ABSTRACT
Mammalian terminal erythropoiesis involves gradual but dramatic chromatin condensation steps that are essential for cell differentiation. Chromatin and nuclear condensation is followed by a unique enucleation process, which is believed to liberate more spaces for hemoglobin enrichment and enable the generation of a physically flexible mature red blood cell. Although these processes have been known for decades, the mechanisms are still unclear. Our recent study reveals an unexpected nuclear opening formation during mouse terminal erythropoiesis that requires caspase-3 activity. Major histones, except H2AZ, are partially released from the opening, which is important for chromatin condensation. Block of the nuclear opening through caspase inhibitor or knockdown of caspase-3 inhibits chromatin condensation and enucleation. We also demonstrate that nuclear opening and histone release are cell cycle regulated. These studies reveal a novel mechanism for chromatin condensation in mammalia terminal erythropoiesis.
KEYWORDS: chromatin condensation, erythropoiesis, enucleation, caspase-3, histone release, nuclear opening
Introduction
Erythropoiesis involves a stepwise process that includes differentiation of committed Burst Forming Units -Erythroid (BFU-Es) to Colony Forming Units -Erythroid (CFU-Es). Differentiation from CFU-Es to mature red blood cells, generally termed terminal erythropoiesis, is driven by multiple erythropoietin (Epo) induced signal transduction pathways. These pathways act individually or collectively to activate or repress genes that regulate cell differentiation, proliferation and inhibit apoptosis.1 During erythropoiesis the chromatin gradually condenses. Nuclear and chromatin condensation is thought to be critical for red cell terminal differentiation and final enucleation.2 Extrusion of the highly condensed nucleus is significant for the development of mammalian erythrocytes. The enucleated red blood cells, therefore, could gain more spaces for hemoglobin enrichment and flexibilities to pass through terminal capillaries with diameters often smaller than those of the red cells.
The mechanisms of mammalian erythroid chromatin condensation are unclear. Recent genetic studies demonstrate that histone tails undergo various modifications during chromatin condensation.3 Enzymatic functions of histone deacetylases are also involved in the condensation process.2,4 Specifically, knockdown of HDAC2 significantly affects chromatin condensation and subsequent enucleation.4 In addition, ectopic expression of Gcn5, a histone acetyltransferase that is normally up-regulated by c-Myc, partially blocks nuclear condensation and enucleation.5 Furthermore, the level of Gcn5 is regulated indirectly by miR-191 whose level decreases during terminal erythropoiesis. MiR-191 targets Riok3 and Mxi1, two erythroid-enriched and developmentally upregulated genes. Riok3 and Mxi1 directly, or indirectly through c-Myc, negatively regulate Gcn5. Overexpression of miR-191, or knockdown of Mxi1 or Riok3, blocks nuclear condensation and enucleation.6 These studies establish the roles of chromatin and histone modifications in condensation and enucleation. However, the genome-wide changes of nucleosomes and how various histones are localized in the condensation process are still not known. Our recent published results reveal that the nucleus and histones may undergo several dynamic and repetitive reorganizations during terminal erythropoiesis, which may shed light on the mechanism of erythroid chromatin condensation.7
Overview of the nuclear opening and histone release processes in mammalian terminal erythropoiesis
To understand the mechanisms of chromatin condensation during erythropoiesis, we started with the analysis of the expression and localization profiles of various histone and nuclear structure proteins using our well-established mouse fetal liver culture system.4,8,9 To our surprise, immunofluorescence experiments revealed that most of the histone proteins, except H2AZ, were partially released out of the nucleus through a large nuclear opening in the early stage of terminal erythropoiesis. We found that caspase-3 was involved in the nuclear opening formation. Treatment of the erythroblasts with caspase inhibitor or knockdown of caspase-3 in the early stage of terminal erythropoiesis blocked nuclear opening formation, chromatin condensation, and led to cell death. In addition, our data support the idea that chromatin condensation during erythropoiesis is mediated through gradual histone nuclear release.7 Our findings also suggest that generation of the nuclear opening could be one of the main functions of caspase-3, whose role in terminal erythropoiesis has been unclear.10,11
Figure 1 schematically illustrates the model. Caspase-3 is activated in the early stage of terminal erythropoiesis to cleave lamin B and nuclear envelope. Major histones, except H2AZ, are partially released from the nuclear opening, which is associated with a dynamic change of nucleosomes and gradual chromatin condensation. These processes start in the early stage of terminal erythropoiesis and are dynamic with an open-and-close cycle for 4-5 rounds during mouse fetal liver erythroid terminal differentiation.
