Abstract
Twice as many women as men suffer from mood and anxiety disorders, yet the biological underpinnings of this phenomenon have been understudied and remain unclear. We and others have shown that the hemodynamic response to subliminally-presented sad or happy faces during fMRI is a robust biomarker of the attentional bias towards negative information classically observed in major depression. Here we used fMRI to compare the performance of healthy females (n=28) and healthy males (n=28) on a backward masking task using a fast event-related design with gradient-recalled, echoplanar imaging with sensitivity encoding. The image data were compared across groups using a region-of-interest analysis with small volume correction to control for multiple testing (pcorrected<0.05, cluster size ≥ 20 voxels). Notably, compared to males, females showed greater BOLD activity in the subgenual anterior cingulate cortex (sgACC) and the right hippocampus when viewing masked sad versus masked happy faces. Further, females displayed reduced BOLD activity in the right pregenual ACC and left amygdala when viewing masked happy versus masked neutral faces. Given that we have previously reported similar findings in depressed participants compared with healthy controls (irrespective of gender), our results raise the possibility that on average healthy females show subtle emotional processing biases that conceivably reflect a subgroup of women predisposed to depression. Nevertheless, we note that the differences between males and females were small and derived from region-of-interest rather than voxel-wise analyses.
Graphical Abstract
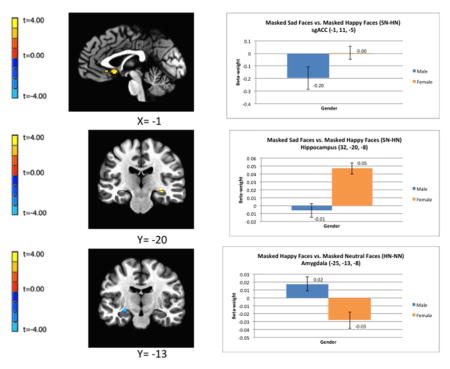
Regional differences in hemodynamic response between healthy males and females while viewing emotionally-expressive face stimuli presented below the level of conscious awareness. Relative to males, females show greater BOLD responses to masked sad versus masked happy faces in the subgenual ACC (top image) and right hippocampus (middle image). Relative to males, females show a reduced BOLD response to masked happy versus masked neutral faces in the left amygdala (bottom image).
INTRODUCTION
Females are more vulnerable than men to developing stress-related disorders such as post-traumatic stress disorder and general anxiety disorder (Kessler et al. 2012; McLean and Anderson 2009), and the lifetime rate of major depressive disorder (MDD) in women has consistently been found to be twice that of men (Kessler 2003; Kessler et al. 1994). Healthy females also experience more negative emotions (Else-Quest et al. 2012), show superior recall of emotional memories (Cahill 2003), and are more sensitive than males to the corporeal manifestations of emotion, including facial expressions (Hall and Matsumoto 2004; Kret and De Gelder 2012). However, it is not yet clear if these phenomena are related to the epidemiological differences between the sexes in mood and anxiety disorders.
What is known is that: a) early-life stress is a potent risk factor for the development of major depression (Kendler et al. 2004; Kessler 1997); b) the neural circuits involved in stress comprise some of the most sexually dimorphic regions in the brain (Goldstein et al. 2001); c) disruption of the hypothalamic-pituitary-adrenal (HPA) axis during development in rodents has sex-specific effects on the hypothalamus, amygdala, hippocampus, and medial prefrontal cortex (mPFC) that compromise stress regulation in adulthood (Goldstein et al. 2014), and d) gonadal hormones also regulate affective responses via the HPA circuitry in healthy human adults (Petersen and Cahill 2015; van Wingen et al. 2008; van Wingen et al. 2009).
Consistent with these data, several functional neuroimaging studies have identified sex differences in the magnitude of corticolimbic responses to different types of emotional stimuli. A recent meta-analysis of these studies revealed that compared to healthy men, negatively-valenced emotional stimuli of all types elicit greater activation of corticolimbic circuits in healthy women including the left amygdala, left medial-dorsal thalamus, left anterior cingulate, and mPFC (Stevens and Hamann 2012). Moreover, compared to healthy women, men displayed greater activity of several brain regions, most notably the subcallosal gyrus, inferior frontal gyrus, and the left amygdala in response to positive stimuli (Stevens and Hamann 2012).
Volume reductions, altered neural activity, and/or altered functional connectivity of components of the stress neurocircuitry are widely reported findings in the depression literature (Hamilton et al. 2015; Price and Drevets 2012; Savitz and Drevets 2009a; Savitz and Drevets 2009b). Further, these structural and functional abnormalities are linked to behavioral abnormalities, notably a fundamental mood-congruent processing bias characteristic of depression; i.e. a greater sensitivity to negative stimuli coupled with reduced attention to and memory for, positive, socially-relevant stimuli (Disner et al. 2011; Harmer et al. 2004; Joormann and Gotlib 2007). For example, depressed patients display exaggerated BOLD responses to explicitly-presented sad faces in the amygdala (Siegle et al. 2002; Surguladze et al. 2005), and this abnormality normalizes after treatment with antidepressant medication (Arnone et al. 2012; Fu et al. 2004).
