Abstract
Oral contraceptive (OC) users typically show a blunted or no cortisol response to psychosocial stress. Although most OC regimens include both an inactive (dummy) and active pill phase, studies have not systematically investigated cortisol responses during these pill phases. Further, high levels of cortisol following a stressor diminish retrieval of emotional material, but the effects of stress on memory among OC users are poorly understood. We examined the effects of a psychosocial stressor, the Trier Social Stress Test, versus a control condition on cortisol responsivity and emotional memory retrieval in women tested either during their active (n=18) or inactive pill phase (n = 21). In secondary analyses, we quantitatively compared OC users to normally cycling women and showed a significant lack of cortisol response during both active and inactive pill phase. Emotional recall did not differ between active and inactive pill phases. Stress differentially diminished recall of negative words compared to positive or neutral words, but cortisol levels were unrelated to memory performance. These findings indicate that OC users have distinct cortisol and memory responses to stress that are similar between the active and inactive pill phases.
Keywords: Sex Steroid Hormones, Oral Contraceptives, Cognition, Emotion
Graphical abstract
In response to a psychosocial stressor, oral contraceptive (OC) users showed no cortisol response and decreased recall of negative stimuli that was maintained during both the active and inactive pill phases. OC use generally may buffer against retrieval of negative memories following stress (see Figure 4).
Introduction
Oral contraceptives (OCs) are the most commonly used form of contraception in the United States; 82% of women aged 15-44 years have ever used OCs and 16% are active users (Daniels and Mosher, 2013; Daniels et al., 2014). Ethinyl-estradiol-containing OCs inhibit ovulation, thereby suppressing endogenous estradiol and progesterone. OC users have blunted salivary cortisol responsivity to laboratory stressors (Kirschbaum et al., 1995b; Kirschbaum et al., 1999; Rohleder et al., 2003; Villada et al., 2013). Recent work has examined the associations among OC use, stress responsivity, and memory (Nielsen et al., 2011; Nielsen et al., 2014; Petersen et al., 2014). Compared to non-users, OC users recalled different aspects of emotional stories (Nielsen et al., 2011) and showed different patterns of recall for emotional images dependent upon their noradrenergic and cortisol reactivity (Nielsen et al., 2013).
To our knowledge, researchers have yet to address how stress responsivity or emotional memory might vary by OC pill phase. OC regimens commonly include an active (21-day) followed by inactive (7-day) pill phase. While endogenous levels of estradiol and progesterone are suppressed during both phases, exposure to exogenous estrogen and progestins is higher during the active pill phase. Notably, we previously demonstrated improved word memory during the active compared to inactive pill phase (Mordecai et al., 2008). In a recent neuroimaging study, Petersen and colleagues (2014) showed differences between the two pill phases in a resting state network important for emotional and cognitive tasks.
The effects of OCs on hypothalamic-pituitary-adrenal (HPA) function has been examined with the Trier Social Stress Test (TSST), a psychosocial stress paradigm involving unanticipated public speaking and mental arithmetic (Kirschbaum et al., 1993). OC users have consistently produced salivary cortisol responses to the TSST that are lower than those of luteal phase women and young men, and lower or comparable to those of follicular phase women (Kirschbaum et al., 1995b; Kirschbaum et al., 1999; Rohleder et al., 2003, Villada et al., 2014). Further investigation is needed to explore whether the effects of OCs on stress responsivity are present during both pill phases. Broadly, the literature suggests that stress responsivity is linked to biological sex and menstrual cycle phase. Many, but not all, studies show a greater cortisol response in men compared to women, and a greater cortisol response during the luteal compared to follicular phase (Kirschbaum et al., 1992; Kirschbaum et al., 1995a and 1995b; Kirschbaum et al., 1999; Rohleder et al., 2003).
The effects of OCs on stress responsivity are also of interest because of the link between stress-related cortisol response and memory performance. Memory consolidation is enhanced when stress exposure occurs during encoding, but retrieval is reduced when exposure occurs immediately prior to retrieval (Roozendaal, 2002; Wolf et al., 2004; Roozendaal et al., 2006). Interestingly, retrieval of emotional stimuli is particularly diminished following the TSST (Domes et al., 2004; Kuhlmann et al., 2005b). We previously reported that emotional memory retrieval following the TSST was related to the magnitude of increase in cortisol in the follicular phase but not the luteal phase (Maki et al., 2015). Thus, in a high-estrogen, high progesterone hormonal milieu, both stress-induced elevations in cortisol and cortisol-related decrements in emotional memory retrieval were blunted.
The overall aim of the current study was to extend this research to OC users. Here we examined the effects of the TSST versus a non-stress control condition on salivary cortisol responsivity and emotional memory retrieval during the active versus inactive pill phases. We hypothesized that following the TSST women tested in the active pill phase would show lower cortisol responsivity and better emotional retrieval compared to women in the inactive pill phase.
Methods
Participants
All methods were reviewed and approved by the University of Illinois at Chicago (UIC) Institutional Review Board. Participants were recruited from UIC and the surrounding community via advertisements on campus and websites. Inclusionary criteria were: 18 to 40 years of age; English as a first language; and consistent use of ethinyl-estradiol containing OCs for the past six months. Exclusionary criteria included: use in the prior six months of glucocorticoids or other prescription or over-the-counter treatments influencing the central nervous system; current smoking; phobia of math or public speaking (per self-report); history of depression, psychiatric illness, or serious medical illness, traumatic brain injury or loss of consciousness > 30 minutes; drug or alcohol dependency or abuse; sensory impairment that would interfere with testing; currently pregnant; child birth or lactation in the previous 6 months; and/or body mass index (BMI) >30. Participants received compensation for their time and travel.
General Procedures
Participants were first screened by phone for general inclusion criteria. They next came to UIC for a screening visit to complete informed consent as well as the Mini-Screen, a 21-item self-report measure used to rule out psychiatric diagnoses (Sheehan et al., 1998). A random number generator was used to randomize each eligible woman to be tested during either the active pill phase (22nd to 24th day of OC cycle where Day 1 is defined as the first day of the inactive pill phase) or inactive pill phase (2nd to 4th day).
