Abstract
Posttraumatic stress disorder and major depression share stress as an etiological contributor and are more common in women than in men. Traditionally, preclinical studies investigating the neurobiological underpinnings of stress vulnerability have used only male rodents, however, recent studies that include females are finding sex-specific mechanisms for responding to stress. Here we review some of this recent literature using a framework developed by McCarthy and colleagues (2012) that highlights different types of sex differences. First, we detail how learned fear responses in rats are sexually dimorphic. Then we contrast this finding to fear extinction, which is similar in males and females at the behavioral level, but at the circuitry level is associated with sex-specific cellular changes and thus exemplifies a sex convergence. Next, sex differences in stress hormones are detailed. Finally, the effects of stress on learning, attention, and arousal are used to highlight the concept of a sex divergence, in which the behavior of males and females is similar at baseline but diverges following stressor exposure. We argue that appreciating and investigating the diversity of sex differences in stress response systems will improve our understanding of vulnerability and resilience to stress-related psychiatric disorders, and likely lead to the development of novel therapeutics to better treat these disorders in both men and women.
Keywords: attention, corticotropin releasing hormone, fear conditioning, sexual dimorphism, posttraumatic stress disorder, depression, glucocorticoids, arousal
Historically, there has been a disconnect between the clinical literature documenting high rates of stress-related psychiatric disorders, such as posttraumatic stress disorder (PTSD) and major depression, in women (Kessler et al. 2012), and the preclinical literature investigating the biological basis for stress vulnerability almost exclusively in male rodents (Beery and Zucker 2011; Lebron-Milad and Milad 2012). However, recently some investigators have begun to include both sexes in preclinical stress studies. These efforts are revealing a variety of sex-specific mechanisms involved in stress responses. Here we use a framework for classifying sex differences developed in the landmark paper by McCarthy and colleagues (2012) to illustrate the myriad of ways that male and female rodents respond differently to stress (Fig. 1). Our goal is to provide some key examples of various types of sex differences. By doing so, we hope to highlight the complexity of the stress response system, as well as provide mechanistic insight into vulnerability and resilience to stress and stress-related psychiatric disorders.
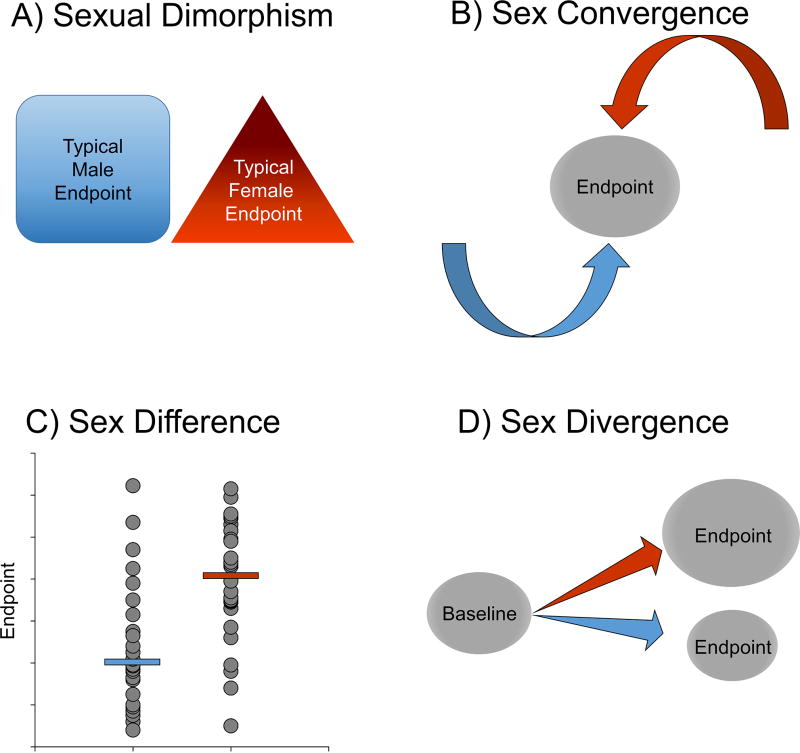
Figure 1.
