Abstract
Dating back to the case of Phineas Gage, decades of neuropsychological research have shown that the ventromedial prefrontal cortex (vmPFC) is crucial to both real-world social functioning and abstract decision-making in the laboratory (e.g., Bechara et al., 1994; Damasio et al., 1994; Stuss et al., 1983). Previous research has found that the relationship between the laterality of individuals’ vmPFC lesions and neuropsychological performance is moderated by their sex, whereby there are more severe social, emotional, and decision-making impairments in men with right-sided vmPFC lesions and in women with left-sided vmPFC lesions (Tranel et al., 2005; Sutterer et al., 2015). We conducted a selective review of studies examining the effect of vmPFC lesions on emotion and decision-making, and found further evidence of sex-related differences in the lateralization of function not only in the vmPFC, but also in other neurological structures associated with decision-making and emotion. Our review suggests that both sex and laterality effects warrant more careful consideration in the scientific literature.
Keywords: sex differences, cognitive function, vmPFC, amygdala, functional lateralization, emotion
Although research has found evidence of consistent sex differences1 in structural and functional neuroanatomy (e.g., Cahill, 2006) and in performance in multiple realms of cognition (e.g., Kimura, 1996), links between these variations in neurological functions and behaviors have remained elusive. For over a century, researchers have documented reliable group differences in cognitive abilities such as verbal fluency and spatial processing (Collins & Kimura, 1997; McGlone, 1980; Woolley, 1910), and the search for neurological correlates of these differences has uncovered sex-related variations in the morphology and functional lateralization of brain areas associated with these abilities (Allen et al., 2003; Shah et al., 2004). Other psychological processes such as emotion and decision-making, however, are still relatively unexamined from this perspective. Nevertheless, there is a growing body of evidence that several key neural correlates of emotion and decision-making show sex-related variations in functional lateralization. In this selective review, we will briefly outline this slowly growing line of research and discuss its implications for neuroscience.
The ventromedial prefrontal cortex
Decades of research on the ventromedial prefrontal cortex (vmPFC) have identified it as a neurological structure critical to decision-making, emotion regulation, and social functioning (Bechara et al., 1994; Damasio et al., 1994; Eslinger & Damasio, 1985). Lesions to the vmPFC can impair individuals’ complex decision-making without significant accompanying impairments in IQ or memory, causing cognitive impairments for patients that are subtle enough that they can be difficult to measure with traditional neuropsychological assessments, yet severe enough that they can cause dramatic problems in patients’ lives. Patients with vmPFC damage may answer questions about normative social and moral behavior quite reasonably in a neuropsychological exam, yet fail to act in accordance with this abstract social knowledge in day-to-day situations (Saver & Damasio, 1991; Anderson et al., 1999). Damasio et al. (1990) also found that vmPFC lesion patients’ emotional and autonomic responses to emotionally salient photographs were severely reduced or even nonexistent, further reinforced by patients’ self-reported lack of emotional reactions while viewing the images.
In order to better assess the subtle cognitive impairments associated with vmPFC lesions on a task simulating the complexity and the blend of uncertainty and risk in real-life decision-making, Bechara and colleagues (1994) developed the Iowa Gambling Task. In this computerized task, participants attempt to maximize their profit on a loan of virtual money by repeatedly choosing a card from one of four decks of cards with different sets of unknown rewards and penalties. Each time participants draw a card, they are informed of both a reward amount and a penalty. Two of the decks, the “disadvantageous decks,” contain cards with consistently high levels of reward but even higher intermittent penalties, such that consistently drawing from them leads to overall losses. The remaining two “advantageous decks” have consistently lower up-front rewards than the disadvantageous decks, but also have reduced loss amounts such that there are overall gains in the long term. The task typically lasts for 100 selections, and neurologically healthy participants tend to gradually learn the optimal strategy of choosing cards from the advantageous decks over the course of the task. In contrast, patients with vmPFC lesions tend to consistently favor the disadvantageous decks throughout the task, losing money (Figure 1; Bechara et al., 1994; 1997). Furthermore, this pattern of disadvantageous choices persists even when patients can verbally state the reward contingencies of all four decks and identify the two advantageous decks (Bechara et al., 1997).
Figure 1.
