Abstract
Sex hormones act throughout the entire brain of both males and females via both genomic and non-genomic receptors. Sex hormones can act through many cellular and molecular processes that alter structure and function of neural systems and influence behavior as well as providing neuroprotection. Within neurons, sex hormone receptors are found in nuclei and are also located near membranes where they are associated with presynaptic terminals, mitochondria, spine apparatus, post-synaptic densities. Sex hormone receptors also are found in glial cells. Hormonal regulation of a variety of signaling pathways as well as direct and indirect effects upon gene expression induce spine synapses, up- or down-regulate and alter the distribution of neurotransmitter receptors, regulate neuropeptide expression and cholinergic and GABAergic activity as well as calcium sequestration and oxidative stress. Many neural and behavioral functions are affected, including mood, cognitive function, blood pressure regulation, motor coordination, pain and opioid sensitivity. Subtle sex differences exist for many of these functions that are developmentally programmed by hormones and by not-yet-precisely-defined genetic factors including the mitochondrial genome. These sex differences and responses to sex hormones in brain regions, and upon functions not previously regarded as subject to such differences, indicates that we are entering a new era of our ability to understand and appreciate the diversity of gender-related behaviors and brain functions.
Keywords: estrogens, hippocampus, prefrontal cortex, cardiovascular, cerebellum, stress
Introduction
In an article in Science in 1964, entitled “Hormones and Sexual Behavior”, Young, Goy and Phoenix (Young et al 1964) reviewed current knowledge at that time as to how gonadal hormones not only affect sexual behavior along with social and cultural factors and individual experiences but also influence sexual differentiation of those behaviors during development. Although there was little mention of the brain, which, after all, controls behavior, they stated the following:
“Few biochemists have been attracted to the problem, but it is they who must clarify the mechanisms of hormonal action in organizing the tissues of the central nervous system during development and in bringing behavior to expression in the adult. They may be helped in such a search by the circumstance that cellular elements in the genital tract, which differentiate and are activated under the influence of these same hormones, are at present more accessible for histophysiological study than those in tissues of the central nervous system
(Young et al 1964).”
When this article was published, there was already ongoing work on gonadal steroid actions in uterus via activation of gene expression by receptors in the cell nucleus; these receptors were detected in reproductive tissues using tritium labeled steroid hormones that allowed assessment of binding to these receptors (Jensen & Jacobson 1962). As will be described, tritiated steroids allowed the identification of putative estrogen receptors (ERs) in cell nuclei of the hypothalamus by steroid autoradiography (Pfaff 1968, Pfaff & Keiner 1973, Stumpf 1968). These were confirmed biochemically as receptors (Zigmond & McEwen 1970) and this led, among other discoveries, to the elegant demonstration of the neural circuitry for sexual behavior (Pfaff 1980b) and demonstrations of the role of testosterone and its metabolites, estradiol and dihydrotestosterone, in brain sexual differentiation (McEwen et al 1978, Naftolin et al 1975).
What was not appreciated at that time was that there are developmentally programmed sex differences throughout the entire brain. Moreover, it was also not known that the entire brain is acted upon by sex hormones via both nuclear and non-nuclear receptors, although this was strongly suggested by the myriad actions of sex hormones on cognitive function, mood and neuroprotection, addiction, blood pressure, fine motor skills, motor coordination, and pain, (Fig. 1) (McEwen et al 2012, McEwen & Alves 1999). After a brief historical overview of sex hormone action and a summary of mechanisms of plasticity of the adult as well as developing brain, this review will highlight some of the key steps in recognizing the broad influence of sex hormones in the brain and their implications for sex differences.
Figure 1.
Estrogens have many effects throughout the brain.
Historical overview
Preceding studies by Young et al. (1964) on sexual behavior, the work of Geoffrey Harris and subsequent pioneers established the connections between the brain and the endocrine system via the hypothalamus and the portal blood vessels that carry releasing factors from the hypothalamus to the pituitary gland (Harris 1948, Meites 1992). After the portal blood supply was shown to carry blood from the hypothalamus to the anterior pituitary (Harris 1948), heroic efforts using hypothalamus tissue from slaughterhouse animals led to the isolation and structural identification of peptide releasing factors (Guillemin 1978, Schally et al 1973). The feedback regulation of hypothalamic and pituitary hormones implied the existence of receptor mechanisms for gonadal, adrenal and thyroid hormones. Then the identification of cell nuclear hormone receptors in peripheral tissues (Jensen et al 1981, Toft & Gorski 1966) using tritiated steroid and iodinated thyroid hormones led to the demonstration by Don Pfaff as well as Walter Stumpf, of similar receptor mechanisms in hypothalamus and pituitary gland (Pfaff & Keiner 1973, Stumpf & Sar 1976). For this, it was necessary to use autoradiographic methods because of the discrete nature of these receptor containing cells, while Richard Zigmond in his Ph.D. thesis work at The Rockefeller University in the McEwen laboratory used more conventional cell fractionation methods, along with sucrose density gradient centrifugation to demonstrate receptors with molecular sizes, like those in the peripheral tissues (Gerlach & McEwen 1972, McEwen & Plapinger 1970, Pfaff & Keiner 1973, Zigmond & McEwen 1970).
What about behavior?
Before the demonstration of nuclear estrogen (ERs) and androgen receptors (ARs) in hypothalamus, some suggested, as noted in the Introduction, that sex hormones acted indirectly to activate sex behavior (Young 1961, Young et al 1964). The demonstration of binding sites and receptors for estrogens in hypothalamus led to studies using discrete hormone implants (Lisk 1962), as well as sophisticated neuroanatomical and neurophysiologic methods that demonstrated that sex hormones facilitate sex behavior via receptors in the hypothalamus (Davis et al 1979, Pfaff 1980a). Yet, in retrospect and even at that time, there were other behaviors and neurological states that were known to be influenced by estrogens involving brain regions besides the hypothalamus, including fine motor control, pain mechanisms, seizure activity, mood, cognitive function and neuroprotection (Bedard et al 1977, McEwen et al 1998, McEwen & Alves 1999, Van Hartesveldt & Joyce 1986). See Figure 1.
Tritiated steroid hormone cell nuclear uptake and retention, as shown by autoradiography, was not all confined to the hypothalamus, although, in the case of sex hormones, the major concentration of such receptors is in the hypothalamic region and amygdala (Pfaff & Keiner 1973). The big surprise was the discovery of receptor sites for steroid hormones outside of the hypothalamus. This finding was first accomplished for glucocorticoids in the hippocampus, not only of rodents, but also monkeys with extension to other species (Gerlach & McEwen 1972, Gerlach et al 1976, McEwen et al 1968). This unexpected finding is a major part of the story because it directed us to brain functions beyond the hypothalamus and, in particular, to the function of the hippocampus, a brain region important for memory and other aspects of behavioral regulation (Eichenbaum & Otto 1992) and it led to finding estrogen receptors in the hippocampus.
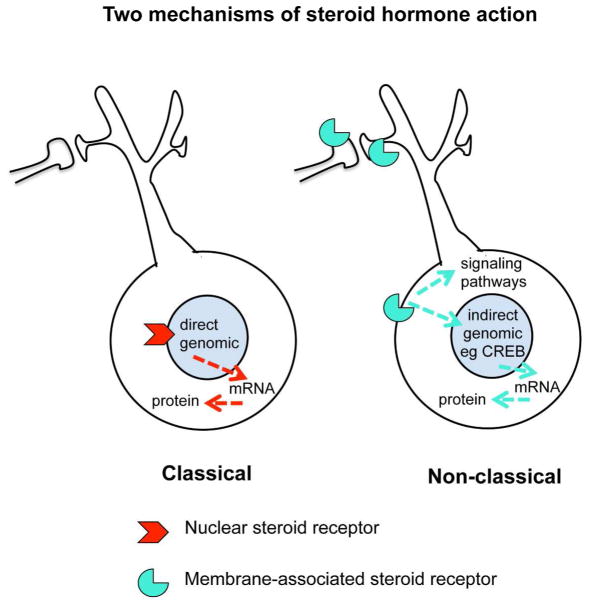
As it turned out, a further serendipitous finding of nuclear ERs in the hippocampus (Loy et al 1988) also represented a turning point in the realization that not all steroid hormone actions occur via cell nuclear receptors, but rather operate via receptors in other parts of the cell via a variety of signaling pathways (Fig. 2) (Kelly & Levin 2001, McEwen & Milner 2007). This is now recognized to be the case for all classes of steroid hormones, including Vitamin D (Huhtakangas et al 2004), aldosterone (Wehling et al 1992), androgens (Tabori et al 2005), as well as estrogens and progestins to be discussed later in this review. First, because structural plasticity of the brain is regulated by hormones, we shall briefly introduce the evolving concepts of structural plasticity in the adult brain.
Figure 2.
Steroid hormones can act via classical (genomic) and non-classical (non-genomic) receptors. In many cases, the same receptor molecule has different functions in the nucleus and non-nuclear sites in the cell.
