benefit in MGMT promoter methylated patients, even among those treated only with radiotherapy, points to its role as a prognostic factor.47 Kim with co-workers compared patients with MGMT un-methylated tumours those with MGMT methylated tumours and reported median survival of 20 and 29 months, and 2-year survival of 31% and 54%, respectively.48
The most important finding of our study is that MGMT promoter methylation was detected in 95% of patients with LTS, and only in 36% of patients in the control group. The difference is highly significant. On the basis of our data, it seems that with the current treatment, MGMT promoter methylation is a condition without which glioblastoma patients with LTS are rarely seen.
In our analysis, methylated MGMT promoter was confirmed in 36/38 and in 12/33 cases for the LTS and control groups, respectively. It appears that without MGMT promoter methylation, LTS unlikely. Even more importantly, an un-methylated genotype is an ominous portent for the majority of patients.
IDH1 mutations are also significantly more common among patients surviving beyond two and half years. IDH1 mutations were present only in the MGMT promoter methylated patients, As IDH1 mutations in glioblastoma are rare and their independent prognostic impact is still unknown, we would not recommend routine IDH1 testing in all glioblastoma patients.49
Treatment of elderly patients with glioblastoma remains a difficult challenge. Recently, it was proposed that elderly patients should be offered treatment, based primarily on the MGMT promoter methylation status: radiotherapy alone for un-methylated and temozolomide or accelerated radiotherapy with temozolomide for patients with MGMT methylated tumours.50 In our survey, age was not a factor when considering patients for conventional tri-modality treatment and we do have some patients with LTS also among the elderly. In our opinion, an elderly patient in good performance status should be offered conventional tri-modality treatment, regardless of MGMT promoter methylation status.
Conclusions
Molecular and genetic testing offers additional prognostic and predictive information for patients with glioblastoma. The most important finding of our analysis is that in the absence of MGMT promoter methylation, long-term survival is very rare. For patients without this mutation, alternative treatments should be explored.
Disclosure: No potential conflicts of interest were disclosed.
References
- 1.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–23. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 2.Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003;30(Suppl 19):10–4. doi: 10.1053/j.seminoncol.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Smrdel U, Kovac V, Popovic M, Zwitter M. Glioblastoma patients in Slovenia from 1997 to 2008. Radiol Oncol. 2014;48:72–9. doi: 10.2478/raon-2014-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartmann C, Hentschel B, Simon M. Long-term survival in primary glioblastoma with versus without isocitrate dehydrogenase mutations. Clin Cancer Res. 2013;19:5146–57. doi: 10.1158/1078-0432.CCR-13-0017. [DOI] [PubMed] [Google Scholar]
- 6.Grabowski MM, Recinos PF, Nowacki AS, Schroeder JL, Angelov L, Barnett GH. et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121:1115–23. doi: 10.3171/2014.7.JNS132449. [DOI] [PubMed] [Google Scholar]
- 7.Chaichana KL, Cabrera-Aldana EE, Jusue-Torres I, Wijesekera O, Olivi A, Rahman M. et al. When gross total resection of a glioblastoma is possible, how much resection should be achieved? World Neurosurg. 2014;82:e257–65. doi: 10.1016/j.wneu.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Vecht CJ, Avezaat CJ, van Putten WL, Eijkenboom WM, Stefanko SZ. The influence of the extent of surgery on the neurological function and survival in malignant glioma. A retrospective analysis in 243 patients. J Neurol Neurosurg Psychiatry. 1990;53:466–71. doi: 10.1136/jnnp.53.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valduvieco I, Verger E, Bruna J, Caral L, Pujol T, Ribalta T. et al. Impact of radiotherapy delay on survival in glioblastoma. Clin Transl Oncol. 2013;15:278–82. doi: 10.1007/s12094-012-0916-x. [DOI] [PubMed] [Google Scholar]
- 10.Sun MZ, Oh T, Ivan ME, Clark AJ, Safaee M, Sayegh ET. et al. Survival impact of time to initiation of chemoradiotherapy after resection of newly diagnosed glioblastoma. J Neurosurg. 2015;122:1144–50. doi: 10.3171/2014.9.JNS14193. [DOI] [PubMed] [Google Scholar]
- 11.Noel G, Huchet A, Feuvret L, Maire JP, Verrelle P, Le Rhun E. et al. Waiting times before initiation of radiotherapy might not affect outcomes for patients with glioblastoma: a French retrospective analysis of patients treated in the era of concomitant temozolomide and radiotherapy. J Neurooncol. 2012;109:167–75. doi: 10.1007/s11060-012-0883-7. [DOI] [PubMed] [Google Scholar]