All test sessions were scheduled on two consecutive days and began at 1:00 pm (thus ending prior to 5:00 pm) to minimize diurnal variation in cortisol. On Day 1, participants met one-on-one with an examiner who was blinded to pill phase. Blood was drawn at the beginning of the session for hormone assays. Height and weight were measured to calculate BMI. A neuropsychological test battery comprised of measures of memory, psychomotor speed, attention, language, and spatial abilities was also administered (data not reported here). An estimate of intelligence (National Adult Reading Test-Revised; Nelson and Willison, 1991) was also obtained at this time. A key procedure at the end of the test session was the acquisition phase of the Emotional Paired Associates Test (see below). On Day 2, participants completed the timeline of procedures as outlined in Figure 1. Of note we choose to implement the timing of the TSST/control condition to be consistent with findings of diminished memory when stress exposure occurs immediately prior to retrieval (Roozendaal, 2002; Wolf et al., 2004; Roozendaal et al., 2006). After arrival on Day 2, participants had a rest period then completed the non-stress control condition consisting of completing questionnaires relating to mood, symptoms, and lifestyle habits (see Measures to Assess Potential Confounds below). Next, retrieval of one of the emotional word pairs learned 24 hours earlier was tested. After another rest period, participants completed the TSST protocol, followed by recall of the other emotional word pair list previously learned. Self-reported anxiety and stress (State-Trait Anxiety Scale, Visual Analog Scale), salivary cortisol levels, and heart rate variability (not reported here) were obtained following each procedure.
Figure 1.
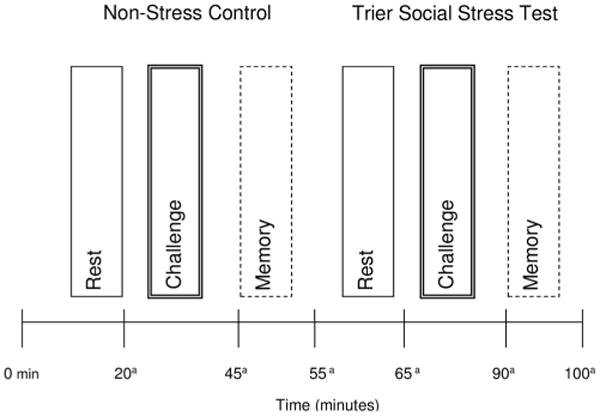
Experimental design.
Note: Condition (Non-Stress versus Stress) and Time (Rest, Challenge, Memory) were within-subject factors and Oral Contraceptive Pill Phase (Active versus Inactive) was a between-subjects factor. aSalivary Cortisol and self-reported anxiety and stress measures were obtained.
Emotional Paired Associates Test
The primary behavioral outcome was the Emotional Paired Associates Test. On Day 1, participants learned two lists (Lists A and B) of 15 emotional word pairs to 100% criterion. The lists were presented in a counterbalanced order across groups (active and inactive pill phase). There were five negative (e.g., “suffocate-loneliness”), five positive (e.g., “champion-laughter”), and five neutral (e.g., “item-passage”) pairs. The two lists were well-matched on overall ratings of valence, arousal, word frequency, and word length based on Affective Norms for English Words (Bradley and Lang, 1999). The word pairs in each list were presented in a random order, and the examiner read one word pair aloud at a rate of one every three seconds. After each list presentation, the examiner prompted the participant with the first member of each pair, and asked the participant to recall the corresponding word. The prompts were also randomly ordered and differed for each recall trial. The examiner responded “that's correct” if the response was correct. If the participant did not respond within 5 seconds or responded incorrectly, the examiner provided the correct answer by saying, “No, ___ goes with ___.” After the first recall trial, the examiner only prompted the participant to recall pairs that she had not successfully recalled. The reading/prompting/learning procedure was repeated until all pairs were correctly recalled once. The list was taught to 100% criterion to ensure equivalent learning across groups, avoid floor effects at recall, and ensure that any errors in recall on the next day were not due to unsuccessful encoding. After both lists were learned to criterion, recall was again measured once for each list and appropriate feedback or corrections were provided as above. Participants were instructed to try to remember the word pairs because they would be asked about them again. On Day 2, 24-28 hours after they learned the two lists to 100% criterion, participants were asked to recall the word pairs of List A (or B) after the control condition and List B (or A) after the stressor condition. The presentation of lists was counter-balanced across conditions (TSST or control) and groups (active pill, inactive pill). For each list recall, participants were prompted with the first member of each pair and asked to recall the corresponding word. Participants received 1 point for each pair recalled (e.g., “What word goes with ‘toxic’?”).
Trier Social Stress Test
Each participant was told that she would take the role of a job applicant for her “ideal job position” and would need to give a speech introducing herself and convincing managers that she is the ideal applicant for a vacant position. She was given 10 minutes to prepare a 5-minute speech and was told that the speech would be recorded and given in front of three experts in the assessment of nonverbal behavior, who were confederate lab personnel. One expert served as the “Chair,” and delivered instructions to the participant. After the preparation period, the participant gave her speech to the “experts.” Those who finished in less than 5 minutes were told sternly to continue the speech for the remainder of the 5 minutes. After the 5-minute speech, the Chair instructed the participant to serially subtract the number 13 from 1,687 as quickly and accurately as possible. If she made an error, she was instructed to start again from 1,687. After 5 minutes of serial subtractions, the task was stopped. Before the stress condition, participants participated in a 20-minute non-stressful control condition where they were instructed to complete a series of questionnaires relating to mood, symptoms and lifestyle habits A slight modification was made to the standard TSST protocol (Kirschbaum et al., 1993), and likewise implemented in the control condition to allow for data collection for an ancillary study. Both conditions were interrupted at 11, 14, and 16 minutes into the condition to complete 1-minute cognitive flexibility tasks at each interruption (data not shown; Alexander et al., 2007). At the end of the test session on Day 2, the examiner debriefed the participant and told her that no analysis of her nonverbal behavior would be performed.
Subjective stress and anxiety
Select measures were obtained at specific time points before and after the TSST and control conditions to measure subjective stress (See Figure 1). At six time points concurrent with saliva sampling, participants completed a two-item Visual Analogue Scale (VAS) measuring how “anxious” and “stressed” they felt on a 10-cm line (maximum distress rating = 10 cm). They also completed the State-Trait Anxiety Inventory: Short Form (STAI), a 6-item questionnaire assessing the extent to which one feels calm, tense, upset, relaxed, content, and worried right now, at this moment (Marteau and Bekker, 1992). Ratings were made on a 4-point Likert scale ranging from “not at all (1)” to “very much so (4)” with higher scores indicating greater state anxiety.