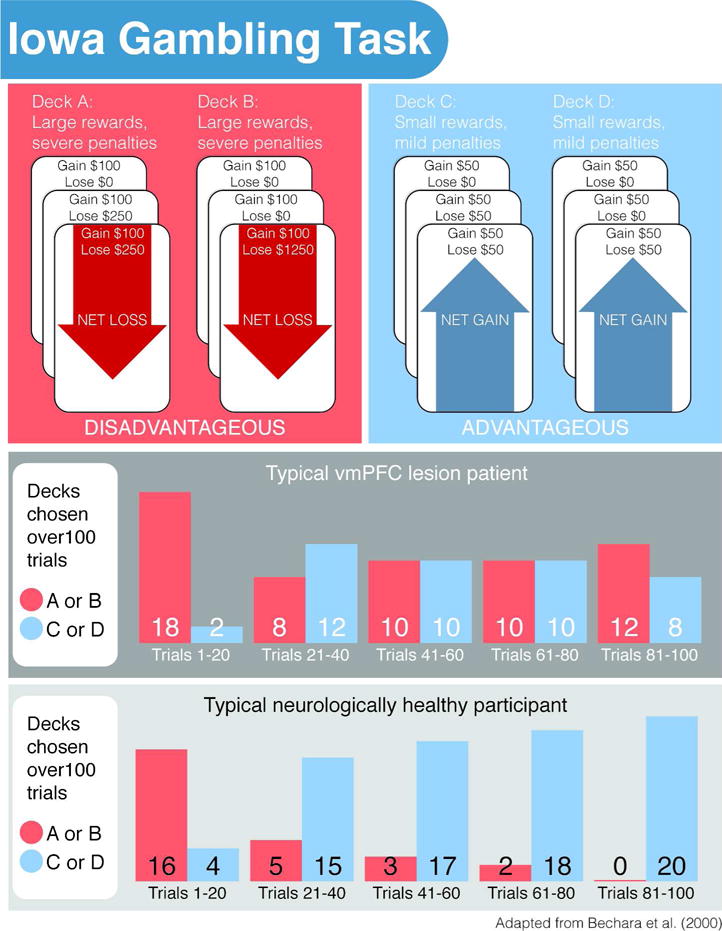
The Iowa Gambling Task. vmPFC = ventromedial prefrontal cortex. Patients with lesions in the vmPFC typically show a preference for cards from the disadvantageous deck throughout the task, leading to a net loss. Neurologically healthy participants typically learn to avoid the disadvantageous decks over the course of the task, eventually drawing exclusively from the advantageous cards for a net gain.
Sex differences on the IGT
The IGT has proven a useful tool for researchers studying sex differences in human decision-making. Although significant sex differences have not been found in performance on a wide variety of decision-making tasks such as the Balloon Analogue Risk Task (Lighthall et al., 2009) and the Cambridge Gambling task (Deakin et al., 1999; van den Bos et al., 2013), researchers have found small but consistent sex differences in IGT performance. For example, Reavis and Overman (2001) found that men consistently performed better than women on the task, with men choosing to draw from the advantageous decks more often over the second and third blocks of trials compared to women. Additionally, significantly more male than female participants were able to explicitly identify the correct choice strategy for the task. Several researchers have replicated this pattern of results, and many have speculated that the early development of the vmPFC—mediated by gonadal hormones—is one of the major mechanisms underlying this sex difference (Bolla et al. 2004; Overman, 2004; Overman et al., 2006; but see Hooper et al., 2004). Research by Welborn and colleagues (2009), for instance, found that sex differences in the relative volume of the vmPFC correlated with behavioral differences in emotion regulation and suppression. Other researchers have argued that men and women use different cognitive strategies to complete the IGT, with women integrating both win-loss frequency and net gains into their deck choices and men relying exclusively upon net gains to make their choices (van den Bos et al., 2013).
Although the hormonal, developmental, and cognitive mechanisms involved remain unclear, Bolla et al. (2004) found evidence that differences in vmPFC activity may contribute to this performance disparity. In addition to recording participants’ behavioral performances on the IGT, Bolla et al. (2004) had participants complete the IGT while in a positron emission tomography (PET) scanner to monitor variations in local brain metabolism during the task. They found that men showed increased task-associated metabolism in the right orbitofrontal cortex and right dorsolateral prefrontal cortex, whereas women showed increased metabolism in the left vmPFC. Although correlational, these findings hinted at differences in the underlying neurological processes of men and women during the IGT. Whether these differences in prefrontal cortex metabolism were due to divergent cognitive strategies limited to the IGT or more overarching differences in general cognitive strategies between the sexes remains an open question. Further research with vmPFC lesion patients, however, has hinted at the latter, and we turn now to some of the research that has been most definitive in this regard.
