ABSTRACT
The nuclear pore complex (NPC) mediates the shuttle transport of macromolecules between the nucleus and cytoplasm in eukaryotic cells. The permeability barrier formed by intrinsically disordered phenylalanine-glycine-rich nucleoporins (FG-Nups) in the NPC functions as the critical selective control for nucleocytoplasmic transport. Signal-independent small molecules (< 40 kDa) passively diffuse through the pore, but passage of large cargo molecules is inhibited unless they are chaperoned by nuclear transport receptors (NTRs). NTRs are capable of interacting with FG-Nups and guide the cargos to cross the barrier by facilitated diffusion. The native conformation of the FG-Nups permeability barrier and the competition among multiple NTRs interacting with this barrier in the native NPCs are the 2 core questions still being highly debated in the field. Recently, we applied high-speed super-resolution fluorescence microscopy to map out the natural structure of the FG-Nups barrier and determined the competition among multiple NTRs as they interact with the barrier in the native NPCs. In this extra-view article, we will review the current understanding in the configuration and function of FG-Nups barrier and highlight the new evidence obtained recently to answer the core questions in nucleocytoplasmic transport.
KEYWORDS: intrinsically disordered proteins, nucleocytoplasmic transport, Nucleoporins, super-resolution fluorescence microcopy
Introduction
Living eukaryotic cells require bidirectional transport of essential materials between nucleus and cytoplasm. Molecules essential for protein synthesis (such as tRNAs, mRNAs, and pre-ribosomal subunits produced in the nucleus) are exported to the cytoplasm, while nuclear proteins synthesized in the cytoplasm (such as histones and transcription factors) are imported into the nucleus.1-4 This bidirectional transport is mediated almost exclusively via the nuclear pore complex (NPC),3,5 a unique machinery that consists of approximately 30 different types of nucleoporins (Nups). In eukaryotic cells, approximately one third of these Nups are intrinsically disordered proteins, with multiple phenylalanine-glycine (FG) repeats that have characteristic unstructured nature.6-9 Over the past years, numerous biochemical, systematic, computational, and microscopy imaging studies have proven that the FG-Nups permeability barrier in the NPC plays an important role in handling the massive nucleocytoplasmic transport. These studies have also shed light on the 2 critical functions of FG-Nups: their role as constituents of the permeability barrier and their role to interact with NTRs to bridge the translocation of macromolecules across the NPC.
In this extra-view paper, first we will briefly describe the major features of FG-Nups in the NPC, including their classification based on our current biochemical and physiological understanding of their properties (Section 1). Specific features of both yeast and human FG-Nups will then be highlighted and discussed (Section 2). Next, our review will focus more on the interactions among FG-Nups and between FG-Nups and NTRs (Section 3). We will devote the next section to discuss the native biophysical configuration of FG-Nups in the native NPCs and their functions as passage routes for the facilitated translocation revealed recently by super-resolution technologies (Section 4). Subsequently, the effective interaction ranges of various NTRs in native NPCs will be demonstrated and discussed (section 5). Finally, we will discuss how the competitions among NTRs occur as they simultaneously interact with the NPCs and when NTRs affect the conformation of FG-Nups barrier in the last section (Section 6).
An overview of the NPCs and FG-Nups
The NPCs
The NPCs are one of the largest protein complexes that perform multiple functions in eukaryotic cells. First, the NPCs remain the essential and very efficient selective gate in transporting thousands of various molecules per NPC per second,2-4 while large ribonucleoprotein complexes can also exit the nucleus via nuclear membrane budding.10 Second, recent evidence suggests that the NPCs are not only essential for nuclear transport, but also play roles in tissue-specific and developmental functions. Several Nups, such as Nup210, Nup45, Nup50, Nup133, and Nup155, express at a different level in different cells and tissues, which suggests that they may be involved in cell- or tissue-specific functions. Mutations in Nup155 and Nup62 induced tissue-specific malfunctions or disease, further supporting the hypothesis that specialized functions in NPCs are composed of pools of unique Nups.10
The size of the NPC is estimated to be ∼60 MD in yeast and ∼120 MD in vertebrates.2,4 This super-structure complex is comprised of 3 functional groups of Nups: transmembrane Nups (Poms) that anchor to the NPC in the nuclear envelope, structural Nups that make up the NPC scaffold, and the intrinsically disordered Nups that constitute the permeability barrier with their disordered regions (Table 1). Each of these Nups is present in 1, 2, or 4 copies on each of the 8 spokes [8-fold rotational symmetry, although 9-fold rotational symmetry is occasionally observed11]. One exception to this grouping is Pom 121 in metazoans, which is a transmembrane protein and a FG-Nup as well.12. In accordance with their name, transmembrane Nups and structural Nups are proteins with secondary structures of β-propeller and α-solenoid motifs, whereas disordered FG-Nups lack typical secondary structure and tertiary structure, displaying a very flexible nature with high net charge and low hydrophobicity as well as low compactness/non-globularity6,8,9,13
Table 1.
Interactions between transport receptors and FG-Nups. √ refers obvious interaction; w represents weak interaction; x means no interaction; n/a means not available. The information in the table is collected from previous publication75 with permission.
| Transport Receptors |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NPC Binding sites |
Import |
Export |
|||||||||
| In NPC Substructure | Yeast Nups | Repeat Motif | Vertebrate Nups | Repeat Motif | Importin β1 | Importin β2 | NTF2 | hCRM1 | CAS | Tap-p15 | |
| Cytoplasmic FG Nups and Filaments | Nup42 | FG | hcG1/NIp1Nup358 | FGFxFG | ✓✓ | ✓W | ✗n/a | ✓✓ | n/an/a | ✓✓ | |
| Nup159 | FG | Nup214 | FG | ✓ | ✗ | W | ✓ | n/a | ✓ | ||
| Symmetric FG Nups with cytoplasmic bias | Nup100Nup116 | GLFG GLFG | Nup98 Nup98 | GLFG GLFG | ✓✓ | ✓✓ | ✗ ✗ | ✓✓ | ✓✓ | ✓✓ | |
| Transmembrane/lumenal ring | Pom121 | FG | n/a | n/a | n/a | n/a | n/a | n/a | |||
| Symmetric FG Nups | Nup49 | GLFG | Nup58/45 | FG | ✓ | W | ✓ | ✓ | n/a | ✓ | |
| Nup57 | GLFG | Nup54 | FG | ✓ | ✓ | ✓ | ✓ | n/a | ✓ | ||
| Nsp1 | FG,FXFG | Nup62 | FxFG | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ||
| Symmetric FG Nups with Nuclear bias | Nup145N | GLFG | Nup98 | GLFG | ✓ | ✓ | ✓ | n/a | ✓ | ||
| Nucleoplasmic FG Nups and Filaments | Nup1 | FXFG | Nup153 | FXFG | ✓ | ✗ | ✓ | ✓ | n/a | ✓ | |
| Nup2 | FXFG | Nup50 | FXFG | ✓ | ✗ | ✓ | n/a | ✓ | ✓ | ||
| Nup60 | FXF | ✓ | ✗ | ✗ | n/a | n/a | n/a | ||||
The NPC central channel is filled with the disordered FG-polypeptides of FG Nups, forming an effective selective permeability barrier. The barrier not only permits the passage of ions and other small molecules (< 40 kDa) via passive diffusion, but also simultaneously blocks the passage of signal-independent, large, inert molecules. Most importantly, the selective barrier allows for the efficient translocation of macromolecules such as proteins and RNAs across the NPC through facilitated diffusion, provided that the molecules carry peptide sequences recognizable by NTRs.1,2 The most abundant NTRs are the karyopherins (Kaps), which are involved in the transport of most proteins and RNAs as both importins and exportins.3,4,12 The direction of cargo transport is controlled by cargo signals/the recognition of signals by NTRs and in many cases, by RanGTP/RanGDP concentration gradient across the nuclear envelope (higher in the nucleus and lower in the cytoplasm for RanGTP, and vice versa for RanGDP). In detail, first, a cargo destined to the nucleus would carry nuclear-localization-sequence (NLS), and a cargo that goes to the cytoplasm would carry nuclear-export-signal (NES). Thus, the signals of the cargos induce the corresponding NTRs for binding and traveling through the NPCs to reach their target sites. Second, the RanGTP/RanGDP controls the final step of transport. RanGTP in the nucleus dissembles NTR-cargo import complexes, releasing the cargo and allowing NTRs to translocate back to the cytoplasm, whereas RanGTP bound exportin-cargo complexes in the cytoplasm are dissembled and simultaneously RanGTP is hydrolyzed to RanGDP by RanGAP and Ran binding proteins.14,15 Therefore, eukaryotic cells evolved this directional controlling mechanism through the initial recognition of cargoes by NTRs and the final disassembly of the NTR-cargo complexes and release of the cargo by RanGTP/RanGDP system for most proteins and RNAs, despite transport directionality could also be controlled by unidentified mechanisms of a few non-Kap NTRs.12
FG-Nups in the NPCs
Among approximately 30 Nups in the NPCs, about one third of them contain multiple FG-repeat domains (Table 1). Assuming an average of 2 copies of each FG-Nup is present in each spoke of the 8 total spokes in the NPC, there are over 200 FG-Nups in a single NPC. If we also assume an average of 20 FG-repeat domains (typically ranging from 5–50) per FG-Nup, there are around 4,000 FG-domains that are available to form an interior structure inside the central channel. These FG-domains also extend to the cytoplasmic and nuclear sides of the NPC as the cytoplasmic filaments and the nuclear basket, respectively. These natively disordered, or unfolded, FG-polypeptides are flexible, extended, and possess no obvious secondary structure.6,8,9,16-18 Although some non FG-Nups are present in the NPC channel, and interacting with FG-Nups, it is generally accepted that the selective barrier is formed mainly by the FG domains of the FG-Nups and only allows the flow-through of small molecules but blocks the passage of all macromolecules unless they are chaperoned by NTRs.
