Abstract
There are increasing numbers of overweight and obese individuals in the US and globally, and correspondingly, the associated healthcare costs are rising dramatically. More than one-third of children are currently considered obese with a predisposition to type 2 diabetes, and it is likely that their metabolic conditions will worsen with age. Physical inactivity has also risen to be the leading cause of many chronic, non-communicable diseases (NCD). Children are more physically inactive now than they were in past decades, which may be due to intrinsic and extrinsic factors. In rodents, the amount of time engaged in spontaneous activity within the home cage is a strong predictor of later adiposity and weight gain. Thus, it is important to understand primary motivators stimulating physical activity (PA). There are normal sex differences in PA levels in rodents and humans. The perinatal environment can induce sex-dependent differences in PA disturbances. This review will consider the current evidence that there are sex differences in PA in rodents and humans. The rodent studies showing that early exposure to environmental chemicals can shape later adult PA responses will be discussed. Next, an exploration of whether there are different motivators stimulating exercise in male vs. female humans will be examined. Finally, the brain regions, genes, and pathways, that modulate PA in rodents, and possibly by translation humans, will be described. A better understanding of why each sex remains physically active through the lifespan could open up new avenues to prevent and treat obesity in children and adults.
Keywords: Sexual Dimorphism, Maternal Effects, Diet, Endocrine Disruptors, Physical Activity, Motivation, Brain, Nucleus Accumbens, Hippocampus, Hypothalamus, Pre-frontal Cortex, Estrogen, Dopamine signaling, and Developmental Origins of Adult Health and Disease
Introduction
At least 35 % of the US population is obese, with costs for treating ailments relating to this condition in 2008 estimated at $147 billion (http://www.cdc.gov/obesity/data/adult.html). More than one-third of children are obese with a predisposition to type 2 diabetes, and most of them will continue to be plagued by metabolic disorders as adults. The World Health Organization (WHO) has estimated that close to 350 million people world-wide already have type 2 diabetes and that this number is increasing annually, particularly in the developing countries as populations gain greater access to a so-called Western-style diet and become more sedentary (Scully 2012). Children are more physically inactive now than they were in past decades, which may be due to a convenient lifestyle with automated transportation, reduced accessibility to parks and other areas to play, and increased amount of time engaged in sedentary activities (Brownson et al. 2005; Gray et al. 2014; Ziviani et al. 2008). However, the increasing rates also suggest that there could be intrinsic disturbances in the normal brain pathways that motivate individuals to exercise. Notably, physical inactivity has risen to be the leading cause of many chronic, non-communicable diseases (NCD) (Bauer et al. 2014; Booth et al. 2008; Booth et al. 2012; Garcia et al. 2014; Goedecke and Micklesfield 2014; Knight 2012; Schottenfeld et al. 2013; Ward 2014). A few examples of the 35 chronic conditions linked with physical inactivity, include obesity, type 2 diabetes, coronary heart disease, other cardiovascular disorders (hypertension, stroke, & congestive heart failure), depression, anxiety, cognitive dysfunction, osteoporosis, and cancer (Bauer et al. 2014; Booth et al. 2008; Booth et al. 2012; Garcia et al. 2014; Goedecke and Micklesfield 2014; Knight 2012; Schottenfeld et al. 2013; Ward 2014).
There is a critical need to understand what motivates individuals to remain PA throughout the lifespan. The underpinning reasons likely differ between males and females. Furthermore, the developmental origins of health and disease (DOHaD) concept also suggests that later PA levels may be influenced by the perinatal environment (Barouki et al. 2012; Gluckman et al. 2007; Hanson and Gluckman 2011; van Deutekom et al. 2013). Early exposure may differentially impact PA in one sex over the other.
This review will discus the current evidence for sex differences in physical activity in several rodent models. Next, the current findings in humans suggesting sex-dependent differences in motivation to exercise or engage in PA throughout the lifesspan will be considered. Finally, the brain regions essential for PA initiation and maintenance in varying rodent models will be discussed. While humans are assuredly influenced by more extrinsic and social factors than rodents, it is likely that such brain regions are also essential in stimulating exercise activities in humans. A greater understanding of these neural mechanisms driving PA and whether they differ between the sexes may open up new avenues to prevent metabolic and other NCD due to physical inactivity.
Sex-dependent differences in voluntary physical activity in rodent models
In this section, we will consider how rodent models have helped elucidate sex-dependent differences in voluntary physical activity levels. Rodent models have also revealed that adult physical activity can be affected by perinatal factors, namely environmental chemicals, and one sex may be more vulnerable than the other depending on the causative factors, as detailed below. The amount of physical activity male and female rodents display may also affect other behavioral patterns, especially voluntary consumption of drugs and alcohol.
Several rodent studies reveal that there are normal sex differences in adult voluntary physical activity levels. Wistar female rats exhibit elevated running rates relative to males regardless of being on ad libitum or food-restricted diet (Jones et al. 1990). Likewise, female mice are more active, as exemplified by running an average of 6.5 ± 1.3 km compared to males who only run 3.9 ± 0.2 km in a 24 hour period, and show increased cardiac hypertrophy due to running than males (females = 15.5 ± 1.9% vs. males = 4.6 ± 0.5%) when both are maintained on a soy-based chow diet (Harlan Teklad 8640) (Konhilas et al. 2015). However, males fed a casein-based diet (Research Diets D10001) demonstrate an increase in running distance and exercise-induced cardiac enlargement to levels approximating those of females maintained on this same diet (females = 17.5 ± 1.6% compared to males = 13.2 ± 2.7%).
Female mice derived from independently bred lines selected over 50 generations show increased voluntary wheel running behavior (S1 and S2) and resistance to high energy (HE) diet-induced obesity at adulthood, even if they are not provided access to running wheel, relative to randomly bred controls (Guidotti et al. 2016). However, perinatal exposure through the maternal diet from two weeks prior to conception through weaning post-natal day 21 results in S1 and S2 becoming susceptible to a HE diet increase in adiposity when provided this same diet at the post-weaning period. Voluntary wheel running activity though remained intact in the S mice exposed to the HE diet during both time periods. This diet also increased circulating leptin concentrations in male and female S mice.
In both sexes of inbred rodent strains, there is a strong correlation between voluntary wheel running and distance traveled in the open-field (Careau et al. 2012). Female Wistar rats demonstrate increased locomotor activity in an open field maze than males (Belviranli et al. 2012). However, increase thigmotaxis behavior (time spent in close contact with the edge of the open field maze) negatively correlates with distance run on the wheels in females but not males. Restraint stress is associated with greater reductions in voluntary wheel-running in female compared to male mice (Yamaura et al. 2013). Sex differences in activity levels in the open-field test may at least be partially mediated by gonadal hormones. As indicated in other studies, male rats are less active than females in this maze (Blizard et al. 1975). Gonadectomy of both sexes reduces the magnitude of this sex-difference, but females continue to outperform males. Estrogen supplementation to ovariectomized females restores activity levels to those observed in intact females, but this treatment has no effect castrated males. However, treatment of neonatal females with testosterone propionate (TP) results in a later masculinized response in the open field maze, in essence to reduced activity levels on par with intact males. Taken together, the findings suggest that early exposure to testosterone may suppress brain regions governing later motivation to engage in physical activity. Estrogen produced in cycling adult females may, however, partially induce activational brain effects resulting in enhanced physical activity. Age and sex may also interact to affect amount of locomotor activity. In several mice strains, young males (60 days of age) have elevated locomotor activity patterns than same age females, but such sex-differences are abolished by 125 days of age (Simmel et al. 1976).
Voluntary physical activity levels may also be impacted by the interaction of sex and genetic background. While it is clear that “pure-bred” high runner (HR) mouse lines run more than those generated from randomly paired mice, heterosis or hybrid vigor due to crossing of a non-purebred to a pure-bred animal may conceivably generate offspring with even greater performance. Such is the case in hybrid male mice that run significantly more and show increased speed on voluntary wheels relative to purebred males; whereas hybrid females obtain intermediate patterns in distance traveled, durations, and speed relative to purebred females (Hannon et al. 2011). The interactive effects of genetic background and sex on physical activity levels could be due to sex hormones, in particular estrogen or testosterone (Lightfoot 2008).