Depiction of various sex differences based on McCarthy et al. (2012). Males are illustrated with blue and females are illustrated with red. A) A sexual dimorphism is when an endpoint takes two forms, one that is prevalent in males and another that is prevalent in females. B) A sex convergence is when the endpoint is similar in males and females, but the underlying mechanisms are different. C) A sex difference is when an endpoint exists on the same continuum in both sexes but the endpoints on average are different for males compared to females. Note that here we have illustrated the female mean as higher than the male mean, but of course the opposite could also be true. D) A sex divergence is when a sex difference emerges after an event, such as a stressor. Although in this depiction the stressor causes an increase in the endpoint in females and a decrease in the endpoint in males, the opposite can also occur. Moreover, in some cases, stress causes the endpoint to change in a similar direction for both males and females, but the magnitude of the effect is greater in one sex than the other.
Sexual dimorphism: Fear responses
Sex differences can be classified as sexually dimorphic when the observed endpoint takes different forms in males and females (Fig. 1a, McCarthy et al. 2012). Copulatory behavior and postpartum aggression are obvious examples of behaviors that meet this definition in many species. However, it is important to note that some sexually dimorphic behaviors are not necessarily so directly related to reproduction. For example, conditioned fear responses are sexually dimorphic (Gruene et al. 2015a).
Fear conditioning is one of the most common tasks used to study the neurobiological underpinnings of learning and remembering distressing events (Blanchard and Blanchard 1969; LeDoux 2000; Maren 2001). It has relevance for understanding aspects of panic disorders and PTSD, which notably are more common in women than men (Fredrikson et al. 1996; Kessler et al. 2012). In the typical rodent fear conditioning task, a neutral stimulus, such as a tone, is played so that it precedes and predicts the administration of a footshock, which serves as the unconditioned stimulus (US). After pairing the tone with the shock, the rodent learns to fear the tone—now considered a conditioned stimulus (CS)—in anticipation of the aversive US. The strength of the CS-US association is often assessed by presenting the tone alone and then measuring the amount of freezing, considered the conditioned response (CR), as an indices of fear to the tone. The greater the freezing to the tone, the stronger the CS-US association is thought to be.
Fear conditioning was developed in male rodents and most studies have assessed this behavior in males (Lebron-Milad and Milad 2012). The few studies that include female rats typically find that females do not freeze to the tone as much as males (Gupta et al. 2001; Maren et al. 1994; Pryce et al. 1999). One interpretation of this result could be that female rodents do not learn the CS-US association as well as males. However, Gruene et al. (2015a) observed that rather than freezing, 40% of female rats dart, or make a brief, high-velocity movement during the CS presentation (Fig. 2). Importantly, in females darting increases as the number of CS-US trials increases, suggesting that, just like freezing, darting reflects a learned response. During extinction trials, when the CS is presented alone and is no longer paired with the US, darting decreases, which again suggests that it is an indication of fear. Interestingly, females that dart also exhibit comparable freezing during extinction, suggesting that darting and freezing are not mutually exclusive responses. Only a small portion (10%) of male rats dart, but their darting does not increase over CS-US training trials. Therefore, in males the darting response may reflect general hyperactivity rather than learning. Together these findings reveal that multiple indices of fear should be evaluated in order to adequately assess fear conditioning in both male and female rats.
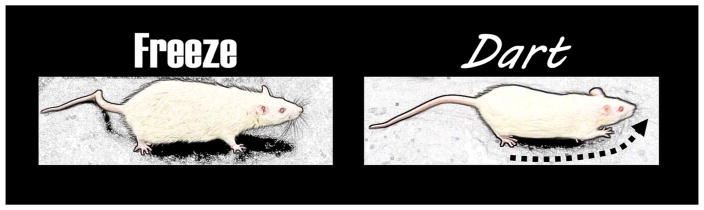
Figure 2.
Illustration depicting the learned fear responses of freezing and darting. These responses are sexually dimorphic because they are two different forms of behavior and darting occurs more frequently in female than male rats.