Plasticity of the adult brain
Long regarded as a rather static and unchanging organ, except for electrophysiological responsivity, such as long-term potentiation (Bliss & Lomo 1973), the brain has gradually been recognized as capable of undergoing rewiring after brain damage (Parnavelas et al 1974) and also able to grow and change as seen by dendritic branching, angiogenesis and glial cell proliferation during cumulated experience (Bennett et al 1964, Greenough & Volkmar 1973). More specific physiological changes in synaptic connectivity were also recognized in relation to hormone action in the spinal cord (Arnold & Breedlove 1985), and in environmentally directed plasticity of the adult songbird brain (DeVoogd & Nottebohm 1981). Seasonally varying neurogenesis in restricted areas of the adult songbird brain is recognized as part of this plasticity (Nottebohm 2002). Indeed, neurogenesis in the adult mammalian brain was initially described (Altman & Das 1965, Kaplan & Bell 1983) and later rediscovered in the dentate gyrus of the hippocampus (Cameron & Gould 1994, Gould & McEwen 1993) in the context of studies of neuron cell death and actions of adrenal steroids and excitatory amino acids in relation to stress. This was further developed to call attention to the generality of neurogenesis across vertebrates (Alvarez-Buylla & Lois 1995), Although the existence of adult neurogenesis in the mammalian central nervous system (CNS) was doubted by some (see(Kaplan 2001)), recent evidence clearly proves that the human hippocampus shows significant neurogenesis in adult life (Spalding et al 2013). Now we turn to mechanisms by which sex hormones alter brain structural as well as functional plasticity, beginning with developmental determinants.
Developmental programming of sex differences
As discussed in this Special Issue, developmentally programmed sex differences arise not only from secretion of sex hormones during sensitive periods in development but there also are contributions of genes on Y and X chromosomes, and in females there is inactivation of one or the other X chromosome in females (McCarthy & Arnold 2011). Moreover, mitochondria derive from the mother and mitochondrial genes make important contributions to brain and body functions (e.g., see (Arnold et al 2004, Gruene et al 2014, McCarthy et al 2012, McEwen & Morrison 2013). We note that there are, in brain, few sexual dimorphisms, that is, complete differences between males and females. The sexually dimorphic nucleus of the preoptic region (SDN-POA) in the rodent brain comes close (Gorski et al 1978) and Witelson has describe an apparent sex dimorphism in the human brain (Witelson et al 1995). However, the vast majority of sex differences are far more subtle and involve patterns of connectivity and brain regional differences that are the subject of controversy (Chekroud et al 2016, Del Giudice et al 2016, Ingalhalikar et al 2014a, Ingalhalikar et al 2014b, Joel et al 2015, Joel & Tarrasch 2014, Rosenblatt 2016). However, at the level of underlying neurochemical and molecular mechanisms there are many surprising and dramatic sex differences in animal model brains and some indications for similar sex differences in the human brain.
Sex differences can emerge throughout the life course via both genetic and epigenetic mechanisms. The following examples delve into different neural systems and processes, involving stress effects on the hippocampus and prefrontal cortex, the dopaminergic system, blood pressure control, the cerebellum and pain sensitivity.. Below we present some examples, by no means exhaustive, to illustrate both the widespread nature of sex hormone influences but also the unexpectedly widespread nature of subtle sex differences. We begin with mechanisms of sex hormone action on structural plasticity of the adult brain, in which estrogen actions have played a major role and where sex differences in hormone action have emerged.
Sex hormone actions beyond the hypothalamus: focus on the hippocampus
First discovered in neuronal cell nuclei of the hypothalamus, receptors for sex hormones are found in virtually every region of the nervous system when they have been looked for, albeit in non-nuclear distributions throughout the cell near membranes (Boulware et al 2007, Kelly & Levin 2001). Recognition of non-nuclear ER in brain was first described in the hypothalamus (Blaustein et al 1992). Later electron microscopic (EM) immunocytochemistry studies revealed that non-nuclear ERs are present in extra-hypothalamic regions as discussed below.
In the original steroid autoradiography studies, a few scattered cells in hippocampus demonstrated strong cell nuclear labeling by 3H estradiol and these have been identified as inhibitory interneurons (Ledoux et al 2009, Loy et al 1988, Nakamura & McEwen 2005). In spite of the paucity of such labeled cells, there was evidence from seizure studies that the threshold for eliciting seizure activity in hippocampus was lowest on the day of proestrus when estrogen levels are elevated (Terasawa & Timiras 1968). Moreover, there were indications that elevated estrogens enhanced memory retention of the type involving the hippocampus (Sherwin 1988). Using the classical Golgi method, a cyclic variation was found the density of spine synapses, on the principal neurons of the CA1 region of the hippocampus, with peak density occurring on the day of proestrus (Woolley et al 1990) (Fig. 3), thus providing a possible structural basis for the lower seizure induction threshold (Terasawa & Timiras 1968). This work in the McEwen lab was led by Catherine Woolley and Nancy Weiland (Weiland 1992, Woolley et al 1997), and Woolley has continued these studies at Northwestern (Huang & Woolley 2012, Smejkalova & Woolley 2010). Since excitatory amino acids are the major neurotransmitter in these neurons and since NMDA receptor activity is involved not only in hippocampal memory functions but also in seizure induction, we used a competitive N-methyl-D-aspartate (NMDA) receptor blocker and discovered that it prevented estradiol induced spine synapse formation. Thus, estradiol does not work alone in causing this synapse formation and the study of underlying mechanisms is revealing some remarkable new aspects not only of hormone action, but also of neuronal plasticity (McEwen et al 2012).
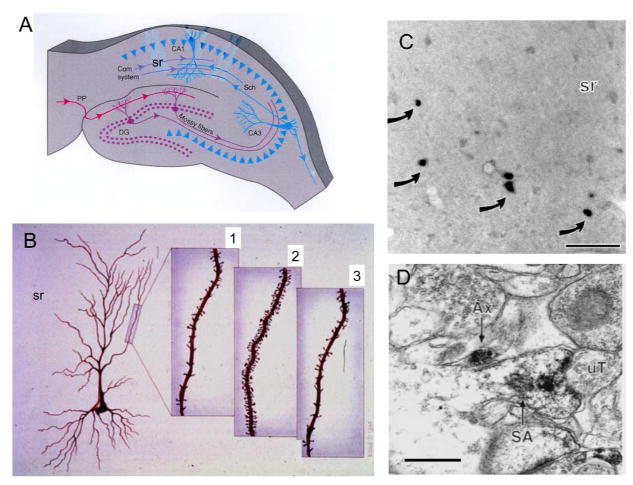
Figure 3.
The discovery of estrogen actions on synapse formation in hippocampus via both genomic and non-genomic mechanisms has opened the way to understanding actions of estrogens and other steroid hormones throughout the brain where nuclear steroid hormone receptors are not evident. A. Schematic of coronal section through the dorsal rat hippocampus. Com, commissural; DG, dentate gyrus; PP, perforant path; Sch, Schaffer collaterals; sr, stratum radiatum. B. Representation of CA1 pyramidal neurons in the female rat hippocampus during the 4–5 day estrous cycle. 1. Diestrus, when estradiol levels are lowest. 2. Proestrus, when estradiol levels are decreasing. 3. Estrous, when estradiol levels are decreasing (A and B from McEwen and Schmeck. The Hostage Brain. Rockefeller Univ. Press, 1994. Drawings by Lidia Kibiuk.) C. Light micrograph shows nuclei with ERα-ir (arrows) in the stratum radiatum (sr) of CA1. D. Electron micrograph shows ERα-ir in a dendritic spine identified by a spine apparatus (SA) that is contacted by an unlabeled terminal (uT). An axon with ERα-ir is nearby. Bar C 40 μm; D, 500nm (C and D modified from Milner et al. (Milner et al 2001).
Multiple cells and mechanisms involved in estrogen induced hippocampal synapse formation
Growing out of the recognition of the existence of non-nuclear ERs, the mechanisms implicated in estrogen-induced synapse formation and maturation have turned out to involve interactions among multiple cell types in the hippocampus, as well as multiple signaling pathways. In addition to NMDA receptors described above, cholinergic modulation of inhibitory interneurons and disinhibition of their input to pyramidal neurons is likely to be involved in estrogen induced synaptogenesis (Murphy et al 1998, Rudick et al 2003). Moreover, inhibitory interneurons and cholinergic activity participate in spine synapse induction (Daniel & Dohanich 2001, Murphy et al 1998, Rudick et al 2003) and estradiol rapidly and non-genomically stimulates acetylcholine release (Packard et al 1996). Electron microscopic studies further revealed that ERα is on cholinergic terminals in the hippocampus (Packard et al 1996, Towart et al 2003).
The paucity of nuclear ERs in hippocampus led to our serendipitous finding, using electron microscopic immunocytochemistry that has the resolution to see more than the cell nuclear sites, that epitopes for the classical ERs can be localized in dendrites, synapses, terminals and glial cell processes (reviewed in (McEwen & Milner 2007). Within these processes, ERs are associated with membranes including endomembranes near mitochondria (Milner et al 2005). These epitopes were then identified as estrogen binding sites by high resolution steroid autoradiograhy with 125I-estradiol (Milner et al 2008). Concurrently, increasing recognition was being given to the so-called non-genomic actions of estrogens and their signaling pathways (Kelly & Levin 2001). Indeed, such non-nuclear membrane-associate ERs have been reported in newly generated dentate gyrus neurons (Herrick et al 2006).
Signaling pathways involving NMDA receptor activation together with estradiol
Although the mechanism is unclear, NMDA receptor activation likely participates in the ability of estradiol to stimulate signaling pathways within cells. In the CA1 pyramidal neurons, non-genomic actions of estrogens via PI3 kinase promote actin polymerization and filopodia outgrowth to form putative synaptic contacts by dendrites with presynaptic elements (Yuen et al 2010). Subsequent PI3 kinase activation via ERs stimulated translation of PSD-95 in dendrites to provide a post-synaptic scaffold for spine synapse maturation (Akama & McEwen 2003, Znamensky et al 2003). Signaling pathways implicated in these events include LIM kinase and cofilin phosphorylation (Yuen et al 2010) and PI3 kinase activation (Akama & McEwen 2003, Znamensky et al 2003), as well as the Rac/Rho signaling system (Kramar et al 2009). It is important to note that the cofilin pathway is implicated in spinogenesis in the ventromedial hypothalamus (Christensen et al 2011) that is part of the induction of lordosis behavior (Frankfurt et al 1990, Frankfurt & McEwen 1991).