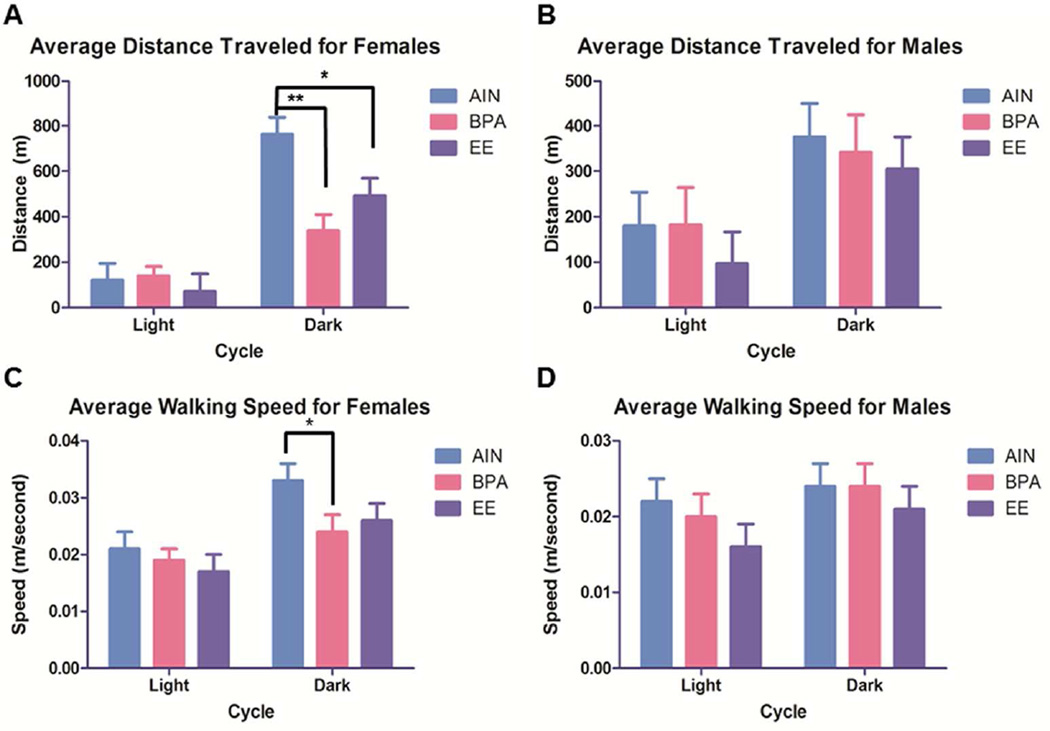
Developmental exposure to select environmental chemicals may suppress later physical activity in one or both sexes. Female California mice (Peromyscus californicus) are highly mobile and show greater exploratory behavior than males of this species, but developmental exposure to BPA abolishes this sex difference, suggesting that this chemical may also masculinize this behavior in exposed females (Williams et al. 2013). In this species, both males and females help rear the pups (Bredy et al. 2004; Dudley 1974a; Dudley 1974b; Gubernick and Alberts 1987; Ribble 1990; Stickel 1968), which is similar to most human societies. California mice are considered an excellent model for human metabolic diseases, in particular type 2 diabetes mellitus (Krugner-Higby et al. 2000). They may also better mirror the genetic diversity present in most human populations. Females but not males developmentally exposed to BPA show less spontaneous activity, sleep more hours, and show disturbances in carbohydrate vs. fat metabolism (Fig. 1) (Johnson et al. 2015). An early study testing behavioral performance in the elevated plus maze and hole board of Sprague-Dawley rats perinatally exposed to BPA suggests that motivation to explore is reduced in both adult males and females, but females also demonstrate reduced motor activity (Farabollini et al. 1999). Gestational exposure of male and female 129/SvSI mice, a common mouse strain used in neurobehavioral studies, to methylmercury (a widespread environmental chemical and well documented neurotoxicant) reduces later exploratory and locomotive behaviors (Spyker et al. 1972). Male Sprague-Dawley rats developmentally exposed to manganese (Mn) are hypoactive when tested as juveniles and adults in an open-field maze, but no effects on activity levels are observed in exposed females (Betharia and Maher 2012). These neurotoxic effects of Mn may be due to impaired dopaminergic, glutamergic, or GARergic transmission, mitochondrial disruptions, oxidative stress, and/or neuroinflammation (Tuschl et al. 2013). Prenatal exposure to dichlorovos, a widely used organophosphate insecticide and acetylcholinesterase inhibitor, also decreases locomotor activity in male but not female rat pups when tested in this maze at 21 days of age (Lazarini et al. 2004).
Fig. 1.
Average distance traveled and walking speed for females and males. A) Average distance traveled for females. B) Average distance traveled for males. C) Average walking speed for females. D) Average walking speed for males. N= 8–12 replicates per group. *p < 0.05 and **p = 0.0003. Adapted from (Johnson et al. 2015).
In rodent models, the physical activity may in a sex-dependent manner alter the amount of self-administered drugs or alcohol. Female mice provisioned with alcohol drink less of this substance when allowed to run on a running wheel; whereas males will drink less alcohol even if the wheels are locked (Ehringer et al. 2009). Male and female rats allowed to self-administer cocaine and then provided an unlocked or locked running wheel show sexually-dimorphic differences (Peterson et al. 2014). While females run more than males, increased access to unlocked running wheels results in a dose-dependent decrease in cocaine intake for males. In contrast, females provided both unlocked and locked wheels, self-administer less cocaine but these effects also depend on estrous cycle phase.
Sex-dependent differences in exercise or leisure-time physical activity in humans
A sampling of the many retrospective and cohort studies examining for sex-differences in leisure time physical activity (LTPA) will be discussed in this section. The studies include participants spanning in age from childhood to elderly individuals. Even with measures designed to provide equitable access to sport activities, such as through Title IX in the United States, a strong disparity still exists between interest and participation with boys significantly outnumbering girls as sport participants (Deaner et al. 2012). These sex-dependent variations in physical activity for children may predispose to later risks for obesity, other metabolic disorders, and NCD. Thus, it is critical to understand why even at these earlier ages and with seemingly equal opportunities available, boys and girls diverge in their motivation to engage in PA.In elementary and adolescent age children, the amount of time engaged in PA is correlated inboys with enjoyment and perceived physical competence, while in girls, PA levels are positively associated withperceived acceptance by peers in games and sports and parental encouragement (Seabra et al. 2013). A survey of high schools students (N = 1163) corroborates these findings as the girls in this study report decreased participation in PA with boys reporting physical exertion and body image, increased muscle mass, as primary attractants to remain physically active (Butt et al. 2011). A study with adolescent boys and girls (N = 812) also found that boys have a higher PA than girls and levels of PA in boys are associated with self-determination and task orientation (Yli-Piipari et al. 2012). In general, adolescent boys are likely motivated to remain physically active because of the competitive aspects, but girls are attracted by the social opportunities that sports may provide (Sirard et al. 2006). In adolescents, engagement of daughters in sports is related the PA habits of both parents; whereas, only the exercise habits of the father is associated with the amount of time sons are PA (Sukys et al. 2014).
Screening of US college students in two studies (n = 621 for one and n= 2199 for another) suggest that motivation to engage in LTPA is primarily governed by internal factors, such as intention, strength, competition, and challenge, in males, but primarily extrinsic factors, including attitude, self-efficacy, body mass index/weight management, physical appearance, i.e. slenderness, and sports participation influence this decision in females (Beville et al. 2014; Egli et al. 2011). Further studies support the notion that motivation drivers for LTPA differ between the sexes in both young and middle age adults (Molanorouzi et al. 2015).
A few comprehensive studies have examined for temporal changes in PA participation from childhood to adulthood. One study sought to determine how pattern of physical activity during the teenage years correlated with later adult activity (Barnekow-Bergkvist et al. 1996). The study encompassed initially 220 boys and 205 girls, who were tested at 16 (1974) and 34 (1992) with 88% retention rate. At 16, more boys than girls participated in some leisure time sports activity (69 vs. 51%). However, when re-examined at adulthood, sex differences in overall physical activity (PA) were absent, but these men exercised more vigorously. Another study with 3500 men and women who were screened regularly from birth to 43 years of age suggests that the type of leisure time physical activity might make a difference as men reported higher rates of sports and recreational activities, gardening, and do-it-yourself; whereas women reported greater rates for bicycling and walking (Kuh and Cooper 1992). Women may increase their physical activity performance if simultaneously listening to their choice in music but the same does not appear to the be case in men (Cole and Maeda 2015).
Cultural norms may underpin sex-differences in PA, as suggested by one study showing Latina men are more PA than women (Marquez and McAuley 2006). A survey of South Asian Punjabi adults (≥ 18 years, N = 204) living in Canada suggests that women are motivated to exercise as a means to reduce weight gain and looking physically like others (Caperchione et al. 2015). In contrast, men are more motivated to exercise to prevent various diseases and because of societal influences. A survey of adult French men and women reveals similar patterns with female participants emphasizing the importance of emotional support, pleasure of doing an activity with others, sense of well-being, and positive body image as primary determinants for physical activity; whereas, male participants emphasized knowledge acquisition, skill development for disease control, and overall health-promoting behaviors as motivators for PA (Ferrand et al. 2008).
Evolutionary theory of intrasexual competition has been postulated to affect exercise behavioral differences between men and women. In first 40,000 years of human evolution, physical activity was tightly associated with energy expenditure and food availability , especially in hunter-gather and early agriculture societies (Lightfoot 2013). However, in modern day Western Societies, where there is overabundance of food sources, has assumingly disrupted this key evolutionary linkage between food acquisition and physical activity levels. Even so, evidence of evolutionary divergence in physical activity levels between the sexes may still remain. In support of this notion, adult male participants in one study focus their energies on building muscle mass and accentuating upper body definition, but females in this study use PA to lose weight and emphasize their lower body (Jonason 2007).
Examination of elderly individuals (≥ 65 years of age) indicates women engage in less LTPA than men (Gardner and Montgomery 2008). Similarly, a self-reported questionnaire with senior men and women (N = 276) also reported decreased overall PA in women, which may be related to personal and environmental limitations (Lee 2005).
