Abstract
Oxytocin (OT) and arginine vasopressin (AVP) exert robust and sexually dimorphic influences on cognition and emotion. How these hormones regulate relevant functional brain systems is not well understood. OT and AVP serum concentrations were assayed in 60 healthy individuals (36 women). Brain functional networks assessed with resting state fMRI (rs-fMRI) were constructed using graph theory-based approaches that characterize brain networks as connected nodes. Sex differences were demonstrated in rs-fMRI. Men showed higher nodal degree (connectedness) and efficiency (information propagation capacity) in left inferior frontal gyrus (IFG) and bilateral superior temporal gyrus (STG) and higher nodal degree in left rolandic operculum. Women showed higher nodal betweenness (being part of paths between nodes) in right putamen and left inferior parietal gyrus (IPG). Higher hormone levels were associated with less intrinsic connectivity. In men, higher AV P was associated with lower nodal degree and efficiency in left IFG (pars orbitalis) and left STG, and less efficiency in left IFG (pars triangularis). In women, higher AVP was associated with lower betweenness in left IPG and higher OT was associated with lower nodal degree in left IFG (pars orbitalis). Hormones differentially correlate with brain networks important for emotion processing and cognition in men and women. AVP in men and OT in women may regulate orbital frontal cortex connectivity, which is important in emotion processing. Hormone associations with STG and pars triangularis in men and parietal cortex in women may account respectively for well-established sex differences in verbal and visuospatial abilities.
Keywords: sex differences, oxytocin, vasopressin, resting state, brain function
Graphical Abstract
Introduction
Sex differences in emotion processing and cognitive functioning are well documented. For instance, women are more emotionally perceptive and reactive to emotional stimuli and show enhanced emotional memory compared to men (Cahill 2003; Gur and Gur 2002; Stevens and Hamann 2012; Whittle et al. 2011). Additionally, women excel at verbal abilities whereas men excel at visuospatial abilities (Andreano and Cahill 2009; de Frias et al. 2006; Gur and Gur 2013; Kimura 1999; Voyer et al. 1995). Meta-analyses show that structural and functional neuroimaging data parallel these behavioral sex differences. Sex differences in human brain morphology (Ruigrok et al. 2014) and in activation during behavioral tasks are apparent in networks related to emotion processing, language, and visuospatial abilities. Sex differences in emotion processing typically include differential activation of prefrontal, temporal, and limbic brain regions (Sacher et al. 2013; Stevens and Hamann 2012). With respect to language, the most common areas showing sex differences are in the prefrontal (inferior frontal gyrus) and temporal (superior temporal gyrus) regions whereas for visuospatial abilities differences are most often demonstrated in posterior parietal cortex (Costafreda et al. 2006; Tomasino and Gremese 2015; Wagner et al. 2014). These sex differences may in part reflect the effects of sex steroid hormones on brain function.
Two sexually dimorphic neurohormones – oxytocin (OT) and arginine vasopressin (AVP) – are believed to contribute to sex differences in brain functioning, particularly regions supporting emotion and cognition. OT, including measurements of basal hormones in plasma, is associated with better emotion processing and worse cognition, whereas AV P is associated with increased emotional reactivity and better cognitive function (Carter et al. 2008; Heinrichs and Domes 2008; Meyer-Lindenberg et al. 2011; Rubin et al. 2014; Rubin et al. 2011). Effects of OT and AVP are impacted by biological sex (Carter 2007; Carter et al. 2009), as endogenous peptides activate their receptors with cascading downstream effects. In men, OT reduces amygdala activation in response to emotional face processing and perception of threatening situations (Domes et al. 2007; Kirsch et al. 2005; Petrovic et al. 2008; Singer et al. 2008) whereas the converse is seen in women (Domes et al. 2010; Lischke et al. 2012). OT administration also influences intrinsic brain connectivity in men, with unknown effects in women. In men, OT is reported to reduce amygdala-precuneus connectivity (Kumar et al. 2015) or enhance amygdala-prefrontal connectivity (Sripada et al. 2013). AVP administration effects are also sex-dependent, with AVP having opposite effects on temporal-limbic and insula activity during cooperative interactions with increased activation in men (Lee et al. 2013; Rilling et al. 2014) and decreased activation in women (Rilling et al. 2014). Importantly, previous studies focus on the consequences of exogenous peptide administration on regional brain activity and behavior, rather than examining relations between resting physiological levels of these hormones and their relation to brain physiology.
OT and AVP may have sex-dependent associations with cognitive and emotional brain networks, particularly in ones showing sex differences (Hjelmervik et al. 2014; Sacher et al. 2013). To our knowledge, no study has examined associations between these peripheral hormones and functional brain connectivity. Therefore, we examined sex differences in functional brain networks and then assessed the relationships of functional connectivity in sex-dependent brain regions with peripheral levels of OT and AVP. Functional brain networks were characterized with graph theory methods (Rubinov and Sporns 2010) which consider brain networks as connected nodes. We predicted that basal levels of OT and AVP would be associated with functional connectivity of sex-dependent brain networks important for cognition and emotion processing.
Materials and Methods
Participants
The study was approved by the University of Illinois at Chicago Institutional Review Board, and all participants provided written informed consent. Participants included 60 healthy individuals (24 men; 36 women) between the ages of 16 and 60 (Table 1). Seven of the 36 women reported using oral contraceptives. All participants were screened using the Structured Clinical Interviews for DSM-IV Non-Patient Edition to rule out current or past psychiatric illness. Exclusion criteria included: history of head injury; pregnant or lactating (for women); positive urine toxicology screen for common drugs of abuse on the day of scanning; diagnosis of substance abuse in the past 30 days or substance dependence in the past 6 months; history of systemic medical or neurological disorder affecting mood or cognition; and age-corrected Wide-Range Achievement Test reading test standard score <65. Anatomic images were inspected by an experienced neuroradiologist, and no gross abnormalities were observed for any subject.
Table 1.