The Gruene et al. (2015a) study highlights a more broad concern with preclinical tests of stress-related behavior. Most common tasks of fear, anxiety-like, and depressive-like behavior were developed for and validated in male subjects (Kokras and Dalla 2014; Shansky 2015). As more females are included in basic research studies—which is necessary to fully understand the mechanisms required for responding stress—these traditional tasks will need to be validated in females, especially given that male and female rodents differ in terms of their size, strength, activity levels, and other characteristics (Bangasser 2015; Fernandes et al. 1999; Kokras and Dalla 2014; Shansky 2015). A failure to do so could lead to inaccurate conclusions and a misunderstanding of female behavior, ultimately hindering scientific progress.
Sex convergence: Circuitry for extinction
Males and females have marked biological differences, such as different sex chromosomes and different levels of gonadal hormones. Yet, often males and females behave similarly. This has led to the proposal that some sex differences in the brain are compensatory, and exist to equate behavior between males and females (De Vries 2004). Thus, sex convergence occurs when the endpoints are the same but the underlying physiology is different in males and females (Fig. 1b, McCarthy et al. 2012). While much of the research into sex differences in stress responding is aimed at identifying differences in endpoints that may help explain increased female vulnerability to certain stress-related disorders, sex convergence is also observed.
Gruene et al. (2015b) investigated differences in learning circuits between rats that are good at extinction, as defined by low freezing (LF) during the extinction retrieval test, and rats that are poor at extinction, as defined by high freezing (HF). Variability in extinction is comparable between males and females, and on average there are no sex differences in extinction (Gruene et al. 2015b). However, there are differences in the neuroanatomical correlates of good versus poor extinction in males and females, specifically in dendritic and spine morphology in the infralimbic (IL) region of the medial prefrontal cortex (mPFC), an area that activates circuitry within the amygdala to suppress fear responses (Herry et al. 2008; Sierra-Mercado et al. 2011). On amygdala projecting neurons in the mPFC, compared to LF male rats, HF male rats have significantly shorter apical dendrites and smaller mushroom spines (Gruene et al. 2015b), which are thought to play a role in the stabilization of memory (Bourne and Harris 2007). Notably, morphological differences are not observed in this neuronal population when HF and LF females are compared. This suggests that, although subsets of males and females have high levels of freezing during extinction, the mechanisms that contribute to poor extinction are, at least in part, sex-specific. Darting behavior was not considered in this study, but recall that even females that dart, freeze during extinction (Gruene et al. 2015a). That said, future studies examining multiple indices of fear are warranted to fully link sex differences in extinction circuitry to behavior.
Sex difference: Corticosterone
In some cases, male and female endpoints take the same form and fall along a continuum, but the group average for each sex is different (Fig. 1c). This phenomenon is defined as a sex difference (McCarthy et al. 2012). A good example of a sex difference in the stress field is circulating levels of the glucocorticoid, corticosterone, in rats. Specifically, female rats, on average, have higher baseline levels of corticosterone than do males (Kitay 1961). These higher corticosterone levels in females track throughout corticosterone’s circadian rhythm (Critchlow et al. 1963). Circulating ovarian hormones appear to contribute to this sex difference, as females in the proestrous phase of the estrous cycle (when ovarian hormones are higher) have higher corticosterone levels than females in other cycle stages (Atkinson and Waddell 1997; Carey et al. 1995). In contrast to the reliably reported sex difference in corticosterone in rodents, similarly reliable sex differences are not found in humans. Levels of cortisol, the glucocorticoid found in humans, are often comparable between healthy men and women (Kirschbaum et al. 1999; Uhart et al. 2006; Young et al. 2004). That said, cortisol levels are sensitive to a variety of variables (e.g., food intake, certain medications, age, etc.) and if it were possible to control for these variables in humans to the degree that they can be controlled for in rats, perhaps consistently higher levels of cortisol would be observed in women compared to men. Alternatively, the regulation of glucocorticoids could be different in rodents versus humans. Even if this is the case, high levels of glucocorticoids are reported in some patients with depression (Deuschle et al. 1997; Holsboer 2001; Plotsky et al. 1998), and this effect is even greater in depressed women (Young and Korszun 2010), therefore understanding the mechanisms that contribute to elevated corticosterone levels in female rats still may have clinical implications.