We (Victor et al. 2010; Victor et al. 2013) and others (Sheline et al. 2001; Stuhrmann et al. 2013; Suslow et al. 2010) have developed a sensitive and reliable probe of the neural correlates of automatic or non-conscious emotional processing biases, i.e. a backward masking fMRI paradigm in which combinations of neutral, sad, and happy faces are displayed in pairs with the first face displaced (masked) by a second face after 17–30 ms so that the participant is only consciously aware of viewing the second face. The advantage of the subliminal presentation of the faces is that they may help to divorce the automatic, early processes underpinning attentional bias from more elaborate conscious processes such as empathy or negative ruminations which may be less reflective of the underlying neurobiology.
We have demonstrated the existence of a “double-dissociation” in which relative to healthy controls, depressed participants on average display increased BOLD responses in the amygdala to sad faces but decreased BOLD responses in the amygdala to masked happy faces (Victor et al. 2010). This effect was reversed by treatment with an SSRI (Victor et al. 2010), consistent with other reports in the literature (Sheline et al. 2001). Subsequently, we reported depression-associated changes in an extended anatomical network - for instance, greater activity to masked sad versus masked happy faces in the left hippocampus and greater activity to masked sad versus masked neutral faces in the left superior temporal cortex and right orbitofrontal cortex (Victor et al. 2012). Notably, we also reported that at baseline, MDD patients displayed an increased hemodynamic response to masked sad versus masked happy faces in the pregenual ACC (pgACC), an effect that was reversed by 8 weeks of treatment with the SSRI, sertraline (Victor et al. 2013).
In sum, it has been previously hypothesized that increased female sensitivity to negative emotional stimuli partly explains the greater prevalence of depression and anxiety disorders in women (Leach et al. 2008; Nolen-Hoeksema 2012). There is indirect evidence to support this hypothesis: (a) healthy women respond more strongly than healthy men to negative emotional stimuli, (b) depressed individuals show a negative emotional processing bias, (c) there is a degree of correspondence in the neural response to emotionally-valenced stimuli (e.g. faces) in healthy women versus men to those in depressed individuals versus healthy controls. Nevertheless, (a) only a modest number of studies have explicitly compared the neural activity between men and women during fMRI scanning with affective paradigms, (b) the implications of these studies are not completely clear since not all of the tasks employed in previous studies have been demonstrated to robustly differentiate depressed from healthy participants, and (c) to our knowledge the neural correlates of sex differences to subliminally-processed masked sad and happy faces has not been studied in healthy individuals.
Here we compare the neurophysiological responses of healthy men and women on the backward masking task, a sensitive and reliable probe of a non-conscious emotional processing bias that is salient in depression. We hypothesized that relative to healthy males, the healthy females would show similarities to patients with depression, i.e. greater BOLD activity to sad versus happy faces in the amygdala, hippocampus, and perigenual ACC.
METHODS
Participants
All participants were recruited from the Laureate Psychiatric Clinic and Hospital (LPCH) or the general community through radio and print advertisements. Participants provided written informed consent after receiving a full explanation of the study procedures and risks as approved by the local IRB.
Participants (n=56; 50% female, ages 18–55) were interviewed with the Structured Clinical Interview for the DSM-IV-TR (First et al. 2002), and additionally completed the Hamilton Depression Rating Scale (HDRS, 25-item) in order to exclude volunteers with psychiatric disorders.
Volunteers were excluded if they had either a personal or a family history (in first degree relatives) of major psychiatric disorders according to the Family Interview for Genetic Studies (Maxwell 1992). Additional exclusion criteria included medical conditions or concomitant medications likely to influence CNS function including cardiovascular, respiratory, endocrine, and neurological diseases, and general MRI exclusions such as paramagnetic implants or claustrophobia.
In accordance with previously published work (Maki et al. 2002; Petersen et al. 2014), females were divided into two principal groups for post hoc exploratory analyses: early follicular phase (days 1–6; low estrogen/progesterone, n=8); mid-luteal phase (days 18–24; high estrogen/progesterone, n=6). Individuals who did not fall into these groups were labeled as “other” (oral contraceptives, n=2; hysterectomy, n=2; different phase, n=10; total n=14).
fMRI Task and Processing
fMRI scanning was performed on a 3T GE MR750 MRI scanner with an eight-channel receive-only head coil. Gradient-recalled, echoplanar imaging (EPI) with sensitivity encoding (SENSE) was used for fMRI with the following parameters: Repetition/echo times (TR/TE)=2000/27ms, SENSE acceleration=2, flip angle=90°, matrix=96×96, field-of-view (FOV)=24cm, thirty-nine axial slices, voxel size=2.5×2.5×2.9mm3. The first three imaging volumes of each run were discarded to allow for steady-state tissue magnetization. High-resolution T1 weighted anatomical MRI scans (TR/TE=6/2.1ms, prep/delay=725/1400ms, FOV 240×192mm, delay=1400ms, flip angle=8°, SENSE acceleration=2, 186 axial slices, voxel-size=0.94×0.94×0.9mm were acquired for co-registration with the EPI series.