The functions of FG-Nups as barrier-forming material and as binding recipients of NTRs are clearly associated with NPC transport, but are largely still not very well understood. For example, deletion of a single FG-domain could undermine the permeability barrier in yeast.19 On the other hand, contradictory to their function, or due to their functional redundancy, half of the disordered regions of the FG-Nups could be mutated, and asymmetrically located FG-Nups' FG domains could also be deleted or mutated, without apparent effect on NPC transport.20,21 Evidence from both in vitro and in vivo suggested that FG-domains of several centrally located FG-Nups could form a cohesive meshwork as a permeability barrier as well as central diffusion route, whereas the FG-domains of some peripherally located FG-Nups might build a non-cohesive (repulsive) peripheral gate as an additional route for diffusion.19 It is also demonstrated that some molecules, such as β-catenin, are imported into the nucleus in the absence of interactions with FG-Nups.22 It is, however, by no means are other proteins excluded from this regulatory function. For example, structural Nups Nup170, Nup188, and Nup192 in yeast may play a role in the integrity of the barrier and thus, the nucleocytoplasmic transport.12,23
Unlike most folded proteins, whose structure and function are closely related to each other, FG-Nups do not have conserved configuration. Therefore, classification or grouping of FG-Nups is mainly based on the biochemical and biophysical nature of FG-Nups and other functionally conserved features.24 Based on the primary core sequence of different FG repeats, FG-Nups can be divided into subfamilies or subtypes. The major types include FxFG (phenylalanine-x-phenylalanine-glycine), GLFG (glycine-leucine-phenylalanine-glycine), and xxFG (x-x-phenylalanine-glycine); and the minor types include PSFG (proline-serine-phenylalanine-glycine), SAFG (serine-alanine-phenylalanine-glycine), and VFG (valine-phenylalanine-glycine). These FG repeat domains are not randomly distributed but occur in certain patches, separated by linker sequences, or so-called spacer sequences.25 Moreover, GLFG-Nups are found predominantly in yeast, whereas FxFG are found in both yeast and high eukaryotes. The FxFG regions are highly charged and more extended, whereas GLFG are largely uncharged and more folded.7,26 In addition, FG-Nups could also be divided into 2 subgroups based on interaction tests between one another: the interacting (cohesive via hydrophobicity) FG-Nups and non-interacting (repulsive) FG-Nups (see Section 3).
Spatially, FG-Nups are assembled in 3 distinct locations in the NPCs (Table 1). For example, in yeast cells, 5 FG-Nups, Nup49, Nup57, Nup100, Nup145, and Nup116, are located in the central transport conduit. While, FG-Nups bearing PSFG/xxFG peptides (Nup159 and Nup42) are located only on the cytoplasmic filaments, and FG-Nups possessing FxFG motifs (Nup60, Nup2, and Nup1) are localized solely on the nuclear basket side. Thus, the centrally located FG-Nups are arranged in a largely symmetric manner, corresponding to the NPC containing 16 copies of each FG-Nup, 8 on each side.5,11 And FG-Nups located on either side likely contribute to the slightly asymmetric structure of the NPC).7,27
Regardless of how FG-Nups are structurally assembled in distinct sub-complexes in the NPCs in different species, several common but unusual features unique to disordered proteins are conserved. First, the long stretch of FG-repeat domains that are distant from their anchor points is separated by variable spacer sequences, hydrophilic and charged, making them a dynamic and flexible structure in occupying the interior of the NPC channel.12 Second, FG-domains contain enriched certain amino acid (AA) residues that are typically associated with structural disorder and flexibility. Third, FG-domains possess high net charge and low hydrophobicity. In addition, unfolded FG domains are in the best position to interact with multiple NTRs and are also in favor of fast association/dissociation rates, which are characteristic of the NPC heavy (busy) transporting nature. The long stretches and flexibility of FG-repeats could make them likely to consistently and simultaneously interact with multiple NTRs.
FG-Nups in eukaryotic cells
FG-Nups in yeast cells
FG-Nups in yeast cells have been most thoroughly and systematically studied, mainly due to the relatively easy manipulation of the organism.5,7,13,16 A total of 11–13 FG-Nups are described in yeast cells, and the number closely represents the total number of FG-Nups in this microorganism (Table 1,5). All FG-Nups in yeast contain enriched charged and polar AAs [such as A, R, Q, E, G, K, P, S (alanine, arginine, glutamine, glutamate, glycine, lysine, proline, serine)] and reduced hydrophobic AAs [such as W, C, I, Y, V, L, N (tryptophan, cysteine, isoleucine, tyrosine, valine, leucine, asparagine)].7,8,13 Disordered content varies among the subfamilies: FxFG Nups have more polar AAs than Nups with GLFG, PSFG, and xxFG repeats.8 Interestingly, the FG-domains of the Nups located in peripheral structure (either on cytoplasmic filaments or on nuclear basket) evolved more divergently in their primary sequence structure than that of the Nups located in the central plane.
Strikingly, the most prevalent ‘F’ residue in FG-Nups is actually an order-promoting residue. Conservation of this residue is necessary since it is the critical determinant for Kap binding.28 As a matter of fact, FG domains in all FG-Nups contain either Kap binding sites (approved or predicted) or other potential functional sites (such as molecular interaction sites, flexible linker sites, or spacers), suggesting that they are involved in receptor-facilitated translocation. One of the major features in FG-Nups of yeast is that all centrally located FG-Nups and one FG-Nup that resides in the cytoplasm, contain GLFG as their distinctive FG-domains, and form cohesive permeability meshwork through hydrophobic interactions, at least in vitro.29 although non-GLFG Nups in higher organisms perform similar functions. Lower eukaryotic cells, such as yeast cells, did not evolve sophisticated post-transcriptional modifications, such as glycosylation of FG-Nups (see 2.2 below), but systematic analysis of NPC proteins in yeast cells has revealed that more than half of the NPC proteins were ubiquitylated. Specifically, FG-Nup159 was mono-ubiquitylated and although this modification affects cell division, it does not affect detectable NPC transport.30
FG-Nups in higher eukaryotic cells
FG-Nups in higher eukaryotic cells are relatively less investigated. Nevertheless, many yeast FG-Nup homologues have been identified in higher eukaryotic cells (Table 1). The overall features of these FG-Nup homologues seem to have been well conserved, AA composition, fold type, and domain structure in particular.4,13,31 The FG-Nups in higher organisms, as those in yeast, also similarly display enriched charged and polar, or “disordered” AAs, and are depleted in hydrophobic, or “order-promoting” AAs.13 Therefore, the structural disordered nature of FG-Nups is persistently conserved during evolution. Although the AA composition of FG-Nups is conserved, the AA sequence between different species differs significantly.13 Interestingly, the Kap-binding domains in FG-Nups have been conserved, despite the overall sequence divergence (Table 1,13). A few of FG-Nup homologues in higher eukaryotic cells (such as Nup62, Nup153, Nup214, and Nup358) have been demonstrated to be able to bind NTRs (such as importin-β), suggesting their critical role in the nucleocytoplasmic transport (Table 1,28,32,33). In addition, unlike FG-Nups centrally located in the NPCs in yeast cells, which contain FG-domains with dominant GLFG features, the FG-Nup homologues in higher organisms (such as hNup54, hNup62, hNup45, and hNup58) contain almost no GLFG motifs. The cohesive interactions between these FG-Nups are not merely due to hydrophobic attractions, but also through a low content of charged residues in between FG-domains and a high content of polar residues.19.
For both higher eukaryotes and yeast cells, several functions can be generally attributed to the entirety of FG-Nups.34 First, the permeability barrier of the NPCs is formed through the collective role of all FG-Nups, even though some FG-Nups seem to play a more important role than others. Second, many FG-Nups may have distinctive roles since they do bind defined and specific NTRs and locate at different sub-regions of NPC. Third, there may be some redundancy in the roles of FG-Nups, but this redundancy may be necessary, for example, to guarantee that some FG-Nups can bind critical NTRs that carry crucial macromolecules cross the NPC in any situation.35-37
Another evolutionary feature present in FG-Nups in higher eukaryotic cells is the post-transcription modification. For example, in metazoans, FG-Nups are often glycosylated via O-linked-N-acetyl-glucosamine transferase, but functions of this modification in cells remain unknown. It is speculated that this O-linked glycosylation may facilitate a binding site for lectins, and may be required for either NPC transport or regulating the FG-Nup phosphorylation that may indirectly affect its function.12
Interactions among FG-Nups and between FG-Nups and NTRs
Interactions among FG-Nups
FG-Nups are the main components in forming the selective nucleocytoplasmic transport gates. The two most important questions in relation to their function are: 1) how do FG-domains interact with one another to form the permeability barrier that blocks the inert macromolecules, and 2) how do FG-domains interact with NTRs to allow the NTR-cargo complexes to pass through. Section 3.2 will discuss the second question in more detail, and the following section will review the first question based on our current understanding. It is particularly difficult to answer the question about the interactions between FG-domains, which is one of the foundations for a particular transport model to build on. Nevertheless, recent studies provide some important insights in terms of interactions in vitro as well as in vivo.