Demographic characteristics, hormone levels, and cognitive data as a function of sex.
| Sex |
|||
|---|---|---|---|
| Variables | Men (n=24) M (SD) |
Women (n=36) M (SD) |
p-value |
| Demographics | |||
| Age (years) | 36.96 (12.03) | 34.67 (12.11) | 0.47 |
| Education (years) | 14.38 (2.75) | 15.47 (2.65) | 0.13 |
| WRAT Reading | 103.48 (9.71) | 107.74 (13.00) | 0.18 |
| Race, n (%) | 0.38 | ||
| Caucasian | 12 (50) | 22 (61) | |
| African-American | 10 (42) | 9 (25) | |
| Other | 2 (8) | 5 (14) | |
| Handedness, n (%) | 20 (83) | 33 (92) | 0.33 |
| Blood draw < noon, n (%) | 23 (95) | 36 (100) | 0.20 |
| Hormone levels | |||
| Mean Log Oxytocin (pg/ml) | 6.10 (0.66) | 6.15 (0.89) | 0.83 |
| Mean Log Vasopressin (pg/ml) | 4.36 (0.65) | 4.53 (0.51) | 0.29 |
| Emotion and cognitive outcomes | |||
| Emotion recognition | 80.88 (8.93) | 84.99 (5.54) | 0.03 |
| Verbal learning | 42.27 (8.36) | 50.30 (8.63) | 0.001 |
| Verbal fluency | 51.73 (13.51) | 55.86 (11.41) | 0.21 |
| Processing speed | 56.64 (13.88) | 62.11 (10.88) | 0.10 |
Note. WRAT, Wide Range Achievement Test-IV, Reading Subtest.
Serum Hormone Assays
Blood samples were drawn in the morning when possible (98% blood draws before noon). Samples were stored in plain tubes, spun at 4°C, divided into 300ul aliquots, and stored at −80°C. Samples were batched, diluted in an assay buffer to give reliable results within the linear portion of the standard curve (OT 1:4; AVP 1:2), and completed in duplicate using unextracted plasma. OT and AVP were quantified with an EIA kit (Enzo Life Sciences/Assay Designs)(Carter et al. 2007). These EIAs are highly sensitive (minimal detection levels <12 pg/ml OT; 4 pg/ml AVP) and specific with cross-reactivity between OT and AVP <0.04%. Samples were batched and assayed simultaneously blind to subject information. The laboratory selected OT values >2000 pg/ml and AVP values >400 pg/ml to be re-run to confirm sample accuracy. This was only the case for one participant’s OT levels. There were no participants with AVP values >400pg/ml. Intra-assay coefficients of variation were less than 10.3% for both assays. Hormone values were log transformed for statistical analysis. Age was not associated with hormone levels (r’s<0.20).
Emotion Processing and Cognition
From the Brief Assessment of Cognition in Schizophrenia (BACS) neuropsychological test battery, we selected three subtests including verbal learning (total words recalled across 5 trials), verbal fluency (total words generated across categories (Animals) and letters (F, S)), and processing speed (symbol-coding). The Penn Emotion Recognition (ER)-40 Test assessed the ability to accurately recognize facial emotions (Gur et al. 2002). The tests were selected based on previous research indicating that the tests demonstrate sex differences in the general population (Kramer et al. 1988; Kramer et al. 1997; Mann 1990; Weiss et al. 2003; Weiss et al. 2006; Williams et al. 2009) or if tests (verbal learning, processing speed) showed a sex difference in favor of women (p’s<0.01) in the large sample of healthy controls from the Bipolar and Schizophrenia Network on Intermediate Phenotypes study (N=304, 55% women) from which the current sample was recruited (subjects from the Chicago site).
Resting State Data Acquisition
Participants underwent 5 minutes of scanning using a GE Signa EXCITE 3.0 Tesla MR imaging system and an 8-channel phased array head coil. Participants fixated on a central crosshair for the duration of the scan. Video monitoring of participants eyes confirmed adherence to this instruction. Soft ear plugs were used to reduce scan noise, and head motion was minimized with head cushions. Echo-planar imaging sensitive to changes in BOLD signals (repetition time= 1775msec, echo time= 27msec, flip angle= 60°) were obtained. The slice thickness was 4 mm (1mm gap) with a matrix size of 64×64 and a field of view of 220×220 mm², resulting in a voxel size of 3.44×3.44×5 mm³. Each brain volume was comprised of 29 axial slices, and each functional run contained 210 image volumes.
Data Processing and Network Construction
Image processing and connectivity network construction for each subject followed that of our prior work (see Zhang et al. 2011). Briefly, the resting state image time series preprocessing was carried out using Statistical Parametric Mapping software (SPM8, http://www.fil.ion.ucl.ac.uk/spm), including slice-time correction, realignment, unwrapping, normalization to Montreal Neurological Institute (MNI) space, resampling to 3×3×3mm³, and bandpass filtering (0.01–0.8 Hz). Head motion artifacts were reduced using a 24 parameter autoregressive model (Friston et al. 1996; Satterthwaite et al. 2013; Yan et al. 2013) and white matter and cerebrospinal fluid signals were regressed out. Movement was < 2.2 mm translation, and < 2° rotation. No significant sex differences were found with respect to head translation or rotation (both p’s > 0.05).
Brain functional networks, comprised of nodes connected by edges, defined below, were constructed from each participant’s preprocessed time series. This was done using graph theoretical approaches using the Brain Connectivity Toolbox (http://www.brain-connectivity-toolbox.net)(Rubinov and Sporns 2010), an approach that is well-established and reliable (Power et al. 2010; Welton et al. 2015; Wig et al. 2011). Nodes were defined as the 90 non-cerebellar regions of the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al. 2002). For each subject, the time series of voxels in each node was averaged and then correlated with each other node’s average time series, creating a 90×90 correlation matrix for each subject that reflects inter-regional functional connectivity strength. These node-to-node correlations represent the initial edges in the network. Networks were then pruned by dropping any edge (correlation) that did not meet statistical significance (p <0.05, uncorrected).