Figure 1.
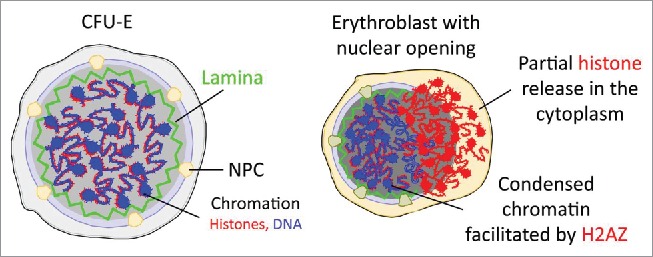
Schematic illustration of chromatin condensation, nuclear opening formation, and histone release during terminal erythropoiesis. NPC: nuclear pore complex; CFU-E: Colony-Forming Units- Erythroid.
Caspase-3 is physiologically activated during terminal erythropoiesis
In addition to physiologic conditions such as erythropoiesis and the development of other haematopoietic lineages, chromatin condensation is also observed during apoptosis. Indeed, apoptotic mechanisms are known to play important roles in erythropoiesis (reviewed in12). Inhibition of the caspase activities blocks terminal erythropoiesis at the basophilic stage.13 Further studies indicate a possible role of caspase-3 in this process by showing a reduced erythroid maturation rate of human CD34 positive cells when caspase-3 was downregulated by shRNA.10 However, the roles of caspases in erythropoiesis remain elusive. Detailed assays in terminal erythropoiesis were not performed in previous reports using animal models.14
Since nuclear lamins are known substrates of caspase-3,15 we asked whether inhibition of the activity of caspase-3 would block the nuclear opening formation. We first confirmed the activation of caspase-3 and cleavage of lamin B in the cultured mouse fetal liver erythroblasts, which was consistent with a previous report.13 We next treated the cultured mouse fetal liver erythroblasts with a caspase-3 inhibitor at the beginning of culture. Indeed, nuclear opening was blocked in caspase-3 inhibitor treated cells compared to the control ones. Caspase-3 inhibitor also significantly blocked nuclear condensation. To demonstrate that caspase-3 directly functions on lamin-B, we generated a caspase-3 noncleavable lamin-B mutant. Ectopic expression of this mutant in erythroid cells indeed blocked nuclear opening formation and chromatin condensation.7
We further studied the role of caspase-3 in vivo using the fetal liver erythroblasts purified from a caspas-3 knockout mouse model in a pure C57BL/6 background. Indeed, these cells exhibited significant defects in nuclear opening formation and chromatin condensation. The caspase-3 knockout mice also showed downward trends of hemoglobin and hematocrit, and statistically significant splenomegaly, indicating mild erythroid defects in vivo. However, these studies cannot rule out the possible compensatory effects of other caspases in nuclear opening formation and chromatin condensation in the absence of caspase-3. Further studies on caspase-1 or caspase-9, which are the upstream regulators of caspase-314, would be helpful to reveal the mechanisms of caspases in vivo in erythroid chromatin condensation.