- 12.Nava F, Tramacere I, Fittipaldo A, Bruzzone MG, Dimeco F, Fariselli L. et al. Survival effect of first- and second-line treatments for patients with primary glioblastoma: a cohort study from a prospective registry, 1997-2010. Neuro Oncol. 2014;16:719–27. doi: 10.1093/neuonc/not316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okita Y, Narita Y, Miyakita Y, Ohno M, Matsushita Y, Fukushima S. et al. IDH1/2 mutation is a prognostic marker for survival and predicts response to chemotherapy for grade II gliomas concomitantly treated with radiation therapy. Int J Oncol. 2012;41:1325–36. doi: 10.3892/ijo.2012.1564. [DOI] [PubMed] [Google Scholar]
- 14.Beiko J, Suki D, Hess KR, Fox BD, Cheung V, Cabral M. et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16:81–91. doi: 10.1093/neuonc/not159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minniti G, Scaringi C, Arcella A, Lanzetta G, Di Stefano D, Scarpino S. et al. IDH1 mutation and MGMT methylation status predict survival in patients with anaplastic astrocytoma treated with temozolomide-based chemoradiotherapy. J Neurooncol. 2014;118:377–83. doi: 10.1007/s11060-014-1443-0. [DOI] [PubMed] [Google Scholar]
- 16.van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MC, Delattre JY. et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31:344–50. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 17.Tran AN, Lai A, Li S, Pope WB, Teixeira S, Harris RJ. et al. Increased sensitivity to radiochemotherapy in IDH1 mutant glioblastoma as demonstrated by serial quantitative MR volumetry. Neuro Oncol. 2014;16:414–20. doi: 10.1093/neuonc/not198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molenaar RJ, Verbaan D, Lamba S, Zanon C, Jeuken JW, Boots-Sprenger SH. et al. The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro Oncol. 2014;16:1263–73. doi: 10.1093/neuonc/nou005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalkan R, Atli Eİ, Özdemir M, Çiftçi E, Aydin HE, Artan S. et al. IDH1 mutations is prognostic marker for primary glioblastoma multiforme but MGMT hypermethylation is not prognostic for primary glioblastoma multiforme. Gene. 2015;554:81–6. doi: 10.1016/j.gene.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Hu F, Zhou Y, Chen W, Shao H, Zhang Y. MGMT promoter methylation and glioblastoma prognosis: a systematic review and meta-analysis. Arch Med Res. 2013;44:281–90. doi: 10.1016/j.arcmed.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Iaccarino C, Orlandi E, Ruggeri F, Nicoli D, Torricelli F, Maggi M. et al. Prognostic value of MGMT promoter status in non-resectable glioblastoma after adjuvant therapy. Clin Neurol Neurosurg. 2015;132:1–8. doi: 10.1016/j.clineuro.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Weller M, Tabatabai G, Kästner B, Felsberg J, Steinbach JP, Wick A. et al. MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res. 2015;21:2057–64. doi: 10.1158/1078-0432.CCR-14-2737. [DOI] [PubMed] [Google Scholar]
- 23.Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK. et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1100–8. doi: 10.1016/S1470-2045(14)70379-1. [DOI] [PubMed] [Google Scholar]
- 24.Sibin MK, Bhat DI, Narasingarao KV, Lavanya C, Chetan GK. CDKN2A (p16) mRNA decreased expression is a marker of poor prognosis in malignant high-grade glioma. Tumour Biol. 2015;36:7607–14. doi: 10.1007/s13277-015-3480-5. [DOI] [PubMed] [Google Scholar]
- 25.Boughton B. BRAF and CDKN2A mutations in secondary high-grade glioma. Lancet Oncol. 2015;16:e201. doi: 10.1016/S1470-2045(15)70145-2. [DOI] [PubMed] [Google Scholar]
- 26.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A. et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yachi K, Watanabe T, Ohta T, Fukushima T, Yoshino A, Ogino A. et al. Relevance of MSP assay for the detection of MGMT promoter hypermethylation in glioblastomas. Int J Oncol. 2008;33:469–75. [PubMed] [Google Scholar]
- 28.Cankovic M, Mikkelsen T, Rosenblum ML, Zarbo RJ. A simplified laboratory validated assay for MGMT promoter hypermethylation analysis of glioma specimens from formalin-fixed paraffin-embedded tissue. Lab Invest. 2007;87:392–7. doi: 10.1038/labinvest.3700520. [DOI] [PubMed] [Google Scholar]
- 29.Shah N, Schroeder B, Cobbs C. MGMT methylation in glioblastoma: tale of the tail. Neuro-Oncology. 2015;17:167–8. doi: 10.1093/neuonc/nou319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeuken J, Cornelissen S, Boots-Sprenger S, Gijsen S, Wesseling P. Multiplex ligation-dependent probe amplification: a diagnostic tool for simultaneous identification of different genetic markers in glial tumors. J Mol Diagn. 2006;8:433–43. doi: 10.2353/jmoldx.2006.060012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker FG 2nd, Chang SM, Larson DA, Sneed PK, Wara WM, Wilson CB. et al. Age and radiation response in glioblastoma multiforme. Neurosurgery. 2001;49:1288–97. doi: 10.1097/00006123-200112000-00002. discussion 1297-8. [DOI] [PubMed] [Google Scholar]
