Abstract
Epilepsy is one of the leading causes of chronic neurological morbidity worldwide. Acquired epilepsy may result from a number of conditions such brain injury, anoxia, tumors, stroke, neurotoxicity, and prolonged seizures. Sex differences have been observed in many seizures types; however there are sex-specific seizure disorders that are much more prevalent in women. Despite some inconsistencies, there is a substantial amount of data which indicates that sensitivity to seizure stimuli differs between the sexes. Men generally exhibit greater seizure susceptibility than women, while many women with epilepsy experience a cyclical occurrence of seizures that tend to center around the menstrual period, which has been termed catamenial epilepsy. Some epilepsy syndromes show gender differences with female predominance or male predominance. Steroid hormones, endogenous neurosteroids, and sexually dimorphic neural networks appear to play a key role in sex differences in seizure susceptibility. Neurosteroids such as allopregnanolone exhibit sex differences in their anticonvulsant activity. This article provides a brief overview of the evidence for sex differences in epilepsy and how sex differences influence the use of neurosteroids in epilepsy and epileptogenesis.
Keywords: Epilepsy, neurosteroids, sex differences, gender, GABA receptor
INTRODUCTION
Epilepsy is one of the leading causes of chronic neurological morbidity worldwide. Unlike stroke, migraine and other neurological diseases, epilepsy diagnosis is associated with a certain degree of stigma as patients experience restrictions with driving, swimming, and certain jobs. As per the new proposed classification of epilepsy, epilepsy of unknown etiology (primary epilepsy) (50%) is characterized by generalized seizures with no apparent cause. Epilepsy due to structural/metabolic etiologies (acquired epilepsy) (50%) may result from a number of circumstances including neurotoxicity, brain injury, anoxia, metabolic imbalances, stroke, status epilepticus, prolonged seizures attributable to drug withdrawal, tumors, or encephalitis (Berg et al., 2010). Sex differences have been observed in many types of seizures (Park et al., 2008; Reddy, 2014); however, there are sex specific seizures disorders that are much more prevalent in women. Neurosteroids have promising potential for the treatment of brain disorders (Reddy and Estes, 2016). This article provides a brief overview of the evidence for sex differences in epilepsy and how sex differences influence the use of neurosteroids in epilepsy and epileptogenesis.
SEX DIFFERENCES IN EPILEPSY AND SEIZURE SUSCEPTIBILITY
Epilepsy is a chronic neurological disorder characterized by the unpredictable occurrence of seizures. Epilepsy affects an estimated 3 million Americans and about 65 million people worldwide in a variety of ways (Jacobs et al., 2009; Hesdorffer et al., 2013). About 150,000 new cases of epilepsy are diagnosed in the United States annually (Hesdorffer et al., 2013). Epileptic seizures are classified into focal and generalized seizures. Focal seizures begin focally and account for approximately 60% of all epilepsies, whereas generalized seizures involve both hemispheres and account for the remaining 40% of epilepsy types (Duncan et al., 2006; Berg et al., 2010; Reddy, 2014). Epilepsy can develop as a result of an abnormality in the connectivity of neural networks, an imbalance in inhibitory and excitatory neurotransmitters, or a combination of these conditions. Epileptogenesis is the process by which a brain becomes progressively epileptic due to an initial precipitating event such as brain injury, stroke, infection, or prolonged seizures. Epileptogenesis includes three distinct stages: (i) the initial precipitating event; (ii) the latent period; and (iii) the chronic period with spontaneous seizures. Neuroinflammation and neurodegeneration appear to trigger epileptogenesis; however, other factors play critical roles in the neuropathology (Pitkänen et al., 2009; Reddy, 2013b).
The incidence of epilepsy is sex-specific as men exhibit relatively greater seizure susceptibility than women (Herzog, 2009; Verotti et al., 2007; Reddy, 2010a; 2013a). Although epilepsy affects more men than women, women exhibit seizure syndromes that much more complex and often intractable. Epilepsy affects an estimated 25 million women worldwide. There are about 1.2 million women with epilepsy of child-bearing age in the United States (El-Sayed, 1998; Kaplan et al, 2007; Harden et al., 2009). Epilepsy is associated with special concerns for women of child-bearing age (Morrell, 2003). There is abundant evidence of hormonal effects on seizures since many women report that their seizure patterns change at puberty, menstrual cycle, and also with menopause. The natural fluctuation in steroid hormones that occurs during the menstrual cycle significantly influences seizure patterns and potentially influences drug therapy. An estimated 10 million women in the United States and 100 million women world-wide use hormonal contraceptives. This is associated with some risks including drug interactions and seizure exacerbations (Reddy, 2010b; Younus and Reddy, 2016). However, there is little evidence for major gender related differences in efficacy of antiepileptic drugs (Perucca et al., 2014a). Gender issues in epilepsy therapy are mainly related to pharmacokinetics and the impact of sex specific physiological factors such as pregnancy, menstrual cycle and drug interactions.
There is a growing interest in sex differences in many neurological disorders. Sex differences in seizure susceptibility is one of the re-emerging issues of epilepsy. Clinical evidence shows gender- and age-related expression in many seizure syndromes. Many studies found that the overall incidence of epilepsy is generally higher in males than in females (Benn et al., 2008; Christensen et al., 2005; 2007; 2009; Hauser, 1993; 1997; Lavados et al., 1992; Tekle-Haimanot et al., 1997). Despite some inconsistences in the reports, there is a considerable amount of data that indicates that men exhibit greater seizure susceptibility than females, while females tend to have greater fluctuation in their susceptibility to seizures, including menstrual cycle-related influences and changes in seizure activity (Joensen, 1986; Kotsopoulos et al., 2002; Perucca et al., 2014b). Many epilepsy syndromes show gender predominance (Savic and Engel, 1998; 2014). Female predominance is found in juvenile myoclonic epilepsy, childhood absence epilepsy, and photosensitive epilepsy, while male predominance is reportedly more common in West syndrome, Dravet syndrome, Landau-Kleffner syndrome, and myoclonic atonic seizures. Using the previous classification scheme, idiopathic generalized epilepsy and cryptogenic localization-related epilepsies (currently classified as unknown etiology) are more frequently diagnosed in women, but localization-related symptomatic epilepsies (currently classified as focal epilepsies due to structural metabolic etiologies) are diagnosed more frequently in men (Hauser, 1997; Christensen et al., 2005). The seizure remission outcomes are similar between men and women; however the risk of sudden unexpected death in epilepsy (SUDEP) is greater in men (Hesdorffer et al., 2011). Some forms of epilepsy affect only women. These conditions are mostly genetically-determined or based on natural fluctuations in hormonal status. For example, PCDH19 pediatric epilepsy is a rare genetic epileptic syndrome that affects females (Tan et al., 2015; Ikeda et al., 2016). One major limitation in most studies of sex differences is limited statistical power due to a small subject cohort.