Prior to each of two 9 min, 8 sec runs, participants were shown two neutral target faces and instructed to remember the faces for the next scan run, and respond as quickly as possible to indicate if the presented face matched one of the target faces based on identity (not emotional expression). Two faces were presented for each stimulus, a “masked” face for 26ms, immediately followed by a “masking face” for 107ms, and a fixation cross for 1866ms. In total, 48 stimuli [6 combinations - (sad/neutral, happy/neutral, neutral/sad, neutral/happy, neutral/neutral female, neutral/neutral male) × 8 presentations each] were presented in each run in a pseudo-randomized, mixed-trial design, with the emotional pairings predetermined (e.g., happy/neutral) and the program Optseq (Dale 1999) used to randomly determine the order of face presentation with an equal number of target faces presented in each position. Each event-type was gender matched. Two different stimulus face sets equivalent on ratings of valence were counterbalanced and used for the two runs. Faces were selected from the NimStim Set of Facial Expressions (Tottenham et al. 2009). A 2–6s inter-stimulus interval served as a baseline comparison.
Image pre-processing and analysis were performed using AFNI (http://afni.nimh.nih.gov) and comprised despiking, slice acquisition time correction, and within-subject realignment. The anatomical image was registered to the first functional image then spatially normalized to the TT_N27 template using Advanced Normalization Tools (ANTs) with SyN method (Avants et al. 2008). The estimated warping parameter was used to normalize the functional images. The template image was resampled to 1.75mm3 isotropic voxels so that the spatially-normalized image had a voxel size of 1.75mm3. Images were smoothed using a 4mm full-width half-maximum Gaussian kernel, and the signal time course was scaled to percent signal change relative to the mean signal across time in each voxel. Using 3dDeconvolve for each participant, the hemodynamic response to each event type was modeled with a delta function at the event onset and convolved with the gamma-variate hemodynamic response function. Regressors modeling the task, motion parameters, and 4th-order polynomial regressors were used in the model. Because the masked and unmasked faces for each stimulus pair were presented too closely in time to model the response to each component separately, the data were modeled as event-related correlates of the combined stimulus pairs – these pairs were the main effects of interest: presentation of sad/neutral (SN), happy/neutral (HN), and neutral/neutral (NN) faces. Events with target faces of sad and happy in the unmasked position were also modeled separately and included in the design matrix.
Regions-of-interest were defined using the Talairach masks provided within AFNI for the amygdala and hippocampus. A modified AFNI mask was utilized to define the region comprising the perigenual ACC. For each participant, the 3dDeconvolve output was resampled and the masks applied to calculate the percent signal change within the regions-of-interest for the SN-HN, SN-NN, and HN-NN contrasts. The significance threshold was set at p<0.05 after small volume correction with a cluster size ≥ 20.
For each individual, the resulting percent signal change values (beta-weights) over the peak voxels of clusters of interest were extracted and used to test for differences across menstrual phase and correlations with depression scores using ANOVA and Pearson’s correlation coefficient, respectively, as implemented by SPSS v17.
RESULTS
Males and females did not differ significantly in age (31±10 versus 34±10 years) or HDRS score (0.6±1.2 versus 0.6±0.9). Further, there was no group difference in self-reported childhood trauma, anxiety, and personality traits (see table 1).
Table 1.
Demographic Data and Psychometric Differences between Males and Females.