Brain regions, genes, and pathways regulating voluntary physical activity/exercise in both sexes
As the above section illustrates, there are a variety of potential motivators for women and men to remain physically active with some being extrinsic-related, such as for social reasons and societal expectations. Thus, it is challenging in humans to dissect how various brain regions stimulate PA and whether the neural responses differ in men vs. women. It is clear though that there is strong conservation in neural-anatomy and function in humans and rodents (Howdeshell 2002; Rice and Barone 2000). Consequently, these species might provide mechanistic insight into the brain regions that are essential in modulating the initiative for PA and the underpinning neural mechanisms. In both sexes, several brain regions are associated with modulating PA. In return, PA can alter brain plasticity, including promoting neuro-generative, -adpative, and – protective responses (Reviewed in (Dishman et al. 2006)). Ex vivo high resolution 3D MR imaging analysis of mice that previously engaged in four weeks of voluntary exercise revealed that this behavior is associated with brain regions required for normal motor function and learning and memory, such as the hippocampus, dentate gyrus, stratum granulosum of the dentate gyrus, cingulate cortex, olivary complex, inferior cerebellar peduncles and other regions of the cerebellum (Cahill et al. 2015). Prior to the initiation of PA, MRI analyses of select brain regions, including the striatum, hippocampus, and pons, are considered excellent predictors of later amount of time engaged in this behavior.
Transgenic knockout mice have been useful in identifying brain transcripts that appear to regulate PA. For instance, mice deficient in G protein-coupled receptor family C group 6 member A (Gprc6a), which acts as a sensing receptor for amino acids, calcium, and steroids, run more on voluntary running wheels than wild-type mice (Clemmensen et al. 2013). By regulating physical activity levels, GPRC6A may affect energy metabolism. The predominant evidence comes from mice or rats bred for several generations to either engage in high or low voluntary PA. Four replicate mouse lines bred for high voluntary wheel running (HR) compared to randomly bred (control) lines suggests that the neurobehavioral profile of HRs is similar to patterns observed in humans with attention deficit hyperactivity disorder (ADHD) (Rhodes et al. 2005). In ADHD individuals and mice that exhibit a high motivation for natural rewards (exercise, food, and sex), and drug-abuse, the responses appear to be modulated to dopamine with chemicals that block dopamine transporter protein (Ritalin and cocaine) decreasing running behavior in High-Runner individuals but not controls. These mice lines also suggest that brain areas including the prefrontal cortex, nucleus accumbens (NAc), caudate-putamen, lateral hypothalamus, and hippocampus are essential in motivation to engage in voluntary physical activity. In the following sections, we will delve more into select brain regions implicated in regulating spontaneous PA and other candidate genes and networks within these regions that may guide this behavior. While it would be optimal if such studies considered effects in both sexes, many of the studies listed below only considered one sex with males predominating. One reason for this disparity is that voluntary PA studies in females generally need to consider the effects of estrous cycle. Thus, vaginal smear cytology may need to be performed, and studies may have to be staggered to accommodate females at various stages of the estrous cycle. Even so, it is highly recommended that going forward all studies consider whether there are differential neural mechanisms regulating PA in males vs. females. With this caveat in mind, the current data to date will be explored.
Striatum (Nucleus Accumbens and Caudate Putamen), Substantia Nigra, and Raphe Nuclei Regulation of PA
The current rodent studies suggest that the striatum region, especially the NAc, is a primary regulator of spontaneous PA in both sexes. Transient inactivation of the NAc or medial prefrontal cortex in male and female Sprague-Dawley rats suppresses running activity during the habitual phase of running (after day 21) but not the acquisition phase (days 1 to 7) in the conditioned place preference testing (Basso and Morrell 2015). These data thus provide strong evidence that both brain regions are essential in the motivation to engage in this behavior. A study with male Long-Evans rats suggests K+-stimulated dopamine release in the striatal region is negatively correlated with average daily running distance (Tarr et al. 2004). ICR male mice selectively bred for high-wheel running activity possess increase concentrations of serotonin (5-HT) in the dorsal striatum but lower concentrations of the serotonin metabolite, 5-hydroxyindoleacetic acid (5-HIAA) in the substantia nigra (Waters et al. 2013). Furthermore, reduced concentrations of dopamine and dopamine metabolite, dihydroxyphenylacetic acid (DOPAC), are observed in the dorsal raphe nucleus and substantia nigra, respectively. A study comparing voluntary wheel running in male C57Bl6J to C3/HeJ mice reveals that C57Bl6J mice use the running wheel more and correspondingly have reduced expression for two dopamine-associated genes, dopamine receptor 1 (Dr1) and tyrosine hydroxylase, in the striatum and NAc. Dr1 is also decreased in highly active interval-specific congenic strains (ISCS) of mice possessing a chromosome 13 substitution (Yang et al. 2012). On this chromosome resides a 3.76 MB interval affects daily voluntary PA, including the Tcfap2a gene that affects promoter activity of Dr1. Cocaine treatment to low-activity ISCS mice elicits a more pronounced response in this pathway than in high-activity individuals.
Comparison of Wistar male and female rats red for high voluntary running (HVR) vs. low voluntary running (LVR) reveals that motivation to engage in voluntary running may be regulated by NAc medium spiny neurons (MSN) and voluntary running early in life stimulates plasticity in neuronal populations and mRNA expression profiles (Roberts et al. 2014). Moreover, a deficiency in plasticity in dopamine-related transcripts occurs in LVR rats. In HVR male and female rats, there is suppression of Oprd1 gene expression, which may relate to differences in dopaminergic signaling relative to LVR rats (Roberts et al. 2013). Injection of dopamine-like 1 (D1) receptor agonist or antagonist lowers total voluntary running distance in HVR female rats (Roberts et al. 2012). However, in the NAc of HVR and LVR rats, there is equal expression of Dr genes (Dr 1, 2, and 5) and other genes examined, N4a2, FosB, and BDNF. Examination of female and male rats bred for high and low-capacity to run on a treadmill (HCR and LCR, respectively) reveals that HCR rats express less encephalin (Enk) in the NAc and olfactory tubules than LCR rats, which is in line with increased dopaminergic tone and increased behavioral motivation in the latter group (Monroe et al. 2014). Additional recent work suggests that estrogen-induced dopamine signaling in the NAc is positively correlated with voluntary PA in female rats bred for high fitness (Park et al. Physiology & Behavior, Resubmitted). Collectively, the studies suggest that at least in females, estrogen might mediate optimal dopamine signaling in the NAc and possibly other brain regions to govern voluntary PA. Administration of estrogen receptor agonists or antagonists to male and female HVR and LVR may help confirm whether these pathways in the NAc are integral in stimulating voluntary PA and whether this response is unique to females.
Mesolimbic DR2 co-localizes with adenosine A2A receptors where the receptor pathways interact in an antagonist manner in regulating motivational behaviors (Salamone et al. 2016). Administration of haloperidol (D2 antagonist) suppresses voluntary wheel running time but increases sucrose consumption; whereas this same compound has no effect in A2A KO mice (Correa et al. 2016). Further, haloperidol increased C-Fos immunoreactivity in the NAc core and anterior cingulate cortex in WT but not KO mice. In mice selectively bred for high wheel running (S) compared to controls, Fos immunoreactivity is greater in the striatum region, along with the lateral hypothalamus and medial frontal cortex, of the former group (Rhodes et al. 2003). Mice that overexpress DeltaFosB in striatal dynorphin-containing neurons show increase daily running compared with control siblings (Werme et al. 2002). Conversely, those that overexpress this protein in striatal encephalin-containing neurons use the running wheels less than controls. In Lewis rats, increased activity on the running wheels corresponds to increase expression of DeltaFosB in the NAc. Taken together, the net effect of DeltaFosB on voluntary activity seems to depend on which neurons are expressing this protein, and there may be a cyclic effect with increased activity upregulating DeltaFosB in neurons that stimulate this behavior. In female rats bred for high voluntary running, increased running distance appears to be regulated by endogenous mu-opioid receptor signaling in the NAc (Ruegsegger et al. 2015). However, it is a fine balance as agonism or antagonism of this receptor pathway diminishes this voluntary behavior. Epigenetic mechanisms within the brain might also impact PA initiation. MicroRNAs (miRs), including miR-466 and miR-342-5p, demonstrate differential expression in the NAc in high (C57L/J) vs. low active inbred (C3H/HeJ) mice with the first miR being downregulated and the second miR upregulated in the highly active mice (Dawes et al. 2015).
Hippocampus
Cannabinoid receptor 1 (CB1) receptors may modulate wheel-running behavior by stimulating hippocampal neurogenesis. Transgenic mice that are homozygous mutant for CB1 receptor travel less distance, spend less time, and show reduced velocity on voluntary wheels and hippocampal neurogenesis is reduced compared to wild-type mice (Dubreucq et al. 2010). However, six weeks of running increases neurogenesis in CB1 KO to comparable levels as controls, indicating CB1 regulates motivation to use the wheels, which could be due to disruptions in hippocampal neurogenesis, but these receptors are not involved in the opposite arm of the pathway, i.e. pro-neurogenic effects of voluntary activity.