To estimate network connectivity properties for group analyses, we applied a range of cost thresholds to the pruned networks (Watts and Strogatz 1998). Cost was defined as the total number of edges divided by the maximum possible number of edges. Instead of selecting a single threshold, we selected a wide range of cost thresholds for the 90 nodes according to the following criteria: 1) the averaged degree (number of edges linked to the node) over all nodes of each thresholded network was larger than 2×log(N), where N=90 (number of maximum possible nodes); 2) the largest size of each individual network was larger than 80 nodes; and 3) the small worldness of the thresholded networks was larger than 1.1, e.g., clustering properties of nodes (connectedness among them) were greater than that of a randomly-generated network (Watts and Strogatz 1998). Based on the criteria above, the network analysis was performed against random network models generated in the small-world interval of 0.12 ≤ cost ≤ 0.49, with step= 0.01.
We calculated nodal network measurements at each cost threshold for each subject and then calculated the area under the curve (AUC) as the value for that network measure for each subject in group comparisons and correlations with hormone levels. AUC of network metrics over a cost threshold range has been found to be sensitive to group differences (Achard and Bullmore 2007; Onnela et al. 2005). Regional measures per node were: nodal degree (number of edges a node has connected to it), nodal efficiency (how quickly information flows between it and other nodes), and nodal betweenness (importance of a node via how many short paths it is part of between nodes). Mathematical descriptions of network measures are as follows:
Nodal degree
The nodal degree quantifies the extent to which a node is relevant to the graph (Rubinov and Sporns 2010). In a network G, the nodal weighed degree is measured as the sum of the weights of all the connections of node i, that is .
Nodal efficiency
The nodal weighed efficiency of a given node i is defined as the inverse of the mean harmonic shortest path length between this node and all other nodes in the network G (Achard and Bullmore 2007), according to the formula: . Nodal efficiency measures the information propagation ability of a node within the network, the node i is more important if the value of is higher.
Nodal betweenness
The betweenness centrality of a node I considers the fraction of all shortest paths in the network that pass through the node (Freeman 1977).
Statistical Analyses
We examined sex differences in network metrics using nonparametric permutation tests (Bullmore et al. 1999). Statistical significance was set at p<0.01, Bonferroni corrected. Brain regions showing significant sex differences in nodal metrics were then correlated with peripheral hormone levels for each sex to assess both brain connectivity-hormone associations and brain connectivity-behavioral performance associations. While age has been shown to be associated with functional brain network connectivity in previous studies (Biswal et al. 2010), age was not correlated with our extracted resting state metrics in our study of primarily midlife adults (all r’s<0.17) and was thus not used as a covariate in our hormone-resting state correlations. Given that this is the first exploratory study to examine these associations, statistical significance was set at p<0.05 uncorrected.
Results
Sex Differences in Network Metrics
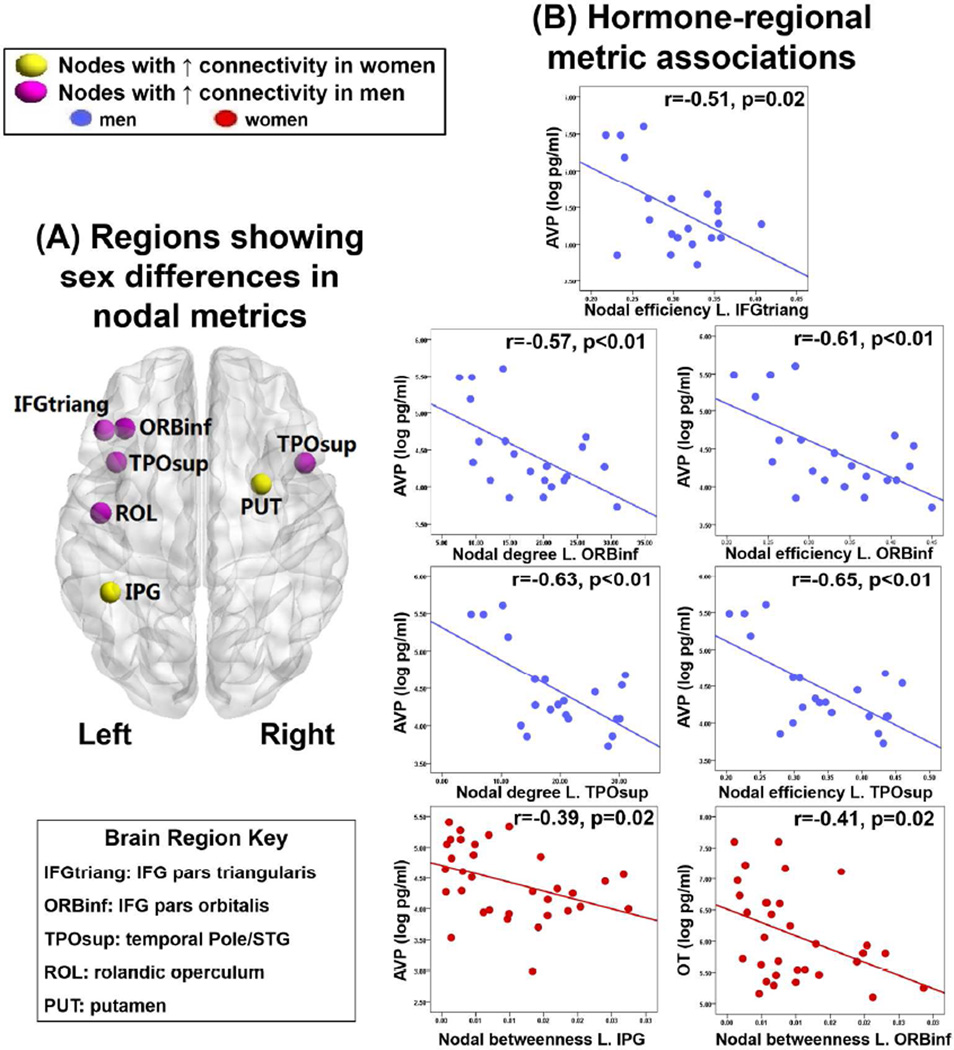
Compared to women, men showed higher nodal degree and nodal efficiency in left inferior frontal gyrus (IFG; pars orbitalis and pars triangularis) and bilateral superior temporal gyrus (STG), as well as higher nodal degree in left rolandic operculum. In comparison, women showed higher nodal betweenness in right putamen and left inferior parietal gyrus (IPG, p<0.01, Bonferroni corrected)(Table 2, Figure 1). Notably, pars triangularis and STG are important for verbal/language processes, pars orbitalis for emotion processing, and IPG for spatial processing.