The mechanisms underlying sexual differences in epilepsy are unknown. Differences in seizure susceptibility between men and women may arise from a myriad of factors including microsomal enzyme activity, steroid hormones, and sexual dimorphisms in the neuronal networks of the brain. Sex-related differences in neurosteroids, neuroendocrine control, steroid hormones and other molecular signaling in inhibitory neurotransmission in the brain could influence seizure sensitivity in males and females (Herzog, 2007; Verotti et al., 2007; Reddy, 2010a; 2013a; Chudomel et al., 2009; Reddy, 2014; Tabatadze et al., 2015).
ROLE OF NEUROSTEROIDS IN SEX-SPECIFIC CATAMENIAL EPILEPSY
In many women with epilepsy, susceptibility to seizures conforms to their menstrual cycle. This sex-specific neuroendocrine disorder is referred to as catamenial epilepsy, and affects up to 70% of women with epilepsy. Three types of catamenial seizures have been defined: perimenstrual (C1), periovulatory (C2), and inadequate luteal-phase (C3) (Herzog et al., 1997). The most commonly diagnosed type of catamenial epilepsy is perimenstrual catamenial epilepsy (C1), in which women experience a sharp increase in seizure activity before, during, or after the onset of menstruation (Reddy, 2009a). These types of catamenial epilepsy have been further described in detail in previous articles (Reddy, 2014). There is no clear evidence of genetic components; therefore, catamenial epilepsy has been categorized an as acquired disorder (Herzog, 2007; Quigg et al., 2009). For this reason, a number of mechanisms have been proposed as possible causes of catamenial epilepsy, including an imbalance of estradiol. It has been known for many years that estradiol plays a key role in the exacerbation of seizures in women (Logothetis et al., 1959; Backstrom, 1976; Jacono and Robinson, 1987), and it has been shown that plasma levels of estradiol increase during both the follicular and luteal phases of the normal menstrual cycle. Thus, an increase or disrupt in the estrogen-to-progesterone ratio during the perimenstrual period might at least partially contribute to the development of perimenstrual seizure exacerbation (Bonuccelli et al., 1989; Herzog et al., 1997).
Progesterone also plays an important role in catamenial epilepsy and has long been known to have antiseizure, anticonvulsant, and antiepileptic characteristics in a variety of animal models of epilepsy (Craig, 1966; Landgren et al., 1978; Reddy, 2009a). The antiseizure mechanisms of progesterone are mediated mostly by its metabolic conversion into neurosteroids (Reddy et al., 2004a; Reddy et al., 2004b; Reddy et al., 2010a; Reddy and Mohan, 2011; Reddy and Ramanathan, 2012). Natural variations in progesterone levels occur during the menstrual cycle which can influence catamenial seizure susceptibility (Tuveri et al., 2008; El-Khayat et al., 2008). In this light, seizures tend to increase when there is a decrease in the serum progesterone-to-estrogen ratio, during the premenstrual phase of the cycle, and seizures start to decrease during the mid-luteal phase while progesterone levels are high (Backstrom, 1976; Bonucelli et al., 1989; Herzog et al., 2001). Recent clinical trials have concluded that administration of progesterone is found to reduce seizures in women with epilepsy (Backstrom et al., 1984; Herzog, 2009). This emerging data indicates that catamenial seizures are linked to a rapid decline in progesterone levels around the time of menstruation.
Neurosteroids can play a critical role in catamenial seizure exacerbation and susceptibility in women with epilepsy. A variety of neurosteroids are synthesized in the brain—the most extensively studied are allopregnanolone (AP) and allotetrahydro-deoxycorticosterone, and androstanediol (Reddy, 2010a). AP and other structurally-related neurosteroids act as positive allosteric modulators and direct activators of GABA-A receptors. AP and related neurosteroids rapidly alter neuronal excitability and therefore act as powerful anticonvulsants (Harrison et al., 1987; Hosie et al., 2007; Carver and Reddy, 2013; van Luijtelaar et al., 2014; Reddy, 2010a; Reddy and Jian, 2010). When neurosteroid levels fluctuate, control of seizures can be lost. It has been suggested that the withdrawal of progesterone-derived neurosteroids can lead to an increase in neuronal excitability, predisposing one to seizures (Reddy et al., 2001; 2012; Reddy, 2013b). Additionally, the plasticity in the GABA-A receptor subunits could also be associated with enhanced seizure susceptibility in perimenstrual catamenial epilepsy, as seen in animal studies (Smith et al., 2007; Gangisetty and Reddy, 2010; Wu et al., 2013). These neuroendocrine changes can result in reduced inhibition resulting in enhanced excitability, which, among other effects, predisposes one to seizures.
Based on neurosteroid physiology, Reddy and colleagues have developed multiple rodent models of catamenial epilepsy which are described in detail in earlier publications (Reddy et al., 2001; Reddy and Zeng, 2007; Reddy et al., 2012). Withdrawal of neurosteroids leads to a decreased seizure threshold and an increase in seizures (Reddy et al., 2001; 2012; Reddy and Zeng, 2007), suggesting that endogenous neurosteroids play a critical role in catamenial seizures. These models were used as tools to investigate novel drug therapies for catamenial epilepsy (Reddy and Rogawski, 2000a,b; 2001; 2009; Reddy et al., 2012). The outcomes of these preclinical studies have led to the proposal for a “neurosteroid replacement” therapy (Reddy and Rogawski, 2009). With this treatment, a neurosteroid could be administered in a “pulse” prior to menstruation and then either withdrawn, or continuously administered at lower doses throughout the month. Low doses of neurosteroid are expected to contribute little to the anticonvulsant activity during the majority of the menstrual cycle, but may be critical in preventing the occurrence of perimenstrual catamenial seizures (Reddy and Rogawski, 2001; Reddy et al., 2012).
SEX DIFFERENCES IN NEUOSTEROID ACTIONS
Emerging experimental data suggests that gender may affect sensitivity to neurosteroids (Reddy et al., 2004; Gorin-Meyer et al., 2007; Reddy, 2009b). Endocrine fluctuations of progesterone and other steroids in plasma levels can influence and mediate the availability of neurosteroids. For this reason, differences are found in the concentration of neurosteroids in the brain between males and females. Furthermore, brain development also differs between genders and also influences the function of neurosteroids (Reddy, 2009b). While neurosteroids are capable of shape inhibition and produce behavioral effects in both genders, regulation of neurosteroid activity may be sex-specific (Gulinello and Smith, 2003). Differences in maximal GABA-A receptor potentiation are observed between male and female rats for THDOC, but not for allopregnanolone or androgenic neurosteroids (Wilson and Biscardi, 1997).