Other studies further support a role for the hippocampus in regulating voluntary physical activity. In female mice (Mus domesticus) selectively bred for enhanced voluntary wheel running, several hippocampal transcripts are altered relative to those derived from random pairings (Bronikowski et al. 2004). Genes associated with immune function and neuronal signaling are suppressed in the latter; whereas, those regulating and transcription and translation and Dr2 and Dr4 are increased in this group. Male and female rats with lesions in the ventral hippocampus due to ibotenate (a neurotoxin and glutatmate receptor antagonist) injections exhibit increased spontaneous and amphetamine-induced locomotor activity, spend greater time the open arms of an elevated plus maze (suggestive of increased exploratory behavior), and correspondingly, potassium evoked l-glutamate from the ventral hippocampus is decreased (Beninger et al. 2009).
Hypothalamus
Voluntary wheel running activity of calorically restricted male Wistar rats compared to ad libitum fed rats suggests that a decrease in adiposity may increase PA by elevating hypothalamic Npy expression, as well as other potential candidate genes in this area, including Lepr, Insr, Ampk, and Sirt1 (Ruegsegger et al. 2016). Overexpression of leptin in F344 X Brown Norway (BN) rats increases voluntary wheel running (Matheny et al. 2009). In contrast, adenovirus-induced overexpression of a leptin receptor antagonist in this rat strain reduces wheel running, but increases circulating concentrations of leptin with resulting greater adiposity.
Amygdala
Pharmacological antagonism of the N-methyl-D-aspartate (NMDA) receptor in the central nucleus of the amygdala (CeA) and the posterior lateral striatum (PLS) reduces spontaneous motor activity in male Sprague-Dawley rats (Andrzejewski et al. 2004). Administration of N-methyl-D-aspartic acid into the basolateral nucleus of the amygdala results in a dose dependent suppression of spontaneous locomotor activity in male Wistar rats, which is reversed by treatment with L-glutamic acid diethyl ester (a glutamatergic antagonist) into the NAc (Yim and Mogenson 1989). Additionally, injection of dopamine into the NAc following amygdala stimulation with NMDA also mitigates the effects on locomotor activity. Bilateral injection of ibotenic acid to induce brain lesions in the nucleus basolateralis amygdalae increases exploratory activity in male Wistar rats (Ambrogi Lorenzini et al. 1991). Bilateral destruction of the medial amygdala in hooded rats results in an initial increase but a later decrease in spontaneous locomotor activity (Korczynski and Fonberg 1979). Voluntary physical activity levels are reduced during the 12 hour dark cycle in ovariectomized female Wistar rats, and these females correspondingly also show reduced serotonin (5-HT) and dopamine levels in the amygdala (Izumo et al. 2012). The data thus suggest that during the dark phase estrogen may increase voluntary physical activity by increasing 5-HT and dopamine within this brain region. It is also possible that estrogen affects the production, packaging, release, re-uptake, and/or degradation of these neurotransmitters in the amygdala and possibly other brain regions.
Other Brain Regions- Prefrontal Cortex, Cerebellum, Midbrain, and Locus Coeruleus in the Pons Region- Regulating Voluntary Physical Activity
Male rats with lesions in the prefrontal cortex have increased wheel running activity (Nonneman and Corwin 1981). As detailed above, suppression of medial prefrontal cortex function in male and female Sprague-Dawley reduces voluntary running activity (Basso and Morrell 2015). Mice bred to engage in increased voluntary physical activity (HR lines) possess larger mid-brain regions but exhibit no differences in the caudate-putamen, hippocampus, cerebellum, forebrain size relative to control mice lines (Kolb et al. 2013). Sexually dimorphic responses are observed in the HR lines with speed varying in females; whereas speed and time differ in HR males relative to control lines (Garland et al. 2011). Testing of obesity-resistant (OR) and Sprague-Dawley rats reveals the locus coerulus (LC) is a primary brain region modulating orexin-induced spontaneous PA in the former group of rats (Teske et al. 2013). Mice with lesions in the dorsal medial habenula (dMHB) exhibit compromised performance in motivational-based locomotor behaviors, including voluntary wheel running and the accelerated rotorod but show mild impairments in gait and balance and exhibit normal basal activity (Hsu and Wang 2014).
Discussion
The current rodent and human studies provide strong evidence that there are normal differences in PA between males and females. Most rodent studies suggest that under normal conditions, females are more active than males. This sex difference could be due to the fact that in most rodent species, females might need to be more active or exploratory in searching for distant food sources needed to provide sufficient energy for themselves and their offspring, i.e. the evolutionary concept that physical activity levels are tightly associated with food acquisition (Lightfoot 2013). Sexually dimorphic differences in this behavior might also be due to variation in sex hormones with increased estrogen and decreased testosterone concentrations in females (Lightfoot 2008). However, early exposure to varying environmental chemicals may suppress PA in one or both sexes. For instance, developmental exposure to BPA suppresses PA in female but not male California mice (Johnson et al. 2015); whereas, early exposure to Mn and dichlorovos reduces PA levels in males (Betharia and Maher 2012; Lazarini et al. 2004). Further studies in this area may reveal that there are complex interactions between genetic background and environmental factors, i.e. the Genes X Environment concept, that ultimately affect motivation to engage in PA, as has been suggested for other endocrine disruptor-induced behavioral deficits (Crews et al. 2014). Rodent studies also suggest that engagement in PA can yield beneficial effects in terms of reducing amount of time spent in deleterious behaviors, such as voluntary consumption of alcohol or drugs, but such effects vary between the sexes. Any conflicting rodent results discussed above may be due to test used to measure voluntary PA (voluntary running wheels, home-cage activity, and/or open field maze), rodent model examined, extrinsic or intrinsic factor that may affect this behavior, and age of the study subjects. To compare across studies, it is important that uniform conditions are employed as much as possible. Different behavioral tests designed to measure voluntary PA, including wheel-running, home-cage activity, and behaviors in an open field test may not always correlate with one another and could even lead to contradictory results (Careau et al. 2012; Copes et al. 2015; Garland et al. 2011).
It is more difficult to pinpoint individual factors that influence PA in humans, where motivation to engage in PA comes under strong social influences. It is possible that conspecifics, especially those of the same sex, may also affect voluntary PA in rodents, although to date, there does not appear to be any studies comparing spontaneous activity levels in socially-housed vs. isolated rodents. In humans, similar patterns across studies with children to elderly individuals emerge in terms of sex differences in PA levels and differential reasons why each sex remain active. At almost all ages studied, human males are more PA than females (Barnekow-Bergkvist et al. 1996; Butt et al. 2011; Deaner et al. 2012; Gardner and Montgomery 2008; Lee 2005; Marquez and McAuley 2006; Sirard et al. 2006; Sukys et al. 2014; Yli-Piipari et al. 2012). For human males, the motivators tend to be more intrinsic, such as improving health, preventing NCD, enhancing body shape, and being competitive (Beville et al. 2014; Butt et al. 2011; Caperchione et al. 2015; Cole and Maeda 2015; Egli et al. 2011; Jonason 2007; Marquez and McAuley 2006; Molanorouzi et al. 2015; Sirard et al. 2006; Yli-Piipari et al. 2012). In contrast, a combination of extrinsic and intrinsic factors, simultaneously listening to music, emotional support, social aspects, sense of well-being, and positive body image, appear to guide PA in females of varying age ranges.
Based on the fact that there are clear sex-differences in humans guiding motivation to engage in PA, this information should be used to help design sex-specific programs to encourage PA. For instance, the structure of a PA program for girls or women may emphasize the social aspects in terms of meeting others, providing a supportive environment, such as various background musical options, and may also include discussions of other measures, namely diet, that can be adapted to promote health and a better body image but without criticizing individual physical appearance. Such an approach is currently being tested with a community based intervention program titled: “Mothers in Motion, MIM” that is aimed at promoting increased PA, healthy eating habits, reducing stress, and preventing weight gain in low-income overweight and obese mothers with the notion that such a strategy may confer positive health benefits to the enrolled women and their children (Chang et al. 2014). In contrast, exercise programs stimulating PA for boys and men may be more competitive-driven and promote the body-shaping and health benefits of engaging in such activities.