Interacting FG-Nups
Biochemically, interacting between FG-Nups can be examined and identified in vitro. Using the immobilized FG-Nups as bait, several groups have developed simple assays to identify other FG-Nups that interact with the bait.7,19,29,38 The sensitive assays, such as bead-halos assay,19,29 can detect low-affinity binding and was employed systematically to test all FG-Nups. Interestingly, the SAFGxPSFG domain of Nup42 and the GLFG domain of 5 centrally located Nups (Nup49, Nup57, Nup100, Nup116, and Nup145N) interacted with each other in pairwise combinations. The peripheral FxFG domains of 3 FG-Nups (Nup1, Nup2, and Nup60) and the peripheral SAFGxPSFG domain of FG-Nup159 did not interact with other GLFG Nups or SAFGxPSFG Nups, or with each other. Others found that several human FG-domains showed no interactions but VFG-domains of Nup214 showed detectable cohesive interactions in a fluorescence based live cell assay.38 Very recently, GLFG of yNup116 and hNup98, FxFG of hNup62, and FG of yNup42 were all found to interact in vivo with many FG-Nups of native human NPCs.75
The data from mutated FG-domains further suggest that LF motifs in GLFG and the overall hydrophobicity are required for the interactions.19 Molecular simulation studies suggest that the backbone-backbone hydrogen bonding also contributes to the interactions of FG-Nups, and hydrophilic linkers may further strengthen the interactions in forming the stabilized permeability barrier.39 In fact, hydrophobic, hydrophilic, and electrostatic interactions were all taken into account for FG-Nup and NPC simulations.40 The interactions of FG-Nups seems to be interesting in at least a few aspects: firstly, the in vitro interactions between GLFG domains and FG-Nups are validated in vivo by the yeast 2-hybrid system; secondly, deletion mutant experiments suggest that the FG-domains and their interactions are required for the NPCs to function as a selective gate; and thirdly, the interactions of FG-Nups may also contribute to the overall geometric orientation of central FG-domains, as observed ordered structure (here not referring to secondary structure) by polarized fluorescence microscopy.41 GLFG repeats (such as from human Nup98) show cohesive interaction in vivo, which is proportional to the number of GLFG repeats.38 The cohesive interactions observed both in vitro and in vivo provide strong evidence for a few transport models that predict that the interactions are necessary for binding NTRs for facilitated diffusion.
It is believed that the hydrophobic (cohesive) interactions between the FG-Domains of the Nups produce the selective permeability barrier, at least in some proposed transport models (discussed further in Section 5). In this respect, it is interesting to note that GLFG containing Nup100 can form amyloid fibers,42 and the FG domains of human Nup 153 and Nup98 and a few yeast FG-Nups are able to form hydrogels in vitro that are visible under microscope.18,34,43,44 The high resolution EM revealed that the hydrogels formed by human FG-Nup153 and yeast FG-Nup49 are in fact cross-linked fibers.45 These hydrogels formed by FG-Nups, assumed via hydrophobic interaction, have been demonstrated to function like permeability barriers.43,45 However, it is unclear if the FG-Nups in vivo could form the hydrogels/fibers as the selective permeability barrier since the in vitro conditions (such as artificially created crowding) may not be the same as the physical conditions in vivo.45
Non-interacting FG-Nups
The FG-Nups located peripherally extend outside of the NPC, on both the cytoplasmic and nuclear side. Possibly due to their unique AA compositions or low ratio of mass to volume, they interact with each other much less or not at all compared to the FG-Nups located centrally,19 and thus, they are non-cohesive. As discussed above, interaction between FG-Nups occurred only with certain combinations, but not in many others, at least in vitro.19 For example, the Nup84 complex binds FG-Nups Nup116, Nup42, and Nup57. However, this interaction is independent on Gsp1-GTP, whose binding actually affects the cargo-transporting interaction between the functional FG-Nups and transport receptors (either importins or exportins). It was speculated that this in vitro biochemically detectable interaction of FG-Nups with the Nup84 complex may be necessary in maintaining the intact permeability barrier of the NPC in vivo.16 Notably, many other FG-Nups (Nup1, Nup2, Nup6, Nsp1, and Nup159) do not bind to the Nup84 complex, and they are located externally on either the cytoplasmic or nuclear side of the NPC. The current in vitro evidence and some in vivo evidence suggests that many FG-Nups, particularly those centrally located, do interact with each other, and other FG-Nups (such as peripheral FG-Nups) may not. Evidently, the spatial distribution of the interactions between FG-Nups in the native NPCs, as recently determined by single-point edge-extraction sub-diffraction (SPEED) microscopy, suggests that not all the individual FG-Nups interact with each other in vivo, and certain FG-Nups are indeed non-interacting FG-Nups.75
Finally, whether FG-Nups are interacting or non-interacting is an important question in considering how and where the permeability barrier is formed. Caution must be exercised in interpreting the data from the above assays. For example, interactions between FG-Nups may not be captured due to very weak signals, or can be detected but due to other factors, such as the NTRs bound to the bait interacting with FG-Nups.7,16
Interactions between FG-Nups and NTRs
Interaction between FG-Nups and NTRs appears to be a requisite in order for large cargos to cross the NPC since they are excluded from passive diffusion due to their size. Such interactions have been documented for many FG-Nups and different types of NTRs (Table 1). The binding sites between FG-Nups and NTRs have been observed by studies in vitro and in vivo28,53 and directly confirmed by crystallography combined with mutation studies.6,28 They can also be predicted using molecular dynamic simulations or through AA composition and AA substitution of FG-Nups.4,7 In yeast, approximately 11 importins and 6 exportins have been described.16 In vitro experimental evidence suggests that in yeast cells, each FG-Nup can bind one or multiple NTRs, and every NTR can bind to one or multiple FG-domains.12 Moreover, more than 50 percent of the FG-domains on the surface of brush-like FG-Nup structures along the NPC central channel could be available for interaction with NTRs based on molecular dynamic simulation studies.46 Apparently, hydrophobic interactions seem to be critical in the binding of FG-Nups to the NTRs and translocation of NTR-cargo complexes.6,28,47 In addition, it was further reported that the negatively charged NTRs easily gain access to the positively charged FG-clusters during the initial interaction.24,61 The initial interaction between FG-Nups and NTRs may behave as a simple place to concentrate NTRs at the entrance of the NPC or as a docking and undocking platform for the cargo-NTR-complexes.48
Specifically, the interactions between some FG-Nups and certain NTRs may play a central role in the selective and efficient transport of large macromolecules with their in-transit signals.49 For example, charged FxFG motifs in FG-Nups on the nuclear basket side (Nup1, Nup2, and Nup60) and mostly uncharged GLFG and PSFG in cytoplasmic Nup42 and in symmetric Nups (Nup49, Nup57, Nup100, and Nup116) may play dominant roles in their differential binding capacity toward distinct groups of NTRs.7 In addition, charged AAs and their electrostatic potential in the spacer interspersed between FG-repeats may contribute to the interactions between FG-Nups and NTRs as well.24,50 Collectively, hydrophobic forces and electrostatic effects may operate in a cooperative and nonequivalent way, depending on the individual transport events, as suggested by molecular theory studies.51
Multiple binding sites of NTRs for FG-Nups
In general, large cargo molecules (> 40–60 kDa) carrying directional signals are translocated in or out of the nucleus via facilitated diffusion through the NPC by NTRs.1 It is clear from the crystallographic observations that the F residue in FG-repeat interacts with hydrophobic pockets of the outer face of the NTRs, including Kaps, NTF2, Mex63-Mtr2, and NXF1-NXT2).4,12, 28,48 Studies with crystallization and x-ray diffraction showed that 5 FxFG repeats of Nsp1p specifically bind the residues 1–442 of Importin-β in the convex face of Ib442 to the primary helices of HEAT repeats 5 and 6 (i.e. hydrophobic pocket).6,28 Interestingly, not only FxFG-Nups, but also GLFG-Nups bind to the Importin-β at similar hydrophobic pockets, and the binding of GLFG to Importin-β and Kap95 is competitively inhibited by soluble FxFG repeats, indicating that the functional difference between these FG-repeats might depend on their spatial localization or organization of FG-Nups.48 Similarly, Msn5 preferentially binds to GLFG-repeats of Nup100 and NxF1 preferentially binds to GLFG-repeats of Nup98 (vertebrate Homolog of yNup116).12,54
Meanwhile, increasing evidence suggests that multiple binding sites likely exist in a single transport receptor. For instance, Kap Msn5 and Kap142 could interact with at least 8 of the 13 yeast FG-Nups, including Nup1, Nup2, Nup42, Nup49, Nup57, Nup100, Nup116, and Nup 145.7,55 Mex67-Mtr2 and NxF1-NxT1 also contain a nuclear transport factor 2 (NTF2)-like FG-binding site and a ubiquitin-associated FG-binding motif.12 In addition, importin-β can bind to FxFG repeats of FG-Nups via α-helices of HEAT repeats 5 and 6 in the N–terminal and outer surface, and can bind to cargo-adaptor-importin-α and RanGTP through helices of HEAT repeats 7–19 or HEATs 1–3, 6, 7, 13, and 14 in the inner surface.28 The mutation studies demonstrated that each of the 4 FG-binding pockets could bind FG-Nups with different affinities and have different sensitivities to RanGTP, and moreover, interactions of FG-Nups, such as Nup153-C (Carboxyl terminal 895–1475 AAs) with full-length Importin-β was severely affected from the binding of RanGTP to the Importin-β N-terminal domain, due to the conformational change of the importin-β induced by RanGTP binding, suggesting a mechanism where RanGTP regulates the interactions between FG-Nups and NTRs for the final release of cargos from the cargo-NTR complexes.56
Multiple binding sites on FG-Nups for NTRs
Likewise, there are likely multiple binding sites in each given FG-Nup for NTRs, suggesting that they are potentially capable of binding to different NTRs or to multiple NTR's simultaneously. For example, sequences of FxFG repeat Nups (Nup62 and Nsp1) binds both NTF2 and importin-β,57 and distinct domains of FG-Nup 116 can interact with Mex67 and Kap95 separately. Moreover, crystals of complexes between 5 FxFG repeats of Nsp1 and yeast NTF2 and between the same 5 FxFG repeats and truncated 1–442 residues of human importin-β were obtained.6 It is also possible that, at least in some cases, a cargo-bound NTR that binds to multiple FG-Nups would enhance their avidity of the interactions, which is likely necessary for their ultimate transporting success.36,58 On the other hand, multiple FG-repeats of each FG-Nup could cope to ease the heavy transporting tasks and also provide cells with some necessary and some redundant functions. Sequence alignment of FG-Nups from 4 species of yeast identified small stretches of conserved AAs (˜6–11 AAs) (called ‘islands’) among divergent FG-domains. Some of these ‘islands’ are coincidently the same or similar sites identified experimentally as Kap binding sites.13 Multiple NTRs are necessary for a cargo modified to carry multiple signals for efficient transport across the NPC.59
Additionally, exceptions exist by using other transport mechanisms, which bypass the typical interactions of FG-domains with NTRs or those controlled by the RanGTP system. For example, some nucleus-directed macromolecules can directly bind to FG-Nups and cross the NPC.12 Transmembrane proteins could be translocated through non-NPC pathway.10 In addition, 60S pre-subunit is exported by yeast Gle2 that bind to the non-FG domains of Nup116.52 Bulk mRNA is also exported through a new non-Kap NTR that is independent of the regulation of the RanGTP/RanGDP system.12
The native conformation of the FG-Nups barrier in the NPCs
Mapping the native FG-Nups barrier by SPEED microscopy
FG-Nups are the key component of the selective permeability barrier for exchanging molecules across the NPC. However, the native conformation of this barrier structure is poorly understood, mainly due to their highly flexible unstructured nature and the transient dynamic interactions with one another and with NTRs in the native NPCs of living cells, which is still a formidable task to map using traditional techniques, such as conventional EM and immune-gold labeling. Recently we accomplished the task by first mapping the spatial locations of FG-FG and FG-NTRs interaction sites in native NPCs with high-speed super-resolution SPEED microscopy.72-75 Then we reconstructed these interactions to form the complete map of the FG-Nup barrier.