Sex divergence: Stress-induced alterations in cognition and arousal
Perhaps the most common type of sex difference in respond to stress is a sex divergence. This occurs when males and females are similar on an endpoint at baseline, but an environmental change, such as stressor exposure, induces a “latent” sex difference (Fig. 1d, McCarthy et al. 2012). Stress can either cause opposing effects in the sexes, or have a greater effect in one sex compared to the other. Here we provide examples of both.
One endpoint that is affected by stress is learning. For example, exposure to an acute stressor improves the acquisition of classical eyeblink conditioning in male rats (Shors 2001; Wood and Shors 1998). However, exposure to this same stressor actually impairs learning on this task in female rats (Wood and Shors 1998). Thus, the patterns of learning diverge between the sexes and are, in fact, opposite following stressor exposure. This effect is a bit more complicated though, because at baseline females tend to learn eyeblink conditioning better than males (Wood and Shors 1998). Thus, in this example of a sex and stress interaction on learning, there are two different types of sex differences: a baseline sex difference in learning and a stress-induced sex divergence.
The circuitry for eyeblink conditioning is well delineated and requires the cerebellum and associated sensory and motor pathways (reviewed in, Christian and Thompson 2003). The regions necessary for stress to regulate conditioning include the hippocampus and amygdala in both male and female rats (Bangasser and Shors 2007; Waddell et al. 2008). Surprisingly, the bed nucleus of the stria terminalis (BNST) mediates the enhancing effect of stress on learning only in males (Bangasser et al. 2005). The BNST does not regulate the impairing effect of stress on learning in females, suggesting that the sex divergence in learning following stress is mediated, at least in part, by different circuits (Bangasser and Shors 2008; Bangasser and Shors 2010). There are notable sex differences in the BNST in terms of the size of subregions, as well as peptide content (del Abril et al. 1987; van Leeuwen et al. 1985). These sex differences are organized by the perinatal testosterone surge that occurs in male rodents (del Abril et al. 1987; Han and De Vries 2003). When that surge is mimicked in female rats by injecting them with testosterone immediately after birth, in adulthood these “masculinized” females have impaired conditioning following stress (Bangasser and Shors 2008; Shors and Miesegaes 2002) and this stress-induced learning impairment in masculinized females requires the BNST (Bangasser and Shors 2008). Taken together, these studies suggest that a BNST that is masculinized by early testosterone exposure is required for this enhanced learning after a stressful experience. Moreover, these results demonstrate that sex divergence can be driven by engaging different circuits in males and females.
Perhaps more frequently, males and females are the same at baseline but then stressor exposure causes an effect in only one sex or a greater effect in one sex than the other (e.g., Conrad et al. 2003; Dalla et al. 2005; Greenberg et al. 2014; Hodes et al. 2015). Recent work from our laboratory exemplifies this (Cole et al. 2016). We were interested in whether the stress neuropeptide, corticotropin releasing factor (CRF), altered sustained attention (i.e., the ability to detect rare and unpredictable events over a prolonged period of time). Under normal, unstressed conditions, male and female rats perform similarly on a standard Sustained Attention Task (SAT), where rats were trained to discriminate signaled from non-signaled trials (Cole et al. 2016). Central administration of CRF causes a dose-dependent decrease in SAT performance in both sexes, but females are more impaired following a moderate CRF dose. Interestingly, the effect of CRF in females is influenced by their estrous cycle stage, such that CRF profoundly impairs sustained attention in females in the diestrous phase of their estrous cycle, characterized by low levels of circulating ovarian hormones (Fig. 3a). However, CRF has no effect on females in the estrous cycle phases where ovarian hormones are higher (estrus and proestrus were combined for this analysis, Fig. 3a). This indicates that high levels of ovarian hormones may be protective against the impairing effect of CRF on attention.
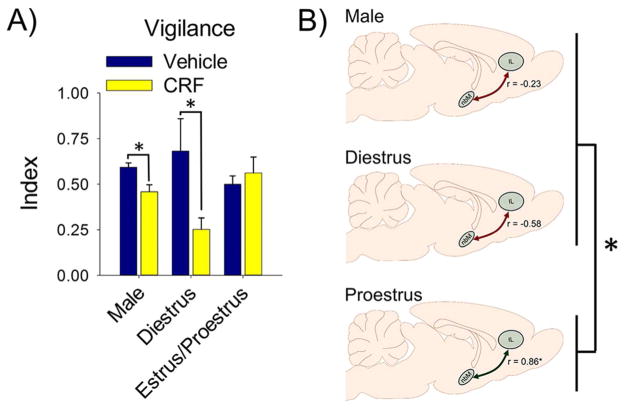
Figure 3.