| Male | Female | Statistic | |
|---|---|---|---|
| N | 28 | 28 | - |
| Age | 30.2±10.6 | 34.1±9.6 | t54=1.5, p=0.152 |
| HDRS | 0.6±1.5 | 0.6±0.9 | t54=0.1, p=0.914 |
| HAM-A | 0.5±0.9 | 0.7±1.0 | t53=0.8, p=0.451 |
| STAI-State | 23.7±3.6 | 24.5±5.5 | t54=0.6, p=0.530 |
| STAI-Trait | 25.6±4.6 | 26.8±5.5 | t54=0.9, p=0.388 |
| TCI-HA | 6.6±5.2 | 8.1±5.8 | t54=1.0, p=0.313 |
| TCI-NS | 18.0±6.9 | 15.7±7.6 | t54=1.2, p=0.234 |
| TCI-P | 5.3±2.1 | 5.2±2.2 | t54=0.3, p=0.803 |
| TCI-RD | 14.8±5.1 | 15.4±6.5 | t54=0.4, p=0.715 |
| TCI-Impulsivity | 3.0±2.0 | 2.8±2.2 | t54=0.5, p=0.610 |
| TCI-C | 35.7±7.8 | 35.2±12.6 | t54=0.2, p=0.849 |
| TCI-SD | 37.4±8.2 | 35.0±6.8 | t54=0.8, p=0.417 |
| TCI-ST | 11.9±5.8 | 13.5±6.8 | t54=1.0, p=0.337 |
| CTQ | 31.0±10.6 | 29.6±5.2 | t40=0.7, p=0.501 |
Abbreviations: HDRS = Hamilton Depression Rating Scale; HAM-A = Hamilton Anxiety Scale; STAI = State-Trait Anxiety Scale; TCI = Temperament and Character Inventory; HA = Harm Avoidance; NS = Novelty Seeking; P = Persistence; RD = Reward Dependence; C = Cooperativeness; SD = Self-Directedness; ST = Self-Transcendence; CTQ = Childhood Trauma Inventory
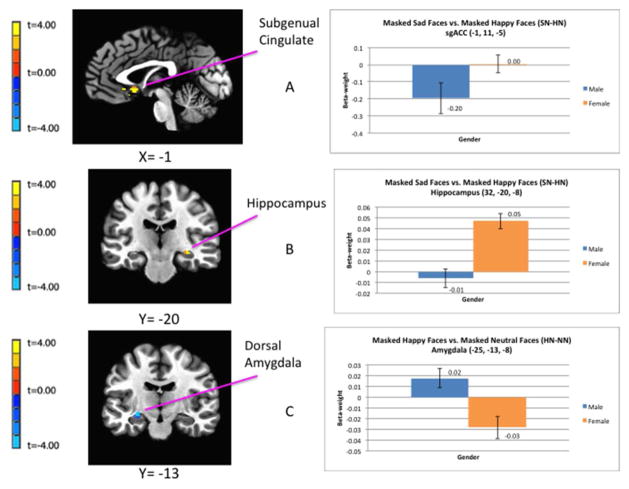
Relative to males, the females showed the following differences in BOLD activity to emotionally-valenced faces (table 2; figure 1):
Table 2.
Regional Differences in BOLD Response to Emotionally-Valenced Faces in Females versus Males.
| Contrast | Region | Talairach Coordinates | Cluster Size | T-value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| SN-HN | ||||||
| F>M | L sgACC (BA25) | −1 | 11 | −5 | 25 | 3.49 |
| F>M | R sgACC (BA24) | 1 | 24 | −3 | 26 | 2.97 |
| F>M | R hippocampus | 32 | −20 | −8 | 23 | 2.86 |
| HN-NN | ||||||
| F<M | L Amygdala | −25 | −13 | −8 | 56 | 3.73 |
| F<M | R sgACC | 1 | 18 | −10 | 27 | 3.81 |
| F<M | R pgACC | 11 | 45 | 11 | 24 | 2.83 |
Note: All results are small volume corrected (p<0.05).
No significant between group differences were found for the SN-NN contrast.
Abbreviations: F = female, M = male, L = left; R = right; sgACC = subgenual anterior cingulate cortex; pgACC = pregenual ACC
Figure 1.
Regional differences in hemodynamic response between males and females while viewing emotionally-expressive face stimuli presented below the level of conscious awareness using the backward masking task. The clusters of voxels for which pcorrected<0.05 in the fMRI contrasts are superimposed on sagittal or coronal sections from co-registered, anatomical MRI images (left column). Adjacent to each image is the corresponding bar graph showing contrast beta-weights plus standard error bars that correspond to the differences between female and male subjects measured over the peak voxel t-value shown in the image data. The figures in A and B show the difference in BOLD activity between females and males in response to masked sad versus masked happy face stimuli (SN-HN) in the left sgACC (A) and right hippocampus (B). The figures in (C) show the regional difference in BOLD activity between females and males in response to happy versus neutral face stimuli (HN-NN) in the left amygdala. Coordinates for the peak T-values correspond to the stereotaxic array of Talairach and Tournoux (1988) as the distance in mm from the anterior commissure (positive x = right, positive y = anterior, positive z = dorsal). Abbreviations: HN-NN = masked happy versus masked neutral faces; SN-HN = masked-sad versus masked-happy faces; sgACC = subgenual anterior cingulate cortex.
An increased BOLD response to masked sad versus masked happy faces (SN-HN) in the right hippocampus and the bilateral sgACC.
A decreased BOLD response to masked happy versus masked neutral faces (HN-NN) in the left amygdala (in the vicinity of the central nucleus), the right sgACC (located in the subcallosal gyrus area that putatively corresponds to infralimbic cortex), and the right pgACC.
There was no significant association between HDRS scores and the magnitude of the regional hemodynamic response measured over the peak voxel within each cluster of significant group difference (N=56, r’s<0.2, p’s>0.1). Further, no significant effect of menstrual phase on activation of the amygdala, hippocampus or sgACC was observed in the female group (N=28, F2,25 < 2, p’s>0.1).
DISCUSSION
The principal results were that relative to males: 1) females showed an increased BOLD response in the SN-HN contrast in the right hippocampus and sgACC, bilaterally, and 2) females showed a reduced BOLD response in the left amygdala and right sgACC in the HN-NN contrast.