In our experiments, first we aimed at detecting thousands of FG-domains present in vivo in the native NPCs in order to observe a complete native configuration of a FG-Nup barrier. To reach the goal, we have used various fluorescently labeled probes to interact with the FG-Nups in native NPCs. These probes, including 6 different FG-segments and 6 different NTRs, together are expected to recognize the FG domains from all the FG-Nups in the native NPCs. Specifically, the FG-segment probes include hNup62 (1–300) and hNup98 (1–466) isolated from human cells and yNup116 (345–458), yNup42 (1–372), yNup159 (441–881) and yNsp1 (1–603) from yeast cells. The NTR probes consist of 3 major importins (Imp-β1, Imp-β2, and NTF2) and 3 major exportins (Crm1, CAS, and Tap-p15). Notably, each of these probes has been reported to bind almost all FG-Nups or only a few of them on its own unique pathways (Table 1). Our high-resolution detection of these probes in the native NPCs largely agreed with the previous reported results, with an exception that Crm1 may only effectively interact with a few FG-Nups that located in the NPC's central scaffold and nuclear basket (more details in Section 5).
By SPEED microscopy, we have obtained 3 topographies of the FG-Nup barrier separately based on FG-FG, FG-importin and FG-exportin interactions.75 We found that these maps have fundamental similarities, suggesting that these different probes may largely bind to same groups of FG-domains or recognize almost all the available FG-domains in the native NPCs. Finally, a complete super-resolution 3D-map of the native configuration of FG-domains in the native NPCs was obtained by merging the above 3 integrated 3D-tomographies from all 3 sets75 (Fig. 1). Four distinct zones are shown across NPCs (Fig. 1 A-B), with the highest density of FG-domains in the central scaffold peripheral regions, barely any FG-domains in the central axial pore, and the medium density of FG-repeats decorating both ends of NPCs. Furthermore, 3 additional layers were observed in the central scaffold regions: a central axial channel pore almost devoid of FG-domains, a belt layer next to the wall with a medium level of FG-domains, and a high density region of FG-domains in between the previous 2 regions (Fig. 1 C-D). This map covers the majority of FG-domains of all native FG-Nups since this spatial distribution of the FG-domains agrees well with the full spectrum of the distribution of all FG-Nups in vivo obtained by SPEED microscopy71-75 (Fig. 1).
Figure 1.
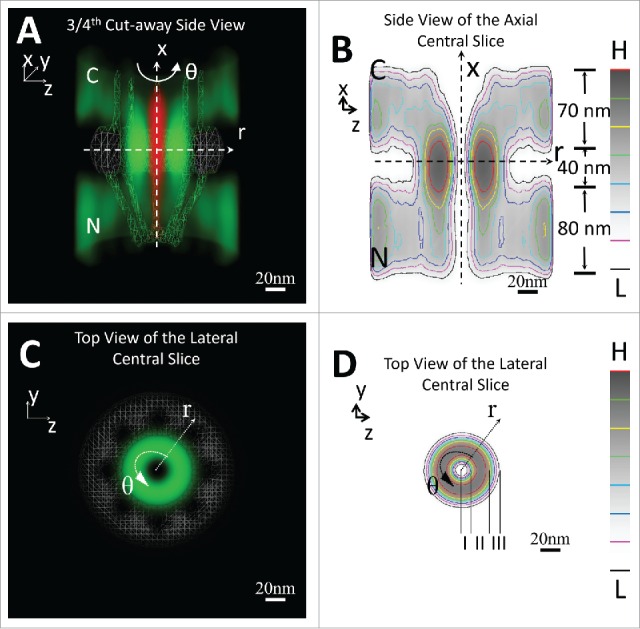
3D Probability Density Map of the FG-Nups barrier in the native NPC. 3D probability density (green clouds) and heat maps of the FG-Nups barrier in the NPC superimposed on the NPC architecture (gray) are shown in both cut-away side view (A-B) and top view (C-D). The passive diffusion route (red clouds) is added in the side view to highlight the relative spatial locations of passive and facilitated diffusion pathways through the native NPC. Both the Cartesian (x, y, z) and cylindrical (x, r, θ) coordinates are shown for these 3D and slice views. Sub-regions in the FG-Nups barrier are labeled in (B) and (D). Red represents the highest density (H) and black the lowest (L) in the color column bar. N, the nucleoplasmic side of the NPC. C, the cytoplasmic side of the NPC. The figures are adapted from previous publication75 with permission.
Computational simulation, cryo-EM and SPEED microscopy in FG-Nups barrier studies
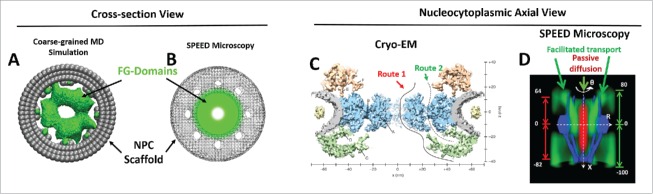
Two other advanced technologies have also recently been employed to study the FG-Nups barrier in the NPC: computer simulation and cryo-EM (Fig. 2). The coarse-grained molecular dynamics simulation uses a one-bead-per-AA model that accounts for interaction forces, such as hydrophobic, hydrophilic, and electrostatic. The model allows to fine-measure the sequence-dependent radius of each FG-Nup and to coarsely determine the collective 3D structure of the FG Nups. The FG-domains from all yeast FG-Nups are distributed inside the NPCs in a doughnut-shaped form, showing a low density zone in the central channel region and a highly charged zone in the scaffold regions, which is in a similar pattern to the 3D map created by SPEED microscopy (76, Fig. 2 A-B). In addition to that, 6 FG-Nups (Nup49, Nup57, Nup46, Nup100, Nup42, and Nup145-2) are present only in the interior of the NPCs, and thus are the main components of the central zone, which is highly charged, while the remaining FG-Nups (Nsp1, Nup159, Nup145-1, Nup60, and Nup1) are extended on either side of the NPC,76 in agreement with biochemical studies (see Section 1).
Figure 2.
FG-Nups barrier obtained from molecular simulation, cryo-EM and SPEED microscopy. FG-domains (green) of the FG-Nups barrier revealed by coarse-grained MD simulation (A) and SPEED microscopy (B) are shown in cross-section view at the NPC scaffold (gray). Shown in nucleocytoplasmic transport axial view, 2 distinct transport routes (route 1 and 2 in C) recently detected by cryo-EM agree with the passive (red) and facilitated (green) transport routes through the native NPCs previously identified by SPEED microscopy (D). Numbers denote nanometers in D. The figures are adapted from previous publications75-77 with permission.
With recent developments, particularly sub-tomogram alignment and averaging, cryo-EM now can preserve the fine structure of macromolecules such as the NPC, via corrected transfer-function in situ.77 The native structure of the nucleocytoplasmic barrier has been visualized: the central channel is occupied by an ordered structure of high-density, which appears to be a ring-like assembly physically attached to the spoke-ring with a porous interface approximately 23 nm from the central channel pore axial. Furthermore, the central channel ring also links extends to the cytoplasmic ring and the entangled filamentous nucleoplasmic ring. In this study, 2 transport routes are suggested for nuclear transport: the central route and the meshwork route, which are almost identical to our proposed passive diffusion route and facilitated diffusion route (see Section 5). This native structure of FG-Nups observed from cryo-EM bears high similarity to the super-resolution-3D-tomograph obtained from SPEED microscopy (77, Fig. 2 C-D) (discussed further below).