Given the important role of caspase-3 in chromatin condensation, it is still mysterious how caspase-3, which is usually triggered active in apoptotic conditions, is activated in terminal erythroid differentiation. We showed in our study that caspase-3 is activated on day one (corresponding to basophilic to polychromatic stages of terminal erythropoiesis) of the two-day mouse fetal liver culture period when the cells are actively responsive to erythropoietin (Epo).7,16 Although Epo is critical for the survival of erythroblasts,17 it is also indicated to paradoxically induce physiologic activation of caspases. Previous studies demonstrated that Epo activates caspase-3 in human erythroid cell line TF-1 cells.18 More recently, we showed that Epo increased the levels of reactive oxygen species (ROS). ROS gradually decreased during terminal differentiation, which was accompanied by the decreased erythroblast response to Epo.16 These data indicate that Epo could potentially induce caspase-3 activation through ROS, which is well known to activate caspases.19
A possible role of H2AZ in chromatin remodeling and histone release during terminal erythropoiesis
We demonstrated that the major histones, except H2AZ, are partially released from the nucleus through the nuclear opening. The role of H2AZ in chromatin remodeling is well studied in yeast (review see.20). In mammalian cells, H2AZ is known to be involved in chromatin remodeling and nucleosome depletion in embryonic stem cell.21 However, its role in erythropoiesis is completely unknown. It is likely that H2AZ plays a role in nucleosome remodeling to facilitate the histone release process in terminal erythropoiesis (Fig. 2). Besides the use of non-canonical histone variants, nucleosome remodeling in mammalian cells is also facilitated through ATP-dependent SWI/SNF chromatin remodeling complexes and histone tail post-translational modifications.22 In supporting of this, replacement of H2A to H2AZ is catalyzed by SWI/SNF-related SWR1 complex in yeast.23,24 Mammalian homology of SWR1, p400/mDomino, plays a critical role in erythropoiesis, which further underlies the significance of chromatin remodeling in terminal erythropoiesis.25,26 In addition, TIP60 histone acetyltransferase complex, which associates with p400, acetylates the canonical histone H4 and H2A that is required for H2AZ incorporation catalyzed by SWR1/p400 histone exchange complex.27 Therefore, these mechanisms could function together to induce chromatin remodeling and histone release in terminal erythropoiesis (Fig. 2).
Figure 2.
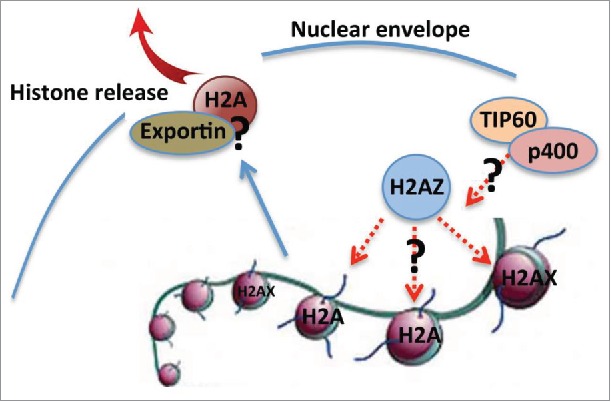
Schematic view of possible mechanisms of H2AZ in chromatin remodeling and histone release. Dashed lines (red) indicate unknown pathways need to be investigated.
Histone nuclear release – passive or active?
A recent study using the same mouse fetal liver culture system identified the cytoplasmic release of histones in the very end stage of terminal erythropoiesis before enucleation, which is mediated by exportin 728. Exportin-7 level is low in the early-stage erythroblasts (Day 0 and Day 1 in our culture system), and peaks in the protein levels on Day 2 in culture. It is mainly located in the nucleus and does not exhibit notable enrichment in the area of the nuclear opening (Fig. 3). There is a significant enrichment of exportin-7 and histone H2A in the enucleating erythroblasts and incipient reticulocytes (unpublished results), which is consistent with the previous study that exportin-7 mediates histone release in the end stages of terminal erythropoiesis.
Figure 3.