To understand motivation to engage in PA or suppression of this behavior by various extrinsic factors, including environmental chemicals, it is also important though to understanding the neurological mechanisms underpinning such behaviors. Rodents models, such as transgenic mice, those with pharmacologically-induced neural lesions, and those selectively bred to engage in high or low amount of wheel running or other PA, have helped elucidate the brain regions and molecular mechanisms regulating PA. Because of the strong conservation in brain structure and physiology between rodents and humans (Howdeshell 2002; Rice and Barone 2000), the same brain areas likely facilitate PA in humans. The brain regions in rodents that are essential in modulating this behavior include the striatum, NAc, hypothalamus, amygdala, hippocampus, prefrontal cortex, locus coeruleus, cerebellum, and pons (Ambrogi Lorenzini et al. 1991; Andrzejewski et al. 2004; Basso and Morrell 2015; Beninger et al. 2009; Bronikowski et al. 2004; Cahill et al. 2015; Dubreucq et al. 2010; Kolb et al. 2013; Korczynski and Fonberg 1979; Monroe et al. 2014; Nonneman and Corwin 1981; Rhodes et al. 2003; Roberts et al. 2014; Ruegsegger et al. 2016; Ruegsegger et al. 2015; Tarr et al. 2004; Teske et al. 2013; Werme et al. 2002; Yim and Mogenson 1989). The currently known identified genes and pathways in the brain that are particularly important in stimulating or suppressing this behavioral response include Fos/DeltaFosB (Correa et al. 2016; Rhodes et al. 2003; Werme et al. 2002), CB1 receptors (Dubreucq et al. 2010), NMDA receptor (Andrzejewski et al. 2004; Yim and Mogenson 1989), dopaminergic (Monroe et al. 2014; Roberts et al. 2013; Roberts et al. 2012; Waters et al. 2013; Yang et al. 2012)(Park et al. Physiology & Behavior, Resubmitted)(Correa et al. 2016), opioid (Ruegsegger et al. 2015), and leptin (Matheny et al. 2009; Ruegsegger et al. 2016) signaling pathways.
In females, estrogen acting via estrogen receptor alpha (ESR1) or estrogen receptor beta (ESR2) is a primary regulator of dopamine transport and signaling in various brain regions, including the NAc (Almey et al. 2015; Chavez et al. 2010; Febo et al. 2003; Hruska and Pitman 1982; Kurling-Kailanto et al. 2010; Le Saux et al. 2006; Menniti and Baum 1981; Quinlan et al. 2013; Tobiansky et al. 2016; Uban et al. 2012). Under normal physiological conditions, estrogen stimulates dopamine release and signaling pathways in the striatum and NAc of females but not males (Becker 1999). This estrogenic response on these brain regions has been postulated to stimulate reproductive success by increasing female pacing behavior. However, administration of estrogen to adult castrated male rats increases locomotor activity (Menniti and Baum 1981).
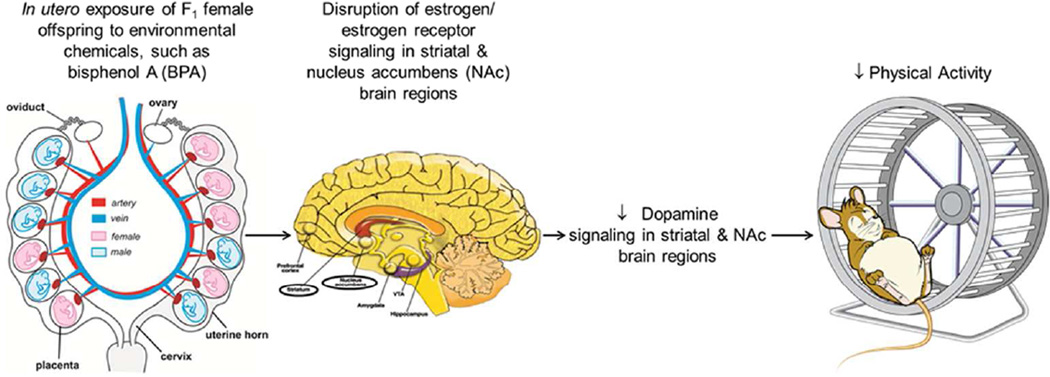
In females, disruption of estrogen through pharmacological inhibition or ovariectomy, results in dopamine-dependent memory deficits (Quinlan et al. 2013) and altered response to psychostimulants and other pleasure seeking behaviors (Febo et al. 2003; Tobiansky et al. 2016; Zhou et al. 2002). Voluntary physical activity or exercise may be considered a hedonistic behavior, as it is stimulates endorphins and other endogenous opioids (Schwarz and Kindermann 1992; Sforzo 1989). Based on these previous findings, it may be hypothesized that anything that disturbs estrogen or estrogen/estrogen receptor signaling pathways in females might suppress dopamine signaling and result in physical inactivity disorders, as illustrated in Fig. 2. The model might explain why developmental exposure to BPA suppressed voluntary PA in female but not male California mice (Johnson et al. 2015). This plausible mechanism might also account for observed sex differences in general PA levels that can be influenced by stage of the estrous or menstrual cycle in females. If this pathway to promote PA is unique to females, then the focus of future studies should be directed at examining for pathways that are unique to males.
Fig. 2.
Diagrammatic illustration of how developmental exposure to endocrine disrupting chemicals, such as BPA, may lead to later physical inactivity in F1 female offspring. The brain diagram is from http://pubs.niaaa.nih.gov/publications/arh314/310-339.htm.
In conclusion, a better understanding the multitude of extrinsic and intrinsic factors that stimulate PA in rodent models and humans is essential in preventing and treating the various NCD, namely metabolic disorders, that have risen dramatically in the past few decades. Sub-optimal perinatal environments, including those where the offspring is exposed to environmental chemicals can result in later voluntary PA disruptions that may affect one or both sexes. It is also clear from childhood through adulthood, human males and females engage in PA for varying reasons, and thus, any environmental or pharmacological intervention strategies to encourage exercise activities need to be tailored for each sex.
Significance Statement.
Obesity and the associated health costs in treating such individuals has become an enormous health concern in the US and other countries. Rates of obesity and diabetes are also rising in children. These diseases may be prevented by eating a healthy diet and remaining physically active. However, more children and adults of both sexes are exercising less and instead leading a sedentary lifestyle, which may be a primary contributor to these diseases. By understanding what motivates human males and females of various ages to engage in physical activity could open up new avenues to prevent and treat these metabolic disorders.
Acknowledgments
The author is grateful to Sarah A. Johnson, Michele Painter, Angela B. Javurek, Mark R. Ellersieck, Charles E. Wiedmeyer, and John P. Thyfault for their assistance with some of the work described herein.
Support: The studies were supported by NIH Grant 5R21ES023150 to Cheryl S. Rosenfeld.
Role of Author
The author takes responsibility for researching, summarizing, and accuracy in reporting the previously published peer-reviewed data: CSR. Drafting of the manuscript: CSR. Critical revision of the manuscript for important intellectual content: CSR. Responding to reviewer’s comments: CSR.
Footnotes
Conflict of Interest Statement
The author declares no conflict of interest.
References
- Almey A, Milner TA, Brake WG. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav. 2015;74:125–138. doi: 10.1016/j.yhbeh.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrogi Lorenzini C, Baldi E, Bucherelli C, Giachetti A, Tassoni G. Effects of nucleus basolateralis amygdalae neurotoxic lesions on some spontaneous activities in the rat. Physiol Behav. 1991;50(6):1215–1219. doi: 10.1016/0031-9384(91)90585-c. [DOI] [PubMed] [Google Scholar]
- Andrzejewski ME, Sadeghian K, Kelley AE. Central amygdalar and dorsal striatal NMDA receptor involvement in instrumental learning and spontaneous behavior. Behav Neurosci. 2004;118(4):715–729. doi: 10.1037/0735-7044.118.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnekow-Bergkvist M, Hedberg G, Janlert U, Jansson E. Physical activity pattern in men and women at the ages of 16 and 34 and development of physical activity from adolescence to adulthood. Scand J Med Sci Sports. 1996;6(6):359–370. doi: 10.1111/j.1600-0838.1996.tb00108.x. [DOI] [PubMed] [Google Scholar]
- Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health. 2012;11:42. doi: 10.1186/1476-069X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso JC, Morrell JI. The medial prefrontal cortex and nucleus accumbens mediate the motivation for voluntary wheel running in the rat. Behav Neurosci. 2015;129(4):457–472. doi: 10.1037/bne0000070. [DOI] [PubMed] [Google Scholar]
- Bauer UE, Briss PA, Goodman RA, Bowman BA. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384(9937):45–52. doi: 10.1016/S0140-6736(14)60648-6. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64(4):803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Belviranli M, Atalik KE, Okudan N, Gokbel H. Age and sex affect spatial and emotional behaviors in rats: the role of repeated elevated plus maze test. Neuroscience. 2012;227:1–9. doi: 10.1016/j.neuroscience.2012.09.036. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Tuerke KJ, Forsyth JK, Giles A, Xue L, Boegman RJ, Jhamandas K. Neonatal ventral hippocampal lesions in male and female rats: effects on water maze, locomotor activity, plus-maze and prefrontal cortical GABA and glutamate release in adulthood. Behav Brain Res. 2009;202(2):198–209. doi: 10.1016/j.bbr.2009.03.044. [DOI] [PubMed] [Google Scholar]
- Betharia S, Maher TJ. Neurobehavioral effects of lead and manganese individually and in combination in developmentally exposed rats. Neurotoxicology. 2012;33(5):1117–1127. doi: 10.1016/j.neuro.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Beville JM, Meyer MR, Usdan SL, Turner LW, Jackson JC, Lian BE. Gender differences in college leisure time physical activity: application of the theory of planned behavior and integrated behavioral model. J Am Coll Health. 2014;62(3):173–184. doi: 10.1080/07448481.2013.872648. [DOI] [PubMed] [Google Scholar]