Table 2.
The comparison nodal network metrics between men and women.
| Nodal metrics |
|||
|---|---|---|---|
|
p-value |
|||
| Brain regions | Nodal degree | Nodal efficiency |
Nodal betweenness |
| Men > Women | |||
| L inferior frontal gyrus (pars orbitalis) | 0.001 | 0.004 | 0.050 |
| R temporal pole: superior temporal gyrus | 0.004 | 0.009 | 0.043 |
| L temporal pole: superior temporal gyrus | 0.004 | 0.009 | 0.097 |
| L inferior frontal gyrus (pars triangularis) | 0.008 | 0.006 | 0.050 |
| L rolandic operculum | 0.008 | 0.017 | 0.321 |
| Women > Men | |||
| L inferior parietal gyrus | 0.455 | 0.304 | 0.005 |
| R putamen | 0.065 | 0.084 | 0.002 |
Note. L=left, R=right. Regions showing significant differences between healthy men and women (p<0.01, bonferroni corrected) in at least one of the three nodal centralities (showed in bold font).
Figure 1.
(A) Differences in nodal metrics between men and women and (B) significant correlations of the metrics in those brain regions with vasopressin (AVP) and oxytocin (OT). Red and blue scatterplots represent men and women, respectively. Brain image is a ventral view. L=left. R=right.
Relationships between Hormones and Network Measures
Nodal network indices showing significant sex differences in a brain region were correlated with peripheral hormone measures stratified by sex. In men, higher AVP was associated with lower nodal degree and lower nodal efficiency in left IFG (pars orbitalis) and left STG and lower nodal efficiency in left IFG (pars triangularis)(p’s<0.05; Figure 1). By comparison, in women, higher AVP was associated with lower betweenness in left IPG and higher OT was associated with lower nodal degree in left IFG (pars orbitalis) (p’s<0.05; Figure 1). No significant associations were found between AVP and nodal degree and efficiency in frontal or temporal regions for women. Similarly, for men, there was no association between OT and betweenness in parietal or frontal areas.
Next, we explored associations between hormone levels and nodal metrics stratified by sex in regions not showing sex differences in regional nodal metrics. In men, higher AVP was associated with lower nodal degree and efficiency in widespread brain regions including regions in frontal (e.g., IFG, superior and middle frontal gyri), temporal (e.g., hippocampus, middle and superior temporal gyri), occipital (e.g., superior, middle, and inferior occipital gyri) cortex. In women, fewer correlations between AVP and nodal metrics were observed, especially in nodal efficiency (Table 3). To supplement this result of a high number of nodal metrics in men correlating with AVP, we evaluated AVP correlations with global efficiency, a summary network efficiency measure across the brain. Efficiency is a relevant metric to describe the brain networks from the perspective of information flow across nodes (Latora and Marchiori 2001). For a network G with N nodes, the global efficiency can be computed as: , where Lij is the shortest weighed path length between nodes i and j in network G As expected, only in men, higher AVP was associated with lower global efficiency (r=−0.50, p=0.01). OT was associated with activity in few brain nodes, and sex differences in OT-related nodal metrics were not as evident as the AVP-related nodal metrics (Table 4).
Table 3.
Brain regions correlating with peripheral arginine vasopressin (AVP) levels in men and women.
| AVP | |||||||
|---|---|---|---|---|---|---|---|
| Men r value |
Women r value |
||||||
| Brain regions | Nodal degree |
Nodal efficiency |
Nodal betweenness |
Brain regions | Nodal degree |
Nodal efficiency |
Nodal betweenness |
| L precentral gyrus | −0.48* | −0.55* | L lingual gyrus | 0.37* | |||
| R middle frontal gyrus, orbital part | −0.46* | R middle occipital gyrus | 0.38* | ||||
| L inferior frontal gyrus, pars orbitalis | −0.57** | −0.61** | R postcentral gyrus | 0.36* | |||
| L anterior cingulate gyri | −0.47* | −0.67** | R pallidum | 0.40* | |||
| L median cingulate gyri | −0.46* | −0.50* | R thalamus | 0.38* | |||
| R median cingulate gyri | −0.47* | −0.51* | L median cingulate gyri | 0.51** | |||
| L superior temporal gyrus | −0.54* | −0.55* | R median cingulate gyri | 0.36* | |||
| R superior temporal gyrus | −0.45* | −0.49* | L fusiform gyrus | 0.51** | |||
| L superior temporal gyrus, temporal pole | −0.63** | −0.65** | L inferior parietal gyrus | −0.39* | |||
| L middle temporal gyrus | −0.64** | −0.65** | L inferior temporal gyrus | −0.36* | |||
| R middle temporal gyrus | −0.44* | −0.52* | |||||
| R inferior temporal gyrus | −0.44* | −0.53* | |||||
| R precentral gyrus | −0.48* | ||||||
| L superior frontal gyrus, dorsolateral | −0.47* | ||||||
| L inferior frontal gyrus, pars opercularis | −0.47* | ||||||
| L inferior frontal gyrus, pars triangularis | −0.51* | ||||||
| R inferior frontal gyrus, pars orbitalis | −0.51* | ||||||
| L rolandic operculum | −0.49* | ||||||
| L supplementary motor area | −0.47* | ||||||
| R supplementary motor area | −0.50* | ||||||
| L superior frontal gyrus, medial | −0.44* | ||||||
| L insula | −0.52* | ||||||
| R anterior cingulate gyri | −0.56** | ||||||
| R hippocampus | −0.49* | ||||||
| L calarine fissure | −0.49* | ||||||
| R calarine fissure | −0.54* | ||||||
| L cuneus | −0.44* | ||||||
| R cuneus | −0.51* | ||||||
| L lingual | −0.51* | ||||||
| R lingual | −0.51* | ||||||
| L superior occipital gyrus | −0.53* | ||||||
| R superior occipital gyrus | −0.57** | ||||||
| L middle occipital gyrus | −0.48* | ||||||
| R middle occipital gyrus | −0.55* | ||||||
| L inferior occipital gyrus | −0.51* | ||||||
| R inferior occipital gyrus | −0.52* | ||||||
| L fusiform gyrus | −0.45* | ||||||
| R postcentral gyrus | −0.49* | ||||||
| L superior parietal gyrus | −0.52* | ||||||
| R superior parietal gyrus | −0.53* | ||||||
| L heschl gyrus | −0.52* | ||||||
| L middle temporal gyrus, temporal pole | −0.52* | ||||||
| L inferior temporal gyrus | −0.50* | ||||||
Note.
p<0.01;
p<0.05.