Sex differences in antiseizure activity progesterone and allopregnanolone
Progesterone is an anticonvulsant hormone. Many studies found that GABA-A receptor-modulating neurosteroids which are synthesized from progesterone mediate protective effects in the brain (Kokate et al., 1999; Reddy et al., 2004). However, the potential sex differences in the anticonvulsant activity or progesterone is not widely studied. We tested the ability of progesterone and neurosteroids to protect against pentylenetetrazol (PTZ)-induced seizures in male and female mice (Reddy et al., 2004). Progesterone protected both male and female mice against PTZ-induced seizures in a dose-dependent fashion. However, in female mice, the dose-response curve for antiseizure activity of progesterone was significantly shifted in a parallel fashion to the left from that of male mice, indicating an increase in the antiseizure potency of progesterone in female mice. Because the antiseizure activity of progesterone is mainly due to its metabolic conversion to the neurosteroid, allopregnanolone, we also evaluated allopregnanolone for protective activity in male and female mice in the PTZ test. Like progesterone, allopregnanolone protected mice in a dose-dependent fashion (Reddy et al., 2014). The dose-response curve for the neurosteroid in male animals was shifted to the right of that for females, indicating reduced potency in the males relative to females. Using progesterone receptor knockout mice, we further confirmed that such gender-related differences in the antiseizure potency of progesterone or allopregnanolone are unlikely to be due to differences in PRs in the brain (Reddy et al., 2004).
These findings are consistent with previous reports that suggest female animals are more sensitive to the CNS actions of progesterone than males (Holmes and Weber, 1984; Finn and Gee, 1994; Mohammad et al., 1998; Reddy and Kulkarni, 1999). The gender-related difference in progesterone protection is not due to known differences in the density or distribution of the PR in the female and male brain (Rainbow et al., 1982). However, the role of sexual dimorphism or brain region involved remains unclear. It is possible, however, that endocrine differences between females and males may be responsible. It has been established that testosterone and related androgens decrease AP’s antiseizure protection (Edwards et al., 2001; Reddy, 2004), which sheds light on the phenomenon of reduced potency of progesterone in males. Alternative explanations include the gender differences in the bioavailability or pharmacokinetics of progesterone and allopregnanolone, or differences in the sensitivity of GABA-A receptors to these compounds.
Sex differences in androgenic neurosteroids
There several androgenic neurosteroids in the brain such as androstanediol and etiocholanone, which are derived from testosterone or other intermediate steroid hormones (Reddy, 2008). Although steroid hormones play a key role in gender differences in susceptibility to epileptic seizures, the functional role of androgenic neurosteroids in gender differences in seizure susceptibility remains unclear. We investigated the efficacy and potency of AP and androstanediol against pilocarpine-induced status epilepticus (SE) in both male and female mice, as well as assessed the potential pharmacokinetic factors (Reddy, 2009b). SE was successfully induced by pilocarpine in adult male and female mice. The protective activity of the two GABAergic neurosteroids was tested in a dose-dependent fashion. Our results show no significant differences in baseline seizure sensitivity to pilocarpine between genders (Reddy, 2009b). Androstanediol was found to have a dose-dependent protection against pilocarpine-induced SE in both male and female mice. However, female mice exhibited significantly enhanced sensitivity to the protective activity of androstanediol as compared to males. Gender differences in the antiseizure potency were also observed with AP (Reddy, unpublished data). Overall, these results strongly support the emerging notion that endogenous GABAergic neurosteroids play a key role in gender differences in seizure susceptibility and that the differences in neurosteroid sensitivity between genders are not related to pharmacokinetic factors.
MOLECULAR MECHANISMS OF SEX DIFFERENCES IN ANTICONVULSANT NEUROSTEROIDS
Despite the growing reports of sex differences in the actions of neurosteroids, limited research has investigated the underlying mechanisms in experimental models. Sex differences in the protective effects of neurosteroids may arise from variations between men and women in factors such as steroid hormones, metabolic activity, and biologic differences in neuronal receptors or neuronal networks in the brain (Finn and Gee, 1994; Li et al, 2007; Wu et al., 2013; Tabatadze et al., 2015). In this section, we review the available evidence on the sexual dimorphism in neuronal circuits and GABAergic receptor targets for neurosteroids.
Sex-related Plasticity in Neurosteroid Target Receptors
Steroid hormones act in discrete regions of the female brain to regulate sex behavior and other aspects of reproduction through classical genomic actions mediated by steroid receptors. Steroid receptors are present in many brain regions. Thus, it is possible that differential distribution of steroid hormones could account for sex differences in seizure susceptibility. It is well known that female mice and rats are more sensitive to the central nervous system actions of progesterone than are males. However, progesterone receptor knockout (PRKO) female mice are also more sensitive to the anticonvulsant effects of progesterone than males (Reddy et al., 2004), indicating that known differences in the density or distribution of the progesterone receptors in the female and male brain are not solely responsible for the sexually dimorphic effects of neurosteroids.
The ovarian cycle modulates behavior and seizure susceptibility in close association with fluctuations in neurosteroid levels (Reddy and Kulkarni, 1999; Molina-Hernandez et al., 2001; Maguire et al., 2005). Neurosteroids have influences on behavioral and excitability changes as well as on inhibition, and therefore, are linked to changes in expression of GABA-A receptors. Furthermore, fluctuations in progesterone and progesterone-derived neurosteroids during the menstrual cycle have been proposed to alter GABA-A receptor-mediated tonic inhibition (Maguire et al., 2005; Gangisetty and Reddy, 2010; Reddy et al., 2012). In a recent study, we discovered a novel role of extrasynaptic δ-containing GABA-A receptors as crucial mediators of the estrous cycle–related changes in neuronal excitability and seizure susceptibility in mice (Wu et al., 2013). We showed estrous cycle–related changes in δ-containing GABA-A receptors expression in different hippocampal subfields and tonic inhibition and seizure susceptibility. These findings are highly relevant to low incidence or severity of seizures in females because extrasynaptic GABA-A receptor are the main targets of neurosteroids. In addition, we sought to determine whether differences in extrasynaptic δGABA-A receptor expression and function underlie the sex differences in seizure sensitivity and the anticonvulsant activity of neurosteroids. Sex differences in neurosteroid protection were unrelated to pharmacokinetic factors because concentrations of neurosteroids associated with seizure protection are similar in male and female groups. Consistent with a greater abundance of extrasynaptic δGABA-A receptors in females, neurosteroids produced a greater potentiation of tonic currents in hippocampal neurons in females than males. Taken together, it is likely that neurosteroids exhibit greater seizure protection in females due to high abundance of extrasynaptic δ-subunit GABA-A receptors in the hippocampus and other brain regions. However, it is likely that additional factors including differences in phosphorylation and subunit trafficking may also contribute to sex differences in the anticonvulsant potency of neurosteroids.