- Blizard DA, Lippman HR, Chen JJ. Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiol Behav. 1975;14(5):601–608. doi: 10.1016/0031-9384(75)90188-2. [DOI] [PubMed] [Google Scholar]
- Booth FW, Laye MJ, Lees SJ, Rector RS, Thyfault JP. Reduced physical activity and risk of chronic disease: the biology behind the consequences. Eur J Appl Physiol. 2008;102(4):381–390. doi: 10.1007/s00421-007-0606-5. [DOI] [PubMed] [Google Scholar]
- Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Comprehensive Physiology. 2012;2(2):1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Lee AW, Meaney MJ, Brown RE. Effect of neonatal handling and paternal care on offspring cognitive development in the monogamous California mouse (Peromyscus californicus) Hormones and Behavior. 2004;46(1):30–38. doi: 10.1016/j.yhbeh.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Bronikowski AM, Rhodes JS, Garland T, Jr, Prolla TA, Awad TA, Gammie SC. The evolution of gene expression in mouse hippocampus in response to selective breeding for increased locomotor activity. Evolution. 2004;58(9):2079–2086. doi: 10.1111/j.0014-3820.2004.tb00491.x. [DOI] [PubMed] [Google Scholar]
- Brownson RC, Boehmer TK, Luke DA. Declining rates of physical activity in the United States: what are the contributors? Annu Rev Public Health. 2005;26:421–443. doi: 10.1146/annurev.publhealth.26.021304.144437. [DOI] [PubMed] [Google Scholar]
- Butt J, Weinberg RS, Breckon JD, Claytor RP. Adolescent physical activity participation and motivational determinants across gender, age, and race. Journal of physical activity & health. 2011;8(8):1074–1083. doi: 10.1123/jpah.8.8.1074. [DOI] [PubMed] [Google Scholar]
- Cahill LS, Steadman PE, Jones CE, Laliberte CL, Dazai J, Lerch JP, Stefanovic B, Sled JG. MRI-detectable changes in mouse brain structure induced by voluntary exercise. Neuroimage. 2015;113:175–183. doi: 10.1016/j.neuroimage.2015.03.036. [DOI] [PubMed] [Google Scholar]
- Caperchione CM, Chau S, Walker GJ, Mummery WK, Jennings C. Gender-Associated Perceptions of Barriers and Motivators to Physical Activity Participation in South Asian Punjabis Living in Western Canada. Journal of physical activity & health. 2015;12(5):686–693. doi: 10.1123/jpah.2013-0208. [DOI] [PubMed] [Google Scholar]
- Careau V, Bininda-Emonds OR, Ordonez G, Garland T., Jr Are voluntary wheel running and open-field behavior correlated in mice? Different answers from comparative and artificial selection approaches. Behav Genet. 2012;42(5):830–844. doi: 10.1007/s10519-012-9543-0. [DOI] [PubMed] [Google Scholar]
- Chang MW, Nitzke S, Brown R, Resnicow K. A community based prevention of weight gain intervention (Mothers In Motion) among young low-income overweight and obese mothers: design and rationale. BMC public health. 2014;14:280. doi: 10.1186/1471-2458-14-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez C, Hollaus M, Scarr E, Pavey G, Gogos A, van den Buuse M. The effect of estrogen on dopamine and serotonin receptor and transporter levels in the brain: an autoradiography study. Brain Res. 2010;1321:51–59. doi: 10.1016/j.brainres.2009.12.093. [DOI] [PubMed] [Google Scholar]
- Clemmensen C, Pehmoller C, Klein AB, Ratner C, Wojtaszewski JF, Brauner-Osborne H. Enhanced voluntary wheel running in GPRC6A receptor knockout mice. Physiol Behav. 2013;118:144–151. doi: 10.1016/j.physbeh.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Cole Z, Maeda H. EFFECTS OF LISTENING TO PREFERENTIAL MUSIC ON SEX DIFFERENCES IN ENDURANCE RUNNING PERFORMANCE. Percept Mot Skills. 2015;121(2):390–398. doi: 10.2466/06.PMS.121c20x9. [DOI] [PubMed] [Google Scholar]
- Copes LE, Schutz H, Dlugosz EM, Acosta W, Chappell MA, Garland T., Jr Effects of voluntary exercise on spontaneous physical activity and food consumption in mice: Results from an artificial selection experiment. Physiol Behav. 2015;149:86–94. doi: 10.1016/j.physbeh.2015.05.025. [DOI] [PubMed] [Google Scholar]
- Correa M, Pardo M, Bayarri P, Lopez-Cruz L, San Miguel N, Valverde O, Ledent C, Salamone JD. Choosing voluntary exercise over sucrose consumption depends upon dopamine transmission: effects of haloperidol in wild type and adenosine A(2)AKO mice. Psychopharmacology (Berl) 2016;233(3):393–404. doi: 10.1007/s00213-015-4127-3. [DOI] [PubMed] [Google Scholar]
- Crews D, Gillette R, Miller-Crews I, Gore AC, Skinner MK. Nature, nurture and epigenetics. Mol Cell Endocrinol. 2014;398(1–2):42–52. doi: 10.1016/j.mce.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes M, Kochan KJ, Riggs PK, Timothy Lightfoot J. Differential miRNA expression in inherently high- and low-active inbred mice. Physiological reports. 2015;3(7) doi: 10.14814/phy2.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaner RO, Geary DC, Puts DA, Ham SA, Kruger J, Fles E, Winegard B, Grandis T. A sex difference in the predisposition for physical competition: males play sports much more than females even in the contemporary U.S. PLoS One. 2012;7(11):e49168. doi: 10.1371/journal.pone.0049168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neurobiology of exercise. Obesity (Silver Spring) 2006;14(3):345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Dubreucq S, Koehl M, Abrous DN, Marsicano G, Chaouloff F. CB1 receptor deficiency decreases wheel-running activity: consequences on emotional behaviours and hippocampal neurogenesis. Exp Neurol. 2010;224(1):106–113. doi: 10.1016/j.expneurol.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Dudley D. Contributions of paternal care to the growth and development of the young in Peromyscus californicus. Behav Biol. 1974a;11(2):155–166. doi: 10.1016/s0091-6773(74)90305-8. [DOI] [PubMed] [Google Scholar]
- Dudley D. Paternal behavior in the California mouse, Peromyscus californicus. Behav Biol. 1974b;11(2):247–252. doi: 10.1016/s0091-6773(74)90433-7. [DOI] [PubMed] [Google Scholar]
- Egli T, Bland HW, Melton BF, Czech DR. Influence of age, sex, and race on college students’ exercise motivation of physical activity. J Am Coll Health. 2011;59(5):399–406. doi: 10.1080/07448481.2010.513074. [DOI] [PubMed] [Google Scholar]
- Ehringer MA, Hoft NR, Zunhammer M. Reduced alcohol consumption in mice with access to a running wheel. Alcohol. 2009;43(6):443–452. doi: 10.1016/j.alcohol.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Farabollini F, Porrini S, Dessi-Fulgheri F. Perinatal exposure to the estrogenic pollutant bisphenol A affects behavior in male and female rats. Pharmacol Biochem Behav. 1999;64:687–694. doi: 10.1016/s0091-3057(99)00136-7. [DOI] [PubMed] [Google Scholar]
- Febo M, Gonzalez-Rodriguez LA, Capo-Ramos DE, Gonzalez-Segarra NY, Segarra AC. Estrogen-dependent alterations in D2/D3-induced G protein activation in cocaine-sensitized female rats. J Neurochem. 2003;86(2):405–412. doi: 10.1046/j.1471-4159.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- Ferrand C, Perrin C, Nasarre S. Motives for regular physical activity in women and men: a qualitative study in French adults with type 2 diabetes, belonging to a patients’ association. Health & social care in the community. 2008;16(5):511–520. doi: 10.1111/j.1365-2524.2008.00773.x. [DOI] [PubMed] [Google Scholar]
- Garcia LM, da LightfootSilva KS, Del Duca GF, da Costa FF, Nahas MV. Sedentary Behaviors, Leisure-Time Physical Inactivity, and Chronic Diseases in Brazilian Workers: A Cross Sectional Study. Journal of physical activity & health. 2014 doi: 10.1123/jpah.2012-0423. [DOI] [PubMed] [Google Scholar]
- Gardner AW, Montgomery PS. DIFFERENCES IN EXERCISE PERFORMANCE AND LEISURE-TIME PHYSICAL ACTIVITY IN OLDER CAUCASIANS AND AFRICAN-AMERICANS. Clinical medicine Geriatrics. 2008;1:1–7. doi: 10.4137/cmger.s664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T, Jr, Kelly SA, Malisch JL, Kolb EM, Hannon RM, Keeney BK, Van Cleave SL, Middleton KM. How to run far: multiple solutions and sex-specific responses to selective breeding for high voluntary activity levels. Proc Biol Sci. 2011;278(1705):574–581. doi: 10.1098/rspb.2010.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol. 2007;19(1):1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- Goedecke JH, Micklesfield LK. The effect of exercise on obesity, body fat distribution and risk for type 2 diabetes. Medicine and sport science. 2014;60:82–93. doi: 10.1159/000357338. [DOI] [PubMed] [Google Scholar]
- Gray CE, Larouche R, Barnes JD, Colley RC, Bonne JC, Arthur M, Cameron C, Chaput JP, Faulkner G, Janssen I, Kolen AM, Manske SR, Salmon A, Spence JC, Timmons BW, Tremblay MS. Are we driving our kids to unhealthy habits? Results of the active healthy kids Canada 2013 report card on physical activity for children and youth. International journal of environmental research and public health. 2014;11(6):6009–6020. doi: 10.3390/ijerph110606009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubernick DJ, Alberts JR. The biparental care system of the California mouse, Peromyscus californicus. J Comp Psychol. 1987;101(2):169–177. [PubMed] [Google Scholar]