Sex differences in the functional lateralization of the vmPFC
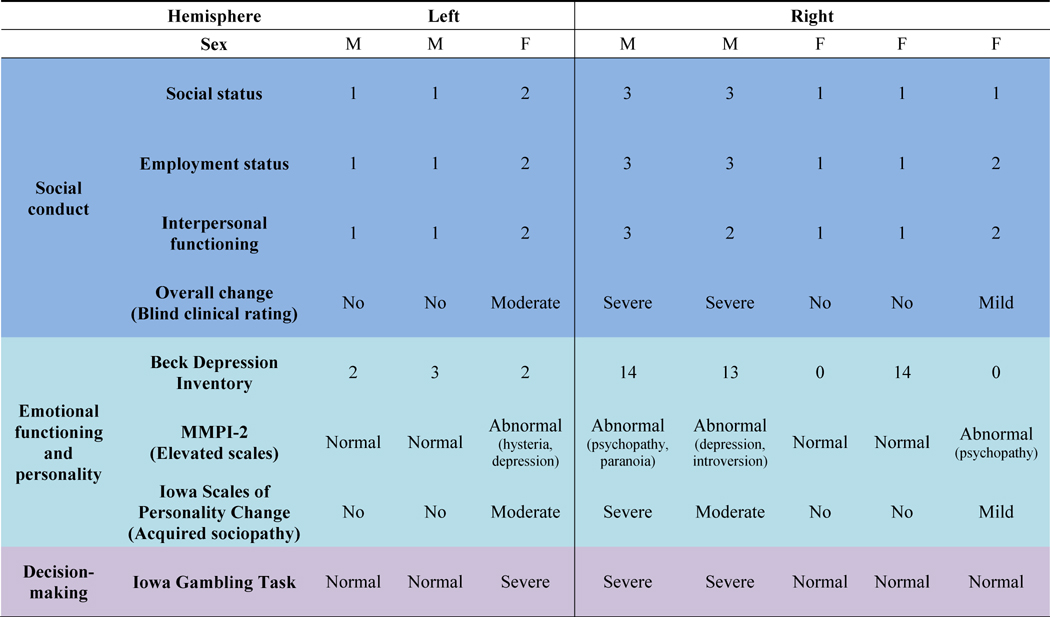
In an experiment assessing the functional lateralization of the vmPFC by contrasting the social, emotional, and decision-making impairments of patients with focal, unilateral brain damage in that region, Tranel and colleagues (2002) noted that patients with lesions of the right vmPFC showed considerably more deficits in all three domains of functioning than those with left-sided lesions. One detail in the results stood out, however: of the seven patients in the study, only the single female participant had broken the typical pattern of results, displaying far fewer deficits in all three behavioral categories than men with similar damage to the right vmPFC. In a further investigation of this inconsistency, Tranel et al. (2005) found evidence that sex modulates the lateralization of vmPFC function relating to social conduct, emotional functioning and personality, and decision-making (Figure 2). In line with the 2002 findings, male patients with right vmPFC lesions showed severe deficits in all three domains (Table 1). This group of men experienced impairments in their social functioning ranging from not being capable of holding down a steady job to having dysfunctional social relationships with family and friends. In the domain of emotion, the men had higher scores on the Beck Depression Inventory (BDI), and their close friends and family reported that their personalities had changed significantly since lesion onset. Men also tended to show significant elevations on several subscales—including paranoia, psychopathic deviation, and introversion—of the Minnesota Multiphasic Personality Inventory-2 (MMPI-2), a widely-used measure of personality and psychopathology.
Figure 2.
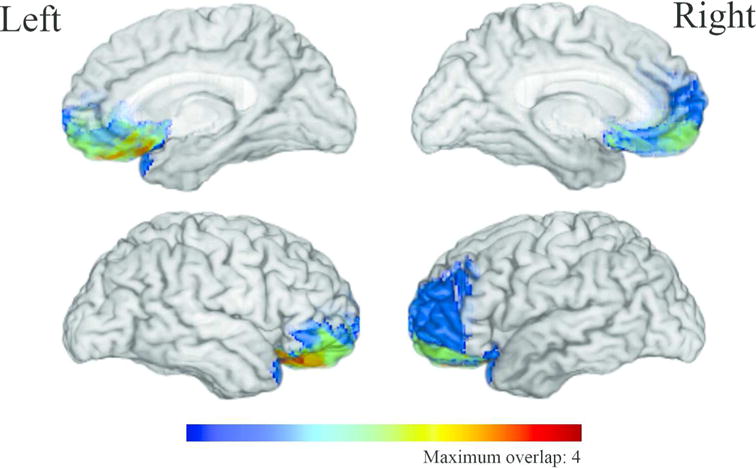
Combined lesion overlap of male and female patients in Tranel et al. (2005). The colors represent the number of overlapping lesions at each voxel. Men with right-sided lesions and women with left-sided lesions displayed moderate to severe impairments in social conduct, emotion, and decision-making. Women with right-sided lesions and men with left-sided lesions showed mild or no impairments in all three domains.
Table 1.
The extent of impairments in social conduct, emotional functioning and personality, and decision-making displayed by patients with unilateral vmPFC lesions. Overall change in social conduct was a composite rating derived from the general assessments of clinical neuropsychologists blinded to the study hypotheses. MMPI-2 = Minnesota Multiphasic Personality Inventory – 2. “Abnormal” scores on the MMPI-2 refer to T scores above 65 on one or more of the clinical scales. Adapted from Tranel et al. (2005).