In addition, sexual dimorphism or distribution of steroid receptors may contribute to sex differences. Progesterone’s physiological actions are mediated by the progesterone receptor (PR) (Li and O’Malley, 2003). PRs are expressed at high levels in the hypothalamus and moderate levels in the limbic areas of the brain and are also widely distributed in the hippocampus (Parsons et al., 1982; Auger and De Vries, 2002; Kato et al., 1994; Alves et al., 2000; Brinton et al., 2008); however, the physiological significance of these receptors remains unclear. P’s seizure protection is undiminished in PR knockout (PRKO) mice and occurs mainly due to its conversion to allopregnanolone (Reddy et al., 2004). It is likely that progesterone regulation of seizure susceptibility occurs via activation of PRs in the hippocampus. We found animals with a targeted deletion of PRs or selective knockdowns of PR expression in the brain are less prone to epileptogenesis (Reddy and Mohan, 2011). The persistence of this epileptic-like excitability condition was also impaired in mice lacking PRs. This data indicates the PR pathway plays an important role in promoting epileptogenesis, long-term stability of this epileptic-like state, and in modulating P’s ability to suppress seizures. PRs may promote epileptogenesis by influencing synaptic plasticity and tonic inhibition in the hippocampus dentate gyrus and also undergo ligand-independent activation by certain neurotransmitters (Mani and Portillo, 2010). It has been proposed that these signaling mechanisms are involved in PR-mediated seizure susceptibility and would, therefore, be relevant to the pathophysiology of epilepsy in women.
Sex-related Plasticity in Chloride Transporters
Another mechanism proposed to account for sex differences in seizure sensitivity is differences in the rate of appearance of hyperpolarizing GABA-A receptor signaling in female brain structures (Galanopoulou, 2008a; Giorgi et al., 2014). During development when intracellular chloride levels are high due to high levels of the Na-K-Cl cotransporter (NKCC1) expression and low levels of KCC2, GABA is depolarizing and neonatal seizures are generally resistant to GABAergic drugs (Fig.1). Bumetanide, a blocker of NKCC1, has been found to be a potential anticonvulsant in models of neonatal seizures. In early stages of nervous system development, GABA-A receptors possess excitatory functions on neurons by chloride channel-mediated depolarization, and this switches to inhibitory function as the brain matures (Cherubini et al., 1991; Rivera et al., 1999). Therefore, differences in the rate of which NKCC1 switches to KCC2 expression may underlie a greater susceptibility to seizures in males (Galanopoulou et al., 2001; 2003). Developmental disparities in neurosteroid activity may be represented by the evidence that the hippocampus in newborn males undergoes a longer period of this GABA-directed excitatory function than in females (Nuñez and McCarthy, 2007; Galanopoulou 2008a; 2008b). Changes in α1, α3, and δ subunit expression can be influenced by fluctuations in intracellular chloride levels. These chloride concentrations can also affect tonic inhibition (Succol et al., 2012). Steroid hormones are shown to modify the expression of KCC2 in specific brain regions in male and females (Galanopoulou and Moshe, 2003; Galanopoulou, 2006; 2008c). Moreover, AP and other protective neurosteroids could have differing effects for males and females experiencing potential excitotoxic or ischemic events; damage to specific neurons could alter function of chloride transporters and thereby the protective effects of benzodiazepines (Kelley et al., 2011). Such alternations in the ratio of NKCC1 and KCC2 have been demonstrated following status epilepticus in adult animals (Sivakumaran and Maguire, 2016). Despite such potential for contribution of chloride transporters to sex differences in seizures in younger populations, the relevance of such a mechanism in adults remains unclear.
Fig.1. Schematic illustration of the mechanism of cation-chloride transporters in the brain.
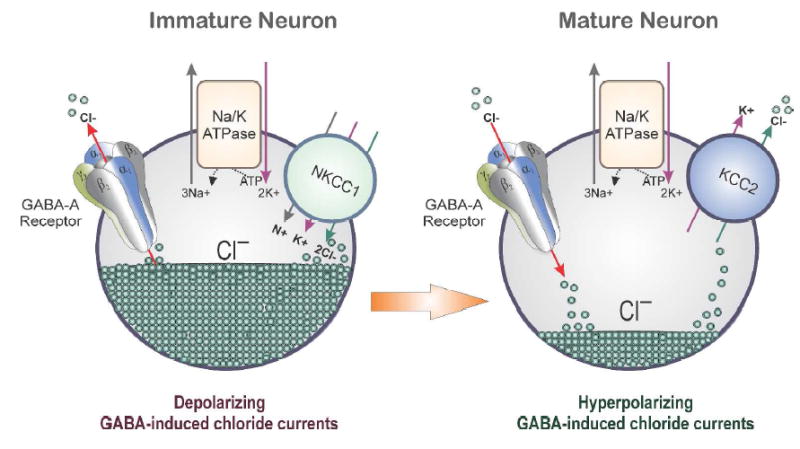
In neurons, KCCs (K—Cl cotransporters) control the reversal potential (E-GABA) of GABA-A receptor-mediated current and voltage responses. Therefore, they influence the efficacy of GABAergic inhibition. The neuron-specific KCC isoform, KCC2, is the main Cl- extruding mechanism in hippocampal and neocortical principal neurons. Activation of GABA-A receptors on the membranes of mature neurons generally results in the influx of Cl− and subsequently membrane hyperpolarization. However, in immature neurons, GABA is depolarizing due to the high intracellular chloride gradient (50 mM). This is attributed to an initially dominant role of Cl− uptake mediated by NKCC1, followed by a developmental up-regulation of the Cl− extruding KCC2, which renders the equilibrium potential of Cl− hyperpolarizing. In adult and immature neurons, the overall chloride gradient actually depends on the relative activity and abundance of NKCC1 and KCC2. Consequently, sex differences in the developmental shift from dominant NKCC1 to dominant KCC2 expression and/or activity may underlie the earlier switch of GABA-A receptor signaling from depolarizing to hyperpolarizing in certain brain regions in the female brain. These changes may ultimately contribute to alteration in GABAergic inhibition and seizure susceptibility.
Sexual Dimorphism in Brain Circuits
There is a clear amount of published evidence for sexual dimorphism in the human brain. A detailed account of sex dimorphism in seizure-controlling network is published recently (Giorgi et al., 2014). During brain development, sex hormones lead to permanent and distinct differences between males and females in various brain regions; therefore, they play a key role in producing sexual dimorphic features (Shah et al., 2012). The presence or absence of testosterone determines the male or female brain phenotype. Furthermore, the youngest age groups have the highest prevalence of epilepsy, though males have a higher incidence of seizures than females. However, the precise mechanisms underlying the sex-dependent differentiation of the specific neuronal circuits, particularly brain regions involved in seizure control are not clear. Sex-specific differences in the development of seizure suppressing neuronal networks may partly account for sex related susceptibility to seizures.