Altered activity of the ventromedial PFC in mood disorders, in particular the region ventral to the genu of the corpus callosum (subcallosal gyrus), is one of the most robust findings in biological psychiatry (Drevets et al. 2008; Savitz and Drevets 2009a). The subcallosal gyrus comprises cytoarchitectonically distinct anterior and posterior components, termed the sgACC and the infralimbic cortex which correspond approximately to BA 24 and 25, respectively, (Ongur et al. 2003). Increased activity in the subcallosal gyrus as well as the region rostral to the genu (pgACC) has been demonstrated to be associated with negative emotional processing biases in depression (Laxton et al. 2013; Victor et al. 2013).
For instance, increased activation of the sgACC in response to sad versus neutral faces was observed in patients with MDD relative to healthy controls (Gotlib et al. 2005). Conversely, greater BOLD activity in the pgACC in response to happy faces is predictive of remission or response to treatment at follow-up (Diler et al. 2013; Opmeer et al. 2016). In depressed patients we previously showed a decrease in the hemodynamic response to masked-sad vs. masked-happy faces following sertraline treatment in the pgACC (Victor et al. 2013). Further this decrement in pgACC activity co-occurred with a positive shift in the emotional processing bias and a reduction in depressive symptoms (Victor et al. 2013). Consistent with these data, in healthy individuals, engagement of the pgACC during the processing of happy faces is positively correlated with emotional stability (Brassen et al. 2011).
The sgACC and the pgACC share substantial, reciprocal anatomical connections with the amygdala (Amaral and Insausti 1992) which is one of the most sexually dimorphic regions of the brain in both rodents (Cooke and Woolley 2005; Hines et al. 1992) and humans (Goldstein et al. 2001). The amygdala is not only activated by negative or aversive stimuli (LeDoux 2000), but is sensitive to the relative intensity of positive stimuli (Bonnet et al. 2015). However, there also is evidence for the lateralization of amygdala function in the context of emotional processing. For instance, masked affective stimuli, regardless of valence, are associated with greater activation of the left compared with the right amygdala (Killgore and Yurgelun-Todd 2004). Conceivably, the lateralized pattern of amygdalar function is partly related to sex differences reported in the literature. The relative decrease in response to happy faces in the left amygdala in women found here is partially consistent with a report of greater left amygdala activation in men than women during the viewing of pleasant International Affective Picture System (IAPS) images (Wrase et al. 2003). Further, a recent meta-analysis reported reduced activation in the left amygdala to a variety of positive emotions in healthy women compared to men (Stevens and Hamann 2012). In addition, Cahill and colleagues found that in women the left amygdala showed greater functional connectivity with the sgACC and hypothalamus at rest; a result the authors propose may be relevant to the sex-related lateralization of amygdala function in the encoding of emotional memories (Kilpatrick et al. 2006). Further evidence for sex-dependent lateralization of amygdala function was provided by Buchel and colleagues in their analysis of amygdalar response to neutral and angry faces in adolescents (Schneider et al. 2011). Compared with females, males showed a stronger activation of the right amygdala compared to the left amygdala and this effect was enhanced when viewing angry faces, suggesting that emotional content enhanced this left-right differential (Schneider et al. 2011).
The reduced left amygdalar response to masked happy faces in healthy females versus males potentially is relevant to understanding the increased prevalence of depression in females. Using a previous iteration of the backward masking task in a fully independent sample of participants, we showed that compared with healthy controls, age and sex-matched unmedicated MDD patients displayed a greater automatic amygdala response to sad faces, and conversely, a reduced amygdala response to happy faces; both of these abnormalities relative to healthy controls were normalized by treatment with sertraline (Victor et al. 2010). The same phenomenon of decreased amygdalar response to subliminally-presented happy faces in depressed individuals also was reported by Suslow, Dannlowski and colleagues (Stuhrmann et al. 2013; Suslow et al. 2010).
The absence of a “normal” positive processing bias is increasingly recognized as a key trait-like component of depression. Behavioral or neurophysiological responses to reward have been demonstrated to remain abnormal in remitted patients with MDD (McCabe et al. 2009; Pechtel et al. 2013) and consistent with these data we previously showed a reduced BOLD response in the left amygdala in remitted MDD individuals in the HN-NN contrast (Victor et al. 2010). Further, several studies have demonstrated that an early increase in positive affect after the initiation of antidepressant treatment is a positive prognostic indicator (Geschwind et al. 2011; Tranter et al. 2009). In fact, Harmer and colleagues have proposed that a common effect of antidepressant pharmacotherapy that cuts across different classes of medication is a shift in the emotional processing bias in the positive direction (Harmer 2008).
Regarding the hippocampus, our finding of an increased hemodynamic response to SN-HN faces in females versus males (XYZ = 32, −20, −8) is noteworthy in light of our previous report of elevated BOLD activity in the SN-HN contrast in the hippocampus (XYZ = −18, −24, −12) in MDD subjects versus healthy controls (Victor et al. 2012).