1 = Mild impairment
2 = Moderate impairment
3 = Severe impairment
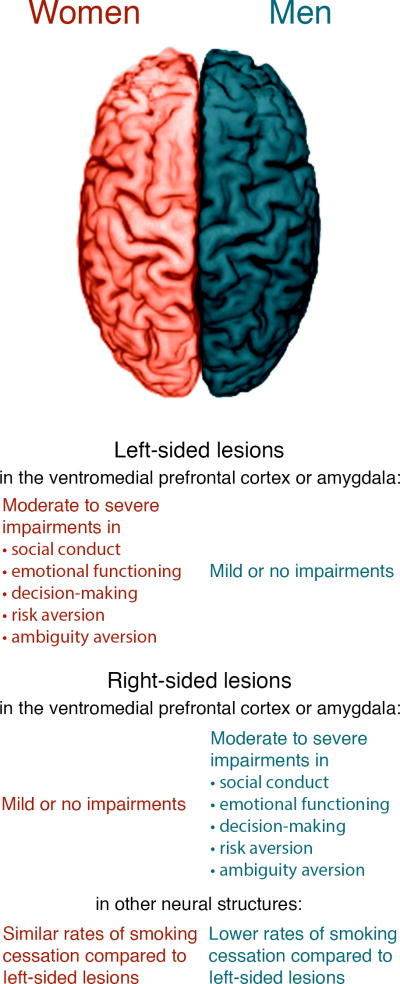
Furthermore, men with right vmPFC lesions consistently showed impairments on the IGT, choosing more disadvantageous cards than advantageous cards and failing to generate anticipatory skin conductance responses (SCRs) to the disadvantageous decks. Male patients with left-sided lesions, conversely, did not show severe impairments in any of these tasks. This lateralization was reversed in women—female patients showed more impairments in emotion and decision-making when they had suffered left hemisphere vmPFC lesions, and minimal or absent impairments when they had right hemisphere lesions.
These findings offer a potential explanation for at least some of the sex differences observed on the IGT in neurologically healthy populations. It may be possible that the right vmPFC is involved in cognitive processes that are better suited to the demands of the IGT than those involving the left vmPFC. If this were true, the differential recruitment of these structures during the IGT observed by Bolla and colleagues (2004) may reflect different cognitive strategies being used by men and women. Men, for instance, may tend to use a value maximization strategy that relies more upon the right vmPFC, while women may use a probability-matching strategy that is more sensitive to loss frequency rates, relying more upon the left vmPFC (Wolford et al., 2000). This would imply that the cognitive processes critical for IGT performance differ by sex, but that the lateralization of the underlying brain structures does not. The variety of behaviors that are differentially impaired by left or right lesions in men and women somewhat belie this viewpoint, but it may well account for at least some of the variations in IGT performance in healthy individuals.
Importantly, however, results from this study indicated that differences in functional lateralization extended beyond tasks like the IGT (where there have been documented differences in performance between men and women) into more qualitative, real-world domains such as emotion or social functioning.
Subsequent research has bolstered this initial evidence that sex may modulate the lateralization of vmPFC function on other decision-making tasks. Sutterer and colleagues (2015) examined the performances of male and female patients with unilateral vmPFC lesions on a task measuring decision-making under risk and uncertainty. Their task, adapted from Ellsberg (1961) and Hsu et al. (2005), had participants choose between pairs of gambles on either a card draw or a knowledge question. On card draw trials, participants chose to predict whether a red or a blue card would be drawn from a deck. Half of the trials were risk trials, where participants were informed of the proportions of red and blue cards, and half of the trials were ambiguous, where no information about the relative card proportions was communicated. The knowledge trials required participants to guess the answer to a Yes or No question, and they were similarly divided into risky and ambiguous questions by varying the degree of knowledge that participants would have about the topic. For instance, a risky trial would have participants gamble on whether the temperature in New York City was higher than 50 Fahrenheit on a specific day, and an ambiguous trial would have participants gamble on the answer to a similar question about Dushanbe, Tajikistan.
The results of this task paralleled Tranel et al.’s (2005) findings: men with right hemisphere vmPFC lesions and women with left hemisphere vmPFC lesions showed severe reductions in normative aversion to risk and ambiguity compared to neurologically healthy comparisons and patients with non-vmPFC brain damage. Furthermore, men with left-sided vmPFC damage and women with right-sided vmPFC damage showed normal levels of risk and ambiguity aversion comparable to the healthy comparisons. Notably, follow-up tests with patients who had bilateral vmPFC lesions found that their scores were similar to the men with right hemisphere and women with left hemisphere lesions. Both men and women with bilateral vmPFC lesions showed impairments in both risk and ambiguity aversion.
