Abstract
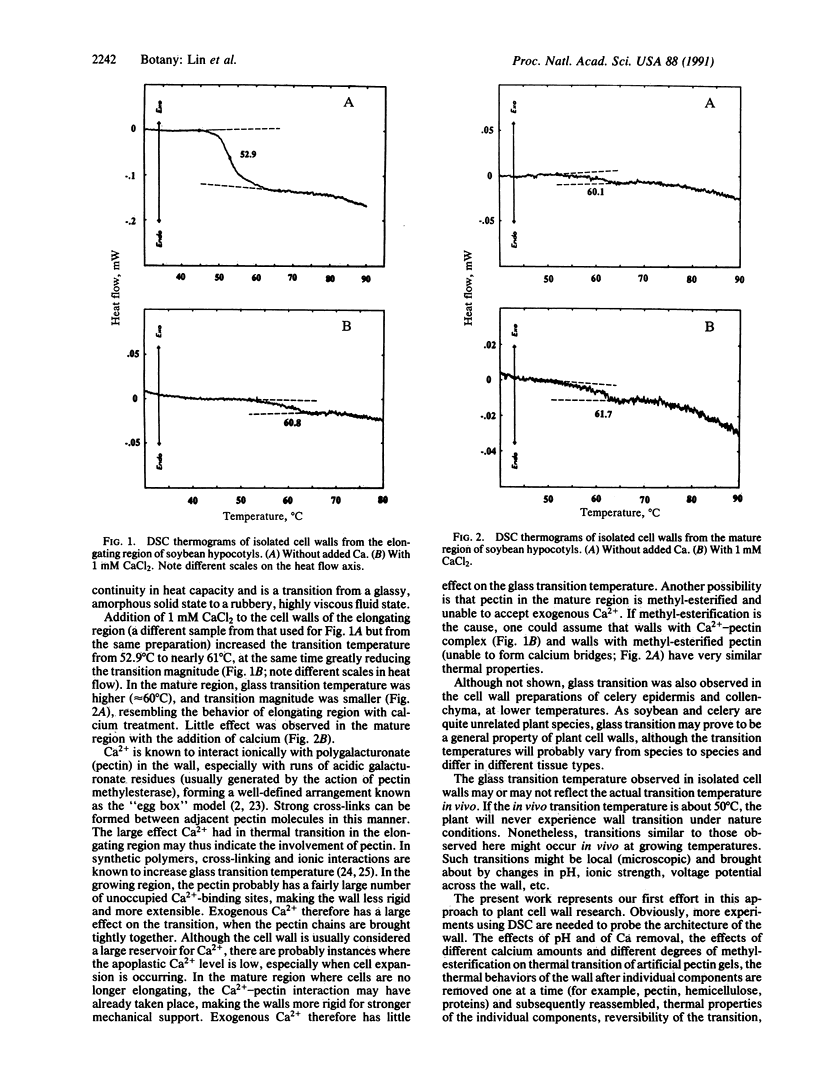
High-sensitivity differential scanning calorimetry has been used to study the phase transition of cell wall preparations of the elongating and mature regions of soybean hypocotyls and of celery epidermis and collenchyma strands. A step-like transition believed to be glass transition was observed in walls isolated from the elongating region of soybean hypocotyls at 52.9 degrees C. Addition of 1 mM CaCl2 to the cell wall preparation increased the transition temperature to 60.8 degrees C and greatly reduced the transition magnitude. In walls from the mature region, the transition was small and occurred at a higher temperature (60.1 degrees C). Addition of calcium to the mature region cell wall had little effect on the transition. Based on the known interactions between calcium and pectin, we propose that calcium affects the glass transition by binding to the polygalacturonate backbone of wall pectin, resulting in a more rigid wall with a smaller transition at a higher temperature. The mature region either has more calcium in the wall or has more methyl-esterified pectin, making it less responsive to added calcium.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertazzon A., Tsong T. Y. High-resolution differential scanning calorimetric study of myosin, functional domains, and supramolecular structures. Biochemistry. 1989 Dec 12;28(25):9784–9790. doi: 10.1021/bi00451a036. [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Lin L. N. Study of strong to ultratight protein interactions using differential scanning calorimetry. Biochemistry. 1990 Jul 24;29(29):6927–6940. doi: 10.1021/bi00481a024. [DOI] [PubMed] [Google Scholar]
- Cassab G. I., Nieto-Sotelo J., Cooper J. B., van Holst G. J., Varner J. E. A developmentally regulated hydroxyproline-rich glycoprotein from the cell walls of soybean seed coats. Plant Physiol. 1985 Mar;77(3):532–535. doi: 10.1104/pp.77.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S., Sturtevant J. M. A scanning calorimetric study of the thermal denaturation of the lysozyme of phage T4 and the Arg 96----His mutant form thereof. Biochemistry. 1989 May 2;28(9):3788–3792. doi: 10.1021/bi00435a024. [DOI] [PubMed] [Google Scholar]
- Ladbrooke B. D., Williams R. M., Chapman D. Studies on lecithin-cholesterol-water interactions by differential scanning calorimetry and X-ray diffraction. Biochim Biophys Acta. 1968 Apr 29;150(3):333–340. doi: 10.1016/0005-2736(68)90132-6. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Sturtevant J. M. Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3862–3866. doi: 10.1073/pnas.73.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marky L. A., Breslauer K. J. Calculating thermodynamic data for transitions of any molecularity from equilibrium melting curves. Biopolymers. 1987 Sep;26(9):1601–1620. doi: 10.1002/bip.360260911. [DOI] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Fry S. C., Albersheim P. Structure and function of the primary cell walls of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- Pace C. N., McGrath T. Substrate stabilization of lysozyme to thermal and guanidine hydrochloride denaturation. J Biol Chem. 1980 May 10;255(9):3862–3865. [PubMed] [Google Scholar]
- Powell D. A., Morris E. R., Gidley M. J., Rees D. A. Conformations and interactions of pectins. II. Influences of residue sequence on chain association in calcium pectate gels. J Mol Biol. 1982 Mar 15;155(4):517–531. doi: 10.1016/0022-2836(82)90485-5. [DOI] [PubMed] [Google Scholar]
- Schwarz F. P. Interaction of cytidine 3'-monophosphate and uridine 3'-monophosphate with ribonuclease a at the denaturation temperature. Biochemistry. 1988 Nov 1;27(22):8429–8436. doi: 10.1021/bi00422a020. [DOI] [PubMed] [Google Scholar]
- Singh J., de La Roche I. A., Siminovitch D. Differential scanning calorimeter analyses of membrane lipids isolated from hardened and unhardened black locust bark and from winter rye seedlings. Cryobiology. 1977 Oct;14(5):620–624. doi: 10.1016/0011-2240(77)90173-0. [DOI] [PubMed] [Google Scholar]
- Steadman B. L., Trautman P. A., Lawson E. Q., Raymond M. J., Mood D. A., Thomson J. A., Middaugh C. R. A differential scanning calorimetric study of the bovine lens crystallins. Biochemistry. 1989 Dec 12;28(25):9653–9658. doi: 10.1021/bi00451a017. [DOI] [PubMed] [Google Scholar]
- Varner J. E., Lin L. S. Plant cell wall architecture. Cell. 1989 Jan 27;56(2):231–239. doi: 10.1016/0092-8674(89)90896-9. [DOI] [PubMed] [Google Scholar]
- Williams R. J., Leopold A. C. The glassy state in corn embryos. Plant Physiol. 1989 Mar;89(3):977–981. doi: 10.1104/pp.89.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]