L=left, R=right.
Table 4.
Brain regions correlating with peripheral oxytocin (OT) levels in men and women.
| OT | |||||||
|---|---|---|---|---|---|---|---|
| Men r value |
Women r value |
||||||
| Brain regions | Nodal degree |
Nodal efficiency |
Nodal betweenness |
Brain regions | Nodal degree |
Nodal efficiency |
Nodal betweenness |
| R superior frontal gyrus, orbital part | 0.47* | L middle temporal gyrus, | −0.41* | −0.37* | |||
| R heschl gyrus | 0.49* | temporal pole | |||||
| L inferior frontal gyrus, pars opercularis | −0.47* | L precentral gyrus | 0.39* | ||||
| L insula | −0.52* | L inferior frontal gyrus, | −0.41* | ||||
| R anterior cingulate gyri | −0.46* | pars orbitalis | |||||
| R superior occipital gyrus | −0.46* | ||||||
| R angular gyrus | −0.56** | ||||||
| L caudate nucleus | 0.48* | ||||||
| R thalamus | −0.51* | ||||||
Note.
p<0.01;
p<0.05.
L=left, R=Right.
Relationships between Network Measures and Behavioral Performance
In men, higher nodal degree and higher nodal efficiency in left IFG (pars orbitalis) and higher nodal efficiency in left IFG (pars triangularis) were associated with better verbal fluency (r=0.47, p=0.02; r=0.43, p=0.04; r=0.44, p=0.03, respectively). In women, higher nodal efficiency in left IFG (pars orbitalis) was associated with better verbal learning (r=0.54, p=0.001) and verbal fluency (r=0.38, p=0.02). However, higher betweenness in left IPG was associated with worse emotion recognition (r=−0.40, p=0.02).
Relationships between Hormones and Beavioral Performance
In women, OT was significantly associated with emotion recognition (r=0.42, p=0.02). There were no other significant or near significant hormone-behavior associations in men or women. However, we note that associations between OT and emotion recognition (r=0.37, p=0.12), OT and verbal learning (r=0.36, p=0.14), AVP and processing speed (r=−0.38, p=0.10), and AVP and verbal fluency (r=−0.34, p=0.15) in men were observed.
Discussion
In this investigation, using a graph theory analytic approach to investigate hormonal associations with resting state brain function, we found sex differences in functional brain networks important for emotion and cognitive processing. Our findings suggest a differential role of OT and AVP in these complex brain networks important in emotion processing and cognition between men and women.
Sex differences in regional functional brain networks
In the present study, left orbital cortex and bilateral superior temporal cortex, neocortical regions central in emotion and language processing, were more connected and efficient in men than women. Right putamen and left inferior parietal cortex, central to visuospatial abilities, had higher betweenness values for women compared to men, indicating greater network integration of these regions. The regional brain network differences found here are consistent with findings from previous structural and functional neuroimaging studies (Biswal et al. 2010; Frederikse et al. 1999; Goldstein et al. 2001; Ruigrok et al. 2014; Sacher et al. 2013; Stevens and Hamann 2012; Tomasino and Gremese 2015; Wagner et al. 2014). However, we did not find sex differences in connectivity of corticolimbic circuitry including amygdala, insula, anterior and posterior cingulate, and dorsolateral and medial prefrontal cortex which are also important for emotion and cognitive processing (Allen et al. 2011; Biswal et al. 2010; Bluhm et al. 2008; Filippi et al. 2013; Kong et al. 2010; Liu et al. 2009; Zuo et al. 2010). While the directionality of our findings in the superior temporal and parietal cortex are consistent with previous studies (Biswal et al. 2010; Filippi et al. 2013; Hjelmervik et al. 2014), there is less consistency with respect to the orbital cortex. Whereas we and others report greater connectivity in men compared to women (Allen et al. 2011; Kong et al. 2010), others report the converse (Biswal et al. 2010; Bluhm et al. 2008; Hjelmervik et al. 2014) or no differences (Weissman-Fogel et al. 2010).
Sex differences in hormone associations with regional functional brain networks
The correlations between hormones and network metrics indicated that higher levels of both OT and AVP were associated with reductions in network connectivity. This is noteworthy because hormone-related connectivity reductions in each sex were in areas where that sex had overall higher connectivity. Thus, AVP and OT may function by dampening connectivity of regions where connectivity was elevated in each sex, with different hormones serving that function in men and women. Higher levels of AVP were generally associated with decreased connectedness and efficiency of connections across the brain for men; this pattern was not seen overall for women’s network metrics and AVP levels. OT associations were fewer and more frequently observed in women. These findings are consistent with previous neuroendocrine studies in animal models suggesting that males are more sensitive to AVP than females (Carter 2007; Carter et al. 2009).
Sex differences in hormonal associations with emotion processing
Our findings in the left IFG (pars orbitalis) highlight different profiles of hormone associations with functional brain connectivity in men and women. This region exerts prominent top-down regulation of limbic regions including the amygdala (Ochsner et al. 2004), and thus the hormone-neural activity may play a role in sex differences in emotion processing and reactivity. Whereas AVP was negatively associated with functional connectivity of left orbital cortex in men, OT was negatively associated in women. With respect to AVP, our findings are in part consistent with previous functional imaging studies. Previous studies report sex-specific associations between plasma AVP levels and neural activity during the processing of negative emotions; however, associations are with the amygdala and not prefrontal cortex (Motoki et al. 2016). Additionally, exogenous AVP increases amygdala-prefrontal connectivity during emotion processing in men (Zink et al. 2010). Although the exact underlying factors driving these differences are unknown, one possibility for the findings in men is that higher AVP, which is in part androgen-dependent (De Vries and Panzica 2006), may modulate activity in orbital frontal cortex and thereby influence emotional reactivity and activity in limbic regions. Consistent with this hypothesis, male rodents show higher AVPR1a receptor density in the medial prefrontal cortex compared to females (Smeltzer et al. 2006). Males have more androgen receptors than females in the paraventricular nucleus of the hypothalamus (Fernandez-Guasti et al. 2000) which is the major site of synthesis of AVP. In humans, androgen relationships with AVP could contribute to greater emotional reactivity in men as AVP is associated with amplifying reactivity to stressors and increasing behavioral or emotional reactivity (Carter et al. 2008; Heinrichs and Domes 2008; Meyer-Lindenberg et al. 2011).