Progesterone actions
Finally, what terminates the estradiol induced synapse formation? Progesterone treatment after estrogen-induced synapse formation caused rapid (12h) down-regulation of spine synapses; moreover, the progesterone receptor antagonist, Ru486, blocked the naturally-occurring down regulation of estradiol induced spines in the estrous cycle (Woolley & McEwen 1993). But where are the progestin receptors that do this? Because Ru486 is effective, it is very likely that the classical progestin receptor is the mediator as opposed to other G-protein coupled progestin receptors that are not affected by Ru486 (Thomas 2008). Curiously, the classical progestin receptor is not detectable in cell nuclei within the rat hippocampus, but it is expressed in non-nuclear sites in hippocampal neurons, and virtually all of the detectable progestin receptor is estrogen inducible (Parsons et al 1982, Waters et al 2008). The mechanism of progesterone action on synapse down-regulation is presently unknown.
Neuroprotective actions of estradiol
It is clear that estrogen has other functions, such as protecting neurons from excitotoxic damage due to seizures and stroke, as well as Alzheimer’s disease (Henderson & Paganini-Hill 1994, McCullough et al 2003). The exact role in this process of cell nuclear ERs found on inhibitory interneurons is unclear, but one clue is the ability of estrogens to enhance neuropeptide Y (NPY) expression and release, since NPY has anti-excitatory actions (Ledoux et al 2009, Nakamura et al 2004). Another facet of estrogen neuroprotection is their ability to translocate ER β to mitochondria and to regulate mitochondrial calcium sequestration, including Bcl-2 translocation (Nilsen & Brinton 2004).
Investigation of the ability of estrogens to protect against stroke damage, as well as Alzheimer’s and Parkinson’s disease, has uncovered the fact that the brain is capable of locally generating estrogens, either from androgens and possibly also directly from cholesterol (Hajszan et al 2007, Hojo et al 2003). Aromatization of androgen precursors produces estrogenic steroids and knock-out of the aromatase enzyme increases ischemic damage even beyond that found after ovariectomy of wild-type mice (McCullough et al 2003).
What about androgens?
The brain also appears to have the capacity to locally generate the androgen, dihydrotestosterone, from as yet unknown precursors, independently of the gonads in an animal model in which mild exercise increases neurogenesis in a manner facilitated by those androgens (Okamoto et al 2012). Moreover, testosterone induces spine synapses in the male rat hippocampus, even though estradiol does not do so, unless genetic male rats are castrated at birth or treated with aromatase blockers to prevent developmental actions of testosterone (Leranth et al 2003, Lewis et al 1995, MacLusky et al 2006). Furthermore, androgens are able to induce spine synapses in the female rat hippocampus (Leranth et al 2004).
Moreover, like estrogens, androgens have neuroprotective effects (Pike et al 2008). In contrast to the ER story, CA1 pyramidal neurons have ample expression of cell nuclear ARs (Fig. 4) (Kerr et al 1995, Tabori et al 2005). In the male, these nuclear ARs may play a pivotal role in spine synapse formation which involves NMDA activity but not cholinergic activity (Romeo et al 2005a, Romeo et al 2005b). There are also extranuclear ARs with epitopes of the nuclear receptor that are found in the hippocampus in membrane associated locations in dendrites, spines and glia cell processes (Tabori et al 2005). However, the role of non-genomic forms of ARs in spine synapse formation and other processes is less clear (Tabori et al 2005).
Fig. 4.
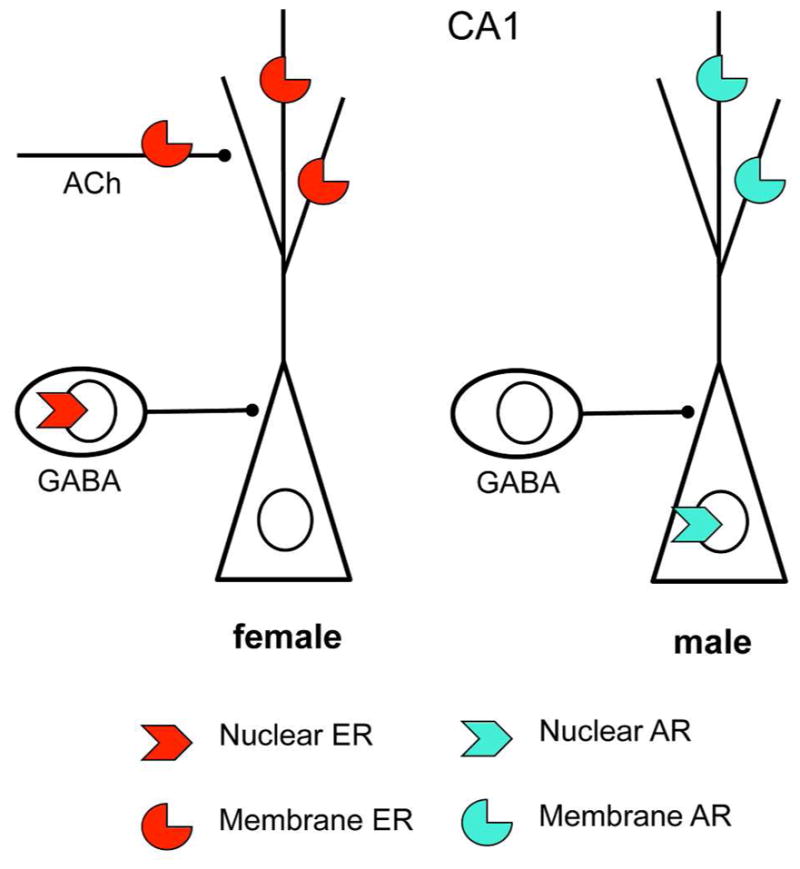
Sex differences in CA1 steroid hormone receptor distribution. Nuclear and nonnuclear steroid receptors are found on different cell types and/or cellular locations in females and males. Both females and males have membrane receptors on dendrites and dendritic spines. However, in females nuclear ERs are in GABAergic neurons whereas in males ARs are in pyramidal cell neurons. Moreover, membrane estrogen receptors are found on cholinergic (ACh) afferents.
Now, with this historical and mechanistic background, we broaden the story to discuss sex differences that are functionally linked to the actions attributed to estrogens in Figure 1.
Sex differences in hippocampal response to stressors
In the hippocampus of male rats, 21 days of chronic restraint stress (CRS) causes apical dendrites of CA3 neurons to retract and a loss of ~30% of the parvalbumin (PARV)-containing neurons in the dentate gyrus; these changes do not occur following CRS in female rats (Galea et al 1997, Milner et al 2013). Moreover, female and male rats show effects in the opposite direction of chronic stress on hippocampal dependent memory, with males showing impairment and females showing enhancement or no effect (Bowman et al 2003, Luine et al 1996, Luine et al 1994). Moreover, exposure of male and female rats to restraint plus intermittent tail shock has opposite effects on classical eyeblink conditioning, inhibiting it in females and enhancing it in males; in females, this effect is abolished by ovariectomy and is therefore estrogen dependent (Shors et al 2001, Wood & Shors 1998). A morphological correlate of this in the hippocampus is the finding that acute stress inhibits estrogen-depending spine formation in CA1 neurons of the hippocampus, whereas the same acute stressors enhance spine density in male CA1 neurons, possibly by increasing testosterone secretion (Shors et al 2001) upon which spine formation in the male CA1 is dependent (Leranth et al 2003). Neonatal masculinizaton of females made them response positively, like genetic males, to the shock stressor (Shors 2016) Moreover, in females, depending on reproductive status and previous experience, the negative stress effect was altered, e.g., it was absent in mothers and virgin females with experience with infants (Shors 2016).
Among possible mechanisms for the sex differences, the corticotrophin releasing factor receptor stands out since in hippocampus as well as in locus coeruleus, there are sex differences in the association of the CRF receptor (CRF1) with the Gs protein and beta-arrestin 2 that make females more responsive to acute stress and less able to adapt to chronic stress as a result of compromised CRF1 internalization (Valentino et al 2013) Thus the failure of female rats and mice to show spine loss and dendrite shrinkage (Galea et al 1997, Pawlak et al 2005) may be related to this sex difference.
There appears to be an important maturational/developmental component to sex differences in chronic stress effects upon dendrite length and branching. This is suggested by the finding that stress in the pubertal transition causes qualitatively similar responses in males and females in hippocampus indicating that full sexual maturation produces the sex differences in responses to stress (Eiland et al 2012). It remains to be determined whether other sex hormone dependent and independent sex differences also show this type of maturation effect.