Effective interaction ranges/transport routes of NTRs in native NPCs
Effective interaction ranges of NTRs
To dissect the transport pathways of NTRs that carry cargoes across the FG-Nups barrier, the interactions of major importins (Impβ1, Impβ2, or NTF2) and exportins (Crm1, CAS, or Tap-p15) with FG-Nups were mapped via SPEED microscopy and the super-resolution 3D-probability density distributions of these NTRs were reconstructed in native NPCs.75 Remarkably, we found that each NTR has its own unique effective strong and weak interaction zones with the FG-Nups barrier in native NPCs (Fig. 3A). For instance, Imp-β1 interacted with FG-Nups located throughout the entire NPC with a single strong interaction zone from −100 nm to 90 nm (0 in the middle plan of the NPC and – at the nucleoplasmic side). In contrast, Imp-β2 and NTF2 contained both strong and weak interaction zones with FG-Nups. As shown in Fig. 3A, the interaction between Imp-β2 and FG-Nups contains a strong-interaction zone from −50 nm to 50 nm around central region and 2 weak-interaction zones on either sides of the NPC, whereas NTF2 interacts with FG-Nups in an even more complex manner, showing 2 strong-interaction zones (from −20 nm to 20 nm in the central scaffold and −70 nm to −40 nm on the nucleoplasmic side) and 3 weak-interaction zones (from −100 nm to −70 nm, −40 nm to −20 nm, and 20 nm to 90 nm).
Figure 3.
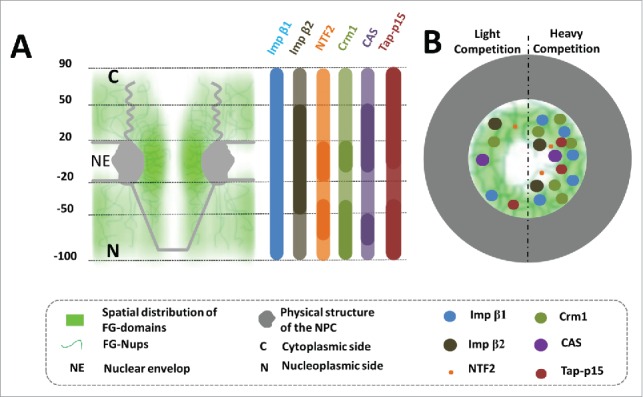
Effective interaction zones for each NTR and competition among multiple NTRs in the FG-Nups barrier of the native NPC. (A) The schematic represents the effective strong (darker color) and weak (lighter color) interaction zones between FG-domains (green clouds containing curved lines) and different NTRs including importins (Impß1, Impß2, and NTF2) and exportins (Crm1, CAS, and Tap-p15). Numbers denote nanometers. C, the cytoplasmic side of NPC; N, the nucleoplasmic side of NPC. (B) This figure is the representation of light (left) and heavy (right) competition among NTRs. Specifically, NTRs could find more available binding sites in the FG-Nups barrier (green clouds containing curved lines) under light competition conditions than heavy competition circumstances.
In a distribution similar to the distribution of Imp-β1-FG interaction, Tap-p15 also interacted with FG-Nups almost completely throughout the whole NPC with a single strong-interaction zone, and very little (less than 1% probability between 0 and −40 nm in Fig. 3A) present in the central axial channel. Interestingly data from CAS and Crm1 displayed complex pictures of NTR-FG-Nup interactions, with patterns of multiple strong- and weak- interactions zones, somewhat similar to TNF2 pattern. Two strong-interaction zones (from −80 nm to −50 nm and −10 nm to 50 nm) and 3 weak-interaction zones (from −100 nm to −80 nm, −50 nm to −10 nm, and 50 nm to 90 nm) were seen for CAS, and 2 strong-interaction regions (from −100 nm to −40 nm and −10 nm to 20 nm) and 2 weak-interaction regions (from −40 nm to −10 nm and 20 nm to 90 nm) for Crm1 (75, Fig. 3A).
Mapping the distribution of the interaction between each of the 6 individual NTRs with FG-Nups is significant for at least 4 reasons: 1) each individual NTR binds to a unique set of FG-Nups on its way to transport through the native NPCs, largely agreeing with previous studies (Table 1); 2) combined maps of the interactions between these individual NTRs with FG-Nups, along with the interactions of FG-FG Nups, provide more a complete picture of the entire FG-Nups barrier; 3) many FG-Nups, especially those in the central scaffold regions, might be used in the interactions with multiple importins and exportins simultaneously during in vivo transport; and 4) the distinctive patterns of the interactions of each NTR with FG-Nups and a merged fuller picture of FG-Nups barrier are solid evidence in supporting a complex mechanism of NPC translocation of cargos with distinctive transport routes (discussed below).
Distinct transport routes and proposed transport models
Transporting thousands of molecules almost simultaneously in and out of nucleus through each NPC is a complex process and could involve multiple mechanisms governing different transport routes for successful translocation of a variety of cargos. The vision on nucleocytoplasmic transport, however, came largely from limited piece of experimental data. In essence, the currently proposed transport models differ in how to view the biochemical or biophysical nature of the FG-domains of FG-Nups in the NPCs. As a matter of fact, the non-FG AAs other than FG-domains may also contribute to the formation of the selective permeability barrier, as suggested by computer simulation studies.60 A critical question concerns whether the FG-Nups interact (through intra- and inter- polypeptides) with each other in order to form the functional selective permeability barrier, and whether this nature can affect its interactions with cargo-NTR complexes during transport. For example, interacting or non-interacting FG-Nups may form the selective permeability gate with distinct physical conditions of the surface, which may suggest a different mechanism for how FG-Nups interact with cargo-NTR complexes. In addition, some models deal more specifically with how cargo-NTR complexes are translocated across the NPC channel. And finally, a few recently proposed models31,43, 62,75 focus more on how the FG-Nups are distributed or organized within NPCs, evenly distributed across the entire NPC or clustered in certain spatial locations.
The Brownian/virtual gate/polymer brushes model assumes that the non-interacting FG-Nups provide an energy/entropy barrier and steric hindrance to the inert molecules that are randomly moving. The proposal is based on the observation that the net positive charge, or ‘entropic exclusion’ of the FG-Nups, which like ‘polymer brushes’ pushes things away in their surroundings.5,62 Interestingly, the repulsive ‘exclusion zone’ containing GLFG-domains was observed by EM in the cytoplasmic opening of the NPC, and the zone area was devoid of ribosomes and other components.26 On the other hand, macromolecule-bound NTRs interact with FG-Nups at specific sites lower the energy level of the barrier, much like how enzymes lower the energy level in chemical reactions.62-64 The model predicts that FG-Nup filaments would collapse as a consequence of binding to a cargo-NTR complex. Experimentally, it demonstrated that FG-Nup153 behaves entropically repulsive or thermally exclusive, and also collapses upon binding to Importin-β, in agreement with the model.63,64 Others observed that FG-Nup153 mediates the translocation of cargo-NTR complex passing through the NPC channel in a manner described as ‘directed molecular motion’, which is analogous to collapse transition.65
In contrast, the selective phase/hydrogel model argues that the multiple FG-domains of Nups interact with one another hydrophobically and predominantly via F residues to form a cohesive meshwork or hydrogel. The cohesive meshwork across the entire NPC channel provides the barrier that blocks all unwanted large molecules from passing, but contains numerous holes in between the connections to allow the passage of small molecules. The binding of the cargo-NTRs to the FG-domains, on the other hand, dissolve through the hydrogel, permitting the cargo-NTRs to pass through the NPCs.43,66 The interactions of FG-domains was indeed observed for a set of FG-Nups, including 5 of the 6 FG-Nups anchored centrally and one FG-Nup anchored on the cytoplasmic side on the NPC.19 The in vivo interactions between added FG-Nup segments of different FG-domains (GLFG, FxFG, or xxFG) and FG-Nups present in the native NPCs were recently further documented.75 More interestingly, FG-Domains (such as FG/FxFG and GLFG) were capable of forming the cohesive meshwork/hydrogel in vitro and could bind to the NTRs.43,44 Similarly, FG-domains were used to coat supported lipid bilayers and to produce a densely attached ultra-thin FG-domain film, to which the NTR could also bind.67 The evidence from these studies supports the selective phase/hydrogel proposal. The cohesive FG-Nups include Nup42, Nup49, Nup57, Nup100, Nup116, and Nup145N.
Alternatively, the reduction of dimensionality (ROD) model suggests that the FG-Nup filaments may interact in such a way that results in FG domains collapsing and collapsed FG-domain coat the central walls in parallel to provide layers for the cargo-NTRs to travel through, much like in 2-dimensional (2-D) working rather than in 3D Brownian movement.68 The 2-D motion, or sliding motion of the NTF2 along the FG-repeat layer, was indeed seen from the molecular dynamic simulations.46 In a slightly different way, it is speculated that the multiple FG-domains located on the cytoplasmic side, the central channel, and the nuclear side may provide step-wise binding sites for NTRs with increasing affinity to pull through the NTRs to translocate across the NPC.48 The ROD, as well as the oily-spaghetti model,3 hypothesize that FG-Nups do interact with each other and FG Nups are arranged in an interconnected way that there is a narrow central channel open for passive diffusion of small molecules.