Salivary Cortisol Assays
To minimize the influence of extra-test factors on cortisol levels, on the day before the TSST, participants were given instructions to refrain from caffeine and physical exertion for three hours prior to their appointment, eat a light lunch low in fat and protein, and refrain from all eating and drinking for one hour before their appointment. During the TSST and control conditions, we collected six saliva samples: one sample per phase (rest, challenge, memory) in each condition (stress, non-stress control; see Figure 1). Participants were asked to use the “passive drool” collection method to collect at least 1.0 mL of saliva (Shirtcliff et al., 2001). Samples were collected in plain Nalgene tubes with no preservatives and were placed into refrigerators set at -80 °C. Unbound cortisol was measured with a commercially available enzyme immunoassay (EIA; Diagnostic Systems Laboratory, Webster, TX). Each of the samples for each participant was run on the same assay plate. Assay sensitivity for cortisol was 0.011 μg/d. At high (4.09 mg/dL), medium (1.41 mg/dL) and low (0.47 mg/dL) concentration of cortisol, the intra-assay coefficients of variation (CV) were 1.9%, 2.8% and, 4.8% respectively. The inter-assay CVs for high (4.12 mg/dL), medium (1.51 mg/dL) and low (0.50 mg/dL) concentrations of cortisol were 3.8%, 2.8% and 7.2% respectively.
Sex Hormone Assays
Blood samples were collected into sterile uncoated blood collection tubes by a registered phlebotomist. Samples were centrifuged and aliquotted for analysis of estradiol, progesterone, and testosterone at Quest Diagnostics (Wood Dale, IL). Serum estradiol was measured using a chemiluminescent immunoassay (Seimens Centaur E2-6 III). Estradiol assay sensitivity was 7 pg/mL. At high, medium, and low concentration of estradiol, intra-assay CV was 8%, 8%, and 10% respectively. Serum progesterone was measured with a chemiluminescent immunoassay (Siemens Centaur Progesterone). Progesterone assay sensitivity was 0.5 ng/mL. At high, medium, and low concentration of progesterone, intra-assay CV was 5%, 5%, and 12% respectively. Serum testosterone was measured with a chemiluminescent immunoassay (Seimens Centaur Testosterone). Testosterone assay sensitivity was determined to be 20 ng/mL. At high, medium, and low concentration of testosterone, intra-assay CV was 8%, 9%, and 15% respectively.
Measures to Assess Potential Confounds
Affective measures
The Center for Epidemiological Studies Depression Scale (CES-D) is a self-administered questionnaire measuring how often (“rarely” to “most of the time”) participants experienced depressive symptoms (e.g., feeling sad, lonely) in the past week (Radloff, 1977). The Beck Anxiety Inventory (BAI) is a 21-item, self-report measure where participants rate on a 4-point scale how much they have been bothered by each symptom (e.g., nervous, shaky) over the prior week (Beck et al., 1988). On the Positive and Negative Affect Schedule (PANAS) participants rate on a 5-point scale the extent to which they have experienced ten pleasant mood states and ten unpleasant mood states during the previous week (Watson et al., 1988).
Stress measures
The Perceived Stress Scale (PSS; Cohen et al., 1983) is a 10-item questionnaire measuring on a 5-point Likert scale the degree to which situations in one's life over the past month are perceived as stressful (e.g., unpredictable, uncontrollable) The Brief Fear of Negative Evaluation (BFNE; Leary, 1983) is a 12-item self-report measure where participants rate on a 5-point scale, the degree to which they experience apprehension at the prospect of being evaluated negatively (e.g., “I am afraid that others will not approve of me”).
Menstrual symptoms measure
The Menstrual Distress Questionnaire (MDQ) assesses 47 perimenstrual symptoms grouped into eight scales including pain, water retention, negative affect, autonomic reaction, impaired concentration, behavioral change, arousal, and control (Moos et al., 1969).
Neuropsychological measure
The revised National Adult Reading Test (NART-R) is an untimed test for estimating premorbid levels of intelligence based on the ability to correctly pronounce 61 words with atypical pronunciation (Nelson and Willison, 1991). A score of 100 is average.
Statistical Analysis
Study design was a 2 (group; active, inactive) × 2 (condition; TSST, control) mixed design. Differences between groups (inactive versus active pill phase) in demographic characteristics, self-reported mood and anxiety symptoms, and hormone levels were examined using independent t-tests for continuous variables and Chisquare tests for categorical variables.
For the first aim addressing salivary cortisol responsivity, a mixed factorial analysis of covariance (ANCOVA) was conducted in which Condition (non-stress control, TSST) and Time (rest, challenge, memory; see Figure 1) were the within subject variables and OC pill phase (inactive versus active) was the between-subject variable and depressive symptoms as the covariate. Support for our primary hypothesis would be evident in a significant Condition by OC pill phase interaction where the difference in cortisol levels between the TSST and control conditions was greater in the inactive versus active pill phase women. For comparison with the previously published TSST studies in women (Schoofs and Wolf 2009; Maki et al., 2015), a responder analysis was also conducted; participants with an increase in cortisol > 2.5 nmol/L from the challenge time-point in control condition to the challenge time-point in the TSST condition were categorized as “responders” and others were categorized as “non-responders”. Group differences in responder rates were analyzed using Chi-square. One woman in the inactive pill phase was missing cortisol levels due to a malfunctioning freezer and, therefore was eliminated from aim 1 analysis. To confirm that women found the TSST to be stressful, the independent and interactive effects of Condition and OC pill phase on subjective ratings of stress and anxiety (VAS, STAI) were also examined in a mixed factorial ANCOVA.
For the second aim regarding emotional retrieval, a mixed factorial ANCOVA was conducted with Condition (non-stress control, TSST) and Valence (negative, positive, neutral words) as the within-subject variables and OC pill phase (inactive pill phase, active pill phase) as the between-subject variable and depressive symptoms as the covariate. Support for our hypothesis would be evident in a significant Condition × OC pill phase interaction where the difference in retrieval between the TSST and stress control condition would be greater in the active compared to the inactive pill phase. All follow-up tests were computed using the appropriate error term from the primary mixed-factorial analysis. Greenhouse-Geisser corrected p-values were used to control for family-wise error.
Lastly, to determine whether the association between salivary cortisol and emotional memory differed by OC pill phase, we conducted a series of multivariable linear regression analyses. Specifically, we examined whether the changes in emotional memory from the TSST to control (i.e., TSST minus control) were related to increases in salivary cortisol (TSST minus control) after controlling for increases in self-reported anxiety (TSST minus control) and depressive symptoms within each OC pill phase group. We also examined partial correlations within OC pill phase group controlling for depressive symptoms during the TSST condition. Significance was set as P < 0.05 (two-sided). Only significant effects are reported, unless otherwise noted.