- Guidotti S, Meyer N, Przybyt E, Scheurink AJ, Harmsen MC, Garland T, Jr, van Dijk G. Diet-induced obesity resistance of adult female mice selectively bred for increased wheel-running behavior is reversed by single perinatal exposure to a high-energy diet. Physiol Behav. 2016;157:246–257. doi: 10.1016/j.physbeh.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Hannon RM, Meek TH, Acosta W, Maciel RC, Schutz H, Garland T., Jr Sex-specific heterosis in line crosses of mice selectively bred for high locomotor activity. Behav Genet. 2011;41(4):615–624. doi: 10.1007/s10519-010-9432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M, Gluckman P. Developmental origins of noncommunicable disease: population and public health implications. Am J Clin Nutr. 2011;94(6 Suppl):1754s–1758s. doi: 10.3945/ajcn.110.001206. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL. A model of the development of the brain as a construct of the thyroid system. Environ Health Perspect. 2002;110(Suppl 3):337–348. doi: 10.1289/ehp.02110s3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska RE, Pitman KT. Distribution and localization of estrogen-sensitive dopamine receptors in the rat brain. J Neurochem. 1982;39(5):1418–1423. doi: 10.1111/j.1471-4159.1982.tb12586.x. [DOI] [PubMed] [Google Scholar]
- Hsu YW, Wang SD. Role of the dorsal medial habenula in the regulation of voluntary activity, motor function, hedonic state, and primary reinforcement. 2014;34(34):11366–11384. doi: 10.1523/JNEUROSCI.1861-14.2014. http://www.cdc.gov/obesity/data/adult.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo N, Ishibashi Y, Ohba M, Morikawa T, Manabe T. Decreased voluntary activity and amygdala levels of serotonin and dopamine in ovariectomized rats. Behav Brain Res. 2012;227(1):1–6. doi: 10.1016/j.bbr.2011.10.031. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Painter MS, Javurek AB, Ellersieck MR, Wiedmeyer CE, Thyfault JP, Rosenfeld CS. Sex-dependent effects of developmental exposure to bisphenol A and ethinyl estradiol on metabolic parameters and voluntary physical activity. Journal of developmental origins of health and disease. 2015:1–14. doi: 10.1017/S2040174415001488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonason PK. An evolutionary psychology perspective on sex differences in exercise behaviors and motivations. J Soc Psychol. 2007;147(1):5–14. doi: 10.3200/SOCP.147.1.5-14. [DOI] [PubMed] [Google Scholar]
- Jones LC, Bellingham WP, Ward LC. Sex differences in voluntary locomotor activity of food-restricted and ad libitum-fed rats. Implications for the maintenance of a body weight set-point. Comparative biochemistry and physiology A, Comparative physiology. 1990;96(2):287–290. doi: 10.1016/0300-9629(90)90694-n. [DOI] [PubMed] [Google Scholar]
- Knight JA. Physical inactivity: associated diseases and disorders. Ann Clin Lab Sci. 2012;42(3):320–337. [PubMed] [Google Scholar]
- Kolb EM, Rezende EL, Holness L, Radtke A, Lee SK, Obenaus A, Garland T., Jr Mice selectively bred for high voluntary wheel running have larger midbrains: support for the mosaic model of brain evolution. J Exp Biol. 2013;216(Pt 3):515–523. doi: 10.1242/jeb.076000. [DOI] [PubMed] [Google Scholar]
- Konhilas JP, Chen H, Luczak E, McKee LA, Regan J, Watson PA, Stauffer BL, Khalpey ZI, McKinsey TA, Horn T, LaFleur B, Leinwand LA. Diet and sex modify exercise and cardiac adaptation in the mouse. American journal of physiology Heart and circulatory physiology. 2015;308(2):H135–H145. doi: 10.1152/ajpheart.00532.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korczynski R, Fonberg E. Spontaneous locomotor activity and food and water intake in rats with medial amygdala lesions. Acta Neurobiol Exp (Wars) 1979;39(4):227–240. [PubMed] [Google Scholar]
- Krugner-Higby L, Shadoan M, Carlson C, Gendron A, Cofta P, Marler C, Wagner J. Type 2 diabetes mellitus, hyperlipidemia, and extremity lesions in California mice (Peromyscus californicus) fed commercial mouse diets. Comp Med. 2000;50(4):412–418. [PubMed] [Google Scholar]
- Kuh DJ, Cooper C. Physical activity at 36 years: patterns and childhood predictors in a longitudinal study. J Epidemiol Community Health. 1992;46(2):114–119. doi: 10.1136/jech.46.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurling-Kailanto S, Kankaanpaa A, Hautaniemi J, Seppala T. Blockade of androgen or estrogen receptors reduces nandrolone’s ability to modulate acute reward-related neurochemical effects of amphetamine in rat brain. Pharmacol Biochem Behav. 2010;95(4):422–427. doi: 10.1016/j.pbb.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Lazarini CA, Lima RY, Guedes AP, Bernardi MM. Prenatal exposure to dichlorvos: physical and behavioral effects on rat offspring. Neurotoxicol Teratol. 2004;26(4):607–614. doi: 10.1016/j.ntt.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Morissette M, Di Paolo T. ERbeta mediates the estradiol increase of D2 receptors in rat striatum and nucleus accumbens. Neuropharmacology. 2006;50(4):451–457. doi: 10.1016/j.neuropharm.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Lee YS. Gender differences in physical activity and walking among older adults. J Women Aging. 2005;17(1–2):55–70. doi: 10.1300/J074v17n01_05. [DOI] [PubMed] [Google Scholar]
- Lightfoot JT. Sex hormones’ regulation of rodent physical activity: a review. International journal of biological sciences. 2008;4(3):126–132. doi: 10.7150/ijbs.4.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoot JT. Why control activity? Evolutionary selection pressures affecting the development of physical activity genetic and biological regulation. BioMed research international. 2013;2013:821678. doi: 10.1155/2013/821678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez DX, McAuley E. Gender and acculturation influences on physical activity in Latino adults. Ann Behav Med. 2006;31(2):138–144. doi: 10.1207/s15324796abm3102_5. [DOI] [PubMed] [Google Scholar]
- Matheny M, Zhang Y, Shapiro A, Tumer N, Scarpace PJ. Central overexpression of leptin antagonist reduces wheel running and underscores importance of endogenous leptin receptor activity in energy homeostasis. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1254–R1261. doi: 10.1152/ajpregu.90449.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menniti FS, Baum MJ. Differential effects of estrogen and androgen on locomotor activity induced in castrated male rats by amphetamine, a novel environment, or apomorphine. Brain Res. 1981;216(1):89–107. doi: 10.1016/0006-8993(81)91280-4. [DOI] [PubMed] [Google Scholar]
- Molanorouzi K, Khoo S, Morris T. Motives for adult participation in physical activity: type of activity, age, and gender. BMC public health. 2015;15:66. doi: 10.1186/s12889-015-1429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe DC, Holmes PV, Koch LG, Britton SL, Dishman RK. Striatal enkephalinergic differences in rats selectively bred for intrinsic running capacity. Brain Res. 2014;1572:11–17. doi: 10.1016/j.brainres.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonneman AJ, Corwin JV. Differential effects of prefrontal cortex ablation in neonatal, juvenile, and young adult rats. J Comp Physiol Psychol. 1981;95(4):588–602. doi: 10.1037/h0077800. [DOI] [PubMed] [Google Scholar]
- Peterson AB, Hivick DP, Lynch WJ. Dose-dependent effectiveness of wheel running to attenuate cocaine-seeking: impact of sex and estrous cycle in rats. Psychopharmacology (Berl) 2014;231(13):2661–2670. doi: 10.1007/s00213-014-3437-1. [DOI] [PubMed] [Google Scholar]
- Quinlan MG, Almey A, Caissie M, LaChappelle I, Radiotis G, Brake WG. Estradiol and striatal dopamine receptor antagonism influence memory system bias in the female rat. Neurobiol Learn Mem. 2013;106:221–229. doi: 10.1016/j.nlm.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Gammie SC, Garland T., Jr Neurobiology of Mice Selected for High Voluntary Wheel-running Activity. Integrative and comparative biology. 2005;45(3):438–455. doi: 10.1093/icb/45.3.438. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Garland T, Jr, Gammie SC. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav Neurosci. 2003;117(6):1243–1256. doi: 10.1037/0735-7044.117.6.1243. [DOI] [PubMed] [Google Scholar]
- Ribble DOaSM. Social organization and nest co-ocuppancy in Peromyscus californicus, a monogamous rodent. Behav Ecol Sociobiol. 1990;26:9–15. [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108 Suppl. 2000;3:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MD, Brown Jd Fau - Company JM, Company Jm Fau - Oberle LP, Oberle Lp Fau - Heese AJ, Heese Aj Fau - Toedebusch RG, Toedebusch Rg Fau - Wells KD, Wells Kd Fau - Cruthirds CL, Cruthirds Cl Fau - Knouse JA, Knouse Ja Fau - Ferreira JA, Ferreira Ja Fau - Childs TE, Childs Te Fau - Brown M, Brown M Fau - Booth FW, Booth FW. 2013. Phenotypic and molecular differences between rats selectively bred to voluntarily run high vs. low nightly distances. (1522-1490 (Electronic)).