The in vitro assay19 detected that some FG-Nups in yeast cells do not exhibit cohesive properties, much like Nup153.63,64 These FG-Nups include FxFG Nups (Nup1, Nup2, Nup60 and Nsp1), and SAFGxPSFG Nups (Nup159). Most of these non-cohesive FG-Nups are located peripherally on the outside of the NPC opening. Therefore, forest/the 2-gate model was proposed: the non-cohesive FG-domains in the peripheral sides of the channel may function as one gate (the repulsive gate) and the cohesive FG-domains in the interior of the central channel may function as another gate (the selective gate).19 The cohesive FG-domains are clustered as ‘shrubs’ from those collapsed-coil domains, and these ‘shrubs’ could be one physical zone (similar to the selective gate above). Whereas the non-cohesive FG-domains are clustered as a ‘tree’ from those extended-coil domains or the combination of extended-coil and collapsed-coil domains, and this represents another physical zone (similar to the repulsive gate above). Therefore, the entire transport channel was decorated with these ‘shrubs and trees’ as transport routes, much like a ‘forest’.31 A molecular dynamic modeling study suggested that Nsp1 FG-domains were able to form brush-like structure with less cross-linked bundles in the NPC channel toward the peripheral opening and a sieve-like structure with more frequently cross-linked bundles in the central region, also suggesting the FG-domains are organized in patterns that vary in different locations of the NPC channel.69
Apparently, each model proposed above can explain some aspects of the NTR-facilitated translocation of large molecules. While, for passive diffusion of small molecules, the models debate whether a single central channel or multiple pores exists in NPCs. To distinguish these models and to provide direct evidence for the location of the transport routes for both passive diffusion of small molecules and for the facilitated diffusion of large molecules, efforts have been taken to map the passive diffusion sites and the interaction sites of FG-Nups with NTRs in real-time in vivo traffic conditions.70,71, 75 The data from our 3D mapping identified that the native FG-Nups are not evenly distributed, but instead clustered in certain spatial locations; and that the interactions of FG-Nups with NTRs as well as the distribution of small molecules along the NPC channel clearly demonstrated that the central channel axial pore is the passive diffusion route and the peripheral regions around the central channel are the sites for facilitated diffusion. These two transport routes are largely distinct, with certain overlap, dependent on the size of transiting molecules.70,71, 75
Competitions between NTRs
The complex mechanisms evolved in eukaryotic cells govern the interaction of cargo-bound NTRs with FG-Nups, as well as the direction of the movement of cargo-bound NTRs across the NPC (briefly discussed in section 1). One factor in NTR-FG-Nups interactions is the competition of binding by multiple NTRs to the same or similar FG-segments. Whether a major NTR that transports essential or a large amount of macromolecules would have a priority of binding (with enhanced affinity) to its FG-Nups over other NTRs performing minor transporting roles remains unknown.
It is well known that NTRs, have substantial differences in their binding affinity toward FG-Nups. Moreover, in living cells, every NTR is able to load its corresponding cargo, find and interact with its FG-Nups, and translocate the cargo through the NPC to reach its target site. The entire translocation process proceeds in a seamless manner without perturbing the integrity of the FG-Nups permeability barrier. In our SPEED microscopy imaging experiments, first to ensure each NTR to have sufficient interactions with the FG-Nups barrier, a tested NTR (importins tested include Imp-β1, Imp-β2, and NTF2; exportins examined include Crm1, CAS, and Tap-p15) was loaded in small amount (1nM, here referred as light competition) into the NPCs of permeabilized cells. On the other hand, as comparisons and to demonstrate competition between NTRs in native NPCs, an excess amount (15uM) of each NTR (unlabeled) was added to NPCs of permeabilized cells, and then its inhibition of labeled NTR with light loading (1nM) on binding to its FG-Nups (heavy competition) was tested. As expected, dominant Imp-β1 almost completely dislocated the interaction of Imp-β2 or TNF2 with their FG-Nups, forming their respective strong interaction zones in the central axial channel. Likewise, the major exportin Crm1 also effectively inhibited CAS and Tap-p15 from binding to their FG-Nups, shifting their unique interaction zones into the central axial channel. In contrast, neither Impβ2 nor NTF2 was able to significantly affect the binding of Impβ1 to its FG-Nup targets under heavy competition conditions. Ineffective competition/inhibition was also seen with the presence of excess amount of CAS or Tap-p15 as Crm1 largely retained effective binding and formed similar unique strong- and weak- reaction zones. However, minor effects were observed, ranging from losing about 5 to 9% of their interactions with FG-Nups for both Imp β1 and Crm1 in their original regions. These minor effects might also be induced, or at least partially, by other factors, such as conformational changes of the FG-Nups caused by the high concentration of NTRs and self-competition (Fig. 3B).75
Interestingly, competition of NTRs in living cells, on the other hand, could be moderate, compared to above heavy loading of NTRS in permeabilized cells. For example, the spatial binding patterns of Imp β1 and Crm1 to FG–Nups in living cells are largely similar to the patterns in permeabilized cells, but also with some difference. Approximately 7% of Imp β1 retained on the cytoplasmic side of NPC in permeabilized cells was now present in the central axial channel, and about 10% of Crm1 located in the nuclear basket moved into the central axial channel as well. It was likely that these alterations might be due to competition from other NTRs present in living cells (with their biological levels, hence medium competition), which are absent in permeabilized cells. Finally, cargo-bound NTRs and the gradient of RanGTP/RanGDP across NE might additionally affect the interaction of tagged Imp β1 or Crm1 with FG-Nups in live cells as well.75
Summary
Understanding the nature and functions of intrinsically disordered FG-Nups in native NPCs is always the key step in unraveling the mechanism of nucleocytoplasmic transport. Over the past years, numerous methodologies, including biochemical, biophysical, computational and microscopy imaging approaches, have shed light on the 2 critical roles that FG-Nups play in mediating nucleocytoplasmic transport: 1) constituents of the NPC's selective permeability barrier to inhibit large macromolecules that should not enter nucleus; and 2) providers of binding sites for NTRs to bridge the translocation of macromolecules across the NPC. In this review paper, we have firstly reviewed the major well-studied features of FG-Nups in the NPC, including their types and spatial locations, the interactions among FG-Nups and the bindings between FG-Nups and NTRs. In the latter sections of this review paper, we have then highlighted the major new findings for the FG-Nups barrier obtained with our high-speed super-resolution SPEED microscopy as follows: 1) the 3D native conformation of FG-Nups in native NPCs is achieved for the first time; 2) each NTR possesses a unique interaction zone within the FG-Nups barrier, in which 2 major NTRs, importin β1 and Crm1, outcompete other NTRs in binding FG-Nups; and 3) NTRs may alter the tomography of the FG-Nups barrier and affect one another's pathways under circumstances of heavy competition.
Abbreviations
- AA
amino acid
- EM
electron microscopy
- Kap
karyopherin
- NES
nuclear export signals
- NLS
nuclear localization sequences
- NPC
nuclear pore complex
- NTF2
nuclear transport factor 2
- NTR
nuclear transport receptor
- Nup
nucleoporin
- SPEED microscopy
single-point edge-extraction sub-diffraction microscopy
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The project was supported by grants from the National Institutes of Health (NIH GM094041, GM097037 and GM116204 to W.Y.).