Hippocampal opioid system and stress
The opioid system in the CA3 region has been implicated in visual-spatial pattern completion (Kesner & Warthen 2010), an important component of contextual associative learning which is a key function of the hippocampal spatial and temporal mapping function (Hartley et al 2014, Moser & Moser 2014). Within the hippocampus, the opioid peptide, enkephalin, is contained in the mossy fiber and lateral perforant path (Drake et al 2007). Enkephalins as well as exogenous opiates (e.g., morphine) predominantly affect excitability and long-term potentiation (LTP) of CA3 pyramidal cells indirectly via activation of μ opioid receptors (MORs) and δ opioid receptors (DORs) which result in inhibition of inhibitory gamma amino butyric acid (GABA)-ergic interneurons (i.e., disinhibition) (Commons & Milner 1995, Derrick et al 1992, Drake et al 2007, Witter 1993, Xie & Lewis 1991). Additionally, enkephalins and exogenous opiates can directly inhibit DORs present on CA3 pyramidal cells (Bao et al 2007). Our light and EM studies have demonstrated notable sex differences in the hippocampal opioid system: for example, at elevated estrogen states, compared to low estrogen states and males, enkephalins, MORs and DORs are subcellularly positioned to enhance excitability and learning processes (Torres-Reveron et al 2008, Torres-Reveron et al 2009b, Williams et al 2011). Moreover, females in proestrus (high estrogen state) compared to diestrus females and males have a lower baseline transmission in the mossy fiber-CA3 pathway that is regulated by MORs and, unlike males and diestrus females, exhibit a LTP evoked by low frequency stimulation of the mossy fibers that is regulated by DORs (Harte-Hargrove et al 2015).
The hippocampal mossy fibers also contain dynorphin that is elevated in the presence of estrogens (Torres-Reveron et al 2009a). The rodent hippocampus contains few kappa opioid receptors (KORs) but in the guinea pig KORs are on inputs from the hypothalamus (Drake et al 2007). However, there are known sex differences in KOR function and their potential impact on addiction (Chartoff & Mavrikaki 2015) making this a recent topic of interest.
Drug addiction, particularly relapse, is often provoked by stress (reviewed by (Bruchas et al 2008, Shalev et al 2000)). Stress has powerful influences on the addictive processes in both males and females (Koob 2008). However, females have a heightened sensitivity to stress (Becker et al 2007, Milner et al 2013) and can show enhanced cognitive performance following stress (Luine et al 2007) that may contribute to their accelerated course of addiction, particularly to opiate analgesics (Elman et al 2001, Hu et al 2004, Lynch et al 2000, Robbins et al 1999).
As described in the section above, chronic stress has the opposite effects on the hippocampal opioid system in males and females. Our studies have shown that 10 days of chronic immobilization stress (CIS) in males essentially “shuts off” the opioid system. Conversely, the opioid system of females regardless of estrogen state is “primed” for even greater excitation of CA3 pyramidal cells after CIS. After CIS females do not display the atrophy of CA3 pyramidal cell dendrites and the loss of PARV-containing GABA interneurons seen in males (McEwen 1999, Milner et al 2013, Vyas et al 2002). Instead, in CIS females, enkephalin levels in mossy fibers are elevated and the distribution of MORs and DORs in hippocampal neurons resembles that seen in unstressed females at high estrogen states (Milner unpublished)(Milner et al 2013, Pierce et al 2014). Moreover, CIS in females traffics DORs to the near plasmalemma of hilar somatostatin/NPY-containing GABAergic interneurons (Milner, unpublished) that project to granule cell dendrites where they converge with entorhinal afferents (Milner & Bacon 1989, Milner & Veznedaroglu 1992). Notably one-hour after a single injection of oxycodone (3mg/kg, i.p.) the DORs in these GABAergic interneurons have moved to the plasmalemma (Milner et al., unpublished) where additional exposure to ligand would promote disinhibition (i.e., excitation) and thus LTP of the entorhinal-granule cell synapses (Sperk et al 2007). Thus, chronic stress “primes” the opioid system in all females in a manner that would promote excitation and learning processes following subsequent exposure either to stress or an opiate ligand (Fig. 5).
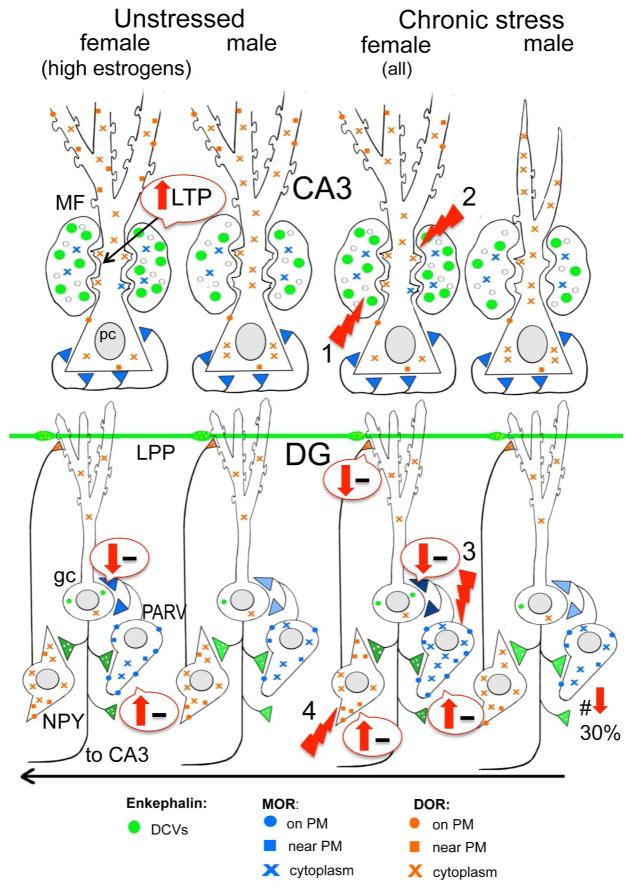
Fig. 5.
Schematic shows sex differences in the hippocampal opioid system in unstressed and CIS rats. Arrows indicate predicted effects of MOR/DOR trafficking changes on inhibition (minus signs). In unstressed conditions, low frequency (1 Hz) stimulation of the granule cells elicits a DOR-dependent LTP in CA3 of proestrus females that is not seen in diestrus females or males (Harte-Hargrove et al 2015). The opioid system in all females after CIS resembles that of females in elevated estrogen states: (1) the pool of available enkephalin is elevated in mossy fibers; (2) DORs are decreased in the dendritic shafts but increased in the spines of CA3 pyramidal cells; and (3) MORs are increased in the dendrites and terminals of PARV GABA interneurons in the dentate gyrus (DG). Moreover, (4) after CIS in females, DORs have mobilized to the near-plasmalemma of the dendrites of GABAergic NPY/SOM interneurons known to project to granule cell dendrites where they converge with entorhinal afferents. Abbreviations: δ opioid receptors (DORs), dense-core vesicles (DCVs), lateral perforant path (LPP), long-term potentiation (LTP), μ opioid receptors (MORs), neuropeptide Y (NPY), plasma membrane (PM), somatostatin (SOM)
Sex differences beyond the hippocampus: some examples
As discussed above, sex hormones affect many regions and functions of the hippocampus, beyond reproduction, through receptors in both males and females that operate via genomic and non-genomic mechanisms (further reading can be found (Dumitriu et al 2010, Hara et al 2015, McEwen et al 2012, McEwen & Milner 2007)]. Besides hippocampus, other brain regions demonstrate estrogen-regulated spine synapse formation and turnover, including the prefrontal cortex (Hao et al 2007) and primary sensory-motor cortex (Chen et al 2009). Indeed, there is likely to be estrogen-regulated spine synapse turnover in other brain regions. Besides estrogen induced spine formation, estrogens are implicated in functions in the nigrostriatal system (Xiao & Becker 1997), cardiovascular nuclei (van Kempen 2016) cerebellum (Smith 1989) and pain circuitry (Loyd & Murphy 2014), suggesting that their effects are widespread in the CNS.
Prefrontal cortex (PFC)
Chronic restraint stress (CRS) for 21d causes neurons in the medial PFC of the male rat to show dendritic debranching and shrinkage (McEwen & Morrison 2013). These neurons project to cortical areas and not to the amygdala, and, in the female, these neurons do not show dendritic changes. However, neurons that project to the amygdala from the medial PFC undergo dendritic expansion in females but not in males; this expansion in the female is dependent on there being estrogens in the system, since ovariectomized females did not show such changes (Shansky et al 2010). Estrogens and stress also interacted in a regionally specific manner in the PFC, in that cortically-projecting PFC neurons, which showed no dendritic changes after CRS in either intact or ovariectomized (OVX) animals, displayed a CRS-induced increase in spine density in OVX animals but not in intact females with circulating estradiol; yet amygdala-projecting PFC neurons showed CRS-induced spine density that was enhanced in intact females to accompany the dendrite expansion (Shansky et al 2010). Regarding function, as shown by lesion studies, contralateral prefrontal to amygdala projection is key to the ability of acute foot shock stress to impair eyeblink conditioning in female rats, whereas male rats normally show enhanced conditioning after the same footshock stress, as is discussed below (Shors 2016).
A subsequent study by Shansky has begun to reveal the consequences for fear learning and extinction in rats exhibiting high (HF) or low (LF) levels of freezing on a extinction retrieval test (Gruene et al 2014). The HF and LF male rats showed neuroanatomical distinctions that were not found in HF or LF female rats, and, though there were no overall sex differences in freezing behavior, HF and LF behavioral differences were evident in males during extinction and in female rats during fear conditioning, the later of which does not involve infralimbic–basolateral amygdala neurons (Gruene et al 2014).
Dopaminergic systems
The study of estrogen actions as well as sex differences on the dopaminergic system has been recently reviewed (Almey et al 2015) and thus will be discussed briefly here. Estradiol stimulation of dopaminergic release was one of the first examples of a rapid estrogen effect apparently independent of nuclear ERs (Mermelstein et al 1996). A sex difference in the ability of estrogen to promote dopamine release has been reported (Bazzett & Becker 1994, Becker et al 1982). Moreover, membrane-associated, non-genomic ERs have been demonstrated in dopamine-terminal areas including the caudate, prefrontal cortex and nucleus accumbens (reviewed in (Almey et al 2015)). Indeed, in the prefrontal cortex local estradiol application mimics the effects of high systemic estrogen in promoting a place memory as opposed to a response memory bias, interacting with the dopaminergic system (Almey et al 2014).