Results
Thirty-nine OC users between 18 and 40 years of age completed the study. Two participants randomized to the active pill phase were not included in analyses (one did not return for Day 2 and one revealed she was not a native English speaker after completion of the study). All received OCs containing ethinyl estradiol, including 20 (51%) on triphasic pills, 17 (43%) on monophasic pills, one (3%) on biphasic pills, and one (3%) with missing data. The daily dose of ethinyl estradiol ranged from .02 mg to .05 mg. The groups did not statistically differ in the types of OCs used. The most common form of progestogen used was norgestimate (49%; see Table 2).
Table 2.
Descriptive Characteristics of Oral Contraceptives by Group.
| Oral Contraceptive Pill Phase | ||
|---|---|---|
|
|
||
| Inactive (n = 21) M (SD) | Active (n = 18) M (SD) | |
| Oral contraceptive use type | ||
| Monophasic | 10 (47) | 7 (39) |
| Biphasic | 1 (5) | - |
| Triphasic | 9 (43) | 11 (61) |
| Unknown | 1 (5) | - |
| Progestin type | ||
| None/Unknown | 1 (4) | - |
| Drospirenone | 3 (14) | 2 (11) |
| Levonorgesterel | 1 (5) | 3 (17) |
| Norgestimate | 10 (48) | 8 (44) |
| Desogesterel | 2 (10) | 2 (11) |
| Norethindrone | 1 (5) | 3 (17) |
| Ethynodiol diacetate | 3 (14) | - |
Note. No significant group differences, Ps > 0.73.
The overall mean age was 26 years, education was 16 years, estimated IQ was 110, and BMI was 23. Mean scores on the psychological outcomes (e.g., depression, anxiety) were within normal limits. Eighty percent of the women were white, 10% were African American, and 10% other. As expected, levels of endogenous estradiol and testosterone were significantly higher in the inactive compared to active phase group, F1,36 = 10.27, P = 0.003, partial η2 = 0.22 and F1,32 = 5.84, P = 0.02, partial η2 = 0.15, respectively. Eighteen women were randomized to complete the task during the active pill phase and 21 during the inactive pill phase. There were no group statistically significant differences in any demographic or psychological outcomes except that the inactive pill group reported more depressive symptoms than active pill group, F1,37 = 4.34, P = 0.04, partial η2 = 0.10; see Table 1) and, thus, depressive symptoms were covaried for in all analyses.
Table 1.
Participant Characteristics by Group.
| Oral Contraceptive Pill Phase | |||
|---|---|---|---|
|
|
|||
| Variables | Inactive (n = 21) M (SD) | Active (n = 18) M (SD) | P-value |
| Demographics | |||
| Age | 25.38 (3.99) | 26.44 (5.19) | 0.48 |
| Years of Education | 16.62 (1.43) | 16.94 (1.92) | 0.55 |
| Full Scale IQ | 108.93 (7.54) | 110.77 (6.30) | 0.42 |
| Body Mass Index | 24.61 (4.46) | 25.14 (5.61) | 0.74 |
| Race (%) | |||
| White (non-Hispanic) | 18 (86) | 13 (72) | 0.45 |
| African-American (non-Hispanic) | 2 (10) | 2 (11) | |
| Other | 1 (5) | 3 (17) | |
| Self-report questionnaires | |||
| CES-D (range: 0-60) | 9.09 (5.03) | 5.83 (4.68) | 0.04* |
| Beck Anxiety Inventory (range: 0-63) | 6.86 (3.80) | 5.72 (4.59) | 0.40 |
| Perceived Stress Scale (range: 0-40) | 13.48 (4.90) | 12.28 (6.37) | 0.51 |
| PANAS (range: 1-5) | |||
| Positive | 3.65 (0.68) | 3.65 (0.69) | 0.99 |
| Negative | 1.77 (0.45) | 1.70 (0.52) | 0.67 |
| BFNE (range: 12-60) | 28.81 (10.02) | 31.22 (7.24) | 0.40 |
| MDQ (range: 0-225) | 26.43 (11.60) | 20.89 (7.47) | 0.09* |
| Hormone levels/factors | |||
| Estradiol (pg/ml) | 58.45 (57.88) | 14.32 (7.68) | 0.003* |
| Progesterone (ng/ml) | 0.77 (0.23) | 0.81 (0.37) | 0.64 |
| Testosterone (ng/dl) | 28.74 (11.70) | 19.80 (9.27) | 0.02* |
Note.
P < 0.05.
Full Scale IQ is an estimate derived from the NAART. CESD = Center for Epidemiologic Studies Depression Scale. PANAS=Positive and Negative Affect Schedule. BFNE=Brief Fear of Negative Evaluation Scale. MDQ= Menstrual Distress Questionnaire.
Figure 2 shows effects of stress condition and OC pill phase on salivary cortisol levels. Overall, levels of salivary cortisol were high across the two conditions. The Condition × Time interaction was significant when depressive symptoms were not controlled for in the analysis, F2, 72 = 3.23, P = 0.04, partial η2 = 0.08 and suggested an increase in cortisol levels only during the TSST condition and only from rest to challenge, F1, 72 = 5.72, P = 0.02. This effect was no longer significant after adjustment for depressive symptoms, F1,35 = 0.66, P = 0.43. The test of the primary hypothesis—the two-way Condition × Group interaction was not significant, F1,35 = 1.27, P = 0.27, partial η2 = 0.03, indicating that changes in cortisol levels from the non-stress control condition to the TSST did not differ between women in the inactive and active pill phase. Consistent with this finding, our responder analysis also indicted no statistical difference in the percent of women in the inactive (n = 6/20, 30%) and active pill (n = 9/18, 50%) who responded to the TSST, χ21, 38 = 1 .59, P = 0.32.
Figure 2.
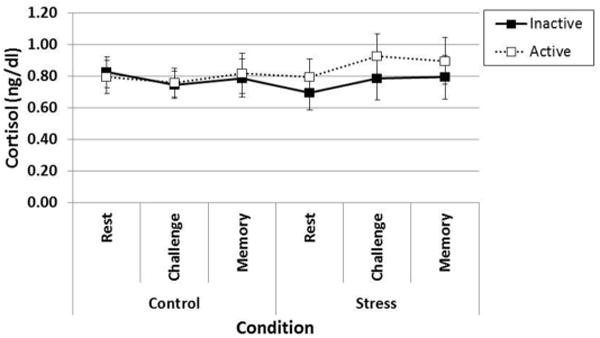
Cortisol levels did not increase during the TSST and did not differ by oral contraceptive pill phase.
Notes. There were no significant main effects or interactions, Ps > 0.10. Error bars indicate Standard Error.