Functional lateralization of decision-making related areas outside the vmPFC
There is evidence that sex-related functional lateralization differences such as the ones reviewed above may extend beyond the vmPFC to other neuroanatomical regions involved in decision-making. For example, multiple studies have revealed consistent sex differences in the functional lateralization of the amygdala during emotional recall tasks (Cahill, 2006). Neuroimaging research by Cahill and colleagues (2001; 2004) revealed that BOLD signal in male participants’ right amygdalae strongly correlated with memory for emotionally arousing stimuli, whereas in female participants, the left amygdala BOLD signal more strongly correlated with memories for emotionally arousing stimuli. Complementing these findings, a study examining resting-state functional connectivity of the amygdala found that men had greater functional connectivity associated with the right amygdala, and women had greater functional connectivity of the left amygdala (Kilpatrick et al., 2006). Finally, using the same neuropsychological measures as Tranel et al. (2005), a lesion study by Tranel and Bechara (2009) examining the effects of unilateral amygdala lesions found a nearly identical pattern of sex-related lateralization in the amygdala. Male patients with right-sided amygdala lesions and women with left-sided amygdala lesions had considerable deficits in social conduct, emotional processing, and decision-making (IGT performance). Men with damage to the left amygdala and women with damage to the right amygdala were relatively unimpaired. These findings are especially notable given both the high degree of anatomical connection between the amygdala and vmPFC (Öngür & Price, 2000), and the critical involvement of the amygdala and vmPFC in many aspects of emotion and decision-making (Damasio, 1994; 1999).
The pattern of predominantly right hemisphere lateralization for men and left hemisphere lateralization for women may even generalize beyond traditional “limbic” regions such as the vmPFC and amygdala. Njemanze (2005), for instance, used bilateral simultaneous transcranial Doppler ultrasound to track mean cerebral blood flow velocities while participants completed Raven’s Progressive Matrices, a standard test of reasoning and general intelligence (Raven, 1938). Male participants showed an increase in blood flow through the right middle cerebral artery (MCA) when correctly answering questions, whereas women exhibited relatively greater flow through the left MCA on correct answers. Another study by Gaznick and colleagues (2014) examined the effects of unilateral brain damage (in several neurological areas including the vmPFC) on smoking cessation, and found that men with right-sided lesions had a significantly lower rate of quitting than men with left-sided lesions. Women, on the other hand, had similar quit rates regardless of lesion laterality. The authors proposed that this difference in outcomes may be due to the functional lateralization of decision-making to right hemisphere structures in men. Damage to men’s right hemispheres would severely impair their typical decision-making abilities, leading to decreased success when attempting to quit smoking. The authors further speculated that women may have a more bilateral distribution of lateralization than men, such that damage to the right or left hemisphere may not produce the same degree of impairment. Not all researchers have observed this pattern, however. An fMRI study by Lee et al. (2009) found that women showed stronger BOLD signal in the right insula and bilaterally in the orbitofrontal cortex (OFC) during a risky gains task. Furthermore, higher signal in the right OFC was correlated with less risky choices for female, but not male participants. Nevertheless, these findings indicate that numerous neurological structures contributing to decision-making and emotion show sex-modulated patterns of functional lateralization similar to the vmPFC, even if they do not necessarily all follow the same pattern.
Conclusion and future directions
In conclusion, there is growing evidence that the functional lateralization of emotion and decision-making in regions such as the vmPFC and amygdala is significantly modulated by sex, such that right-sided lesions in men and left-sided lesions in women cause the greatest impairments (Figure 3). Reminiscent of functional lateralization patterns of more well-studied cognitive phenomena such as verbal fluency (where women tend to outperform men at population level) and spatial processing (where men tend to outperform women at population level), it appears that there are reliable sex-related differences in the organization of the neurological substrates underlying decision-making and emotion. This is not to say that all neurological structures involved in decision-making and emotion, or even all of the myriad cognitive phenomena that fall under the umbrella of decision-making and emotion, share this pattern of sex-related lateralization. A great deal of work remains to be done if we ever hope to even grasp the countless factors that influence decision-making and emotion, let alone understand how sex differences and neurological organization interact with these factors.
Figure 3.
Summary of sex-related differences in effects of unilateral lesions (Gaznick et al., 2014; Sutterer et al., 2015; Tranel et al., 2005).