In contrast to men, higher levels of OT in women were associated with decreased connectivity in the IFG and better emotion recognition. These findings are consistent with some previous neuroimaging studies. Whereas plasma levels of OT were not found to be associated with brain activation during the processing of negative emotions in men or women (Motoki et al. 2016), there are sex-dependent associations between exogenous OT and amygdala-prefrontal connectivity (Ebner et al. 2016). In particular, exogenous OT has robust effects on amygdala-prefrontal connectivity among young women with no effects in young men. OT receptor binding can be comparatively sparse in socially monogamous primates (Freeman et al. 2014), females have a higher densities of OT receptors than males in the medial prefrontal cortex (Smeltzer et al. 2006). Such findings may in part explain the capacity of chronic exposure to OT to dampen emotion reactivity, and suggest that the mechanism for this effect may be its impact on reducing functional connectivity among targeted brain regions with sharing circuit-level integration.
Sex differences in brain substrates of cognition
Our findings, particularly with the left IFG (pars triangularis), STG, and IPG, suggest that OT and AVP may modify brain physiology in ways that might contribute to sex differences in cognitive abilities, as these regions support verbal and visuospatial abilities respectively. In men, higher AVP was associated with less connectedness and efficiency in left IFG (pars triangularis) and left STG, two important regions for verbal abilities (Costafreda et al. 2006; Wagner et al. 2014; Wang et al. 2014). Consistent with this idea is that higher connectedeness and efficiency in left pars triangularis was associated with better verbal fluency in men. Thus, AVP levels may in part explain why men on average perform worse than women on verbal abilities, particularly verbal fluency. Although not significant, higher AVP was trending towards relating to worse performance on verbal abilities in men. However, in women higher AVP was associated with lower efficiency in the left parietal cortex, which is important for visuospatial abilities (Tomasino and Gremese 2015). Consequently, AVP levels may in part explain why women on average perform worse than men on visuospatial abilities. However, it is important to note that because higher levels of horomones were associated with lowered connectivity which overall was increased in men or women, it remains to be determined in future experimental studies whether hormones were partially compensating for heightened functional connectivity to enhance adaptive neural function.
Limitations
The present study has limitations including the cross-sectional study design and a single measurement of hormone levels. Second, we measured peripheral hormone levels of OT and AVP. However, animal data indicate that central and peripheral hormone levels are correlated (Landgraf and Neumann 2004) and peripheral measures of OT are strongly associated with neural activation in response to animacy in humans (Lancaster et al. 2015). Third, choices in methodology of network construction including anatomical parsing scheme and network construction and pruning may have impacted our findings. The choices made were similar to prior work, but there are a range of approaches for assessing neural connectivity. Fourth, while we did not control for parity, menopausal stage, endogenous hormone cycling (menstrual cycle) or exogenous hormone administration (e.g., oral contraceptive pills). Some (Petersen et al. 2014), but not all studies (Hjelmervik et al. 2014), demonstrate the effects of sex steroid hormones on brain connectivity. However, there was only a small proportion of women in the present study on oral contraceptives. Finally, a more thorough behavioral association of emotional reactivity and sexually dimorphic cognitive abilities. particularly tasks typically showing a male advantage (visuospatial abilities), may enhance the detection of associations between network measures, hormones and behavior.
Conclusions
Understanding the interplay between biological sex, hormones, and neural networks serves as a foundation upon which to model the mechanisms of behavioral and cognitive differences between males and females. This is fundamentally important, and it has applied implications given that alterations hormones and neural systems may underlie differences between males and females in psychological manifestations of psychiatric disorders (Goldstein 2002; Ross and Pearlson 1996). There are sex differences in intrinsic brain activity particularly in regions important in emotion processing and cognition including prefrontal, temporal, and parietal regions. Our data suggest that OT and AVP may modify brain physiology in these regions to contribute to or diminish these differences, rather to increase them. Notably, our findings suggest a differential role of OT and AVP in these complex brain networks important in emotion processing and cognition in men and women.
Significance Statement.
Oxytocin and arginine vasopressin are hormones that contribute to sex differences in brain systems supporting emotion and cognition. We examined sex differences in the association between these hormones and functional brain connectivity. Findings suggest that different hormones modulate brain systems supporting emotion processing in men and women, and may account for well-established sex differences in verbal and visuospatial abilities.
Hormones differentially correlate with brain networks important for cognition and emotion processing in men and women. Vasopressin and oxytocin may in part account for sex differences in verbal and visuospatial abilities and emotion processing.
Acknowledgments
Support/Grant Information: This work was supported in part by a 2012 NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation to Dr. Rubin, and by the National Institute of Health (K12HD055892, K08MH083888, MH083126, MH077851, MH078113, MH077945, MH077852, and MH077862), the National Natural Science Foundation of China (Grant No. 81371527), Program for Changjiang Scholars and Innovative Research Team in University of China. The funding agencies had no role in the design and conduct of the study collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Conflict of interest statement
Dr. Bishop has served as an advisory board member for Physician Choice Laboratory Services (PCLS). Dr. Sweeney has consulted to Eli Lilly, Roche, and Takeda. The other authors have nothing to disclose.