We next examined subjective ratings of stress and anxiety on three outcome measures during the TSST and non-stress control conditions (See Figure 3). Similar patterns of results were evident on all three outcomes. On the STAI, subjective ratings of anxiety were higher during the stress (M = 9.81, SE = 0.43) compared to the control condition (M = 8.03, SE = 0.33), F1,35 = 12.27, P = 0.001, partial η2 = 0.26. Ratings also differed over time, F2,70 = 12.16, P < 0.001, partial η2 = 0.26, and the magnitude of change over time differed by Condition, Condition × Time: F2,70 = 14.20, P < 0.001, partial η2 = 0.29. Specifically, STAI scores increased significantly over time during the stress condition, F2,70 = 29.76, P < 0.001, but not the control condition, F1,72 = 1.70, P = 0.19. STAI scores increased from rest to the challenge time point, F1,70 = 56.20, P < 0.001, and decreased from challenge to the memory time point, F1,70 = 4.71, P = 0.04.There was no effect of pill phase on STAI scores, nor did phase interact with condition or time on this outcome. Similarly, on the VAS measure of stress, there was a significant main effect of Condition, F1,36 = 7.82, P = 0.008, partial η2 = 0.18, and Time, F2,72 = 10.29, P < 0.001, partial η2 = 0.22, and a significant Condition × Time Interaction, F2,72 = 7.30, P < 0.001, partial η2 = 0.17, but no effect of pill phase. On the VAS measure of anxiety, there was also a significant main effect of Condition, F1,36 = 12.00, P = 0.001, partial η2 = 0.25, and Time, F2,72 = 16.40, P < 0.001, partial η2 = 0.31, and a significant Condition × Time Interaction, F2,72 = 10.82, P < 0.001, partial η2 = 0.23, but no effect of pill phase.
Figure 3.
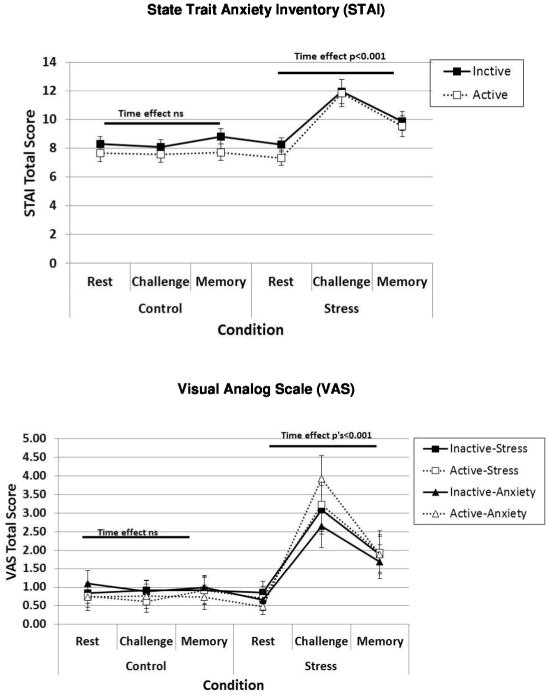
Subjective anxiety and stress measured with the State-Trait Anxiety Inventory (STAI) and Visual Analog Scales (VAS) increase during the TSST but are not influenced by OC pill phase.
Notes. Significant main effects of Condition and Time (i.e., Rest, Challenge, Memory) and a significant Condition × Time interaction were found on each measure (covariate: CES-D). See text for details. Error bars indicate Standard Error.
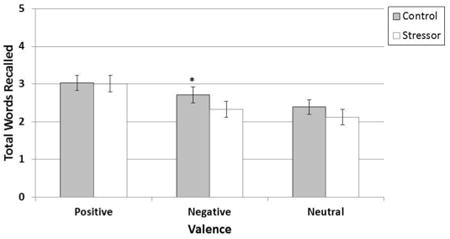
For the second aim, we compared memory performance for emotionally-valent and neutral words between Conditions (TSST vs. non-stress control) and between pill phase (active vs. inactive; see Figure 4). Although there was no significant main effect of condition or pill phase, there was a significant Valence × Condition interaction, F2,72 = 3.24, P = 0.04, partial η2 = 0.08. The interaction was driven by worse recall of negative words following the TSST, F1,72 = 7.75, P = 0.01, whereas there was no group difference in recall of positive or neutral words between the control and TSST conditions, F1,72 = 0.55, P = 0.46 and F1,72 = 0.01, P = 0.91, respectively. Cortisol levels were not correlated with recall performance in any condition, rs < -0.24, Ps > 0.14.
Figure 4. Performance on the emotional retrieval task during stress and non-stress control conditions for a combined sample of women in the inactive pill phase and active pill phase.
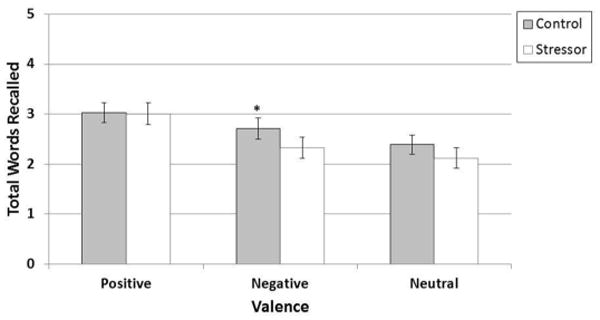
Notes. Significant Valence × Condition interaction. F2,72 = 3.24, P = 0.04, due to decreased retrieval of negatively-valent words (M = 2.71, SE = 0.21) following TSST compared to non-stress control condition (M = 2.33, SE = 0.21), E1,72 = 7.75, P = 0.01. Retrieval of positive words following the TSST (M = 3.01, SE = 0.22) and non-stress control condition (M = 3.01, SE = 0.22) did not differ. Similarly, retrieval of neutral words following the TSST (M = 2.12, SE = 0.20) and non-stress control condition did not differ (M = 2.39, SE = 0.19). Error bars indicate Standard Error.
Comparison between OC pill use (inactive vs. active pill phase) and the naturally cycling women (follicular vs. midluteal phase) reported by Maki et al., 2015
Our statistical analyses thus far have failed to demonstrate a salivary cortisol response to the TSST in a sample of 39 OC users despite demonstrating a subjective response with respect to stress and anxiety to the TSST. The results are in contrast to our previous findings obtained in 40 naturally cycling women tested either in the follicular (n = 20; low estradiol/low progesterone; days 2-4) or midluteal phase (n = 20; high estradiol/high progesterone; days 22-24) where we demonstrated both a salivary cortisol and subjective response to the TSST (Maki et al., 2015). To characterize possible differences between the neuroendocrine and behavioral findings between these two studies, we directly compared the results obtained as the methods were identical across reports.