References
- [1].Görlich D, Kutay U. Transport between the cell's nucleus and the cytoplasm. Annu Rev Cell Dev Biol 1999; 15:607-60; PMID:10611974; http://dx.doi.org/ 10.1146/annurev.cellbio.15.1.607 [DOI] [PubMed] [Google Scholar]
- [2].Floch A, Palancade B, Doye V. Fifty years of nuclear pores and nucleocytoplasmic transport studies: multiple tools revealing complex rules. Methods Cell Biol 2014; 122:1-40; PMID:24857723; http://dx.doi.org/ 10.1016/B978-0-12-417160-2.00001-1 [DOI] [PubMed] [Google Scholar]
- [3].Macara I. Transportation into and out of the nucleus. Microbiol Mol Biol Rev 2001; 65:570-94; PMID:11729264; http://dx.doi.org/ 10.1128/MMBR.65.4.570-594.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tetenbaum-Novatt J, Rout M. The mechanism of nucleocytoplasmic transport through the nuclear pore complex. Cold Spring Harbor Symposia on Quantatative Biol 2014; 75:567-84; http://dx.doi.org/ 10.1101/sqb.2010.75.033 [DOI] [PubMed] [Google Scholar]
- [5].Rout M, Aitchison J, Suprapto A, Hjertaas K, Zhao Y, Chait B. The yeast nuclear pore complex: composition, architecture, and transportation mechanism. J Biol Chem 2000; 148:635-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bayliss R, Kent H, Corbett A, Stewart M. Crystallization and initial x-ray diffraction characterization of complexes of FxFG nucleoporin repeats with nuclear transport factors. J Struct Biol 2000; 131:240-7; PMID:11052897; http://dx.doi.org/ 10.1006/jsbi.2000.4297 [DOI] [PubMed] [Google Scholar]
- [7].Allen N, Huang L, Burlingame A, Rexach M. Proteomic analysis of nucleoporin interacting proteins. Biol Chem J 2001; 276:29268-74; http://dx.doi.org/ 10.1074/jbc.M102629200 [DOI] [PubMed] [Google Scholar]
- [8].Denning D, Patel S, Uversky V, Fink A, Rexach M. Disorder in the nuclear pore complex: the FG repeat regions of nucleoporins are natively unfolded. Proc Natl Acad Sci USA 2003; 100:2450-5; PMID:12604785; http://dx.doi.org/ 10.1073/pnas.0437902100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Denning D, Uversky V, Patel S, Fink A, Rexach M. The Saccharomyces cerevisiae Nucleoporin Nup2p is a natively unfolded protein. Biol Chem J 2002; 277:33447-55; http://dx.doi.org/ 10.1074/jbc.M203499200 [DOI] [PubMed] [Google Scholar]
- [10].Raices M, D'Angelo MA. Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions. Nature Rev 2012; 13:687-99; http://dx.doi.org/ 10.1038/nrm3461 [DOI] [PubMed] [Google Scholar]
- [11].Löschberger A, Frank C, Krohne G, van de Linde S, Sauer M. Correlative super-resolution fluorescence and electron microscopy of the nuclear pore complex with molecular resolution. Cell Sci J 2014; 127:277-89; http://dx.doi.org/ 10.1242/jcs.137596 [DOI] [PubMed] [Google Scholar]
- [12].Terry L, Wente S. Flexible gates: dynamic topologies and functions for FG nucleoporins in nucleocytoplasmic transport. Eukaryot Cell 2009; 8:1814-27; PMID:19801417; http://dx.doi.org/ 10.1128/EC.00225-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Denning D, Rexach M. Rapid evolution exposes the boundaries of domain structure and function in natively unfolded FG Nucleoporins. Mol Cell Proteomics 2007; 6:272-282; PMID:17079785; http://dx.doi.org/ 10.1074/mcp.M600309-MCP200 [DOI] [PubMed] [Google Scholar]
- [14].Rexach M, Blobel G. Protein import into nuclei: Association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell 1995; 8:683-92; http://dx.doi.org/ 10.1016/0092-8674(95)90181-7 [DOI] [PubMed] [Google Scholar]
- [15].Delphin G, Guan T, Melchior F, Gerace L. RanGTP targets p97 to RanBP2, a filamentous protein localized at the cytoplasmic periphery of the nuclear pore complex. Mol Biol 1997; Cell 8:2379-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Allen N, Patel S, Huang L, Chalkley R, Burlingame A, Lutzman M, Hurt E, Rexach M. Deciphering networks of protein interactions at the nuclear pore complex. Mol Cell Proteomics 2002; 1:920-46; http://dx.doi.org/ 10.1074/mcp.T200012-MCP200 [DOI] [PubMed] [Google Scholar]
- [17].Peleg O, Lim R. Converging on the function of intrinsically disordered nucleoporins in the nuclear pore complex. Biol Chem J 2010; 291:719-30 [DOI] [PubMed] [Google Scholar]
- [18].Milles S, Lemke E. Single molecule study of the intrinsically disordered FG-repeat nucleoporins. Biophys J 2011; 101:1710-29; PMID:21961597; http://dx.doi.org/ 10.1016/j.bpj.2011.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Patel S, Belmont B, Sante J, Rexach M. Natively unfolded nucleoporins gate protein diffusion across the nuclear pore complex. Cell 2007; 129:83-96; PMID:17418788; http://dx.doi.org/ 10.1016/j.cell.2007.01.044 [DOI] [PubMed] [Google Scholar]
- [20].Strawn L, Shen T, Shulga N, Goldfarb DS, Wente SR. Minimal nuclear pore complexes define FG repeat domains essential for transport. Nature Cell Biol 2004; 6:197-206; PMID:15039779; http://dx.doi.org/ 10.1038/ncb1097 [DOI] [PubMed] [Google Scholar]
- [21].Zeitler B, Weis K. The FG-repeat asymmetry of the nuclear pore complex is dispensible for bbulk nucleocytoplasmic transport in vivo. Cell Biol 2004; 167:583-90; http://dx.doi.org/ 10.1083/jcb.200407156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Suh E, Gumbiner B. Translocation of ß-catenin into the nucleus independent of interactions with FG-rich nucleoporins. Exp Cell Res 2003; 290:447-56; PMID:14568002; http://dx.doi.org/ 10.1016/S0014-4827(03)00370-7 [DOI] [PubMed] [Google Scholar]
- [23].Sampathkumar P, Kim S, Upla P, Rice W, Phillips J, Timney B, Pieper U, Bonanno J, Fernandez-Martinez J, Hakhverdyan Z, Ketaren N, Matsui T, Weiss T, Stokes D, Sauder J, Burley S, Sali A, Rout M, Almo S. Structure, dynamics, evolution, and function of a major scaffold component in the nuclear pore complex. Structure 2013; 21:560-71; PMID:23499021; http://dx.doi.org/ 10.1016/j.str.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ando D, Colvin M, Gopinathan A. Physical motif clustering within intrisically disordered nucleoporin sequences reveals universal functional features. PLOS One 2013; 8:e73831; PMID:24066078; http://dx.doi.org/ 10.1371/journal.pone.0073831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rout M, Wente S. Pores for thought: nuclear pore complex proteins. Trends Cell Biol 1994; 4:357-65; PMID:14731624; http://dx.doi.org/ 10.1016/0962-8924(94)90085-X [DOI] [PubMed] [Google Scholar]
- [26].Fiserova J, Spink M, Richards S, Saunter C, Goldberg M. Entry into the nuclear pore complex is controlled by a cytoplasmic exclusion zone containing dynamic GLFG-repeat nucleoporin domains. J Cell Sci 2014; 127:124-36; PMID:24144701; http://dx.doi.org/ 10.1242/jcs.133272 [DOI] [PubMed] [Google Scholar]
- [27].Rout M, Aitchison J. The nuclear pore complex as a transport machine. Biol Chem J 2001; 276:16593-6; http://dx.doi.org/ 10.1074/jbc.R100015200 [DOI] [PubMed] [Google Scholar]
- [28].Bayliss R, Littlewood T, Stewart M. Structural basis for the interaction between FxFG nucleoporin repeats and importin-β in nuclear trafficking. Cell 2000; 102:99-108; PMID:10929717; http://dx.doi.org/ 10.1016/S0092-8674(00)00014-3 [DOI] [PubMed] [Google Scholar]
- [29].Patel S, Rexach M. Discovering novel interactions at the nuclear pore complex using bead halo. Mol Cell Proteomics 2008; 7:121-31; PMID:17897934; http://dx.doi.org/ 10.1074/mcp.M700407-MCP200 [DOI] [PubMed] [Google Scholar]
- [30].Hayakawa A, Babour A, Senggmanivong L, Dargemont C. Ubiquitylation of the nuclear pore complex controls nuclear migration during mitosis in S. cerevisiae. Cell Biol 2012; J 196:19-27; http://dx.doi.org/ 10.1083/jcb.201108124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yamada J, Phillips J, Patel S, Goldfien G, Calestagne-Morelli A, Huang H, Reza R, Acheson J, Krishnan V, Newsam S, Gopinathan A, Lau E, Colvin M, Uversky V, Rexach M. A bimodal distribution of two distinct categories of intrinsically-disordered structures with separate functions in FG nucleoporins. Mol Cell Proteomics 2010; 9:2205-24; PMID:20368288; http://dx.doi.org/ 10.1074/mcp.M000035-MCP201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Finlay D, Forbes D. Reconstitution of biochemically altered nuclear pores:transport can be eliminated and restored. Cell 1990; 60:17-20; PMID:2295087; http://dx.doi.org/ 10.1016/0092-8674(90)90712-N [DOI] [PubMed] [Google Scholar]
- [33].Bonitaci N, Moroianu J, Radu A, Blobel G. Karyopherin b2 mediates nuclear import of a mRNA binding protein. Proc Natl Acad Sci USA 1997; 94:5055-60; PMID:9144189; http://dx.doi.org/ 10.1073/pnas.94.10.5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hülsmann B, Labokha A, Görlich D. The permeability of reconstituted nuclear pores provides direct evidence for the selective phase model. Cell 2012; 150:738-51; PMID:22901806; http://dx.doi.org/ 10.1016/j.cell.2012.07.019 [DOI] [PubMed] [Google Scholar]
- [35].Bernad R, Engelsma D, Sanderson H, Pickersgill H, Fornerod M. Nup214-Nup88 nucleoporin subcomplex is required for CRM1 mediated 60 S peribosomal nuclear export. J Biol Chem 2006; 281:19378-86; PMID:16675447; http://dx.doi.org/ 10.1074/jbc.M512585200 [DOI] [PubMed] [Google Scholar]