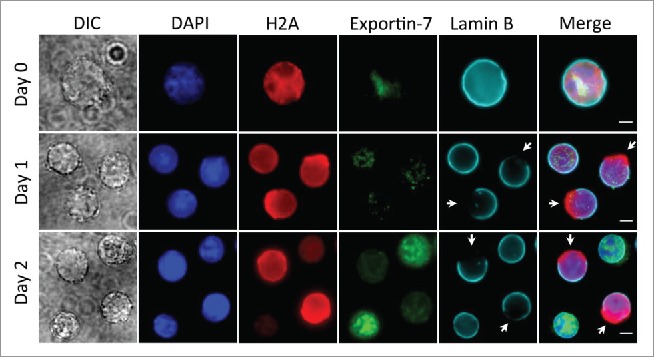
Localization of exportin-7 in different stages of mouse fetal liver erythropoiesis. Ter119-negative E13.5 mouse fetal liver erythroblasts were purified and cultured for indicated days in an erythropoietin-containing medium. Immunofluorescence stains for exportin-7, H2A, Lamin B and DNA (DAPI) were performed. Arrows indicate nuclear opening. Scale bars: 5 μM.
To determine whether the histone release out of the nuclear opening in the early stage of terminal erythropoiesis is also nuclear exportin dependent, we performed studies in which we treated the Ter119 negative mouse fetal liver erythroblasts with exportin inhibitor leptomycin B in the early stage of terminal differentiation but with a visible nuclear opening. We demonstrate that neither nuclear opening formation nor histone release was affected eight hours after the treatment of leptomycin B. Cells treated longer than eight hours underwent apoptosis as nuclear transport is crucial for cell survival. The same result was also obtained when leptomycin B was added in the relatively late stage of terminal erythropoiesis. These results indicate that histone nuclear release at different stages of terminal erythropoiesis could be exportin independent. However, leptomycin B may not be effective on all the exportin family proteins, especially exportin 7. Therefore, knocking down experiment of exportin 7 at different developmental stages in mouse and human terminal erythropoiesis would be necessary to determine whether exportin 7 is involved in the histone release process.
Conclusions
While enucleation is unique in mammals, erythroid chromatin condensation occurs in a wide variety of species. In addition, chromatin condensation is also present during terminal differentiation of many other haematopoietic lineages. Therefore, understanding the mechanisms of chromatin condensation in mammalian terminal erythropoiesis has a broad impact beyond the red cell biology. Our unpublished studies demonstrate that human erythroblasts also exhibit nuclear opening and histone release although with a different kinetics and in different developmental stages. These studies may have a clinical impact since many red cell related diseases that are resistant to erythropoiesis-stimulating agents, such as megaloblastic anemia, congenital dyserythropoietic anemia, and myelodysplastic syndromes, often show a failure of chromatin condensation as one of the dysplastic changes in the erythroid lineage.29-31
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Work in the Ji lab is supported by National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) grant DK102718 (P.J.), Department of Defense grant CA140119 (P.J.).
References
- [1].Hattangadi SM, Wong P, Zhang L, Flygare J, Lodish HF. From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood 2011; 118:6258-68; PMID:21998215; http://dx.doi.org/ 10.1182/blood-2011-07-356006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ji P, Murata-Hori M, Lodish HF. Formation of mammalian erythrocytes: chromatin condensation and enucleation. Trends Cell Biol 2011; 21:409-15; PMID:21592797; http://dx.doi.org/ 10.1016/j.tcb.