To determine possible confounders and thus necessary covariates for analyses, we first conducted a series of ANOVAs with Phase (where Days 2-4 were inactive pill phase or follicular phase, Days 22-24 were active pill phase or luteal phase) and Group (OC user vs. naturally cycling women) as the between-subject variables for continuous outcomes and Chi-square tests for categorical variables. OC users and naturally cycling women significantly differed on race and BMI. There was a higher proportion of White non-Hispanic among OC users (79%, n = 35) compared to naturally cycling women (50%, n = 20), Χ21 = 7.50, P = 0.006. Additionally, OC users (M = 22.92, SE = 0.67) had a lower BMI compared to naturally cycling women (M = 24.88, SE = 0.66), F1,75 = 4.27, P = 0.04. Although the Phase × Group interaction did not quite meet statistical significance for depressive symptoms, F1,75 = 2.27, P = 0.14, race (White non-Hispanic vs. other), BMI, and depressive symptoms were included as covariates in the analyses. There were no other significant differences.
We first examined the independent and interactive associations of stress Condition (TSST vs. non-stress control), Phase (Days 2-4 vs. Days 22-24), and Group (OC user vs. naturally cycling women) on cortisol levels using a mixed factorial ANCOVA in which Condition (non-stress control, TSST) and Time (rest, challenge, memory) were the within-subjects variables, Phase and Group were the between-subject variables and race, BMI, and depressive symptoms were the covariates. There were two notable findings. First, there was a significant effect of Group whereby OC users (M = 0.83, SE = 0.07) had significantly higher cortisol levels compared to naturally cycling women (M = 0.53, SE = 0.07), F1,65 = 8.59, P = 0.005, partial η2 = 0.12. Importantly, there was a significant Condition × Phase × Group interaction, F1,65 = 7.22, P = 0.009, partial η2 = 0.10, which suggests that cortisol responsivity to the TSST depended on the combined influence of Phase and Group (See Figure 5). When restricting the analyses in OC users, the Condition × Phase interaction was not significant, F1,65 = 0.77, P = 0.37, which again suggests that neither active nor inactive pill phase users show a cortisol response to the TSST. In alignment with our previous findings, when restricting analyses in naturally cycling women, the Condition × Phase interaction was significant, F1,65 = 1179, P = 0.002, suggesting that the cortisol response to the TSST differs for women in the follicular and midluteal phase of the menstrual cycle. Specifically, the follicular group showed a significant increase in cortisol levels from the non-stress control to the TSST condition, F1,65 = 4.57, P = 0.04, whereas the Luteal group did not show a significant increase in cortisol levels across conditions, F1,65 = 0.04, P = 0.85.
Figure 5.
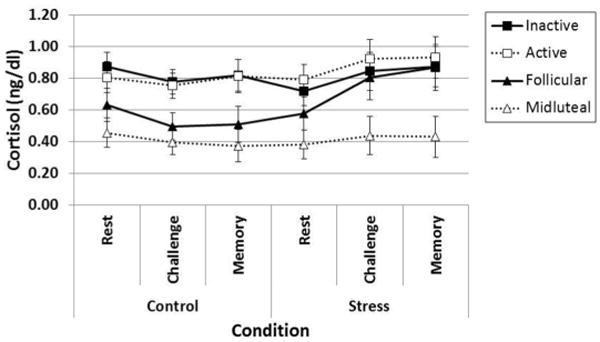
Cortisol responsivity depends on the combined influence of Group (Oral Contraceptive Users, Naturally Cycling Women) and Phase (Day 2-4, Day 22-24).
Notes. Significant Condition × Phase × Group interaction, F1,65 = 7.22, P = 0.009 (covariates: race, BMI, CES-D). Error bars indicate Standard Error.
Discussion
In this investigation, we replicated findings of no salivary cortisol responsivity to a laboratory-induced stressor in OC users compared to naturally cycling women (Bouma et al., 2009) and provided new evidence that this lack of a cortisol response is maintained during both the active and inactive pill phases. Notably, salivary cortisol levels were high across the control and stressor conditions in OC users during both the active and inactive pill phases. Self-reported stress and anxiety were significantly increased following the stressor for both pill phases. Following the stressor, similar patterns of emotional recall were observed in OC users during the active and inactive pill phases; stress differentially diminished recall of negative words compared to positive or neutral words. Cortisol levels and subjective stress/anxiety were unrelated to emotional memory performance in OC users.
A secondary analysis comparing OC users in the current study and naturally cycling women from our previous study (Maki et al., 2015) revealed that OC users had overall higher salivary cortisol levels. A recent cold pressor study also showed no salivary cortisol response in the subset of OC users with high baseline cortisol (Nielsen et al., 2013). In contrast, earlier studies show lower levels of salivary cortisol response in OC users compared to naturally cycling women (Kirschbaum et al., 1995b; Kirschbaum et al., 1999), a difference that was attributed to ethinyl estradiol, which increases corticosteroid binding globulin (CBG) and decreases levels of free cortisol. A study of 115 users of ethinyl estradiol-containing OCs found that CBG was an important regulator of the HPA axis in that higher levels of CBG were negatively associated with salivary cortisol levels overall but positively associated with salivary cortisol levels following the TSST (Kumsta et al., 2007). Although ethinyl estradiol is the most widely used form of estrogen in OCs, the type of progestin used varies across OC regimens and might contribute to differences in cortisol responsivity across studies. The most common form of progestogen used in the current study was norgestimate (46%). Notably, the combination of ethinyl estradiol plus norgestimate increases CBG as well as serum cortisol during the active and inactive pill phase (Wiegratz et al., 1995; Wiegratz et al., 1998). Thus, the current findings of elevated cortisol across conditions might reflect the use of norgestimate among OC users in this particular study. Norgestimate also has low androgenic activity, particularly compared to levonorgesterol, and cortisol levels might be influenced by the androgenic activity of the progestin used in OC formulations. Further study of OC formulation is needed.
The higher levels of cortisol in the OC users observed throughout both stress and non-stress conditions raise the possibility that the lack of cortisol response to the TSST was the result of baseline cortisol levels, an effect consistent with a previous finding in OC users with high baseline salivary cortisol levels (Nielsen et al., 2013). Although small sample sizes in human studies can limit the reliability of findings and the ability to detect group differences, the current findings in our relatively small sample are in line with a recent study of stress responsivity in 644 adolescents (Bouma et al., 2009). Among 125 adolescent OC users aged 15-17 years, there was no significant salivary cortisol response evident after a public speaking and math stressor and this differed from a significant cortisol response in 167 naturally cycling girls (Bouma et al., 2009). Importantly, we were also able to validate the subjective success of the TSST protocol in producing a psychological stress response as increases in stress and anxiety on self-report measures were found despite the lack of salivary cortisol response.