The nigrostriatal system is interesting because of its role in Parkinson’s disease. The Parkinson’s connection arose with the observation that high dose estrogen treatment used in the initial contraceptive preparations exacerbated symptoms of Parkinson’s Disease in women (Bedard et al 1977). This was very unexpected for those who believed in the nuclear ER story because there are almost no cell nuclei with ERα or ERβ in the rodent striatum (Almey et al 2012), and yet tiny unilateral implants of estradiol in the rodent striatum elicited unilateral rotation associated with imbalanced dopaminergic function (Van Hartesveldt & Joyce 1986). Now we know that estradiol regulates dopamine release from striatum in a sexually-dimorphic manner via non-genomic estrogen receptors of the type first identified in hippocampus and now elsewhere in the brain (Castner et al 1993). Moreover, with lower doses of estradiol, there is evidence for neuroprotection in Parkinson’s disease (Currie et al 2004, Leranth et al 2000) and the involvement of multiple signaling pathways (Bourque et al 2012), including the G-protein coupled ER (GPER1) (Almey et al 2012, Almey et al 2015).
Interestingly, besides dopamine, beta 1 adrenergic receptors in the striatum, and possibly elsewhere in the brain, are up-regulated after ovariectomy and there is a sex difference that is programmed by exposure to testosterone early in life so that males are not responsive to estrogens and genetic females given testosterone at birth do not show these effects of ovariectomy (Meitzen et al 2013)
CNS cardiovascular regulation
Sex differences in blood pressure control have been extensively reviewed recently (van Kempen 2016) and thus will only be discussed briefly here. Light and electron microscopic immunocytochemical studies in rodents have revealed that gonadal steroid receptors are anatomically poised to influence the regulation of blood pressure in a number of brain regions involved in cardiovascular regulation. In particular, nuclear and extranuclear ER’s, PR’s and AR’s are complementary and overlapping in three major autonomic regions: the rostral ventrolateral medulla), nucleus of the solitary tract (NTS) and paraventricular nucleus of the hypothalamus (PVN) (McEwen 2012). Within this circuitry, there are many potential sites by which gonadal steroid receptors interact with angiotensin II and related signaling molecules critical for neuronal activation and plasticity in brain cardiovascular regulatory circuits.
There is a significant sex difference in both the slow-pressor response to AngII and changes in NMDA and related signaling pathways in PVN neurons. Like humans, a sex-dependent susceptibility to hypertension is seen in rodents following systemic administration of low doses of angiotensin II (AngII) via osmotic minipumps. In young male mice, but not cycling young female mice, systemic low-dose AngII-infusion results in a slowly developing increase in blood pressure (Girouard et al 2009, Li et al 2008, Marques-Lopes et al 2015a, Marques-Lopes et al 2014, Marques-Lopes et al 2015b, Pinkerton & Stovall 2010, Tiwari et al 2009, Van Kempen et al 2015b, Xue et al 2013, Xue et al 2005). However, slow-pressor AngII-infusion induces hypertension in OVX mice that model surgical menopause (Hay et al 2014, Xue et al 2013) and in aged rodents (Fortepiani et al 2003, Marques-Lopes et al 2015b, Tiwari et al 2009) that model the acyclicity (Nelson et al 1995) seen in post-menopause. Using a mouse model of Accelerated Ovarian Failure (AOF) that uniquely recapitulates hormonal changes seen in human menopause (Van Kempen et al 2014, Van Kempen et al 2011), we showed that the susceptibility to slow-pressor AngII hypertension begins at emerges at a timepoint that mimics perimenopause (i.e., when estrogens are present but erratically fluctuating) [9].
The PVN, predominantly through projections to the spinal cord, is a primary source of the excitatory drive that supports the elevation of sympathetic vasomotor tone critical for the emergence of slow-pressor AngII hypertension (Benarroch 2005). In particular, up-regulation of postsynaptic NMDA receptor function in PVN neurons that project to the spinal cord play a pivotal role in enhancing excitatory drive (Li et al 2008). Activation of PVN-spinal neurons results in NADPH oxidase (NOX) activity, generating reactive oxygen species (ROS), leading to the activation of voltage-gated L-type Ca2+ currents (Girouard et al 2009, Wang et al 2006, Wang et al 2004). Importantly, spatiotemporal deletion of the obligatory GluN1 subunit of the NMDA receptor in the PVN attenuates hypertension in males (Glass et al 2015). Our recent electron microscopy studies (Marques-Lopes et al 2015a, Marques-Lopes et al 2015b, Van Kempen et al 2015a) revealed that alterations in the subcellular distributions of GluN1 in ERβ-containing PVN neurons (but not those containing angiotensin type A receptors or CRF1 receptors (Marques-Lopes et al 2015a, Van Kempen et al 2015a) reflect the hypertensive responses of male and female mice following slow-pressor AngII. In particular, the density of GluN1 is elevated in hypertensive male and aged female mice but decreased in non-hypertensive young females (Marques-Lopes et al 2014). Using the AOF model, we showed that the AngII-induced hypertension is accompanyied by an increase in plasma membrane GluN1 receptors in ERβ-containing PVN neurons (Marques Lopes 2016). Importantly, the increase in plasma membrane GluN1 receptors was not seen in hypertensive AOF mice from a “post-menopausal” timepoint (e.g., post-AOF). These findings are consistent with accumulating clinical evidence (Hodis & Mack 2011, Pinkerton & Stovall 2010) that perimenopause is a “window of opportunity” for gonadal steroids to modulate hypertension susceptibility.
Hypertension linked to AngII is strongly associated with superoxide production by NOX neurons in the PVN (Coleman et al 2013, Wang et al 2013, Zimmerman et al 2004). The NOX2 isoform requires mobilization of cytoplasmic p47phox to dock with the membrane bound proteins for superoxide production (Brandes et al 2014). In males, slow-pressor AngII hypertension results in a repartitioning of p47phox to the plasmalemma of PVN neurons that do not express arginine vasopressin (AVP), along with a concomitant increase in ROS production (Coleman et al 2013). At baseline, young females have a greater density of p47phox SIG near the plasma membrane of AVP-containing dendrites than males m(Van Kempen et al 2015b). Compared to young females, post-AOF females have increased plasmalemmal p47phox SIG particles on non-AVP dendrites (Van Kempen et al 2015b). Like males, slow pressor AngII elevates blood pressure in post-AOF females. However, opposite to males, slow pressor AngII decreases plasmalemmal p47phox SIG in non-AVP dendrites and increases near plasmalemmal p47phox SIG in AVP-containing dendrites in post-AOF females (Van Kempen et al 2015b). These findings provide evidence for fundamental sex differences in the hypothalamic changes underlying the neurohumoral regulation of blood pressure (Fig. 6).
Fig. 6.
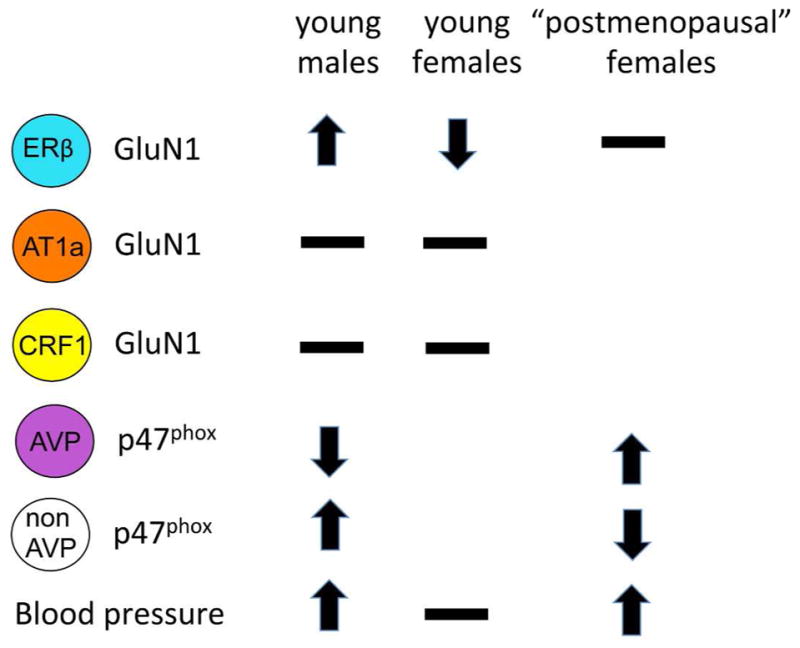
Sex differences in PVN neuron responses to slow pressor AngII. Fourteen days following slow pressor Angiotensin II delivered through osmotic minipumps, blood pressor increases in young males and in accelerated ovarian failure females from a timepoint that corresponds to “postmenopause” in humans. The trafficking of GluN1 and p47phox within PVN neurons varies depending on sex, ovarian hormone status and cell type. Arrows indicate direction of movement towards the plasma membrane. Receptors on the plasma membrane are available for ligand binding. Thus, an increase of GluN1 would indicate potential for greater excitability.