The effect of CRF on sustained attention and its underlying circuitry is mediated by ovarian hormones. (A) CRF (0.5 μg, intracerebroventricular administration) impairs the vigilance index, an overall measure of sustained attention, in males and diestrous females, but not estrous/proestrous females. Reproduced with permission from Cole et al., (2016). (B) CRF activates the nucleus basalis of Meynet (nbM) and infralimbic cortex (IL) sustained attention circuitry in all groups, but the correlation for neuronal activation between these regions, as assessed with Fisher’s z-tests, is significantly different in proestrous females compared to other hormonal conditions (Bangasser et al. 2015). Asterisks indicate a significant difference (p < .05).
We are still trying to elucidate the mechanisms by which CRF impairs attention in males and cycling females. However, some recent evidence suggests that ovarian hormones regulate the circuit required for sustained attention (Bangasser et al. 2015). This circuit includes cholinergic neurons in the basal forebrain region of the nucleus basalis of Meynet (nbM) that project to the cortex (McGaughy et al. 1996; Sarter et al. 2001). In turn, the PFC sends afferents to the basal forebrain, and by working together, these regions coordinate attentional processes (Sarter et al. 2001). Our laboratory used cFOS as a marker of neuronal activation to determine whether central administration of CRF activated different circuits in males, diestrous females, and proestrous female rats (Bangasser et al. 2015). Indeed CRF’s regulation of many regions differs across hormonal conditions, however, of relevance to sustained attention is the relationship between neuronal activation in the nbM and IL region of the PFC. While both regions are activated by CRF in all conditions, the correlation for neuronal activation between the nbM and IL, which was only positive in proestrous females, was significantly different, as assessed with Fisher’s z-tests (Maras et al. 2014), in proestrous females compared to males and diestrous females (Fig. 3b). This finding suggests that ovarian hormones alter the way that brain regions that are critical for attention work together in response to CRF, although future studies are needed to understand precisely how this process occurs.
Divergent responses to stress mediators are also observed at the cellular level. For example, although noradrenergic neurons in the locus coeruleus (LC) arousal center have similar electrophysiological properties in males and females under most conditions, their response to stress is markedly different (Curtis et al. 2006). Specifically, hypotensive stress causes a sex divergence, such that female LC neurons fire faster than those of males (Curtis et al. 2006). The effect of hypotensive stress on LC physiology is mediated by CRF1 receptors (Curtis et al. 1994; Valentino et al. 1991; Valentino and Wehby 1988). Therefore, it is not surprising that female LC neurons are more sensitive to intra-LC administration of CRF, as evidenced by the fact that the CRF dose-response curve for LC activation is shifted to the left in female compared to male rats (Curtis et al. 2006).
Divergent electrophysiological responses of LC neurons to CRF are linked to sex differences in the CRF1 receptor (Bangasser et al. 2010; Bangasser et al. 2013). CRF1 receptors are G-protein coupled receptors that preferentially bind to the GTP binding protein, Gs, to activate the cAMP/PKA signaling pathway (Chen et al. 1986; Grammatopoulos et al. 2001; Hillhouse and Grammatopoulos 2006). It is through this pathway that CRF increases LC neuronal firing (Jedema and Grace 2004). Thus, increased activation of this pathway in females could explain their heightened electrophysiological responses to CRF. To begin to test this idea, sex differences in CRF1 receptor coupling to Gs were evaluated (Bangasser et al. 2010). Female rats, in fact, have greater coupling of Gs to the CRF1 receptor than male rats (Bangasser et al. 2010). This suggests that female CRF1 receptors would signal more through the cAMP/PKA pathway. In support of this idea, pharmacological approaches utilizing an antagonist for cAMP revealed that the electrophysiological response of LC neurons to CRF in females is completely cAMP mediated, while this response is only partially mediated by cAMP in males (Bangasser et al. 2010).