There is increasing evidence for sex differences in certain brain regions implicated in human epilepsy (see Savic and Engel, 2014). It is likely that sex differences in cerebral morphology, structural and functional connections can contribute to gender differences in seizure susceptibility and other brain diseases. Sex differences are described primarily in the limbic and motor networks and hence might be relevant to temporal lobe epilepsy and some genetic generalized epilepsies. A detailed account of cerebral sexual dimorphism and possible implications of these differences to sex related differences in epilepsies is published elsewhere (Savic and Engel, 2014).
The substantia nigra pars reticulata (SNR), a midbrain structure with a high expression of GABAergic neurons, plays a key role in seizure control in an age- and sex-dependent manner (Veliskova et al., 1996a; 1996b; Veliskova and Moshe, 2001). GABAergic activation of SNR at P15 has sex-specific features on seizure control. For example, bilateral SNR microinjections of the GABA-A receptor agonist muscimol have been associated with proconvulsant effects on flurothyl-induced seizures in males but not in females. Continuing studies have further revealed that postnatal testosterone is responsible for male proconvulsant phenotypes of SNR muscimol effects on seizures. GABAergic synaptic transmission is often excitatory early in development and then later shifts to the mature hyperpolarizing type. The adjustment occurs in females around postnatal day (PN) 10 and in males around PN17. This sex dimorphism may influence several other highly recognized sex differences in the development of SNR and in its regulatory role in seizures. Overall, the pathophysiological relevance of sex related differences in extra-limbic structures is still far from being clear, the dimorphism of seizure-controlling structures (e.g. SNR) appear to exert a key role in sex differences in seizures and the modulating effects of GABAergic drugs.
CONCLUSIONS
In contrast to clinical studies that require the inclusion of women, most experimental studies have been usually done using males only. In 2014, the National Institutes of Health issued a policy statement to encourage the inclusion of both males and females in all preclinical studies. This has led to a greater interest in sex differences in neurological disorders. Males are more likely to have greater risk of epilepsy than females. The developmental susceptibility to seizures varies in females at puberty, menstrual cycle and menopause. In experimental studies, male animals exhibit a greater susceptibility to acute seizures and epileptogenesis than females. Steroid hormones and neurosteroids are believed to play an important role in the sex specific forms of epilepsies and gender-related seizure susceptibility—sex hormones in both males and females can influence hyperexcitability in the brain (Tauboll et al., 2015). Menstrual and stress related fluctuations in seizures may be related to alterations in the concentration of neurosteroids in the brain. The current and emerging data clearly supports the notion that sexual dimorphism in brain structure and development contribute to differences in seizure susceptibility and sensitivity between sexes in congruence with hormonal milieu. Neurosteroids exhibit greater anticonvulsant sensitivity in females than males. However, the potential mechanisms of sex differences in the anticonvulsant activity of neurosteroids are poorly understood. In addition to neurosteroid fluctuations, there is emerging evidence that plasticity in GABA-A receptor structure and function plays a role in seizure exacerbation in gender-specific forms of epilepsy such as catamenial epilepsy. Such neuroendocrine changes can result in reduced inhibition, resulting in enhanced excitability, which, among other effects, predisposes to catamenial seizures. Neurosteroids hold great promise for treatment of epilepsy. A greater understanding of the mechanisms of sex differences in neurosteroid actions may offer improvements in treatment strategies for gender-specific forms of epilepsy.
SIGNIFICANCE.
Epilepsy affects an estimated 65 million people worldwide. Men and women exhibit differences in sensitivity to seizures and epileptogenic events. Despite some limitations, the incidence of epilepsy is strongly sex-specific as men exhibit greater seizure susceptibility than women. Women exhibit epilepsies and seizure syndromes that are much more complex and often intractable including menstrual-cycled linked catamenial epilepsy. Neurosteroids and other signaling molecules in inhibitory neurotransmission in the brain could influence seizure protection in males and females differently.
Acknowledgments
This work was supported by NIH grants NS052158 and NS051398] (to DSR). Dr. Reddy research work was supported by the CounterACT Program, National Institutes of Health, Office of the Director and the National Institute of Neurologic Disorders and Stroke [Grant U01 NS083460]. The author thanks Victoria Golub for editing the manuscript.
Footnotes
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alves SE, McEwen BS, Hayashi S, Korach KS, Pfaff DW, Ogawa S. Estrogen-regulated progestin receptors are found in the midbrain raphe but not hippocampus of estrogen receptor alpha (ER-α) gene-disrupted mice. J Comp Neurol. 2000;427:185–195. doi: 10.1002/1096-9861(20001113)427:2<185::aid-cne2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Auger CJ, De Vries GJ. Progestin receptor immunoreactivity within steroid-responsive vasopressin-immunoreactive cells in the male and female rat brain. J Neuroendocrinol. 2002;14:561–567. doi: 10.1046/j.1365-2826.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- Bäckström T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol Scand. 1976;54:321–347. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Bäckström T, Zetterlund B, Blom S, Romano M. Effect of intravenous progesterone infusions on the epileptic discharge frequency in women with partial epilepsy. Acta Neurol Scand. 1984;69:240–248. doi: 10.1111/j.1600-0404.1984.tb07807.x. [DOI] [PubMed] [Google Scholar]
- Bazan AC, Montenegro MA, Cendes F, Min LL, Guerreiro CA. Menstrual cycle worsening of epileptic seizures in women with symptomatic focal epilepsy. Arg Neuro-Psiquiatria. 2005;63(3B):751–756. doi: 10.1590/s0004-282x2005000500006. [DOI] [PubMed] [Google Scholar]
- Benn EK, et al. Estimating the incidence of first unprovoked seizure and newly diagnosed epilepsy in the low-income urban community of Northern Manhattan, New York City. Epilepsia. 2008;49:1431–1439. doi: 10.1111/j.1528-1167.2008.01564.x. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshé SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Bonuccelli U, Melis GB, Paoletti AM, Fioretti P, Murri L, Muratorio A. Unbalanced progesterone and estradiol secretion in catamenial epilepsy. Epilepsy Res. 1989;3:100–106. doi: 10.1016/0920-1211(89)90037-5. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABA-A receptors: Regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacol. 2013;230:151–188. doi: 10.1007/s00213-013-3276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Christensen J, Kjeldsen MJ, Anderson H, Friis ML, Sidenius P. Gender differences in epilepsy. 2005;46:956–960. doi: 10.1111/j.1528-1167.2005.51204.x. [DOI] [PubMed] [Google Scholar]