Cerebellum
The cerebellum is responsive to estrogens and generates both estradiol and progesterone during its development, and, in humans, it is implicated in disorders that show sex differences (Dean & McCarthy 2008, Hedges et al 2012). Estrogens direct the growth of dendrites in the developing cerebellum and regulate both excitatory and inhibitory balance, affect not only motor coordination but also memory and mood regulation (Hedges et al 2012). Although there are few described sex differences except possibly more neurons in the male in the cerebellum there are sex differences in the disorders associated with the cerebellum such as autism, attention deficit hyperactivity disorder (ADHD), and schizophrenia males (Dean & McCarthy 2008, Hedges et al 2012).. Associative learning related to pain is also mediated in part by the cerebellum. Men and women showed different functional connectivity in cerebellar lobules: for women, the lobules mostly represent somatomotor networks, while in men, activity in the lobules showed enhanced neural activation that are representative of frontoparietal and ventral attention networks (Labrenz et al 2015). The authors conclude “ that the cerebellum is involved in associative learning processes of conditioned anticipatory safety from pain and mediates sex differences in the underlying neural processes” (Labrenz et al 2015). They suggest that the high prevalence of chronic pain conditions in women may be due in part to these sex differences in cerebellum (Labrenz et al 2015)
Pain sensitivity and circuitry
Clinical studies indicate that morphine is less potent women compared to men in alleviating pain. Within the brain, the periagueductal gray (PAG) and its descending projections to the rostral medial medulla and spinal cord comprise the essential neural circuit for both the endogenous and exogenous opioid-mediated analgesia. Gonadal steroids, primarily through ERα and ARs in the PAG, exert a sexually dimorphic regulation of spinal antinociception (Loyd & Murphy 2014, Mogil 2012). Recent evidence (Sorge et al 2015) indicates that microglial inhibitors reduce allodynia, a form of pain hypersensitivity to touch, in males but not females and that this sex-specific response depends on testosterone levels.
Migraine is a pain condition that is more frequent in women than in men and Increased pain sensitivity in women (Maleki et al 2012). Women with migraine had thicker posterior insula and precuneus cortices compared with male migraineurs as well as healthy controls of both sexes. Furthermore, responses to heat within the migraine groups revealed a sex-specific pattern of functional connectivity of the posterior insula and precueus with the rest of the brain, pointing to a ‘sex phenotype’ in migraine and indicate that brains are differentially affected by migraine in females compared with males. Furthermore, the authors not that their “ results also support the notion that sex differences involve both brain structure as well as functional circuits, in that emotional circuitry compared with sensory processing appears involved to a greater degree in female than male migraineurs” (Maleki et al 2012).
Indeed, in relation to emotional circuitry, assessments of empathy in male and female volunteers, in which both sexes perform equally well on 3 separate tests, reveal different brain regional patterns of activation using fMRI (Derntl et al 2010). One way to generalize this is that, in their daily lives, men and women use different “strategies” in their relationships and approaches to solving problems and yet, on the average and with considerable overlap, do most things equally well ((Derntl et al 2010, McEwen & Lasley 2005).
Conclusions
While the brain was for many years not regarded as a target for estrogens and other hormones, except the hypothalamus for regulation of reproductive function, we now know that the entire brain is a target for gonadal, as well as for stress and other steroid hormones and metabolic hormones (McEwen 2007). While gonadal hormone actions on many brain functions were suspected for many years, this new view was made possible at the level of mechanism by the identification of membrane associated receptors that appear to be post-translational modifications of the same receptors that work at the cell nuclear level. Moreover, subtle sex differences are now being recognized in brain regions and functions not previously regarded as subject to such differences, indicating that we are entering a new era of our ability to understand and appreciate the diversity of gender-related behaviors and brain functions.
Significance statement.
Sex hormones act throughout the entire brain of both males and females via both genomic and non-genomic receptors and through many cellular and molecular processes that alter structure and function of neural systems and influence behavior as well as providing neuroprotection. Developmentally programmed, subtle sex differences and responses to sex hormones that influence functions and in brain regions not previously regarded as subject to such differences, indicates that we are entering a new era of our ability to understand and appreciate the diversity of gender-related behaviors and brain functions.
Acknowledgments
Supported by: NIH grants HL098351, DA08259 & HL096571 (TAM), AG016765 (TAM & BSM), MH41256 (BSM) and NS07080 (BSM).
We thank Ms. Sanoara Mazid for preparation of Figure 5.
Footnotes
Conflict of interests: The authors have no conflicts to declare.
Author Contributions: Bruce McEwen and Teresa Milner co-designed and co-wrote the manuscript and contributed figures. Dr. Milner created the final figures.
References
- Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci. 2003;23:2333–39. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Cannell E, Bertram K, Filardo E, Milner TA, Brake WG. Medial prefrontal cortical estradiol rapidly alters memory system bias in female rats: ultrastructural analysis reveals membrane-associated estrogen receptors as potential mediators. Endocrinology. 2014;155:4422–32. doi: 10.1210/en.2014-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Filardo EJ, Milner TA, Brake WG. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology. 2012;153:5373–83. doi: 10.1210/en.2012-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Milner TA, Brake WG. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav. 2015;74:125–38. doi: 10.1016/j.yhbeh.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–36. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lois C. Neuronal stem cells in the brain of adult vertebrates. Stem Cells. 1995;13:263–72. doi: 10.1002/stem.5530130307. [DOI] [PubMed] [Google Scholar]
- Arnold A, Breedlove S. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–98. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Xu J, Grisham W, Chen X, Kim Y-H, Itoh Y. Minireview: Sex chromosomes and brain sexual differentiation. Endocrinology. 2004;145:1057–62. doi: 10.1210/en.2003-1491. [DOI] [PubMed] [Google Scholar]
- Bao G, Kang L, Li H, Li Y, Pu L, et al. Morphine and heroin differentially modulate in vivo hippocampal LTP in opiate-dependent rat. Neuropsychopharmacology. 2007;32:1738–49. doi: 10.1038/sj.npp.1301308. [DOI] [PubMed] [Google Scholar]
- Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D dopamine receptor binding. Brain Res. 1994;637:163–72. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- Becker JB, Monteggia LM, Perrot-Sinal TS, Romeo RD, Taylor JR, et al. Stress and disease: is being female a predisposing factor? J Neurosci. 2007;27:11851–5. doi: 10.1523/JNEUROSCI.3565-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Robinson TE, Lorenz KA. Sex differences and estrous cycle variations in amphetamine-elicited rotational behavior. Eur J Pharmacol. 1982;80:65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- Bedard PJ, Langelier P, Villeneuve A. Estrogens and the extrapyramidal system. The Lancet. 1977;2:1367–68. doi: 10.1016/s0140-6736(77)90429-9. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Paraventricular nucleus, stress response, and cardiovascular disease. Clin Auton Res. 2005;15:254–63. doi: 10.1007/s10286-005-0290-7. [DOI] [PubMed] [Google Scholar]
- Bennett E, Diamond M, Krech D, Rosenzweig M. Chemical and anatomical plasticity of brain. Science. 1964;146:610–19. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Lehman MN, Turcotte JC, Greene G. Estrogen receptors in dendrites and axon terminals in guinea pig hypothalamus. Endocrinology. 1992;131:281–90. doi: 10.1210/endo.131.1.1612006. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiology. 1973;232:331–56. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–50. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque M, Dluzen DE, Di Paolo T. Signaling pathways mediating the neuroprotective effects of sex steroids and SERMs in Parkinson’s disease. Front Neuroendocrinol. 2012;33:169–78. doi: 10.1016/j.yfrne.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm & Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Weissmann N, Schroder K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic Biol Med. 2014;76:208–26. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Xu M, Chavkin C. Repeated swim stress induces kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport. 2008;19:1417–22. doi: 10.1097/WNR.0b013e32830dd655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–09. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Castner SA, Xiao L, Becker JB. Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res. 1993;610:127–34. doi: 10.1016/0006-8993(93)91225-h. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Mavrikaki M. Sex Differences in Kappa Opioid Receptor Function and Their Potential Impact on Addiction. Frontiers in neuroscience. 2015;9:466. doi: 10.3389/fnins.2015.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekroud AM, Ward EJ, Rosenberg MD, Holmes AJ. Patterns in the human brain mosaic discriminate males from females. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1523888113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-R, Yan Y-T, Wang T-J, Chen L-J, Wang Y-J, Tseng G-F. Gonadal hormones modulate the dendritic spine densities of primary cortical pyramidal neurons in adult female rat. Cerebral Cortex. 2009;19:2719–27. doi: 10.1093/cercor/bhp048. [DOI] [PubMed] [Google Scholar]
- Christensen A, Dewing P, Micevych P. Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity. J Neurosci. 2011;31:17583–9. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CG, Wang G, Faraco G, Marques Lopes J, Waters EM, et al. Membrane trafficking of NADPH oxidase p47(phox) in paraventricular hypothalamic neurons parallels local free radical production in angiotensin II slow-pressor hypertension. J Neurosci. 2013;33:4308–16. doi: 10.1523/JNEUROSCI.3061-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Milner TA. Ultrastructural heterogeneity of enkephalin-containing terminals in the rat hippocampal formation. J Comp Neurol. 1995;358:3224–342. doi: 10.1002/cne.903580303. [DOI] [PubMed] [Google Scholar]