Hippocampal lesions interrupt afferent neurotransmission to the amygdala which typically conveys information regarding the broader environmental context (e.g. place or time) of an emotionally salient stimulus (Phillips and LeDoux 1994). Thus, we previously hypothesized that the hippocampus sets the context for the negative emotional processing bias observed in depression by driving the amygdala to respond differently to sad versus happy stimuli (Victor et al. 2012).
Estradiol plays an important role in regulating hippocampus-dependent learning and memory under stress by modulating dendritic spine density and synaptic number within the hippocampus (Luine 2015). For instance, estradiol replacement in ovariectomized rats may increase contextual fear memory formation and decrease fear extinction learning (McDermott et al. 2015) - although some studies have reported that blockade of estradiol production impairs rather than enhances fear extinction (Graham and Milad 2013). Conceivably, estradiol and/or other gonadal hormones may affect hippocampal-dependent memory formation during emotionally-salient events and thus influence the tendency to develop negative (or positive) emotional processing biases.
One important limitation of our study is that the reported differences between males and females were relatively small and were derived from region-of-interest analyses with a relatively liberal threshold for statistical significance, i.e. p<0.05 after small volume correction with a minimum cluster size of 20 contiguous voxels.
Secondly, our study was likely underpowered to detect neurophysiological differences between women according to the phase of the menstrual cycle. Thus the absence of a significant association between menstrual phase and the hemodynamic response to masked faces in females should be treated with caution as other studies have suggested that estrogen and progesterone may play opposing roles in modulating the brain’s stress circuitry (Andreano and Cahill 2010; Goldstein et al. 2005; Ossewaarde et al. 2010; Petersen et al. 2014).
Noteworthy strengths of the methods include the rapid event-related, backward masking design and the high sensitivity of the imaging methods for regions prone to BOLD signal susceptibility artifact. Compared to the slow-event related backward masking task we described in Victor et al. (2010), in the current study we employed a rapid event-related design which allowed more trials during a shorter scan time. An advantage of this shorter task is that head motion during scanning is minimized. In addition, the improved imaging hardware, acquisition speed and BOLD signal sensitivity we employed herein afforded higher temporal signal-to-noise ratios than the scanning technology used in Victor et al. (2010), particularly in areas such as the basotemporal and basofrontal cortices. This greater sensitivity may explain our ability to detect BOLD changes during task performance in the subgenual prefrontal cortex, an area that is susceptible to BOLD signal dropout because of its proximity to the sphenoid sinus. As reported above, Gotlib et al. (2005) previously reported activation of the sgACC region in response to sad versus neutral faces in patients with MDD relative to healthy controls. Moreover, in depressed patients being prepared for deep brain stimulation, Laxton et al. (2013) performed electrophysiological recordings from neurons in the sgACC region, and showed that these neurons preferentially responded to emotional pictorial stimuli, with more neurons increasing their firing activity while viewing affectively negative versus positive stimuli. The technical improvements implemented herein to increase sensitivity to BOLD activity in this region proved advantageous for characterizing sex differences in the neural response of the subgenual prefrontal cortical region to implicitly presented sad versus happy face stimuli.
Significance Statement.
The prevalence of depression is approximately 2-fold greater in women than men but the biological mechanisms underlying this phenomenon remain unclear. Depressed individuals reliably differ from healthy controls in activation of several cortico-limbic regions when viewing emotionally-expressive faces during fMRI, a finding that is thought to reflect an attentional bias towards negative stimuli. We report an overlap in the neural circuits that differentiate healthy females from males and the circuits that differentiate depressed from non-depressed subjects during the viewing of emotionally-valenced faces. Interrogation of these circuits may help to elucidate the biological basis of the female preponderance in depression.
Acknowledgments
Support was received from The William K. Warren Foundation, the National Institute of Mental Health (K01MH096077) and the Oklahoma Center for the Advancement of Science and Technology (HR10-172).
Footnotes
CONFLICT OF INTEREST STATEMENT
TAV, MM, JB, and JS have no disclosures. WCD is an employee of Janssen Research & Development, LLC, of Johnson & Johnson.
ROLE OF AUTHORS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: TAV, WCD, JS. Acquisition of data: TAV, JB. Analysis and interpretation of data: TAV, WCD, MM, JB, JS. Drafting of the manuscript: JS. Critical revision of the manuscript for important intellectual content: TAV, WCD, MM, JB, JS. Statistical analysis: TAV, JS. Obtained funding: WCD, JS. Administrative, technical, and material support: JB, JS. Study supervision: TV, JS.