Role of authors
Drs. Rubin and Sweeney conceived the neuroendocrine study idea. Dr. Sweeney coordinated the Chicago B-SNIP study and the sample presented here was ascertained as part of the B-SNIP study at the Chicago site. Drs. Rubin, Yao, Lui, Carter, Drogos, Sweeney, and Keedy wrote the first draft of the manuscript. Dr. Rubin and Dr. Yao take responsibility for the integrity of the hormone data and the MRI data analyses respectively. Drs. Yao, Lui, Liao, and Ji planned and conducted the graph theory based connectivity analyses. Dr. Sweeney provided oversight as principle investigator of the study. Dr. Carter developed the methodology to examine oxytocin and vasopressin, and assays were done in her laboratory by Dr. Pournajafi-Nazarloo and Dr. Lauren Drogos. Dr. Bishop collected and managed the biological samples. All authors contributed to the writing of the manuscript and approved the final version.
References
- Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS computational biology. 2007;3(2):e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R, Michael AM, Caprihan A, Turner JA, Eichele T, Adelsheim S, Bryan AD, Bustillo J, Clark VP, Feldstein Ewing SW, Filbey F, Ford CC, Hutchison K, Jung RE, Kiehl KA, Kodituwakku P, Komesu YM, Mayer AR, Pearlson GD, Phillips JP, Sadek JR, Stevens M, Teuscher U, Thoma RJ, Calhoun VD. A baseline for the multivariate comparison of resting-state networks. Frontiers in systems neuroscience. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16(4):248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Osuch EA, Lanius RA, Boksman K, Neufeld RW, Theberge J, Williamson P. Default mode network connectivity: effects of age, sex, and analytic approach. Neuroreport. 2008;19(8):887–891. doi: 10.1097/WNR.0b013e328300ebbf. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. Ieee T Med Imaging. 1999;18(1):32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Cahill L. Sex-related influences on the neurobiology of emotionally influenced memory. Ann N Y Acad Sci. 2003;985:163–173. doi: 10.1111/j.1749-6632.2003.tb07080.x. [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res. 2007;176(1):170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Carter CS, Boone EM, Pournajafi-Nazarloo H, Bales KL. Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev Neurosci. 2009;31(4):332–341. doi: 10.1159/000216544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, Schwertz D. Oxytocin: behavioral associations and potential as a salivary biomarker. Ann N Y Acad Sci. 2007;1098:312–322. doi: 10.1196/annals.1384.006. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 2006;27(10):799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias CM, Nilsson LG, Herlitz A. Sex differences in cognition are stable over a 10-year period in adulthood and old age. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2006;13(3–4):574–587. doi: 10.1080/13825580600678418. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138(3):947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62(10):1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35(1):83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Ebner NC, Chen H, Porges E, Lin T, Fischer H, Feifel D, Cohen RA. Oxytocin’s effect on resting-state functional connectivity varies by age and sex. Psychoneuroendocrinology. 2016;69:50–59. doi: 10.1016/j.psyneuen.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Kruijver FP, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol. 2000;425(3):422–435. doi: 10.1002/1096-9861(20000925)425:3<422::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Filippi M, Valsasina P, Misci P, Falini A, Comi G, Rocca MA. The organization of intrinsic brain activity differs between genders: a resting-state fMRI study in a large cohort of young healthy subjects. Hum Brain Mapp. 2013;34(6):1330–1343. doi: 10.1002/hbm.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederikse ME, Lu A, Aylward E, Barta P, Pearlson G. Sex differences in the inferior parietal lobule. Cereb Cortex. 1999;9(8):896–901. doi: 10.1093/cercor/9.8.896. [DOI] [PubMed] [Google Scholar]
- Freeman L. A set of measures of centrality based upon betweenness. Sociometry. 1977;40(1):35–41. [Google Scholar]
- Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, Young LJ. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neuroscience. 2014;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Goldstein Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging (vol 59, pg 154, 2002) Arch Gen Psychiat. 2002;59(5):480–480. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11(6):490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gur RE. Memory in health and in schizophrenia. Dialogues Clin Neurosci. 2013;15(4):399–410. doi: 10.31887/DCNS.2013.15.4/rgur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115(2):137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Gur RE, Gur RC. Gender differences in aging: cognition, emotions, and neuroimaging studies. Dialogues Clin Neurosci. 2002;4(2):197–210. doi: 10.31887/DCNS.2002.4.2/rgur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog Brain Res. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Hjelmervik H, Hausmann M, Osnes B, Westerhausen R, Specht K. Resting states are resting traits--an FMRI study of sex differences and menstrual cycle effects in resting state cognitive control networks. PLoS One. 2014;9(7):e103492. doi: 10.1371/journal.pone.0103492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura D. Sex and Cognition. London: MIT Press; 1999. [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25(49):11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Tu PC, Zyloney C, Su TP. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res. 2010;211(2):215–219. doi: 10.1016/j.bbr.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Delis DC, Daniel M. Sex differences in verbal learning. Journal of Clinical Psychology. 1988;44:907–915. [Google Scholar]
- Kramer JH, Delis DC, Kaplan E, O’Donnell L, Prifitera A. Developmental sex differences in verbal learning. Neuropsychology. 1997;11(4):577–584. doi: 10.1037//0894-4105.11.4.577. [DOI] [PubMed] [Google Scholar]