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wong P, Hattangadi SM, Cheng AW, Frampton GM, Young RA, Lodish HF. Gene induction and repression during terminal erythropoiesis are mediated by distinct epigenetic changes. Blood 2011; 118:e128-38; PMID:21860024; http://dx.doi.org/ 10.1182/blood-2011-03-341404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ji P, Yeh V, Ramirez T, Murata-Hori M, Lodish HF. Histone deacetylase 2 is required for chromatin condensation and subsequent enucleation of cultured mouse fetal erythroblasts. Haematologica 2010; 95:2013-21; PMID:20823130; http://dx.doi.org/ 10.3324/haematol.2010.029827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jayapal SR, Lee KL, Ji P, Kaldis P, Lim B, Lodish HF. Down-regulation of Myc is essential for terminal erythroid maturation. J Biol Chem 2010; 285:40252-65; PMID:20940306; http://dx.doi.org/ 10.1074/jbc.M110.181073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang L, Flygare J, Wong P, Lim B, Lodish HF. miR-191 regulates mouse erythroblast enucleation by down-regulating Riok3 and Mxi1. Genes Dev 2011; 25:119-24; PMID:21196494; http://dx.doi.org/ 10.1101/gad.1998711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhao B, Mei Y, Schipma MJ, Roth EW, Bleher R, Rappoport JZ, Wickrema A, Yang J, Ji P. Nuclear condensation during mouse erythropoiesis requires caspase-3-mediated nuclear opening. Dev Cell 2016; 36:498-510; PMID:26954545; http://dx.doi.org/ 10.1016/j.devcel.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ji P, Jayapal SR, Lodish HF. Enucleation of cultured mouse fetal erythroblasts requires Rac GTPases and mDia2. Nat Cell Biol [Internet] 2008; 10:314-21. Available from: http://www.nature.com/ncb/journal/v10/n3/full/ncb1693.html; PMID:18264091 [DOI] [PubMed] [Google Scholar]
- [9].Zhao B, Keerthivasan G, Mei Y, Yang J, McElherne J, Wong P, Doench JG, Feng G, Root DE, Ji P. Targeted shRNA screening identified critical roles of pleckstrin-2 in erythropoiesis. Haematologica 2014; 99:1157-67; PMID:24747950; http://dx.doi.org/ 10.3324/haematol.2014.105809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Carlile GW, Smith DH, Wiedmann M. Caspase-3 has a nonapoptotic function in erythroid maturation. Blood 2004; 103:4310-6; PMID:14976035; http://dx.doi.org/ 10.1182/blood-2003-09-3362 [DOI] [PubMed] [Google Scholar]
- [11].Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F, Varet B, Solary E, Hermine O. Caspase activation is required for terminal erythroid differentiation. J Exp Med 2001; 193:247-54; PMID:11208865; http://dx.doi.org/ 10.1084/jem.193.2.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nhan TQ, Liles WC, Schwartz SM. Physiological functions of caspases beyond cell death. Am J Pathol 2006; 169:729-37; PMID:16936249; http://dx.doi.org/ 10.2353/ajpath.2006.060105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F, Varet B, Solary E, Hermine O. Caspase activation is required for terminal erythroid differentiation. J Exp Med 2001; 193:247-54; PMID:11208865; http://dx.doi.org/ 10.1084/jem.193.2.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene 2008; 27:6194-206; PMID:18931687; http://dx.doi.org/ 10.1038/onc.2008.297 [DOI] [PubMed] [Google Scholar]
- [15].Dechat T, Adam SA, Taimen P, Shimi T, Goldman RD. Nuclear lamins. Cold Spring Harbor Perspectives Biol 2010; 2:a000547-7; PMID:20826548; http://dx.doi.org/ 10.1101/cshperspect.a000547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhao B, Mei Y, Yang J, Ji P. Erythropoietin-regulated oxidative stress negatively affects enucleation during terminal erythropoiesis. 2016. Oct; 44(10):975-81. http://dx.doi.org/ 10.1016/j.exphem.2016.06.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rhodes MM, Kopsombut P, Bondurant MC, Price JO, Koury MJ. Bcl-xL prevents apoptosis of late-stage erythroblasts but does not mediate the antiapoptotic effect of erythropoietin. Blood 2005; 106:1857-63; PMID:15899920; http://dx.doi.org/ 10.1182/blood-2004-11-4344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lui JC, Kong SK. Erythropoietin activates caspase-3 and downregulates CAD during erythroid differentiation in TF-1 cells - A protection mechanism against DNA fragmentation. FEBS Letters 2006; 580:1965-70; PMID:16529748; http://dx.doi.org/ 10.1016/j.febslet.2006.02.059 [DOI] [PubMed] [Google Scholar]