Taken together, these cellular studies suggest that, compared to males, increased CRF1 receptor-Gs coupling in females leads to greater activation of the cAMP/PKA signaling pathway to increase LC neuronal firing. Behaviorally, this sex difference is likely to lead to increased arousal following stress in females compared to males. This may be adaptive in certain situations. However, if the LC-arousal system is activated inappropriately or persistently, it could lead to the dysregulated state of hyperarousal (Bangasser and Valentino 2014). Hyperarousal is a key feature of PTSD and can contribute to the restlessness, sleep disturbance, and lack of concentration observed in some patients with depression (American Psychiatric Association 2013). Thus, if a similar heightened sensitivity of LC neurons to CRF is present in women, it could contribute, in part, to their higher rates of stress-related psychiatric disorders that have hyperarousal as a key feature. Finally, it is noteworthy that sex differences in CRF1 receptors are not limited to their signaling. There are differences in CRF1 receptor trafficking in the LC, CRF receptor binding throughout the forebrain, and CRF receptor co-localization with specific neuronal populations in the hippocampus and dorsal raphe (Bangasser et al. 2010; Bangasser et al. 2013; Howerton et al. 2014; Williams et al. 2011). Although detailing these findings is beyond the scope of this review, collectively they reveal a variety of mechanisms by which stress can cause divergent responses in the sexes.
Conclusions
Sex differences in responses to stress are not all created equally; they can range from an endpoint that is similar in both sexes achieved via sex-specific underlying processes, to an opposing endpoint in males versus females following stressor exposure. As more researchers begin to include female subjects, they will need to consider the possibility of finding sexual dimorphisms, sex convergences, sex differences, and sex divergences in their studies. Despite the complexity of studying sex differences, the potential benefits range from gaining a better understanding of female neurobiology to developing better pharmacotherapies to treat stress-related psychiatric disorders, as well as other psychiatric and neurological disorders, such as Alzheimer’s disease which is more common in women and exacerbated by stress (Gao et al. 1998; Wilson et al. 2006). In fact, because the underlying mechanisms for responding to stress often differ in males compared to females, it is likely that in the future different drugs will be required in men and women to optimally treat a variety of psychiatric and neurological disorders that are influenced by stress. This treatment approach is consistent with the move towards personalized medicine, in which sex certainly needs to be considered.
Significance Statement.
Women are more likely than men to suffer from stress-related psychiatric disorders, including posttraumatic stress disorder and major depression. Preclinical studies in rodents are identifying sex differences in stress response systems that predispose females to stress and certain aspects of stress-related psychiatric disorders. Here we review this literature and highlight the wide variety of sex-specific mechanisms for responding to stress. A better understanding of these mechanisms should improve the development of new medications to treat stress-related psychiatric disorders so that they are effective in both men and women.
Acknowledgments
We would like to thank David Waxler for his helpful comments on the manuscript.
Footnotes
Conflict of interest statement
None.