- 32.Mangiola A, Maira G, De Bonis P, Porso M, Pettorini B, Sabatino G. et al. Glioblastoma multiforme in the elderly: a therapeutic challenge. J Neurooncol. 2006;76:159–63. doi: 10.1007/s11060-005-4711-1. [DOI] [PubMed] [Google Scholar]
- 33.Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003;30(6 Suppl 19):10–4. doi: 10.1053/j.seminoncol.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 34.Orringer D, Lau D, Khatri S, Zamora-Berridi GJ, Zhang K, Wu C. et al. Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg. 2012;117:851–9. doi: 10.3171/2012.8.JNS12234. [DOI] [PubMed] [Google Scholar]
- 35.Dea N, Fournier-Gosselin MP, Mathieu D, Goffaux P, Fortin D. Does extent of resection impact survival in patients bearing glioblastoma? Can J Neurol Sci. 2012;39:632–7. doi: 10.1017/s0317167100015377. [DOI] [PubMed] [Google Scholar]
- 36.Marina O, Suh JH, Reddy CA, Barnett GH, Vogelbaum MA, Peereboom DM. et al. Treatment outcomes for patients with glioblastoma multiforme and a low Karnofsky Performance Scale score on presentation to a tertiary care institution. Clinical article. J Neurosurg. 2011;115:220–9. doi: 10.3171/2011.3.JNS10495. [DOI] [PubMed] [Google Scholar]
- 37.Stark AM, van de Bergh J, Hedderich J, Mehdorn HM, Nabavi A. Glioblastoma: clinical characteristics, prognostic factors and survival in 492 patients. Clin Neurol Neurosurg. 2012;114:840–5. doi: 10.1016/j.clineuro.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 38.Chambless LB, Kistka HM, Parker SL, Hassam-Malani L, McGirt MJ, Thompson RC. The relative value of postoperative versus preoperative Karnofsky Performance Scale scores as a predictor of survival after surgical resection of glioblastoma multiforme. J Neurooncol. 2015;121:359–64. doi: 10.1007/s11060-014-1640-x. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Wang M, Won M, Shaw EG, Coughlin C, Curran WJ Jr. et al. Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81:623–30. doi: 10.1016/j.ijrobp.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paravati AJ, Heron DE, Landsittel D, Flickinger JC, Mintz A, Chen YF. et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma and anaplastic astrocytoma: validation of Radiation Therapy Oncology Group-Recursive Partitioning Analysis in the IMRT and temozolomide era. J Neurooncol. 2011;104:339–49. doi: 10.1007/s11060-010-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6:227–35. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ardebili SY, Zajc I, Gole B, Campos B, Herold-Mende C, Drmota S, Lah TT. CD133/prominin1 is prognostic for GBM patient’s survival, but inversely correlated with cysteine cathepsins’ expression in glioblastoma derived spheroids. Radiol Oncol. 2011;45:102–15. doi: 10.2478/v10019-011-0015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mesti T, Moltara ME, Boc M, Rebersek M, Ocvirk J. Bevacizumab and irinotecan in recurrent malignant glioma, a single institution experience. Radiol Oncol. 2015;49::80–5. doi: 10.2478/raon-2014-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M, Dignam JJ, Won M, Curran W, Mehta M, Gilbert MR. Variation over time and interdependence between disease progression and death among patients with glioblastoma on RTOG 0525. Neuro Oncol. 2015;17:999–1006. doi: 10.1093/neuonc/nov009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brandes AA, Franceschi E, Ermani M, Tosoni A, Albani F, Depenni R. et al. Pattern of care and effectiveness of treatment for glioblastoma patients in the real world: results from a prospective population-based registry. Could survival differ in a high-volume center? Neurooncol Pract. 2014;1:166–71. doi: 10.1093/nop/npu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weller M, Tabatabai G, Kästner B. MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res. 2015;21:2057–64. doi: 10.1158/1078-0432.CCR-14-2737. [DOI] [PubMed] [Google Scholar]
- 47.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M. et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 48.Kim YS, Kim SH, Cho J, Kim JW, Chang JH, Kim DS. et al. MGMT gene promoter methylation as a potent prognostic factor in glioblastoma treated with temozolomide-based chemoradiotherapy: a single-institution study. Int J Radiat Oncol Biol Phys. 2012;84:661–7. doi: 10.1016/j.ijrobp.2011.12.086. [DOI] [PubMed] [Google Scholar]
- 49.Ogura R, Tsukamoto Y, Natsumeda M, Isogawa M, H, Kobayashi T. et al. Immunohistochemical profiles of IDH1, MGMT and P53: practical significance for prognostication of patients with diffuse gliomas. Neuropathology. 2015;35:324–35. doi: 10.1111/neup.12196. [DOI] [PubMed] [Google Scholar]
- 50.Lévy S, Chapet S, Mazeron JJ. [Management of gliomas]. [Article in French] Cancer Radiother. 2014;18:461–7. doi: 10.1016/j.canrad.2014.07.147. [DOI] [PubMed] [Google Scholar]