- [19].Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radical Biol Medic 2010; 48:749-62; PMID:20045723; http://dx.doi.org/ 10.1016/j.freeradbiomed.2009.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zlatanova J, Thakar A. H2A.Z: view from the top. Structure 2008; 16:166-79; PMID:18275809; http://dx.doi.org/ 10.1016/j.str.2007.12.008 [DOI] [PubMed] [Google Scholar]
- [21].Li Z, Gadue P, Chen K, Jiao Y, Tuteja G, Schug J, Li W, Kaestner KH. Foxa2 and H2A.Z mediate nucleosome depletion during embryonic stem cell differentiation. Cell 2012; 151:1608-16; PMID:23260146; http://dx.doi.org/ 10.1016/j.cell.2012.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Segal E, Fondufe-Mittendorf Y, Chen L, Thåström A, Field Y, Moore IK, Wang J-PZ, Widom J. A genomic code for nucleosome positioning. Nature 2006; 442:772-8; PMID:16862119; http://dx.doi.org/ 10.1038/nature04979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mizuguchi G, Shen X, Landry J, Wu WH, Sen S, Wu C. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 2004; 303:343-8; PMID:14645854; http://dx.doi.org/ 10.1126/science.1090701 [DOI] [PubMed] [Google Scholar]
- [24].Luk E, Ranjan A, FitzGerald PC, Mizuguchi G, Huang Y, Wei D, Wu C. Stepwise histone replacement by SWR1 requires dual activation with histone H2A.Z and canonical nucleosome. Cell 2010; 143:725-36; PMID:21111233; http://dx.doi.org/ 10.1016/j.cell.2010.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fujii T, Ueda T, Nagata S, Fukunaga R. Essential role of p400/mDomino chromatin-remodeling ATPase in bone marrow hematopoiesis and cell-cycle progression. J Biol Chem 2010; 285:30214-23; PMID:20610385; http://dx.doi.org/ 10.1074/jbc.M110.104513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ueda T, Watanabe-Fukunaga R, Ogawa H, Fukuyama H, Higashi Y, Nagata S, Fukunaga R. Critical role of the p400/mDomino chromatin-remodeling ATPase in embryonic hematopoiesis. Genes Cells 2007; 12:581-92; PMID:17535249; http://dx.doi.org/ 10.1111/j.1365-2443.2007.01080.x [DOI] [PubMed] [Google Scholar]
- [27].Altaf M, Auger A, Monnet-Saksouk J, Brodeur J, Piquet S, Cramet M, Bouchard N, Lacoste N, Utley RT, Gaudreau L, et al.. NuA4-dependent acetylation of nucleosomal histones H4 and H2A directly stimulates incorporation of H2A.Z by the SWR1 complex. J Biol Chem 2010; 285:15966-77; PMID:20332092; http://dx.doi.org/ 10.1074/jbc.M110.117069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hattangadi SM, Martinez-Morilla S, Patterson HC, Shi J, Burke K, Avila-Figueroa A, Venkatesan S, Wang J, Paulsen K, Görlich D, et al.. Histones to the cytosol: exportin 7 is essential for normal terminal erythroid nuclear maturation. Blood 2014; 124:1931-40; PMID:25092175; http://dx.doi.org/ 10.1182/blood-2013-11-537761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011; 117:5019-32; PMID:21300984; http://dx.doi.org/ 10.1182/blood-2011-01-293050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lindsley RC, Ebert BL. Molecular pathophysiology of myelodysplastic syndromes. Annu Rev Pathol 2013; 8:21-47; PMID:22934674; http://dx.doi.org/ 10.1146/annurev-pathol-011811-132436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wickramasinghe SN, Wood WG. Advances in the understanding of the congenital dyserythropoietic anaemias. Br J Haematol 2005; 131:431-46; PMID:16281933; http://dx.doi.org/ 10.1111/j.1365-2141.2005.05757.x [DOI] [PubMed] [Google Scholar]