- Roberts MD, Gilpin L, Parker KE, Childs TE, Will MJ, Booth FW. Dopamine D1 receptor modulation in nucleus accumbens lowers voluntary wheel running in rats bred to run high distances. Physiol Behav. 2012;105(3):661–668. doi: 10.1016/j.physbeh.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Roberts MD, Toedebusch RG, Wells KD, Company JM, Brown JD, Cruthirds CL, Heese AJ, Zhu C, Rottinghaus GE, Childs TE, Booth FW. Nucleus accumbens neuronal maturation differences in young rats bred for low versus high voluntary running behaviour. J Physiol. 2014;592(Pt 10):2119–2135. doi: 10.1113/jphysiol.2013.268805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegsegger GN, Speichinger KR, Manier JB, Younger KM, Childs TE, Booth FW. Hypothalamic Npy mRNA is correlated with increased wheel running and decreased body fat in calorie-restricted rats. Neurosci Lett. 2016;618:83–88. doi: 10.1016/j.neulet.2016.02.037. [DOI] [PubMed] [Google Scholar]
- Ruegsegger GN, Toedebusch RG, Will MJ, Booth FW. Mu opioid receptor modulation in the nucleus accumbens lowers voluntary wheel running in rats bred for high running motivation. Neuropharmacology. 2015;97:171–181. doi: 10.1016/j.neuropharm.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Pardo M, Yohn SE, Lopez-Cruz L, SanMiguel N, Correa M. Mesolimbic Dopamine and the Regulation of Motivated Behavior. Current topics in behavioral neurosciences. 2016;27:231–257. doi: 10.1007/7854_2015_383. [DOI] [PubMed] [Google Scholar]
- Schottenfeld D, Beebe-Dimmer JL, Buffler PA, Omenn GS. Current perspective on the global and United States cancer burden attributable to lifestyle and environmental risk factors. Annu Rev Public Health. 2013;34:97–117. doi: 10.1146/annurev-publhealth-031912-114350. [DOI] [PubMed] [Google Scholar]
- Schwarz L, Kindermann W. Changes in beta-endorphin levels in response to aerobic and anaerobic exercise. Sports Med. 1992;13(1):25–36. doi: 10.2165/00007256-199213010-00003. [DOI] [PubMed] [Google Scholar]
- Scully T. Diabetes in numbers. Nature. 2012;485(7398):S2–S3. doi: 10.1038/485s2a. [DOI] [PubMed] [Google Scholar]
- Seabra AC, Seabra AF, Mendonca DM, Brustad R, Maia JA, Fonseca AM, Malina RM. Psychosocial correlates of physical activity in school children aged 8–10 years. European journal of public health. 2013;23(5):794–798. doi: 10.1093/eurpub/cks149. [DOI] [PubMed] [Google Scholar]
- Sforzo GA. Opioids and exercise. An update. Sports Med. 1989;7(2):109–124. doi: 10.2165/00007256-198907020-00003. [DOI] [PubMed] [Google Scholar]
- Simmel EC, Haber SB, Harshfield G. Age, sex and genotype effects on stimulus exploration and locomotor activity in young mice. Exp Aging Res. 1976;2(3):253–269. doi: 10.1080/03610737608257180. [DOI] [PubMed] [Google Scholar]
- Sirard JR, Pfeiffer KA, Pate RR. Motivational factors associated with sports program participation in middle school students. J Adolesc Health. 2006;38(6):696–703. doi: 10.1016/j.jadohealth.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Spyker JM, Sparber SB, Goldberg AM. Subtle consequences of methylmercury exposure: behavioral deviations in offspring of treated mothers. Science. 1972;177(4049):621–623. doi: 10.1126/science.177.4049.621. [DOI] [PubMed] [Google Scholar]
- Stickel L. Home range and travels. In: King J, editor. Biology of Peromyscus (Rodentia) Stillwater: American Society of Mammalogists; 1968. pp. 373–411. [Google Scholar]
- Sukys S, Majauskiene D, Cesnaitiene VJ, Karanauskiene D. Do parents’ exercise habits predict 13–18-year-old adolescents’ involvement in sport? Journal of sports science & medicine. 2014;13(3):522–528. [PMC free article] [PubMed] [Google Scholar]
- Tarr BA, Kellaway LA, St Clair Gibson A, Russell VA. Voluntary running distance is negatively correlated with striatal dopamine release in untrained rats. Behav Brain Res. 2004;154(2):493–499. doi: 10.1016/j.bbr.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Teske JA, Perez-Leighton CE, Billington CJ, Kotz CM. Role of the locus coeruleus in enhanced orexin A-induced spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2013;305(11):R1337–R1345. doi: 10.1152/ajpregu.00229.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiansky DJ, Will RG, Lominac KD, Turner JM, Hattori T, Krishnan K, Martz JR, Nutsch VL, Dominguez JM. Estradiol in the Preoptic Area Regulates the Dopaminergic Response to Cocaine in the Nucleus Accumbens. Neuropsychopharmacology. 2016;41(7):1897–1906. doi: 10.1038/npp.2015.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuschl K, Mills PB, Clayton PT. Manganese and the brain. Int Rev Neurobiol. 2013;110:277–312. doi: 10.1016/B978-0-12-410502-7.00013-2. [DOI] [PubMed] [Google Scholar]
- Uban KA, Rummel J, Floresco SB, Galea LA. Estradiol modulates effort-based decision making in female rats. Neuropsychopharmacology. 2012;37(2):390–401. doi: 10.1038/npp.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deutekom AW, Chinapaw MJ, Vrijkotte TG, Gemke RJ. Study protocol: the relation of birth weight and infant growth trajectories with physical fitness, physical activity and sedentary behavior at 8–9 years of age - the ABCD study. BMC pediatrics. 2013;13:102. doi: 10.1186/1471-2431-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PW. Inactivity, not gluttony, causes obesity. BMJ. 2014;348:g2717. doi: 10.1136/bmj.g2717. [DOI] [PubMed] [Google Scholar]
- Waters RP, Pringle RB, Forster GL, Renner KJ, Malisch JL, Garland T, Jr, Swallow JG. Selection for increased voluntary wheel-running affects behavior and brain monoamines in mice. Brain Res. 2013;1508:9–22. doi: 10.1016/j.brainres.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler EJ, Brene S. Delta FosB regulates wheel running. J Neurosci. 2002;22(18):8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Jasarevic E, Vandas GM, Warzak DA, Geary DC, Ellersieck MR, Roberts RM, Rosenfeld CS. Effects of developmental bisphenol A exposure on reproductive-related behaviors in California mice (Peromyscus californicus): A monogamous animal model. PLoS ONE. 2013;8(2):e55698. doi: 10.1371/journal.pone.0055698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaura K, Bi Y, Ishiwatari M, Oishi N, Fukata H, Ueno K. Sex differences in stress reactivity of hippocampal BDNF in mice are associated with the female preponderance of decreased locomotor activity in response to restraint stress. Zoolog Sci. 2013;30(12):1019–1024. doi: 10.2108/zsj.30.1019. [DOI] [PubMed] [Google Scholar]
- Yang HS, Shimomura K, Vitaterna MH, Turek FW. High-resolution mapping of a novel genetic locus regulating voluntary physical activity in mice. Genes Brain Behav. 2012;11(1):113–124. doi: 10.1111/j.1601-183X.2011.00737.x. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Low doses of accumbens dopamine modulate amygdala suppression of spontaneous exploratory activity in rats. Brain Res. 1989;477(1–2):202–210. doi: 10.1016/0006-8993(89)91408-x. [DOI] [PubMed] [Google Scholar]
- Yli-Piipari S, Leskinen E, Jaakkola T, Liukkonen J. Predictive role of physical education motivation: the developmental trajectories of physical activity during grades 7–9. Res Q Exerc Sport. 2012;83(4):560–569. doi: 10.1080/02701367.2012.10599253. [DOI] [PubMed] [Google Scholar]
- Zhou W, Cunningham KA, Thomas ML. Estrogen regulation of gene expression in the brain: a possible mechanism altering the response to psychostimulants in female rats. Brain Res Mol Brain Res. 2002;100(1–2):75–83. doi: 10.1016/s0169-328x(02)00134-1. [DOI] [PubMed] [Google Scholar]
- Ziviani J, Wadley D, Ward H, Macdonald D, Jenkins D, Rodger S. A place to play: socioeconomic and spatial factors in children’s physical activity. Australian occupational therapy journal. 2008;55(1):2–11. doi: 10.1111/j.1440-1630.2006.00646.x. [DOI] [PubMed] [Google Scholar]