References
- Amaral DG, Insausti R. Retrograde transport of D-[3H]-aspartate injected into the monkey amygdaloid complex. Experimental brain research. 1992;88(2):375–388. doi: 10.1007/BF02259113. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. Neuroimage. 2010;53(4):1286–1293. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, McKie S, Elliott R, Thomas EJ, Downey D, Juhasz G, Williams SR, Deakin JF, Anderson IM. Increased amygdala responses to sad but not fearful faces in major depression: relation to mood state and pharmacological treatment. Am J Psychiatry. 2012;169(8):841–850. doi: 10.1176/appi.ajp.2012.11121774. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical image analysis. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet L, Comte A, Tatu L, Millot JL, Moulin T, Medeiros de Bustos E. The role of the amygdala in the perception of positive emotions: an “intensity detector”. Frontiers in behavioral neuroscience. 2015;9:178. doi: 10.3389/fnbeh.2015.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassen S, Gamer M, Buchel C. Anterior cingulate activation is related to a positivity bias and emotional stability in successful aging. Biol Psychiatry. 2011;70(2):131–137. doi: 10.1016/j.biopsych.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Cahill L. Sex-related influences on the neurobiology of emotionally influenced memory. Ann N Y Acad Sci. 2003;985:163–173. doi: 10.1111/j.1749-6632.2003.tb07080.x. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Sexually dimorphic synaptic organization of the medial amygdala. J Neurosci. 2005;25(46):10759–10767. doi: 10.1523/JNEUROSCI.2919-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8(2–3):109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diler RS, Ladouceur CD, Segreti A, Almeida JR, Birmaher B, Axelson DA, Phillips ML, Pan LA. Neural correlates of treatment response in depressed bipolar adolescents during emotion processing. Brain imaging and behavior. 2013;7(2):227–235. doi: 10.1007/s11682-012-9219-7. [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12(8):467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS spectrums. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else-Quest NM, Higgins A, Allison C, Morton LC. Gender differences in self-conscious emotional experience: a meta-analysis. Psychol Bull. 2012;138(5):947–981. doi: 10.1037/a0027930. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York, NY: New York State Psychiatric Institute, Biometrics Research; 2002. [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61(9):877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Nicolson NA, Peeters F, van Os J, Barge-Schaapveld D, Wichers M. Early improvement in positive rather than negative emotion predicts remission from depression after pharmacotherapy. Eur Neuropsychopharmacol. 2011;21(3):241–247. doi: 10.1016/j.euroneuro.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Holsen L, Handa R, Tobet S. Fetal hormonal programming of sex differences in depression: linking women’s mental health with sex differences in the brain across the lifespan. Front Neurosci. 2014;8:247. doi: 10.3389/fnins.2014.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25(40):9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11(6):490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Sivers H, Gabrieli JD, Whitfield-Gabrieli S, Goldin P, Minor KL, Canli T. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005;16(16):1731–1734. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol Psychiatry. 2013;73(4):371–378. doi: 10.1016/j.biopsych.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Matsumoto D. Gender differences in judgments of multiple emotions from facial expressions. Emotion. 2004;4(2):201–206. doi: 10.1037/1528-3542.4.2.201. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol Psychiatry. 2015;78(4):224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ. Serotonin and emotional processing: does it help explain antidepressant drug action? Neuropharmacology. 2008;55(6):1023–1028. doi: 10.1016/j.neuropharm.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry. 2004;161(7):1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579(2):321–326. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol. 2007;116(1):80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry. 2004;161(4):631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74(1):5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International journal of methods in psychiatric research. 2012;21(3):169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. Neuroimage. 2004;21(4):1215–1223. doi: 10.1016/j.neuroimage.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30(2):452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kret ME, De Gelder B. A review on sex differences in processing emotional signals. Neuropsychologia. 2012;50(7):1211–1221. doi: 10.1016/j.neuropsychologia.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Laxton AW, Neimat JS, Davis KD, Womelsdorf T, Hutchison WD, Dostrovsky JO, Hamani C, Mayberg HS, Lozano AM. Neuronal coding of implicit emotion categories in the subcallosal cortex in patients with depression. Biol Psychiatry. 2013;74(10):714–719. doi: 10.1016/j.biopsych.2013.03.029. [DOI] [PubMed] [Google Scholar]
- Leach LS, Christensen H, Mackinnon AJ, Windsor TD, Butterworth P. Gender differences in depression and anxiety across the adult lifespan: the role of psychosocial mediators. Social psychiatry and psychiatric epidemiology. 2008;43(12):983–998. doi: 10.1007/s00127-008-0388-z. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Luine V. Estradiol: Mediator of memories, spine density and cognitive resilience to stress in female rodents. The Journal of steroid biochemistry and molecular biology. 2015 doi: 10.1016/j.jsbmb.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rich JB, Rosenbaum RS. Implicit memory varies across the menstrual cycle: estrogen effects in young women. Neuropsychologia. 2002;40(5):518–529. doi: 10.1016/s0028-3932(01)00126-9. [DOI] [PubMed] [Google Scholar]