Regarding our secondary aim of examining emotional memory following a stressor, we provided new evidence that emotional memory is similar across the active and inactive pill phases. Interestingly, in OC users, memory performance was worse for negative words following the TSST but remained stable for positive and neutral words. This finding is consistent with prior work showing that administration of cortisol before recall in healthy young women differentially diminished recall of negative compared to neutral items (Kuhlmann et al., 2005a) and extends that work to suggest worse recall of negative compared to positive emotional items. Given previous findings that individual differences in cortisol responsivity affect emotional recall (Buchanan et al., 2006; Buchanan and Tranel, 2008), it is striking that recall of negative stimuli in the current study was diminished despite no increase in cortisol level. Indeed, the absolute level of cortisol in this study was not associated with recall performance. In contrast, the bias for negative memories in depression, a condition associated with high levels of cortisol, is well-documented (see Wolf, 2008 for a review). Enhanced retrieval of negative material was also found for young men and women who lacked a cortisol response following a stressor (Buchanan and Tranel, 2008). Our findings raise the possibility that OCs might provide protection against this bias and future studies should examine the possibility that OCs play a protective role against recall of negative memories in psychiatric disorders. Such studies should take into consideration noradrenergic mechanisms, as elevated noradrenergic response has been found to improve emotional memory whereas blocking noradrenergic response with beta blockers attenuates this effect (for Review see Tully and Bolshakov, 2010; van Stegeren, 2008). Work by Bemelmens and colleagues (2003) suggests that norepinephrine and cortisol may have opposing effects on cognitive processes, underscoring the need for its measurement in studies where cortisol responsivity is blunted. Likewise, other factors that can influence emotional recall such as length of memory delay (Sharot and Yonelinas, 2008) and attentional resources available during encoding (Kennsinger and Corkin, 2004) should be taken into account in future study designs.
Our study has several weaknesses. Given the lack of research on stress effects across OC pill phases and the somewhat limited sample size, these results require replication. Two limitations come from the choice of design. First, similar to previous studies that used a between-subjects design to study menstrual phase effects (Kirschbaum et al., 1999; Kuhlmann and Wolf, 2005; Maki et al., 2015), we used a between-subjects design to study OC pill phase effects because cortisol responsivity to the TSST decreases significantly on the second administration (Kirschbaum et al., 1995a). Although a within-subjects design would control for individual differences in cortisol responsivity, which are generally large, in this sample cortisol levels were uniformly high and cortisol responses were not apparent in OC users. Second, to prevent carry over effects while controlling for day of the OC regimen, and to allow comparison with naturally cycling women in our previous study (Maki et al., 2015), the stress condition always came after the control condition. It is possible that outcomes in the stress condition could be compromised by interference from the first retrieval, lack of motivation, increased tiredness or other factors during the preceding control condition. However, that aspect of the experimental design was similar in the active and inactive pill groups and therefore does not bias that interpretation. Other limitations apply generally to the body of work on OCs and stress responsivity, but are nonetheless important. These include the lack of control over the varying types and dosages of ethinyl estradiol and progestins used and the use of an observational design. Although unrealistic, a randomized, double-blind, placebo-controlled trial of OCs would provide the needed control over confounds and bias to optimally investigate OCs' effects on cognitive and hormonal responses to stress. The OC users in observational studies adhere to OCs, creating a potential “survivor effect” in that only those that can tolerate the drug are ultimately studied (Oinonen and Mazmanian, 2002). It has been suggested that the survivor effect has induced systematic error into studies of the effects of OCs on venous thromboembolism (Spitzer, 1998; Lewis, 1999). A “healthy user” bias may also influence the results given that OC users seek regular medical care, are typically more educated than naturally cycling women, and show no contraindications to OCs (Daniels et al., 2013; Daniels, et al., 2014). Although OC users and nonusers in this study were similar on a number of measures, the OC users were more predominantly White, had a lower BMI, and endorsed fewer depressive symptoms.
To our knowledge, this is the first study to compare how women respond to psychosocial stress during the active and inactive phases of OC regimens. We did not find statistical differences between pill phases for salivary cortisol responsivity, subjective stress and anxiety ratings, or emotional memory. These findings coupled with findings of distinct patterns of stress and cognitive responses among OC users and nonusers following an identical stressor protocol, suggest that OCs have specific effects on HPA axis responsivity that persist during both the active and the inactive pill phase. The lack of differences between the active and inactive pill phases provides new evidence that it might not be critical to account for pill phase in studies of stress responsivity in OC users. However, the two related studies that compared OC users across pill phases found differences in declarative memory (Mordecai et al., 2008) and resting state connectivity (Petersen et al., 2014) suggesting that affective and mnemonic processes can vary with pill phase.
In summary, the current study supports the hypothesis that OCs can influence cortisol and cognitive responses to stress. The widespread use of OCs underscores the need to continue to investigate the effects of OCs on affective and cognitive outcomes, particularly those relating to PTSD, anxiety states, and depressive disorders.
Significance Statement.
Over 82% of American women use oral contraceptives (OCs) during their lifetime. OCs and menstrual phase influence stress responsivity and memory but little is known about how OC effects may differ in active and inactive pill phases. In this study, OC users had generally high cortisol levels. In response to psychosocial stress, they showed increased anxiety, no increase in cortisol, and diminished recall of negative stimuli. These effects did not differ by pill phase. Overall, OC pill phase does not appear to alter stress responsivity in women, but OC use generally may buffer against retrieval of negative memories following stress.
Acknowledgments
Grant information: L. Rubin's effort was supported by grant number 1K01MH098798-01 from the National Institute of Mental Health (NIMH) and grant number K12HD055892 from the National Institute of Child Health and Human Development (NICHD), and the National Institutes of Health Office of Research on Women's Health (ORWH).
Footnotes
Role Of Authors: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: KLM, LHR, PMM
Acquisition of data: KLM, LHR, EA, ES, LD, EA, PMM
Analysis and interpretation of data: KLM, LHR, PMM
Drafting of the manuscript: KLM, LHR, PMM
Critical revision of the manuscript for important intellectual content: KLM, LHR, EA, ES LD, EA, PMM
Statistical analysis: LHR
Obtained funding: PMM
Administrative, technical, and material support: KLM, LHR, EA, ES, LD, EA, PMM
Study supervision: PMM
Conflict Of Interest Statement: There are no conflicts of interest to be declared.