- Christensen J, et al. Incidence and prevalence of epilepsy in Denmark. Epilepsy Res. 2007;76:60–65. doi: 10.1016/j.eplepsyres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Christensen J, et al. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet. 2009;373:1105–1110. doi: 10.1016/S0140-6736(09)60214-2. [DOI] [PubMed] [Google Scholar]
- Chudomel O, Herman H, Nair K, Moshé SL, Galanopoulou AS. Age- and gender-related differences in GABAA receptor-mediated postsynaptic currents in GABAergic neurons of the substantia nigra reticulata in the rat. Neurosci. 2009;163:155–167. doi: 10.1016/j.neuroscience.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CR. Anticonvulsant activity of steroids: Separability of anticonvulsant from hormonal effects. J Pharmacol Exp Ther. 1966;153:337–343. [Google Scholar]
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367(9516):1087–100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- Edwards HE, Mo V, Burnham WM, MacLusky NJ. Gonadectomy unmasks an inhibitory effect of progesterone on amygdala kindling in male rats. Brain Res. 2001;889:260–263. doi: 10.1016/s0006-8993(00)03147-4. [DOI] [PubMed] [Google Scholar]
- El-Khayat HA, Soliman NA, Tomoum HY, Omran MA, El-Wakad AS, Shatla R. Reproductive hormonal changes and catamenial pattern in adolescent females with epilepsy. Epilepsia. 2008;49:1619–1626. doi: 10.1111/j.1528-1167.2008.01622.x. [DOI] [PubMed] [Google Scholar]
- El-Sayed YY. Obstetric and gynecologic care of women with epilepsy. Epilepsia. 1998;39(Suppl 8):S17–S25. doi: 10.1111/j.1528-1157.1998.tb02603.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Gee KW. The estrus cycle, sensitivity to convulsants and the anticonvulsant effect of a neuroactive steroid. J Pharm Exp Ther. 1994;271:164–170. [PubMed] [Google Scholar]
- Galanopoulou AS. Sex- and cell-type-specific patterns of GABA-A receptor and estradiol-mediated signaling in the immature rat substantia nigra. Eur J Neurosci. 2006;23:2423–2430. doi: 10.1111/j.1460-9568.2006.04778.x. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS. Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABAA receptors. J Neurosci. 2008a;28:1557–1567. doi: 10.1523/JNEUROSCI.5180-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. GABAA receptors in normal development and seizures: friends or foes. Curr Neuropharmacol. 2008b;6:1–20. doi: 10.2174/157015908783769653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. Sexually dimorphic expression of KCC2 and GABA function. Epilepsy Res. 2008c;80:99–113. doi: 10.1016/j.eplepsyres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS, Liptakova S, Velíšková J, Moshé SL. Sex and regional differences in the time and pattern of neurogenesis of the rat substantia nigra. Epilepsia. 2001;42(Suppl 7):109. [Google Scholar]
- Galanopoulou AS, Moshé SL. Role of sex hormones in the sexually dimorphic expression of KCC2 in rat substantia nigra. Exp Neurol. 2003;184:1003–1009. doi: 10.1016/S0014-4886(03)00387-X. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Kyrozis A, Claudio OI, Stanton PK, Moshé SL. Sex-specific KCC2 expression and GABA-A receptor function in rat substantia nigra. Exp Neurol. 2003;183:628–637. doi: 10.1016/s0014-4886(03)00213-9. [DOI] [PubMed] [Google Scholar]
- Gangisetty O, Reddy DS. Neurosteroid withdrawal regulates GABA-A receptor α4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neurosci. 2010;170:865–880. doi: 10.1016/j.neuroscience.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad R, Sadeh M, Rapoport A, Dabby R, Lampl Y. Lamotrigine and catamenial epilepsy. Seizure. 2008;17:531–534. doi: 10.1016/j.seizure.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Giorgi FS, Galanopoulou AS, Moshé SL. Sex dimorphism in seizure-controlling networks. Neurobiol Dis. 2014;72(Pt B):144–152. doi: 10.1016/j.nbd.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin-Meyer RE, Wiren KM, Tanchuck MA, Long SL, Yoneyama N, Finn DA. Sex differences in the effect of finasteride on acute ethanol withdrawal severity in C57BL/6J and DBA/2J mice. Neurosci. 2007;146:1302–1315. doi: 10.1016/j.neuroscience.2007.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J Pharm Exp Ther. 2003;305:541–548. doi: 10.1124/jpet.102.045120. [DOI] [PubMed] [Google Scholar]
- Harden CL, Pennell PB, Koppel BS, et al. Management issues for women with epilepsy--focus on pregnancy (an evidence-based review): III. Vitamin K, folic acid, blood levels, and breast-feeding: Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50:1247–1255. doi: 10.1111/j.1528-1167.2009.02130.x. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure-activity relationships for steroid interactions with the γ-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1987;241:346–353. [PubMed] [Google Scholar]
- Hauser WA. Incidence and prevalence. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. Lippincott-Raven Publishers; Philadelphia: 1997. pp. 47–57. [Google Scholar]
- Hauser WA, et al. Incidence of epilepsy and unprovoked seizures in Rochester Minnesota: 1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Is there a lateralized asymmetry in the sensitivity of the brain to hormones in epilepsy? Epilepsy Behav. 2007;11:157–9. doi: 10.1016/j.yebeh.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Hormonal therapies: progesterone. Neurotherapeutics. 2009;6:383–391. doi: 10.1016/j.nurt.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM, Smithson SD, Kalayjian LA, Heck CN, Sperling MR, Liporace JD, Harden CL, Dworetzky BA, Pennell PB, Massaro JM Progesterone Trial Study Group. Progesterone vs placebo therapy for women with epilepsy: A randomized clinical trial. Neurol. 2012;78:1959–1966. doi: 10.1212/WNL.0b013e318259e1f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Friedman MN, Freund S, Pascual-Leone A. Transcranial magnetic stimulation evidence of a potential role for progesterone in the modulation of premenstrual corticocortical inhibition in a woman with catamenial seizure exacerbation. Epilepsy Behav. 2001;2:367–369. doi: 10.1006/ebeh.2001.0232. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Harden CL, Liporace J, Pennell P, Schomer DL, Sperling M, Fowler K, Nikolov B, Shuman S, Newman M. Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Ann Neurol. 2004;56:431–434. doi: 10.1002/ana.20214. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Begley CE. Surveillance of epilepsy and prevention of epilepsy and its sequelae: lessons from the Institute of Medicine report. Curr Opin Neurol. 2013;26:168–173. doi: 10.1097/WCO.0b013e32835ef2c7. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Logroscino G, Benn EK, Katri N, Cascino G, Hauser WA. Estimating risk for developing epilepsy: A population-based study in Rochester, Minnesota. Neurol. 2011;76:23–27. doi: 10.1212/WNL.0b013e318204a36a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GL, Weber DA. The effect of progesterone on kindling: a developmental study. Brain Res. 1984;318:45–53. doi: 10.1016/0165-3806(84)90061-0. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABAA receptors. Pharmacol Ther. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Imai K, Ikeda H, Shigematsu H, Takahashi Y, Inoue Y, Higurashi N, Hirose S. Characteristic phasic evolution of convulsive seizure in PCDH19-related epilepsy. Epileptic Disord. 2016;18:26–33. doi: 10.1684/epd.2016.0803. [DOI] [PubMed] [Google Scholar]