Additionally, given the paucity of research on the subject, it is difficult to speculate upon the biological, evolutionary, or psychosocial mechanisms driving the sex differences outlined in this review. Perhaps the best evidence for a hormonal account comes from primate literature, where hormonal influences on the early development of the OFC have been shown to drive sex differences on a reversal learning task (Clark & Goldman-Rakic, 1989). Although male monkeys tend to outperform female monkeys on the task, androgenized female monkeys perform as well as males. As the monkeys mature, the performance gap disappears. Furthermore, bilateral lesions to the OFC impair reversal learning in male and androgenized female, but not female monkeys. Importantly, human infants also show similar patterns of sex differences in performance on reversal learning tasks, lending credence to the hypothesis that hormonal differences may be at least partially underlie the development of sex differences in functional lateralization of brain structures (Overman et al., 1996). It is still difficult, however, to gauge how much these effects generalize from primates to humans. Human research has yet to document clear effects of menstrual cycle variations on IGT performance, and the effects of gonadal hormones on functional lateralization in the human prefrontal cortex remain unclear (Reavis & Overman, 2001).
Beyond the nonspecific speculation that hormones may influence patterns of functional lateralization in the vmPFC during development, there is a distinct lack of empirical support for other mechanistic explanations for the phenomenon. From an evolutionary and psychosocial standpoint, one may speculate that women’s biological and social roles in giving birth and childrearing may play a role in the development and organization of brain structures related to decision-making and emotion, but there is precious little evidence for or against that supposition. It is also important to realize that, although most research upon sex effects couches these differences as dichotomous and biologically determined—men are right-lateralized, women are left-lateralized—there is evidence that continuous variables measuring constructs such as self-rated gender role identity are actually more predictive of functional brain lateralization than a dichotomous biological sex variable (Bourne & Maxwell, 2010). These findings underscore the sheer complexity of biological and social factors that influence the function and physical development of the brain throughout life. There is, unfortunately, no genetic “blueprint” for the lateralization of the brain.
Although these differences in functional configuration are not necessarily detectable on a behavioral level in neurologically healthy individuals, they can play a significant role in neuropsychological outcomes after brain damage and have important implications for the way we approach the study of the brain. From a clinical perspective, these findings have substantial consequences for the study and treatment of psychological disorders involving dysfunction of the prefrontal cortex or difficulties in decision-making. Many psychological disorders—including post-traumatic stress disorder, obsessive-compulsive disorder, anxiety disorders, and psychopathy—have been strongly linked to vmPFC and amygdala dysfunction (Koenigs & Grafman, 2009; Irle et al., 1998; Greenberg et al., 2013; Blair, 2007). Research on the neural correlates of these disorders could observe dramatically different results if they study a predominantly male or female sample. Given the large sex differences in the prevalence of many of these psychological disorders (a factor that also could be partially accounted for by sex differences in the underlying neurological organization of involved structures), it would not be unusual for researchers to examine a sample that is predominantly male or female, potentially drawing conclusions that do not generalize to both men and women. Furthermore, the findings that we have reviewed help underscore the importance of considering sex differences when developing effective treatments for psychological disorders involving decision-making or emotion.
The importance of considering sex differences, however, extend beyond clinical research and treatment and into basic neuroscience. Tranel et al. (2005), for instance, identified a study by Manes et al. (2002) in which patients with unilateral lesions to the OFC showed minimal impairment on the IGT that potentially could be explained by the sex and lesion laterality of the sample. The participants of that study—predominantly men with left-sided lesions and women with right-sided lesions—would, based upon the patterns in the research above, would likely show minimal impairment on the IGT and other decision-making tasks. This surprising failure to replicate the well-documented effect of vmPFC lesions upon IGT performance in Manes et al.’s (2002) study may well be the result of testing relatively asymptomatic patients. Failing to account for sex differences in functional lateralization can wash out otherwise reliable effects, or lead to unwarranted inferences, such as Tranel et al.’s (2002) preliminary conclusion that the right vmPFC was more critical to decision-making and emotion than the left vmPFC based upon a patient sample of six males and one female.
Other researchers have noted a distinct lack of behavioral results when ignoring sex differences, yet observed robust effects of brain damage on a psychophysiological measure of emotion when including the variable of sex in their analysis (Belfi et al., 2016). Although damage to different brain structures appeared to have little to no effect on individuals’ skin conductance responses to music, Belfi et al.’s (2016) analysis revealed that men with brain damage had reduced SCRs while listening to music, but women with brain damage showed no significant reductions. Yet despite the evidence that similar brain damage can affect men and women in markedly divergent ways, precious little attention is paid to sex effects in neuropsychological research. These differences often go unreported in the literature at best, and remain entirely unexamined and unaccounted for at worst. These problems are not exclusive to lesion research—lateralization effects might average out in functional neuroimaging group analyses in studies with equal numbers of male and female participants if sex is not included as a variable. Conversely, studies with mostly male or mostly female sample groups can lead to overgeneralizations about the functional lateralization of specific neural correlates.