Role of authors
D.A.B. and B.W. developed and drafted sections of the manuscript. They also have contributed to and approved the final manuscript.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, D.C: American Psychiatric Publishing; 2013. [Google Scholar]
- Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology. 1997;138(9):3842–3848. doi: 10.1210/endo.138.9.5395. [DOI] [PubMed] [Google Scholar]
- Bangasser D. To freeze or not to freeze. eLife. 2015:4. doi: 10.7554/eLife.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D, Wiersielis K, Cohen S, Van Buskirk G, Losen D, Keita H, Bergmann J, Baksh N, Wicks B. Corticotropin-Releasing Factor Activates Different Circuits in Male and Female Rats. Neuropsychopharmacology. 2015:S446–S447. [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15(9):877, 896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Reyes BA, Piel D, Garachh V, Zhang XY, Plona ZM, Van Bockstaele EJ, Beck SG, Valentino RJ. Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol Psychiatry. 2013;18(2):166–173. doi: 10.1038/mp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Santollo J, Shors TJ. The bed nucleus of the stria terminalis is critically involved in enhancing associative learning after stressful experience. Behav Neurosci. 2005;119(6):1459–1466. doi: 10.1037/0735-7044.119.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. The hippocampus is necessary for enhancements and impairments of learning following stress. Nat Neurosci. 2007;10(11):1401–1403. doi: 10.1038/nn1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. The bed nucleus of the stria terminalis modulates learning after stress in masculinized but not cycling females. J Neurosci. 2008;28(25):6383–6387. doi: 10.1523/JNEUROSCI.0831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. Critical brain circuits at the intersection between stress and learning. Neurosci Biobehav Rev. 2010;34(8):1223–1233. doi: 10.1016/j.neubiorev.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35(3):303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35(3):565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. Journal of comparative and physiological psychology. 1969;68(1):129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Current opinion in neurobiology. 2007;17(3):381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144(2):311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- Chen FM, Bilezikjian LM, Perrin MH, Rivier J, Vale W. Corticotropin releasing factor receptor-mediated stimulation of adenylate cyclase activity in the rat brain. Brain Res. 1986;381(1):49–57. doi: 10.1016/0006-8993(86)90688-8. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10(6):427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Cole RD, Kawasumi Y, Parikh V, Bangasser DA. Corticotropin releasing factor impairs sustained attention in male and female rats. Behav Brain Res. 2016;296:30–34. doi: 10.1016/j.bbr.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol Learn Mem. 2003;79(1):32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. The American journal of physiology. 1963;205(5):807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31(3):544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Grigoriadis DE, Page ME, Rivier J, Valentino RJ. Pharmacological comparison of two corticotropin-releasing factor antagonists: in vivo and in vitro studies. J Pharmacol Exp Ther. 1994;268(1):359–365. [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z. Chronic mild stress impact: Are females more vulnerable? Neuroscience. 2005;135(3):703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Minireview: Sex Differences in Adult and Developing Brains: Compensation, Compensation, Compensation. Endocrinology. 2004;145(3):1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- del Abril A, Segovia S, Guillamón A. The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Developmental Brain Research. 1987;32(2):295–300. doi: 10.1016/0165-3806(87)90110-6. [DOI] [PubMed] [Google Scholar]
- Deuschle M, Schweiger U, Weber B, Gotthardt U, Korner A, Schmider J, Standhardt H, Lammers CH, Heuser I. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. The Journal of clinical endocrinology and metabolism. 1997;82(1):234–238. doi: 10.1210/jcem.82.1.3689. [DOI] [PubMed] [Google Scholar]
- Fernandes C, González MI, Wilson CA, File SE. Factor Analysis Shows That Female Rat Behaviour Is Characterized Primarily by Activity, Male Rats Are Driven by Sex and Anxiety. Pharmacology Biochemistry and Behavior. 1999;64(4):731–736. doi: 10.1016/s0091-3057(99)00139-2. [DOI] [PubMed] [Google Scholar]
- Fredrikson M, Annas P, Fischer H, Wik G. Gender and age differences in the prevalence of specific fears and phobias. Behaviour Research and Therapy. 1996;34(1):33–39. doi: 10.1016/0005-7967(95)00048-3. [DOI] [PubMed] [Google Scholar]
- Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55(9):809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Randeva HS, Levine MA, Kanellopoulou KA, Hillhouse EW. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. Journal of neurochemistry. 2001;76(2):509–519. doi: 10.1046/j.1471-4159.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, Trainor BC. Title: Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front Behav Neurosci. 2014:7. doi: 10.3389/fnbeh.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM. Sexually divergent expression of active and passive conditioned fear responses in rats. eLife. 2015a:4. doi: 10.7554/eLife.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene TM, Roberts E, Thomas V, Ronzio A, Shansky RM. Sex-Specific Neuroanatomical Correlates of Fear Expression in Prefrontal-Amygdala Circuits. Biological Psychiatry. 2015b;78(3):186–193. doi: 10.1016/j.biopsych.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1) Brain Res. 2001;888(2):356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- Han TM, De Vries GJ. Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. Journal of neurobiology. 2003;54(3):502–510. doi: 10.1002/neu.10157. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454(7204):600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27(3):260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, Menard C, Aleyasin H, Koo JW, Lorsch ZS, Feng J, Heshmati M, Wang M, Turecki G, Neve R, Zhang B, Shen L, Nestler EJ, Russo SJ. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci. 2015;35(50):16362–16376. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disorders. 2001;62(1–2):77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- Howerton AR, Roland AV, Fluharty JM, Marshall A, Chen A, Daniels D, Beck SG, Bale TL. Sex differences in corticotropin-releasing factor receptor-1 action within the dorsal raphe nucleus in stress responsivity. Biol Psychiatry. 2014;75(11):873–883. doi: 10.1016/j.biopsych.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci. 2004;24(43):9703–9713. doi: 10.1523/JNEUROSCI.2830-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International journal of methods in psychiatric research. 2012;21(3):169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- Kokras N, Dalla C. Sex differences in animal models of psychiatric disorders. Br J Pharmacol. 2014;171(20):4595–4619. doi: 10.1111/bph.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biology of mood & anxiety disorders. 2012;2(1):3. doi: 10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion Circuits in the Brain. Annu Rev Neurosci. 2000;23(1):155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Maras PM, Molet J, Chen Y, Rice C, Ji SG, Solodkin A, Baram TZ. Preferential loss of dorsal-hippocampus synapses underlies memory impairments provoked by short, multimodal stress. Mol Psychiatry. 2014;19(7):811–822. doi: 10.1038/mp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661(1–2):25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex Differences in the Brain: The Not So Inconvenient Truth. J Neurosci. 2012;32(7):2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110(2):247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. The Psychiatric clinics of North America. 1998;21(2):293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Lehmann J, Feldon J. Effect of sex on fear conditioning is similar for context and discrete CS in Wistar, Lewis and Fischer rat strains. Pharmacol Biochem Behav. 1999;64(4):753–759. doi: 10.1016/s0091-3057(99)00147-1. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35(2):146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Shansky RM. Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning. Neurobiology of stress. 2015;1:60–65. doi: 10.1016/j.ynstr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. Acute Stress Rapidly and Persistently Enhances Memory Formation in the Male Rat. Neurobiol Learn Mem. 2001;75(1):10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G. Testosterone in utero and at birth dictates how stressful experience will affect learning in adulthood. Proc Natl Acad Sci U S A. 2002;99(21):13955–13960. doi: 10.1073/pnas.202199999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable Roles of Prelimbic and Infralimbic Cortices, Ventral Hippocampus, and Basolateral Amygdala in the Expression and Extinction of Conditioned Fear. Neuropsychopharmacology. 2011;36(2):529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, Chong RY, Oswald L, Lin PI, Wand GS. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31(5):642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Curtis AL. Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res. 1991;555(1):25–34. doi: 10.1016/0006-8993(91)90855-p. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Wehby RG. Corticotropin-releasing factor: evidence for a neurotransmitter role in the locus ceruleus during hemodynamic stress. Neuroendocrinology. 1988;48(6):674–677. doi: 10.1159/000125081. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FW, Caffe AR, Vries GJd. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Research. 1985;325(1–2):391–394. doi: 10.1016/0006-8993(85)90348-8. [DOI] [PubMed] [Google Scholar]
- Waddell J, Bangasser DA, Shors TJ. The basolateral nucleus of the amygdala is necessary to induce the opposing effects of stressful experience on learning in males and females. J Neurosci. 2008;28(20):5290–5294. doi: 10.1523/JNEUROSCI.1129-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Akama KT, Knudsen MG, McEwen BS, Milner TA. Ovarian hormones influence corticotropin releasing factor receptor colocalization with delta opioid receptors in CA1 pyramidal cell dendrites. Exp Neurol. 2011;230(2):186–196. doi: 10.1016/j.expneurol.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proceedings of the National Academy of Sciences. 1998;95(7):4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E, Korszun A. Sex, trauma, stress hormones and depression. Mol Psychiatry. 2010;15(1):23–28. doi: 10.1038/mp.2009.94. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson JL, Cameron OG. Effect of comorbid anxiety disorders on the hypothalamic-pituitary-adrenal axis response to a social stressor in major depression. Biol Psychiatry. 2004;56(2):113–120. doi: 10.1016/j.biopsych.2004.03.017. [DOI] [PubMed] [Google Scholar]