- Kumar J, Vollm B, Palaniyappan L. Oxytocin affects the connectivity of the precuneus and the amygdala: a randomized, double-blinded, placebo-controlled neuroimaging trial. Int J Neuropsychopharmacol. 2015;18(5) doi: 10.1093/ijnp/pyu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster K, Carter CS, Pournajafi-Nazarloo H, Karaoli T, Lillard TS, Jack A, Davis JM, Morris JP, Connelly JJ. Plasma oxytocin explains individual differences in neural substrates of social perception. Frontiers in human neuroscience. 2015;9:132. doi: 10.3389/fnhum.2015.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Efficient behavior of small-world networks. Physical review letters. 2001;87(19):198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Coccaro EF, Cremers H, McCarron R, Lu SF, Brownstein MJ, Simon NG. A novel V1a receptor antagonist blocks vasopressin-induced changes in the CNS response to emotional stimuli: an fMRI study. Frontiers in systems neuroscience. 2013;7:100. doi: 10.3389/fnsys.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischke A, Gamer M, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC, Domes G. Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology. 2012;37(9):1431–1438. doi: 10.1016/j.psyneuen.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Liu H, Stufflebeam SM, Sepulcre J, Hedden T, Buckner RL. Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proc Natl Acad Sci U S A. 2009;106(48):20499–20503. doi: 10.1073/pnas.0908073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann VA, Sasanuma S, Sakuma N, Masaki S. Sex differences in cognitive abilities: A cross-cultural perspective. Neuropsychologia. 1990;28(10):1063–1077. doi: 10.1016/0028-3932(90)90141-a. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Motoki K, Sugiura M, Takeuchi H, Kotozaki Y, Nakagawa S, Yokoyama R, Kawashima R. Are Plasma Oxytocin and Vasopressin Levels Reflective of Amygdala Activation during the Processing of Negative Emotions? A Preliminary Study. Frontiers in psychology. 2016;7:480. doi: 10.3389/fpsyg.2016.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Onnela JP, Saramaki J, Kertesz J, Kaski K. Intensity and coherence of motifs in weighted complex networks. Physical review E, Statistical, nonlinear, and soft matter physics. 2005;71(6 Pt 2):065103. doi: 10.1103/PhysRevE.71.065103. [DOI] [PubMed] [Google Scholar]
- Petersen N, Kilpatrick LA, Goharzad A, Cahill L. Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. Neuroimage. 2014;90:24–32. doi: 10.1016/j.neuroimage.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28(26):6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67(5):735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Demarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R, Pagnoni G. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–248. doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Pearlson GD. Schizophrenia, the heteromodal association neocortex and development: potential for a neurogenetic approach. Trends in neurosciences. 1996;19(5):171–176. doi: 10.1016/s0166-2236(96)10022-9. [DOI] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Bishop JR, Pournajafi-Nazarloo H, Drogos LL, Hill SK, Ruocco AC, Keedy SK, Reilly JL, Keshavan MS, Pearlson GD, Tamminga CA, Gershon ES, Sweeney JA. Reduced levels of vasopressin and reduced behavioral modulation of oxytocin in psychotic disorders. Schizophr Bull. 2014;40(6):1374–1384. doi: 10.1093/schbul/sbu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Jamadar R, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Sex-specific associations between peripheral oxytocin and emotion perception in schizophrenia. Schizophr Res. 2011;130(1–3):266–270. doi: 10.1016/j.schres.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Okon-Singer H, Gotowiec S, Villringer A. Sexual dimorphism in the human brain: evidence from neuroimaging. Magn Reson Imaging. 2013;31(3):366–375. doi: 10.1016/j.mri.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Snozzi R, Bird G, Petrovic P, Silani G, Heinrichs M, Dolan RJ. Effects of oxytocin and prosocial behavior on brain responses to direct and vicariously experienced pain. Emotion. 2008;8(6):781–791. doi: 10.1037/a0014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394(2):146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Sripada CS, Phan KL, Labuschagne I, Welsh R, Nathan PJ, Wood AG. Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. Int J Neuropsychopharmacol. 2013;16(2):255–260. doi: 10.1017/S1461145712000533. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50(7):1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Tomasino B, Gremese M. Effects of Stimulus Type and Strategy on Mental Rotation Network: An Activation Likelihood Estimation Meta-Analysis. Frontiers in human neuroscience. 2015;9:693. doi: 10.3389/fnhum.2015.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol Bull. 1995;117(2):250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Wagner S, Sebastian A, Lieb K, Tuscher O, Tadic A. A coordinate-based ALE functional MRI meta-analysis of brain activation during verbal fluency tasks in healthy control subjects. BMC Neurosci. 2014;15:19. doi: 10.1186/1471-2202-15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mathalon DH, Roach BJ, Reilly J, Keedy SK, Sweeney JA, Ford JM. Action planning and predictive coding when speaking. Neuroimage. 2014;91:91–98. doi: 10.1016/j.neuroimage.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393(6684):440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Kemmler G, Deisenhammer EA, Fleischhacker WW, Delazer M. Sex differences in cognitive function. Pers Individ Dif. 2003:863–875. [Google Scholar]
- Weiss EM, Ragland JD, Brensinger CM, Bilker WB, Deisenhammer EA, Delazer M. Sex differences in clustering and switching in verbal fluency tasks. J Int Neuropsychol Soc. 2006;12(4):502–509. doi: 10.1017/s1355617706060656. [DOI] [PubMed] [Google Scholar]
- Weissman-Fogel I, Moayedi M, Taylor KS, Pope G, Davis KD. Cognitive and default-mode resting state networks: do male and female brains “rest” differently? Hum Brain Mapp. 2010;31(11):1713–1726. doi: 10.1002/hbm.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welton T, Kent DA, Auer DP, Dineen RA. Reproducibility of graph-theoretic brain network metrics: a systematic review. Brain connectivity. 2015;5(4):193–202. doi: 10.1089/brain.2014.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Yucel M, Yap MB, Allen NB. Sex differences in the neural correlates of emotion: evidence from neuroimaging. Biol Psychol. 2011;87(3):319–333. doi: 10.1016/j.biopsycho.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Wig GS, Schlaggar BL, Petersen SE. Concepts and principles in the analysis of brain networks. Ann N Y Acad Sci. 2011;1224:126–146. doi: 10.1111/j.1749-6632.2010.05947.x. [DOI] [PubMed] [Google Scholar]
- Williams LM, Mathersul D, Palmer DM, Gur RC, Gur RE, Gordon E. Explicit identification and implicit recognition of facial emotions: I. Age effects in males and females across 10 decades. J Clin Exp Neuropsychol. 2009;31(3):257–277. doi: 10.1080/13803390802255635. [DOI] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang J, Wu Q, Kuang W, Huang X, He Y, Gong Q. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry. 2011;70(4):334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Zink CF, Stein JL, Kempf L, Hakimi S, Meyer-Lindenberg A. Vasopressin modulates medial prefrontal cortex-amygdala circuitry during emotion processing in humans. J Neurosci. 2010;30(20):7017–7022. doi: 10.1523/JNEUROSCI.4899-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zang YF, Castellanos FX, Milham MP. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 2010;30(45):15034–15043. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]