- [36].Roloff S, Spillner C, Kehlenbach R. Several phenylalanine-glycine motives in the nuclearporin Nup214 are essential for binding of the nuclear export receptor CRM1. Biol Chem J 2013; 288:3952-63; http://dx.doi.org/ 10.1074/jbc.M112.433243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hamada M, Haeger A, Jeganathan K, Van Ree J, Matureanu L, Walde S, Joseph J, Kehlenbach R, van Deursen J. Ran-dependant docking of importin-ß to RanBP2/Nap358 filaments is essential for protein import and cell viability. J Cell Biol 2011; 194:597-612; PMID:21859863; http://dx.doi.org/ 10.1083/jcb.201102018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xu S, Powers M. In vivo analysis of human nucleoporin repeat domain interactions. Mol Biol Cell 2013; 24:1222-31; PMID:23427268; http://dx.doi.org/ 10.1091/mbc.E12-08-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dölker N, Zachariae U, Grubmüller H. Hydrophillic linkers and polar contacts affect aggregation of FG repeat peptides. Biophys J 2010; 98:26532661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Popken P, Ghavami A, Onck P, Poolman B, Veenhoff L. Size-dependent leak of soluble and membrane proteins through the yeast nuclear pore complex. Mol Biol Cell 2015; 26:1386-894; PMID:25631821; http://dx.doi.org/ 10.1091/mbc.E14-07-1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Atkinson C, Mattheyes A, Kampmann M, Sanford S. Conserved spatial organization of FG domains in the nuclear pore complex. Biophys J 2013; 104:37-50; PMID:23332057; http://dx.doi.org/ 10.1016/j.bpj.2012.11.3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Halfmann R, Wright J, Alberti S, Lindquist S. Prion formation by a yeast GLFG nucleoporin. Prion 2012; 6:391-9; PMID:22561191; http://dx.doi.org/ 10.4161/pri.20199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Frey S, Görlich D. A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. . Cell 2007; 130:512-23; PMID:17693259; http://dx.doi.org/ 10.1016/j.cell.2007.06.024 [DOI] [PubMed] [Google Scholar]
- [44].Frey S, Görlich D. FG/FxFG as well as GLFG repeats form a selective permeability barrier with self-healing properties. EMBO J 2009; 58:2554-67; http://dx.doi.org/ 10.1038/emboj.2009.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Milles S, Bui K, Koehler C, Eltsov M, Beck M, Lemke E. Facilitated aggregation of FG nucleoporins under molecular crowding conditions. EMBO Rep 2013; 14:178-83; PMID:23238392; http://dx.doi.org/ 10.1038/embor.2012.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Miao L, Schulten K. Transport-related structures and processes of the nuclear pore complex studied through molecular dynamics. Structure 2009; 17:449-59; PMID:19278659; http://dx.doi.org/ 10.1016/j.str.2008.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Naim B, Zbaida D, Dagan S, Kapon R, Reich Z. Cargo surface hydrophobicity is sufficient to overcome the nuclear pore complex selective barrier. EMBO J 2009; 28:2697-705; PMID:19680225; http://dx.doi.org/ 10.1038/emboj.2009.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bayliss R, Littlewood T, Strawn L, Wente S, Stewart M. GLFG and FxFG nucleoporins bind to overlapping sites and importin-β. J Biol Chem 2002; 277:50597-606; PMID:12372823; http://dx.doi.org/ 10.1074/jbc.M209037200 [DOI] [PubMed] [Google Scholar]
- [49].Zilman A, Di Talia S, Chait B, Rout M., Magnasco M. Efficiency, selectivity, and robustness of nucleocytoplasmic transport. PLoS Comput Biol 2007; 3:1281-90; http://dx.doi.org/ 10.1371/journal.pcbi.0030125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Colwell I, Brenner M, Ribber K. Charge as a selection criterion for transportation through the nuclear pore complex. PLoS Comput Biol 2010; 6:e1000747; PMID:20421988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tagliazucchi M, Peleg O, Kroger M, Rabin Y, Szleifer I. Effect of charge, hydrophobicity, and sequence of nucleoporins on the translocation of model particles through the nuclear pore complex. Proc Natl Acad Sci USA 2013; 110(9):3363-8; PMID:23404701; http://dx.doi.org/ 10.1073/pnas.1212909110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Occhipinti L, Chang Y, Altvater M, Menet A, Kemmler S, Panse VG. Non-FG mediated transport of the large pre-ribosomal subunit through the nuclear pore complex by the mRNA export factor Gle2. Nucleic Acids Res 2013; 41:8266-79; PMID:23907389; http://dx.doi.org/ 10.1093/nar/gkt675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yaseem N, Blobel G. GTP hydrolsis links initiation and termination of nuclear import on the nucleoporin Nup358. J Biol Chem 1999; 274:26493-502; PMID:10473610; http://dx.doi.org/ 10.1074/jbc.274.37.26493 [DOI] [PubMed] [Google Scholar]
- [54].Finn E, DeRoo E, Clement G, Rao S, Kruse S, Kokanovich K, Belanger K. A subset of FG nucleoporins is necessary for efficient Msn5-mediated nuclear protein export. Biochim Biophys Acta 2013; 1833:1096-103; PMID:23295456; http://dx.doi.org/ 10.1016/j.bbamcr.2012.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Damelin M, Silver P. Mapping Interactions between nuclear transport factors in living cells reveals pathways through the nuclear pore complex. Mol Cell 2000; 5:133-40; PMID:10678175; http://dx.doi.org/ 10.1016/S1097-2765(00)80409-8 [DOI] [PubMed] [Google Scholar]
- [56].Otsuka S, Iwasaka S, Yoneda Y, Takeyasu K, Yoshimura SH. Individual binding pockets of importin-β for FG-Nucleoporins have different binding properties and different sensitivities to RanGTP. PNAS 2008; 41:8266-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Clarkson W, Kent H, Stewart M. Separate binding sites on nuclear transport factor 2 (NTF2) for GDP-Ran and the phenylalanine-rich repeat regions of nucleoporins p62 and Nsp1p. J Mol Biol 1996; 263:517-24; PMID:8918934; http://dx.doi.org/ 10.1006/jmbi.1996.0594 [DOI] [PubMed] [Google Scholar]
- [58].Shah S, Forbes D. Separate nuclear import pathways converge on the nucleoporin Nup153 and can be dissected with dominant negative inhibitors. Curr Biol 1998; 8:1376-86; PMID:9889100; http://dx.doi.org/ 10.1016/S0960-9822(98)00018-9 [DOI] [PubMed] [Google Scholar]
- [59].Tu L, Fu G, Zilman A, Musser S. Large cargo transport by nuclear pores: implications for the spatial organization of Fg-nucleoporins. EMBO J 2013; 32:3220-30; PMID:24213245; http://dx.doi.org/ 10.1038/emboj.2013.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Miao L, Schulten K. Probing a structural model of the nuclear pore complex studied through molecular dynamics. Biophys J 2010; 98:1658-67; PMID:20409487; http://dx.doi.org/ 10.1016/j.bpj.2009.12.4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Goryaynov A, Yang W. Role of molecular charge in nucleocytoplasmic transport. PLOS One 2014; 9:e88792; PMID:24558427; http://dx.doi.org/ 10.1371/journal.pone.0088792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rout M, Aitchison J, Magnasco M, Chait B. Virtual gating and nuclear transport: the whole picture. Trends Cell Biol 2003; 13:622-8; PMID:14624840; http://dx.doi.org/ 10.1016/j.tcb.2003.10.007 [DOI] [PubMed] [Google Scholar]
- [63].Lim R, Köser J, Huang N, Schwarz-Herion K, Aebi U. Nanomechanical interactions of phenylalanine-glycine nucleoporins studied by single molecule force-volume spectroscopy. Struct Biol J 2007; 159:277-89; http://dx.doi.org/ 10.1016/j.jsb.2007.01.018 [DOI] [PubMed] [Google Scholar]
- [64].Lim R, Huang N, Koser J, Deng J, Lau K, Schwart-Herion K, Aebi U. Flexible phenylalanine-glycine nuceloporins as entropic barriers to nucleocytoplasmic transport. Proc Natl Acad Sci USA 2006; 103:9512-17; PMID:16769882; http://dx.doi.org/ 10.1073/pnas.0603521103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Caradelli F, Lanzano L, Graton E. Capturing directed molecular motion in the nuclear pore complex of live cells. Proc Natl Acad Sci USA 2012; 109:9863-8; PMID:22665783; http://dx.doi.org/ 10.1073/pnas.1200486109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ribbeck K, Görlich D. Kinetic analysis of translocation through nuclear pore complexes. EMBO J 2001; 20:1320-30; PMID:11250898; http://dx.doi.org/ 10.1093/emboj/20.6.1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Eisele N, Frey S, Piehler J, Görlich D, Richter R. Ultrathin nucleoporin phenylalanine-glycine repeat films and their interaction with nuclear transport receptors. EMBO Rep 2012; 11:366-72; http://dx.doi.org/ 10.1038/embor.2010.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Peters R. Translocation through the nuclear pore complex: selectivity and speed by reduction-of-dimensionality. Traffic 2005; 6:421-427; PMID:15813752; http://dx.doi.org/ 10.1111/j.1600-0854.2005.00287.x [DOI] [PubMed] [Google Scholar]
- [69].Gamini R, Han W, Stone J, Schulten K. Assembly of Nsp1 nucleoporins provides insight into nuclear pore complex gating. PLoS Comput Biol 2014; 10:e1003488; PMID:24626154; http://dx.doi.org/ 10.1371/journal.pcbi.1003488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yang W. ‘Natively unfolded’ nucleporins in nucleocytoplasmic transport. Nucleus 2011; 2:10-16; PMID:21647294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yang W. Distinct, but not completely separate spatial transport routes in the nuclear pore complex. Nucleus 2013; 4:166-75; PMID:23669120; http://dx.doi.org/ 10.4161/nucl.24874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ma J, Yang W. Three-dimentional distribution of transient interactions in the nuclear pore comple obtained from single-molecule snapshots. Proc Natl Acad Sci USA 2010; 107:7305-10; PMID:20368455; http://dx.doi.org/ 10.1073/pnas.0908269107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ma J, Goryanov A, Sarma A, Yang W. Self-regulated viscous channel in the nuclear pore complex. Proc Natl Acad Sci USA 2012; 109:7326-31; PMID:22529346; http://dx.doi.org/ 10.1073/pnas.1201724109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Schnell S, Ma J, Yang W. Three-dimensional mapping of mRNA export through the nuclear pore compex. Genes 2014; 5:1032-1049; PMID:25393401; http://dx.doi.org/ 10.3390/genes5041032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ma J, Goryanov A, Yang W. Super-resolution 3D tomography of interactions and competition in the nuclear pore complex. Nat Struct Mol Biol 2016; 23:239-47; PMID:26878241; http://dx.doi.org/ 10.1038/nsmb.3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ghavami A, Veenhoff L, Giessen E, Onck P. Probing the disordered domain of the nulear pore complex through coarse-grained molecular dynomics simulations. Biophys J 2014; 107:1393-1402; PMID:25229147; http://dx.doi.org/ 10.1016/j.bpj.2014.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Eibauer M, Pellanda M, Turgay Y, Dubrovsky A, Wild A, Medalia O. Structure and gating of the nuclear pore complex. Nat Commun 2015; 6:7532-40; PMID:26112706; http://dx.doi.org/ 10.1038/ncomms8532 [DOI] [PMC free article] [PubMed] [Google Scholar]