Although it is tempting to assume that similar behaviors and levels of performance on emotion and decision-making tasks is evidence that the neurological correlates of these behaviors are similarly organized, research in other cognitive areas with behavioral similarities—such as language and emotional memory—has indicated otherwise (Piefke et al., 2005). A neuroimaging study by Grabowski et al. (2003), for instance, examined the neural correlates of both a visual naming task and a face orientation task using PET. Although men and women did not differ significantly in performance on either task, they had divergent patterns of neural activity during the tasks. During the naming task, men showed significantly greater metabolic activity (or less decrease in metabolic activity) in the left inferotemporal areas relative to women, and women had greater metabolic activity in the right inferior frontal gyrus as well as the right precentral cortex. As evidenced by the research we have reviewed above, this type of pattern—subtle differences or even indistinguishable performances on behavioral tasks, accompanied by considerable disparities in their neurological correlates—appears to extend beyond the domains of language and perception, and it clearly warrants further research. Sex differences cannot remain unexamined if we hope to understand the neural mechanisms of emotion and decision-making.
Significance Statement.
In this review, we find evidence that several neurological structures critical for emotion and decision-making follow different patterns of functional lateralization for men and women. This is especially evident in structures such as the ventromedial prefrontal cortex and the amygdala, in which men with right-sided lesions and women with left-sided lesions display significant behavioral impairments, yet men with left-sided lesions and women with right-sided lesions display relatively unimpaired performance on emotion and decision-making tasks.
Acknowledgments
Supported by a McDonnell Foundation Collaborative Award to D.T. (#220020387)
Footnotes
Associate editor: Larry Cahill
The authors declare no conflict of interest.
Both authors participated in the writing of this manuscript.
The terms “sex differences” and “sex-related differences” have been used in various ways, often interchangeably. We acknowledge that the terms may not be entirely interchangeable, but for ease of expression, we will use the terms interchangeably.
References
- Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W. Sexual dimorphism and asymmetries in the gray–white composition of the human cerebrum. Neuroimage. 2003;18(4):880–894. doi: 10.1016/s1053-8119(03)00034-x. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in the human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304):1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Belfi AM, Chen KH, Schneider B, Tranel D. Neurological damage disrupts normal sex differences in psychophysiological responsiveness to music. Psychophysiology. 2016;53(1):14–20. doi: 10.1111/psyp.12453. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences. 2007;11(9):387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Cerebral Cortex. 2004;14(11):1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- Bourne VJ, Maxwell AM. Examining the sex difference in lateralisation for processing facial emotion: Does biological sex or psychological gender identity matter? Neuropsychologia. 2010;48(5):1289–1294. doi: 10.1016/j.neuropsychologia.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nature Reviews Neuroscience. 2006;7(6):477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Cahill L, et al. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiology of Learning and Memory. 2001;75(1):1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an FMRI investigation. Learning & Memory. 2004;11(3):261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AS, Goldman-Rakic PS. Gonadal hormones influence the emergence of cortical function in nonhuman primates. Behavioral Neuroscience. 1989;103(6):1287. doi: 10.1037//0735-7044.103.6.1287. [DOI] [PubMed] [Google Scholar]
- Collins DW, Kimura D. A large sex difference on a two-dimensional mental rotation task. Behavioral Neuroscience. 1997;111(4):845. doi: 10.1037//0735-7044.111.4.845. [DOI] [PubMed] [Google Scholar]
- Damasio A. Descartes’ error: Passion, reason, and the human brain. London, England: Penguin Press; 1994. [Google Scholar]
- Damasio AR. The feeling of what happens. New York: Harcourt Brace; 1999. [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: Clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioural Brain Research. 1990;41(2):81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Deakin J, Aitken M, Robbins T, Sahakian BJ. Risk taking during decision-making in normal volunteers changes with age. Journal of the International Neuropsychological Society. 1999;10(4):590–598. doi: 10.1017/S1355617704104104. [DOI] [PubMed] [Google Scholar]
- Ellsberg D. Risk, ambiguity, and the Savage axioms. The Quarterly Journal of Economics. 1961:643–669. [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation Patient EVR. Neurology. 1985;35(12):1731–1731. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Gaznick N, Bechara A, Tranel D. Hemispheric side of damage influences sex-related differences in smoking cessation in neurological patients. Journal of Clinical and Experimental Neuropsychology. 2014;36(5):551–558. doi: 10.1080/13803395.2014.915012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Eichhorn GR, Tranel D. Effects of gender on blood flow correlates of naming concrete entities. NeuroImage. 2003;20(2):940–954. doi: 10.1016/S1053-8119(03)00284-2. [DOI] [PubMed] [Google Scholar]
- Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica-Parodi LR. Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depression and Anxiety. 2013;30(3):242–250. doi: 10.1002/da.22016. [DOI] [PubMed] [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents’ performance on the Iowa Gambling Task: implications for the development of decision making and ventromedial prefrontal cortex. Developmental Psychology. 2004;40(6):1148. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer C. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310(5754):1680. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Irle E, Exner C, Thielen K, Weniger G, Rüther E. Obsessive-compulsive disorder and ventromedial frontal lesions: Clinical and neuropsychological findings. American Journal of Psychiatry. 1998;155(2):255–263. doi: 10.1176/ajp.155.2.255. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30(2):452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kimura D. Sex, sexual orientation and sex hormones influence human cognitive function. Current Opinion in Neurobiology. 1996;6(2):259–263. doi: 10.1016/s0959-4388(96)80081-x. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. Posttraumatic stress disorder: The role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15(5):540–548. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TM, Chan CC, Leung AW, Fox PT, Gao JH. Sex-related differences in neural activity during risk taking: An fMRI study. Cerebral Cortex. 2009;19(6):1303–1312. doi: 10.1093/cercor/bhn172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall NR, Mather M, Gorlick MA. Acute stress increases sex differences in risk seeking in the balloon analogue risk task. PloS One. 2009;4(7):e6002. doi: 10.1371/journal.pone.0006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125(3):624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- McGlone J. Sex differences in human brain asymmetry: A critical survey. Behavioral and Brain Sciences. 1980;3(02):215–227. [Google Scholar]
- Njemanze PC. Cerebral lateralization and general intelligence: Gender differences in a transcranial Doppler study. Brain and Language. 2005;92(3):234–239. doi: 10.1016/j.bandl.2004.06.104. [DOI] [PubMed] [Google Scholar]
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Overman WH. Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex. Brain and Cognition. 2004;55(1):134–147. doi: 10.1016/S0278-2626(03)00279-3. [DOI] [PubMed] [Google Scholar]
- Overman WH, Bachevalier J, Schuhmann E, Ryan P. Cognitive gender differences in very young children parallel biologically based cognitive gender differences in monkeys. Behavioral Neuroscience. 1996;110(4):673–684. doi: 10.1037//0735-7044.110.4.673. [DOI] [PubMed] [Google Scholar]
- Overman W, Graham L, Redmond A, Eubank R, Boettcher L, Samplawski O, Walsh K. Contemplation of moral dilemmas eliminates sex differences on the Iowa gambling task. Behavioral Neuroscience. 2006;120(4):817. doi: 10.1037/0735-7044.120.4.817. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Markowitsch HJ, Fink GR. Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Human Brain Mapping. 2005;24(4):313–324. doi: 10.1002/hbm.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JC. Progressive matrices: A perceptual test of intelligence. London: HK Lewis; 1938. [Google Scholar]
- Reavis R, Overman WH. Adult sex differences on a decision-making task previously shown to depend on the orbital prefrontal cortex. Behavioral Neuroscience. 2001;115(1):196. doi: 10.1037/0735-7044.115.1.196. [DOI] [PubMed] [Google Scholar]
- Saver JL, Damasio AR. Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia. 1991;29(12):1241–1249. doi: 10.1016/0028-3932(91)90037-9. [DOI] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43(3):313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF, Weir WS, Naeser MA, Lieberman I, Ferrill D. The involvement of orbitofrontal cerebrum in cognitive tasks. Neuropsychologia. 1983;21(3):235–248. doi: 10.1016/0028-3932(83)90040-4. [DOI] [PubMed] [Google Scholar]
- Sutterer MJ, Koscik TR, Tranel D. Sex-related functional asymmetry of the ventromedial prefrontal cortex in regard to decision-making under risk and ambiguity. Neuropsychologia. 2015;75:265–273. doi: 10.1016/j.neuropsychologia.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Bechara A. Sex-related functional asymmetry of the amygdala: preliminary evidence using a case-matched lesion approach. Neurocase. 2009;15(3):217–234. doi: 10.1080/13554790902775492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38(4):589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128(12):2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Van den Bos R, Homberg J, de Visser L. A critical review of sex differences in decision-making tasks: Focus on the Iowa Gambling Task. Behavioural Brain Research. 2013;238:95–108. doi: 10.1016/j.bbr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Welborn BL, Papademetris X, Reis DL, Rajeevan N, Bloise SM, Gray JR. Variation in orbitofrontal cortex volume: relation to sex, emotion regulation and affect. Social Cognitive and Affective Neuroscience. 2009;4(4):328–339. doi: 10.1093/scan/nsp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolford G, Miller MB, Gazzaniga M. The left hemisphere’s role in hypothesis formation. Journal of Neuroscience. 2000;20(6):1–4. doi: 10.1523/JNEUROSCI.20-06-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley HT. A review of the recent literature on the psychology of sex. Psychological Bulletin. 1910;7(10):335. [Google Scholar]