- Younus I, Reddy DS. Seizure facilitating activity of the oral contraceptive ethinyl estradiol. Epilepsy Res. 2016;121:29–32. doi: 10.1016/j.eplepsyres.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacono JJ, Robinson J. The effects of estrogen, progesterone, and ionized calcium on seizures during the menstrual cycle in epileptic women. Epilepsia. 1987;28:571–577. doi: 10.1111/j.1528-1157.1987.tb03690.x. [DOI] [PubMed] [Google Scholar]
- Joensen P. Prevalence, incidence, and classification of epilepsy in the Faroes. Acta Neurol Scand. 1986;74:150–155. doi: 10.1111/j.1600-0404.1986.tb04642.x. [DOI] [PubMed] [Google Scholar]
- Kaplan PW, Norwitz ER, Ben Menachem E, Pennell PB, Druzin M, Robinson JN, Gordon JC. Obstetric risks for women with epilepsy during pregnancy. Epilepsy Behav. 2007;11:283–291. doi: 10.1016/j.yebeh.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Kato J, Hirata S, Nozawa A, Yamada-Mouri N. Gene expression of progesterone receptor isoforms in the rat brain. Horm Behav. 1994;28:454–463. doi: 10.1006/hbeh.1994.1043. [DOI] [PubMed] [Google Scholar]
- Kelley MH, Kuroiwa M, Taguchi N, Herson PS. Sex difference in sensitivity to allopregnanolone neuroprotection in mice correlates with effect on spontaneous inhibitory post synaptic currents. Neuropharmacology. 2011;61:724–729. doi: 10.1016/j.neuropharm.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokate TG, Juhng KN, Kirkby RD, Llamas J, Yamaguchi S, Rogawski MA. Convulsant actions of the neurosteroid pregnenolone sulfate in mice. Brain Res. 1999;831:119–24. doi: 10.1016/s0006-8993(99)01287-1. [DOI] [PubMed] [Google Scholar]
- Kotsopoulos IA, et al. Systematic review and meta-analysis of incidence studies of epilepsy and unprovoked seizures. Epilepsia. 2002;43:1402–1409. doi: 10.1046/j.1528-1157.2002.t01-1-26901.x. [DOI] [PubMed] [Google Scholar]
- Landgren S, Bäckström T, Kalistratov G. The effect of progesterone on the spontaneous interictal spike evoked by the application of penicillin to the cat’s cerebral cortex. J Neurol Sci. 1978;36:119–133. doi: 10.1016/0022-510x(78)90166-1. [DOI] [PubMed] [Google Scholar]
- Lavados J, et al. A descriptive study of epilepsy in the district of El Salvador, Chile, 1984–1988. Acta Neurol Scand. 1992;85:249–256. doi: 10.1111/j.1600-0404.1992.tb04040.x. [DOI] [PubMed] [Google Scholar]
- Li X, O’Malley BW. Unfolding the actions of progesterone receptors. J Biol Chem. 2003;278:39261–39264. doi: 10.1074/jbc.R300024200. [DOI] [PubMed] [Google Scholar]
- Li H, Huguenard JR, Fisher RS. Gender and age differences in expression of GABA-A receptor subunits in rat somatosensory thalamus and cortex in an absence epilepsy model. Neurobiol Dis. 2007;25:623–630. doi: 10.1016/j.nbd.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis J, Harner R, Morrel F. The role of estrogens in catamenial exacerbation of epilepsy. Neurol. 1959;9:352–360. doi: 10.1212/wnl.9.5.352. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA-A receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Mani S, Portillo W. Activation of progesterone receptors in female reproductive behavior: Interactions with neurotransmitters. Front Neuroendocrinol. 2010;31:157–171. doi: 10.1016/j.yfrne.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad S, Abolhassan A, Pourgholami MH. Evaluation of the anticonvulsant profile of progesterone in male amygdala-kindled rats. Epilepsy Res. 1998;30:195–202. doi: 10.1016/s0920-1211(98)00004-7. [DOI] [PubMed] [Google Scholar]
- Molina-Hernández M, Contreras CM, Téllez-Alcántara P. Diazepam increases the number of punished responses in a conflict-operant paradigm during late proestrus and estrus in the Wistar rat. Neuropsychobiol. 2001;43:29–33. doi: 10.1159/000054862. [DOI] [PubMed] [Google Scholar]
- Morrell MJ. Reproductive and metabolic disorders in women with epilepsy. Epilepsia. 2003;44(suppl 4):11–20. doi: 10.1046/j.1528-1157.44.s4.2.x. [DOI] [PubMed] [Google Scholar]
- Nuñez JL, McCarthy MM. Evidence for an extended duration of GABA-mediated excitation during the developing male versus female hippocampus. Dev Neurobiol. 2007;67:1879–1890. doi: 10.1002/dneu.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YS, Kim DS, Shim KW, Kim JH, Choi JU. Factors contributing to resectability and seizure outcomes in 44 patients with gnaglioglioma. Clin Neurol Neurosurg. 2008;110:667–73. doi: 10.1016/j.clineuro.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Parsons B, Rainbow TC, MacLusky NJ, McEwen BS. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci. 1982;2:1446–1452. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucca E, Battino D, Tomson T. Gender issues in antiepileptic drug treatment. Neurobiol Dis. 2014a;72(Pt B):217–223. doi: 10.1016/j.nbd.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Perucca P, Camfield P, Camfield C. Does gender influence susceptibility and consequences of acquired epilepsies? Neurobiol Dis. 2014b;72(Pt B):125–130. doi: 10.1016/j.nbd.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Immonen RJ, Gröhn OH, Kharatishvili I. From traumatic brain injury to posttraumatic epilepsy: what animal models tell us about the process and treatment options. Epilepsia. 2009;50(Suppl 2):21–29. doi: 10.1111/j.1528-1167.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- Quigg M, Smithson SD, Fowler KM, Sursal T, Herzog AG the Progesterone Trial Study Group. Laterality and location influence catamenial seizure expression in women with partial epilepsy. Neurol. 2009;73:223–227. doi: 10.1212/WNL.0b013e3181ae7adf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, McEwen BS. Sex differences in rat brain estrogen and progestin receptors. Nature. 1982;300(5893):648–649. doi: 10.1038/300648a0. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Anticonvulsant activity of the testosterone-derived neurosteroid 3α -androstanediol. Neuroreport. 2004a;15:515–518. doi: 10.1097/00001756-200403010-00026. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3α -androstanediol and 17β-estradiol. Neurosci. 2004b;129:195–207. doi: 10.1016/j.neuroscience.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Mass spectrometric assay and physiological-pharmacological activity of androgenic neurosteroids. Neurochem Int. 2008;52:541–53. doi: 10.1016/j.neuint.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res. 2009a;85:1–30. doi: 10.1016/j.eplepsyres.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Steroid hormones and sex differences in seizure susceptibility. In: Philip Schwartzkroin., editor. Encyclopedia of Basic Epilepsy Res. Vol. 1. Oxford: Academic Press; 2009b. pp. 526–533. [Google Scholar]