- Maxwell E. Family Interview for Genetic Studies (FIGS): A Manual for FIGS. Bethesda, MD: Clinical Neurogenetics Branch, Intramural Research Program, National Institute of Mental Health; 1992. [Google Scholar]
- McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology (Berl) 2009;205(4):667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott CM, Liu D, Ade C, Schrader LA. Estradiol replacement enhances fear memory formation, impairs extinction and reduces COMT expression levels in the hippocampus of ovariectomized female mice. Neurobiol Learn Mem. 2015;118:167–177. doi: 10.1016/j.nlm.2014.12.009. [DOI] [PubMed] [Google Scholar]
- McLean CP, Anderson ER. Brave men and timid women? A review of the gender differences in fear and anxiety. Clin Psychol Rev. 2009;29(6):496–505. doi: 10.1016/j.cpr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Emotion regulation and psychopathology: the role of gender. Annu Rev Clin Psychol. 2012;8:161–187. doi: 10.1146/annurev-clinpsy-032511-143109. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460(3):425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Opmeer EM, Kortekaas R, van Tol MJ, Renken RJ, Demenescu LR, Woudstra S, Ter Horst GJ, van Buchem MA, van der Wee NJ, Veltman DJ, Aleman A. Changes in Regional Brain Activation Related to Depressive State: A 2-Year Longitudinal Functional Mri Study. Depress Anxiety. 2016;33(1):35–44. doi: 10.1002/da.22425. [DOI] [PubMed] [Google Scholar]
- Ossewaarde L, Hermans EJ, van Wingen GA, Kooijman SC, Johansson IM, Backstrom T, Fernandez G. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology. 2010;35(1):47–55. doi: 10.1016/j.psyneuen.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA. Blunted reward responsiveness in remitted depression. J Psychiatr Res. 2013;47(12):1864–1869. doi: 10.1016/j.jpsychires.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Cahill L. Amygdala reactivity to negative stimuli is influenced by oral contraceptive use. Soc Cogn Affect Neurosci. 2015;10(9):1266–1272. doi: 10.1093/scan/nsv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Kilpatrick LA, Goharzad A, Cahill L. Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. Neuroimage. 2014;90:24–32. doi: 10.1016/j.neuroimage.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem. 1994;1(1):34–44. [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neuroscience and biobehavioral reviews. 2009a;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience. 2009b;164(1):300–330. doi: 10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Peters J, Bromberg U, Brassen S, Menz MM, Miedl SF, Loth E, Banaschewski T, Barbot A, Barker G, Conrod PJ, Dalley JW, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Mallik C, Mann K, Artiges E, Paus T, Poline JB, Rietschel M, Reed L, Smolka MN, Spanagel R, Speiser C, Strohle A, Struve M, Schumann G, Buchel C consortium I. Boys do it the right way: sex-dependent amygdala lateralization during face processing in adolescents. Neuroimage. 2011;56(3):1847–1853. doi: 10.1016/j.neuroimage.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51(9):693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50(7):1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Stuhrmann A, Dohm K, Kugel H, Zwanzger P, Redlich R, Grotegerd D, Rauch AV, Arolt V, Heindel W, Suslow T, Zwitserlood P, Dannlowski U. Mood-congruent amygdala responses to subliminally presented facial expressions in major depression: associations with anhedonia. J Psychiatry Neurosci. 2013;38(4):249–258. doi: 10.1503/jpn.120060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57(3):201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schoning S, Ohrmann P, Bauer J, Pyka M, Kersting A, Arolt V, Heindel W, Dannlowski U. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry. 2010;67(2):155–160. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranter R, Bell D, Gutting P, Harmer C, Healy D, Anderson IM. The effect of serotonergic and noradrenergic antidepressants on face emotion processing in depressed patients. J Affect Disord. 2009;118(1–3):87–93. doi: 10.1016/j.jad.2009.01.028. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar JK, Fernandez G. Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry. 2008;13(3):325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, Zylicz SA, Pieters S, Mattern C, Verkes RJ, Buitelaar JK, Fernandez G. Testosterone increases amygdala reactivity in middle-aged women to a young adulthood level. Neuropsychopharmacology. 2009;34(3):539–547. doi: 10.1038/npp.2008.2. [DOI] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Bellgowan PS, Ohman A, Drevets WC. The extended functional neuroanatomy of emotional processing biases for masked faces in major depressive disorder. PLoS One. 2012;7(10):e46439. doi: 10.1371/journal.pone.0046439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Archives of General Psychiatry. 2010;67(11):1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Changes in the neural correlates of implicit emotional face processing during antidepressant treatment in major depressive disorder. Int J Neuropsychopharmacol. 2013;16(10):2195–2208. doi: 10.1017/S146114571300062X. [DOI] [PubMed] [Google Scholar]
- Wrase J, Klein S, Gruesser SM, Hermann D, Flor H, Mann K, Braus DF, Heinz A. Gender differences in the processing of standardized emotional visual stimuli in humans: a functional magnetic resonance imaging study. Neurosci Lett. 2003;348(1):41–45. doi: 10.1016/s0304-3940(03)00565-2. [DOI] [PubMed] [Google Scholar]