References
- Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. J Cogn Neurosci. 2007;19(3):468–478. doi: 10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of consulting and clinical psychology. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Bouma EM, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. Adolescents' cortisol responses to awakening and social stress; effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology. 2009;34(6):884–93. doi: 10.1016/j.psyneuen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words (ANEW): Instruction manual and affective ratings. Technical Report C-1 1999 [Google Scholar]
- Buchanan TW, Tranel D. Stress and emotional memory retrieval: effects of sex and cortisol response. Neurobiol Learn Mem. 2008;89(2):134–41. doi: 10.1016/j.nlm.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learn Mem. 2006;13(3):382–7. doi: 10.1101/lm.206306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Daniels K, Daugherty J, Jones J. NCHS Data Brief 2014. 2014. Current contraceptive status among women aged 15-44: United States, 2011-2013; pp. 173pp. 1–8. [PubMed] [Google Scholar]
- Daniels K, Mosher WD. Contraceptive methods women have ever used: United States, 1982-2010. Natl Health Stat Report 2013. 2013:62, 1–15. [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Rimmele U, Reichwald U, Hautzinger M. Acute stress impairs recognition for positive words--association with stress-induced cortisol secretion. Stress. 2004;7(3):173–181. doi: 10.1080/10253890412331273213. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1-2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. Preliminary evidence for reduced cortisol responsivity to psychological stress in women using oral contraceptive medication. Psychoneuroendocrinology. 1995a;20(5):509–514. doi: 10.1016/0306-4530(94)00078-o. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D, Schommer N, Hellhammer DH. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosomatic medicine. 1995b;57(5):468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54(6):648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29(1):83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. J Neurosci. 2005;25(11):2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumsta R, Entringer S, Hellhammer DH, Wust S. Cortisol and ACTH responses to psychosocial stress are modulated by corticosteroid binding globulin levels. Psychoneuroendocrinology. 2007;32(8-10):1153–1157. doi: 10.1016/j.psyneuen.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Leary M. A Brief Version of the Fear of Negative Evaluation Scale. Personality and Social Psychology Bulletin. 1983;9(3):371–375. [Google Scholar]
- Lewis MA. The Transnational Study on Oral Contraceptives and the Health of Young Women. Methods, results, new analyses and the healthy user effect. Hum Reprod Update. 1999;5(6):707–20. doi: 10.1093/humupd/5.6.707. [DOI] [PubMed] [Google Scholar]
- Maki PM, Mordecai KL, Rubin LH, Sundermann E, Savarese A, Eatough E, Drogos L. Menstrual cycle effects on cortisol responsivity and emotional retrieval following a psychosocial stressor. Horm Behav. 2015;74:201–208. doi: 10.1016/j.yhbeh.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI) Br J Clin Psychol. 1992;31(Pt 3):301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- Moos RH, Kopell BS, Melges FT, Yalom ID, Lunde DT, Clayton RB, Hamburg DA. Fluctuations in symptoms and moods during the menstrual cycle. Journal of psychosomatic research. 1969;13(1):37–44. doi: 10.1016/0022-3999(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Mordecai KL, Rubin LH, Maki PM. Effects of menstrual cycle phase and oral contraceptive use on verbal memory. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Nelson H, Willison J. The revised national adult reading test - Test manual. Windsor, UK: NFER-Nelson; 1991. [Google Scholar]
- Nielsen SE, Ahmed I, Cahill L. Sex and menstrual cycle phase at encoding influence emotional memory for gist and detail. Neurobiol Learn Mem. 2013;106:56–65. doi: 10.1016/j.nlm.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Ahmed I, Cahill L. Postlearning stress differentially affects memory for emotional gist and detail in naturally cycling women and women on hormonal contraceptives. Behav Neurosci. 2014;128(4):482–493. doi: 10.1037/a0036687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Ertman N, Lakhani YS, Cahill L. Hormonal contraception usage is associated with altered memory for an emotional story. Neurobiol Learn Mem. 2011;96(2):378–384. doi: 10.1016/j.nlm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oinonen KA, Mazmanian D. To what extent do oral contraceptives influence mood and affect? J Affect Disord. 2002;70(3):229–40. doi: 10.1016/s0165-0327(01)00356-1. [DOI] [PubMed] [Google Scholar]
- Petersen N, Kilpatrick LA, Goharzad A, Cahill L. Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. Neuroimage. 2014;90:24–32. doi: 10.1016/j.neuroimage.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rohleder N, Wolf JM, Piel M, Kirschbaum C. Impact of oral contraceptive use on glucocorticoid sensitivity of pro-inflammatory cytokine production after psychosocial stress. Psychoneuroendocrinology. 2003;28(3):261–273. doi: 10.1016/s0306-4530(02)00019-7. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem. 2002;78(3):578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138(3):901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Schoofs D, Wolf OT. Stress and memory retrieval in women: no strong impairing effect during the luteal phase. Behav Neurosci. 2009;123(3):547–554. doi: 10.1037/a0015625. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl. 1998;20:22–33. quiz 34-57. [PubMed] [Google Scholar]
- Spitzer WO. Bias versus causality: interpreting recent evidence of oral contraceptive studies. Am J Obstet Gynecol. 1998;179(3 Pt 2):S43–50. doi: 10.1053/ob.1998.v179.a93059. [DOI] [PubMed] [Google Scholar]
- Villada C, Hidalgo V, Almela M, Mastorci F, Sgoifo A, Salvador A. Coping with an Acute Psychosocial Challenge: Behavioral and Physiological Responses in Young Women. PLoS ONE. 2014;9(12):e114640. doi: 10.1371/journal.pone.0114640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark L, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1062–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wiegratz I, Jung-Hoffmann C, Gross W, Kuhl H. Effect of two oral contraceptives containing ethinyl estradiol and gestodene or norgestimate on different lipid and lipoprotein parameters. Contraception. 1998;58(2):83–91. doi: 10.1016/s0010-7824(98)00074-2. [DOI] [PubMed] [Google Scholar]
- Wiegratz I, Jung-Hoffmann C, Kuhl H. Effect of two oral contraceptives containing ethinyl estradiol and gestodene or norgestimate upon androgen parameters and serum binding proteins. Contraception. 1995;51(6):341–346. doi: 10.1016/0010-7824(95)00098-u. [DOI] [PubMed] [Google Scholar]
- Wolf OT. The influence of stress hormones on emotional memory: relevance for psychopathology. Acta Psychol (Amst) 2008;127(3):513–31. doi: 10.1016/j.actpsy.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kuhlmann S, Buss C, Hellhammer DH, Kirschbaum C. Cortisol and memory retrieval in humans: influence of emotional valence. Ann N Y Acad Sci. 2004;1032:195–197. doi: 10.1196/annals.1314.019. [DOI] [PubMed] [Google Scholar]