- Reddy DS. Neurosteroids: Endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010a;186:113–137. doi: 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Clinical pharmacokinetic interactions between antiepileptic drugs and hormonal contraceptives. Expert Rev Clin Pharmacol. 2010b;3:183–192. doi: 10.1586/ecp.10.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Frontiers in Endocrinol. 2011;2(38):1–11. doi: 10.3389/fendo.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Neuroendocrine aspects of catamenial epilepsy. Hormones & Behav. 2013a;63:254–266. doi: 10.1016/j.yhbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Role of hormones and neurosteroids in epileptogenesis. Front Cell Neurosci. 2013b;7(115):1–20. doi: 10.3389/fncel.2013.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Neurosteroids and their role in sex-specific epilepsies. Neurobiol Dis. 2014;72(Pt B):198–209. doi: 10.1016/j.nbd.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Estes WA. Clinical potential of neurosteroids for CNS disorders. Trends Pharmacol Sci. 2016;37(7):543–561. doi: 10.1016/j.tips.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Jian K. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABA-A receptors. J Pharmacol Exp Ther. 2010;334:1031–1041. doi: 10.1124/jpet.110.169854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Sex and estrous cycle-dependent changes in neurosteroid and benzodiazepine effects on food consumption and plus-maze learning behaviors in rats. Pharmacol Biochem Behav. 1999;62:53–60. doi: 10.1016/s0091-3057(98)00126-9. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Mohan A. Development and persistence of limbic epileptogenesis are impaired in mice lacking progesterone receptors. J Neurosci. 2011;31:650–658. doi: 10.1523/JNEUROSCI.4488-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Ramanathan G. Finasteride inhibits the disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Epilepsy Behav. 2012;25:92–97. doi: 10.1016/j.yebeh.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther. 2000a;294:909–915. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000b;295:1241–1248. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:303–310. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics. 2009;6:392–401. doi: 10.1016/j.nurt.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Zeng YC. Effect of neurosteroid withdrawal on spontaneous recurrent seizures in a rat model of catamenial epilepsy. FASEB J. 2007;21:A1179–A11179. [Google Scholar]
- Reddy DS, Castaneda DC, O’Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310:230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Gangisetty O, Briyal S. Disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Neuropharmacol. 2010;59:573–581. doi: 10.1016/j.neuropharm.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Gould J, Gangisetty O. A mouse kindling model of perimenstrual catamenial epilepsy. J Pharmacol Exp Therap. 2012;341:784–793. doi: 10.1124/jpet.112.192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Savic I, Engel J., Jr Structural and functional correlates of epileptogenesis—does gender matter? Neurobiol Dis. 2014;70:69–73. doi: 10.1016/j.nbd.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I, Engel J., Jr Sex differences in patients with mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 1998;65:910–912. doi: 10.1136/jnnp.65.6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NM, Jessell TM, Sanes JR. Sexual differentiation of the nervous system. In: Kandell ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ, editors. Principles of Neural Science. Fifth edition. McGraw-Hill; New York: 2012. pp. 1306–1328. [Google Scholar]
- Sivakumaran S, Maguire J. Bumetanide reduces seizure progression and the development of pharmacoresistant status epilepticus. Epilepsia. 2016;57:222–232. doi: 10.1111/epi.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA-A receptors: focus on the alpha4 and delta subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Succol F, Fiumelli H, Benfenati F, Cancedda L, Barberis A. Intracellular chloride concentration influences the GABAA receptor subunit composition. Nat Commun. 2012;3:738. doi: 10.1038/ncomms1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatadze N, Huang G, May RM, Jain A, Woolley CS. Sex differences in molecular signaling at inhibitory synapses in the hippocampus. J Neurosci. 2015;35:11252–11265. doi: 10.1523/JNEUROSCI.1067-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C, Shard C, Ranieri E, Hynes K, Pham DH, Leach D, Buchanan G, et al. Mutations of protocadherin 19 in female epilepsy (PCDH19-FE) lead to allopregnanolone deficiency. Hum Mol Genet. 2015;24:5250–5259. doi: 10.1093/hmg/ddv245. [DOI] [PubMed] [Google Scholar]
- Tauboll E, Sveberg L, Svalheim S. Interactions between hormones and epilepsy. Seizure. 2015;28:3–11. doi: 10.1016/j.seizure.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Tekle-Haimanot R, et al. Incidence of epilepsy in rural central Ethiopia. Epilepsia. 1997;38:541–546. doi: 10.1111/j.1528-1157.1997.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Tuveri A, Paoletti AM, Orrù M, Melis GB, Marotto MF, Zedda P, Marrosu F, Sogliano C, Marra C, Biggio G, Concas A. Reduced serum level of THDOC, an anticonvulsant steroid, in women with perimenstrual catamenial epilepsy. Epilepsia. 2008;49:1221–1229. doi: 10.1111/j.1528-1167.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar G, Onat FY, Gallagher MJ. Animal model of absence epilepsies: what do they model and do sex and sex hormones matter? Neurobiol Dis. 2014;72(Pt B):167–79. doi: 10.1016/j.nbd.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veliskova J, Moshe SL. Sexual dimorphism and developmental regulation of substantia nigra function. Ann Neurol. 2001;50:596–601. doi: 10.1002/ana.1248. [DOI] [PubMed] [Google Scholar]
- Velísková J, Velísek L, Moshé SL. Subthalamic nucleus: a new anticonvulsant site in the brain. Neuroreport. 1996a;7:1786–1788. [PubMed] [Google Scholar]
- Velísková J, Velísek L, Nunes ML, Moshé SL. Developmental regulation of regional functionality of substantial nigra GABAA receptors involved in seizures. Eur J Pharmacol. 1996b;309:167–173. doi: 10.1016/0014-2999(96)00341-x. [DOI] [PubMed] [Google Scholar]
- Verrotti A, Greco R, Giannuzzi R, Chiarelli F, Latini G. Old and new antiepileptic drugs for the treatment of idiopathic generalized epilepsies. Curr Clin Pharmacol. 2007;2:249–59. doi: 10.2174/157488407781668794. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Biscardi R. Influence of gender and brain region on neurosteroid modulation of GABA responses in rat. Life Sci. 1997;60:1679–1691. doi: 10.1016/s0024-3205(97)00110-0. [DOI] [PubMed] [Google Scholar]
- Wu X, Gangisetty O, Carver CM, Reddy DS. Estrous cycle regulation of extrasynaptic δ-containing GABAA receptor-mediated tonic inhibition and limbic epileptogenesis. J Pharmacol Exp Ther. 2013;346:146–160. doi: 10.1124/jpet.113.203653. [DOI] [PMC free article] [PubMed] [Google Scholar]