- Currie LJ, Harrison MB, Trugman JM, Bennett JP, Wooten GF. Postmenopausal estrogen use affects risk for Parkinson disease. Arch Neurol. 2004;61:886–88. doi: 10.1001/archneur.61.6.886. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–56. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P, McEwen BS, Pfaff DW. Localized behavioral effects of tritiated estradiol implants in the ventromedial hypothalamus of female rats. Endocrinology. 1979;104:898–903. doi: 10.1210/endo-104-4-898. [DOI] [PubMed] [Google Scholar]
- Dean SL, McCarthy MM. Steroids, sex and the cerebellar cortex: implications for human disease. Cerebellum. 2008;7:38–47. doi: 10.1007/s12311-008-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Lippa RA, Puts DA, Bailey DH, Bailey JM, Schmitt DP. Joel et al.’s method systematically fails to detect large, consistent sex differences. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1525534113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Eickhoff S, Kellermann T, Falkenberg DI, et al. Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology. 2010;35:67–82. doi: 10.1016/j.psyneuen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Derrick BE, Rodriguez SB, Lieberman DN, Martinez JL., Jr Mu opioid receptors are associated with the induction of hippocampal mossy fiber long-term potentiation. J Pharmacol Exp Ther. 1992;263:725–33. [PubMed] [Google Scholar]
- DeVoogd T, Nottebohm F. Gonadal hormones induce dendritic growth in the adult avian brain. Science. 1981;214:202–04. doi: 10.1126/science.7280692. [DOI] [PubMed] [Google Scholar]
- Drake CT, Chavkin C, Milner TA. Opioid systems in the dentate gyrus. Prog Brain Res. 2007;163:245–63. doi: 10.1016/S0079-6123(07)63015-5. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Rapp PR, McEwen BS, Morrison JH. Estrogen and the aging brain: an elixir for the weary cortical network. Ann N Y Acad Sci. 2010;1204:104–12. doi: 10.1111/j.1749-6632.2010.05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T. The hippocampus - what does it do? Behav Neural Biol. 1992;57:2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37:39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Karlsgodt KH, Gastfriend DR. Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. The American journal of drug and alcohol abuse. 2001;27:193–202. doi: 10.1081/ada-100103705. [DOI] [PubMed] [Google Scholar]
- Fortepiani LA, Zhang H, Racusen L, Roberts LJ, 2nd, Reckelhoff JF. Characterization of an animal model of postmenopausal hypertension in spontaneously hypertensive rats. Hypertension. 2003;41:640–5. doi: 10.1161/01.HYP.0000046924.94886.EF. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, Gould E, Wolley C, McEwen BS. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: a golgi study in the adult rat. Neuroendo. 1990;51:530–35. doi: 10.1159/000125387. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, McEwen BS. Estrogen increases axodendritic synapses in the VMN of rats after ovariectomy. NeuroReport. 1991;2:380–82. doi: 10.1097/00001756-199107000-00006. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–97. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Gerlach J, McEwen BS. Rat brain binds adrenal steroid hormone: radioautography of hippocampus with corticosterone. Science. 1972;175:1133–36. doi: 10.1126/science.175.4026.1133. [DOI] [PubMed] [Google Scholar]
- Gerlach J, McEwen BS, Pfaff DW, Moskovitz S, Ferin M, et al. Cells in regions of rhesus monkey brain and pituitary retain radioactive estradiol, corticosterone and cortisol differently. Brain Res. 1976;103:603–12. doi: 10.1016/0006-8993(76)90463-7. [DOI] [PubMed] [Google Scholar]
- Girouard H, Wang G, Gallo EF, Anrather J, Zhou P, et al. NMDA receptor activation increases free radical production through nitric oxide and NOX2. J Neurosci. 2009;29:2545–52. doi: 10.1523/JNEUROSCI.0133-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass MJ, Wang G, Coleman CG, Chan J, Ogorodnik E, et al. NMDA Receptor Plasticity in the Hypothalamic Paraventricular Nucleus Contributes to the Elevated Blood Pressure Produced by Angiotensin II. J Neurosci. 2015;35:9558–67. doi: 10.1523/JNEUROSCI.2301-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex differences within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–46. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS. Neuronal birth and death. Current opinion in neurobiology. 1993;3:676–82. doi: 10.1016/0959-4388(93)90138-o. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Volkmar FR. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exper Neurol. 1973;40:491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- Gruene TM, Roberts E, Thomas V, Ronzio A, Shansky RM. Sex-Specific Neuroanatomical Correlates of Fear Expression in Prefrontal-Amygdala Circuits. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin R. Peptides in the brain: the new endocrinology of the neuron. Science. 1978;202:390–402. doi: 10.1126/science.212832. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Milner TA, Leranth C. Sex steroids and the dentate gyrus. Progress in brain research. 2007;163:399–415. doi: 10.1016/S0079-6123(07)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WGM, Lou W, Lasley BL, et al. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci USA. 2007;104:11465–70. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Waters EM, McEwen BS, Morrison JH. Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol Rev. 2015;95:785–807. doi: 10.1152/physrev.00036.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GW. Electrical stimulation of the hypothalamus and the mechanism of neural control of the adenohypophysis. J Physiol. 1948;107:418–29. doi: 10.1113/jphysiol.1948.sp004286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Varga-Wesson A, Duffy AM, Milner TA, Scharfman HE. Opioid receptor-dependent sex differences in synaptic plasticity in the hippocampal mossy fiber pathway of the adult rat. J Neurosci. 2015;35:1723–38. doi: 10.1523/JNEUROSCI.0820-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Lever C, Burgess N, O’Keefe J. Space in the brain: how the hippocampal formation supports spatial cognition. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2014;369:20120510. doi: 10.1098/rstb.2012.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay M, Xue B, Johnson AK. Yes! Sex matters: sex, the brain and blood pressure. Curr Hypertens Rep. 2014;16:458. doi: 10.1007/s11906-014-0458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges VL, Ebner TJ, Meisel RL, Mermelstein PG. The cerebellum as a target for estrogen action. Front Neuroendocrinol. 2012;33:403–11. doi: 10.1016/j.yfrne.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Paganini-Hill A. Oestrogens and alzheimer’s disease. Annual Progress in Reporductive Medicine. 1994;2:1–21. [Google Scholar]
- Herrick SP, Waters EM, Drake CT, McEwen BS, Milner TA. Extranuclear estrogen receptor beta immunoreactivity is on doublecortin-containing cells in the adult and neonatal rat dentate gyrus. Brain Res. 2006 doi: 10.1016/j.brainres.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ. A “window of opportunity:” the reduction of coronary heart disease and total mortality with menopausal therapies is age- and time-dependent. Brain Res. 2011;1379:244–52. doi: 10.1016/j.brainres.2010.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Hattori T-a, Enami T, Furukawa A, Suzuki K, et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc Natl Acad Sci USA. 2003 doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–5. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol Acutely Suppresses Inhibition in the Hippocampus through a Sex-Specific Endocannabinoid and mGluR-Dependent Mechanism. Neuron. 2012;74:801–8. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1α,25(OH)2-vitamin D3 in vivo and in vitro. Mol Endocrin. 2004;18:2660–71. doi: 10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, et al. Reply to Joel and Tarrasch: On misreading and shooting the messenger. Proc Natl Acad Sci U S A. 2014a;111:E638. doi: 10.1073/pnas.1323601111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, et al. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A. 2014b;111:823–8. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E, Geoffrey L, Greene L, Closs LED, Nadji M. Receptors reconsidered a 20- year perspective. In: Greep R, editor. Recent progress in hormone research. New York: Academic Press; 1981. pp. 1–40. [DOI] [PubMed] [Google Scholar]
- Jensen E, Jacobson H. Basic guides to the mechanism of estrogen action. Rec Prog Horm Res. 1962;18:387–408. [Google Scholar]
- Joel D, Berman Z, Tavor I, Wexler N, Gaber O, et al. Sex beyond the genitalia: The human brain mosaic. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1509654112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Tarrasch R. On the mis-presentation and misinterpretation of gender-related data: the case of Ingalhalikar’s human connectome study. Proc Natl Acad Sci U S A. 2014;111:E637. doi: 10.1073/pnas.1323319111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS. Environment complexity stimulates visual cortex neurogenesis: death of a dogma and a research career. Trends Neurosci. 2001;24:617–20. doi: 10.1016/s0166-2236(00)01967-6. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Bell DH. Neuronal proliferation in the 9-month-old rodentradioautographic study of granule cells in the hippocampus. Exp Brain Res. 1983;52:1–5. doi: 10.1007/BF00237141. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endo & Metab. 2001;12:152–56. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and Hormonal Regulation of Androgen Receptor (AR) and AR Messenger Ribonucleic Acid in the Rat Hippocampus. Endocrinology. 1995;136:3213–21. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Warthen DK. Implications of CA3 NMDA and opiate receptors for spatial pattern completion in rats. Hippocampus. 2010;20:550–7. doi: 10.1002/hipo.20676. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Brandon NJ, Rex CS, Liu F, et al. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29:12982–93. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrenz F, Icenhour A, Thurling M, Schlamann M, Forsting M, et al. Sex differences in cerebellar mechanisms involved in pain-related safety learning. Neurobiol Learn Mem. 2015;123:92–9. doi: 10.1016/j.nlm.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Ledoux VA, Smejkalova T, May RM, Cooke BM, Woolley CS. Estradiol facilitates the release of neuropeptide Y to suppress hippocampus-dependent seizures. J Neurosci. 2009;29:1457–68. doi: 10.1523/JNEUROSCI.4688-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004;24:495–99. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–92. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Roth RH, Elswoth JD, Naftolin F, Horvath TL, Redmond DEJ. Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: Implications for Parkinson’s disease and memory. J Neurosci. 2000;20:8604–09. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, McEwen BS, Frankfurt M. Estrogen-induction of dendritic spines in ventromedial hypothalamus and hippocampus: effects of neonatal aromatase blockade and adult castration. Devel Brain Res. 1995;87:91–95. doi: 10.1016/0165-3806(95)00052-f. [DOI] [PubMed] [Google Scholar]
- Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol. 2008;586:1637–47. doi: 10.1113/jphysiol.2007.149732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisk R. Diencephalic placement of estradiol and sexual receptivity in female rat. Am J Physiol. 1962;203:493–96. doi: 10.1152/ajplegacy.1962.203.3.493. [DOI] [PubMed] [Google Scholar]
- Loy R, Gerlach J, McEwen BS. Autoradiographic localization of estradiol-binding neurons in rat hippocampal formation and entorhinal cortex. Dev Brain Res. 1988;39:245–51. doi: 10.1016/0165-3806(88)90028-4. [DOI] [PubMed] [Google Scholar]
- Loyd DR, Murphy AZ. The neuroanatomy of sexual dimorphism in opioid analgesia. Exp Neurol. 2014;259:57–63. doi: 10.1016/j.expneurol.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Martinez C, Villegas M, Magarinos AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiology & Behavior. 1996;59:27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–70. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, MacLusky NJ. Chronic stress and neural function: Accounting for sex and age. J Neuroendocrinology. 2007;19:743–51. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152:132–9. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Johansen JA, Jordan CL, Leranth C. Androgen effects on hippocampal CA1 spine synapse numbers are retained in Tfm male rats with defective androgen receptors. Endocrinology. 2006;147:2392–98. doi: 10.1210/en.2005-0673. [DOI] [PubMed] [Google Scholar]
- Maleki N, Linnman C, Brawn J, Burstein R, Becerra L, Borsook D. Her versus his migraine: multiple sex differences in brain function and structure. Brain: a journal of neurology. 2012;135:2546–59. doi: 10.1093/brain/aws175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques Lopes J, Tesfaye E, Israilov S, van Kempen TA, Wang G, Glass MJ, Pickel VM, Iadecola C, Waters EM, Milner TA. Redistribution of NMDA receptors in estrogen receptor beta-containing paraventricular hypothalamic neurons folloowing slow pressor Angiontensin II hypertension in female mice with accelerated ovarian failure. Neuroendocrinology. 2016 doi: 10.1159/000446073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Lopes J, Lynch MK, Van Kempen TA, Waters EM, Wang G, et al. Female protection from slow-pressor effects of angiotensin II involves prevention of ROS production independent of NMDA receptor trafficking in hypothalamic neurons expressing angiotensin 1A receptors. Synapse. 2015a;69:148–65. doi: 10.1002/syn.21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Lopes J, Van Kempen T, Waters EM, Pickel VM, Iadecola C, Milner TA. Slowpressor angiotensin II hypertension and concomitant dendritic NMDA receptor trafficking in estrogen receptor beta-containing neurons of the mouse hypothalamic paraventricular nucleus are sex and age dependent. J Comp Neurol. 2014;522:3075–90. doi: 10.1002/cne.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Lopes J, Van Kempen T, Waters EM, Pickel VM, Iadecola C, Milner TA. Slow-pressor angiotensin II hypertension and concomitant dendritic NMDA receptor trafficking in estrogen receptor beta-containing neurons of the mouse hypothalamic paraventricular nucleus are sex and age dependent. The Journal of comparative neurology. 2015b;69:148–65. doi: 10.1002/cne.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14:677–83. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 2012;32:2241–7. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003;23:8701–05. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The ever-changing brain: cellular and molecular mechanisms for the effects of stressful experiences. Dev Neurobiol. 2012;72:878–90. doi: 10.1002/dneu.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126:4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE, Bulloch K, Weiland NG. Clinically relevant basic science studies of gender differences and sex hormone effects. Psychopharmacology Bulletin. 1998;34:251–59. [PubMed] [Google Scholar]
- McEwen BS, Alves SH. Estrogen Actions in the Central Nervous System. Endocrine Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Davis P, Krey L, Lieberburg I, MacLusky N, Roy E. Steroid hormone receptors in brain and pituitary. In: Fuxe K, Hokfelt T, Luft R, editors. Central Regulation of the Endocrine System. New York: Plenum Press; 1978. pp. 261–71. [Google Scholar]
- McEwen BS, Lasley EN. The Dana Forum on Brain Science. Dana Press; 2005. The End of Sex as We Know It In Cerebrum. [Google Scholar]
- McEwen BS, Milner TA. Hippocampal formation: Shedding light on the influence of sex and stress on the brain. Brain Res Rev. 2007;55:343–55. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH. The Brain on Stress: Vulnerability and Plasticity of the Prefrontal Cortex over the Life Course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Plapinger L. Association of corticosterone-1,2 3H with macromolecules extracted from brain cell nuclei. Nature. 1970;226:263–64. doi: 10.1038/226263a0. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss J, Schwartz L. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–12. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- Meites J. Short history of neuroendocrinology and the International Society of Neuroendocrinology. Neuroendocrinology. 1992;56:1–10. doi: 10.1159/000126201. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Perry AN, Westenbroek C, Hedges VL, Becker JB, Mermelstein PG. Enhanced striatal beta1-adrenergic receptor expression following hormone loss in adulthood is programmed by both early sexual differentiation and puberty: a study of humans and rats. Endocrinology. 2013;154:1820–31. doi: 10.1210/en.2012-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, et al. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, Bacon CE. GABA-ergic neurons in the rat hippocampal formation: Ultrastructure and synaptic relationships with catecholaminergic terminals. J Neurosci. 1989;9:3410–27. doi: 10.1523/JNEUROSCI.09-10-03410.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Burstein SR, Marrone GF, Khalid S, Gonzalez AD, et al. Stress differentially alters mu opioid receptor density and trafficking in parvalbumin-containing interneurons in the female and male rat hippocampus. Synapse. 2013;67:757–72. doi: 10.1002/syn.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Lubbers LS, Alves SE, McEwen BS. Nuclear and extranuclear estrogen binding sites in the rat forebrain and autonomic medullary areas. Endocrinology. 2008;149:3306–12. doi: 10.1210/en.2008-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagen L, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–71. [PubMed] [Google Scholar]
- Milner TA, Veznedaroglu E. Ultrastructural localization of neuropeptide Y-like immunoreactivity in the rat hippocampal formation. Hippocampus. 1992;2:107–26. doi: 10.1002/hipo.450020204. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13:859–66. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB. Mapping your every move. Cerebrum: the Dana forum on brain science. 2014;2014:4. [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–59. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, et al. The formation of estrogens by central neuroendocrine tissues. Recent progress in hormone research. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- Nakamura NH, McEwen BS. Changes in interneuronal phenotypes regulated by estradiol in the adult rat hippocampus: A potential role for neuropeptide Y. Neuroscience. 2005;136:357–69. doi: 10.1016/j.neuroscience.2005.07.056. [DOI] [PubMed] [Google Scholar]
- Nakamura NH, Rosell DR, Akama KT, McEwen BS. Estrogen and ovariectomy regulate mRNA and protein of glutamic acid decarboxylases and cation-chloride cotransporters in the adult rat hippocampus. Neuroendocrinology. 2004;80:308–23. doi: 10.1159/000083657. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Karelus K, Bergman MD, Felicio LS. Neuroendocrine involvement in aging: evidence from studies of reproductive aging and caloric restriction. Neurobiol Aging. 1995;16:837–43. doi: 10.1016/0197-4580(95)00072-m. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Mitochondria as therapeutic targets of estrogen action in the central nervous system. Curr Drug Targets - CNS & Neurol Disorders. 2004;3:297–313. doi: 10.2174/1568007043337193. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Why are some neurons replaced in adult brain? J Neurosci. 2002;22:624–28. doi: 10.1523/JNEUROSCI.22-03-00624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Hojo Y, Inoue K, Matsui T, Kawato S, et al. Mild exercise increases dihydrotestosterone in hippocampus providing evidence for androgenic mediation of neurogenesis. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1210023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Kohlmaier JR, Alexander GM. Posttraining Intrahippocampal Estradiol Injections Enhance Spatial Memory in Male Rats: Interaction With Cholinergic Systems. Behav Neurosci. 1996;110:626–32. doi: 10.1037//0735-7044.110.3.626. [DOI] [PubMed] [Google Scholar]
- Parnavelas J, Lynch G, Brecha N, Cotman C, GLobus A. Spine loss and regrowth in hippocampus following deafferentation. Nature. 1974;248:71–73. doi: 10.1038/248071a0. [DOI] [PubMed] [Google Scholar]
- Parsons B, Rainbow TC, MacLusky N, McEwen BS. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci. 1982;2:1446–52. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak R, Rao BSS, Melchor JP, Chattarji S, McEwen B, Strickland S. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc Natl Acad Sci USA. 2005;102:18201–06. doi: 10.1073/pnas.0509232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW. Autoradiographic localization of radioactivity in rat brain after injection of tritiated sex hormones. Science. 1968;161:1355–6. doi: 10.1126/science.161.3848.1355. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Estrogens and brain function. Springer-Verlag 1980a [Google Scholar]
- Pfaff DW, editor. Estrogens and Brain Function. New York: Springer-Verlag; 1980b. [Google Scholar]
- Pfaff DW, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–58. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Kelter DT, McEwen BS, Waters EM, Milner TA. Hippocampal mossy fiber leu-enkephalin immunoreactivity in female rats is significantly altered following both acute and chronic stress. Journal of chemical neuroanatomy. 2014;55:9–17. doi: 10.1016/j.jchemneu.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ, Nguyen T-VV, Ramsden M, Yao M, Murphy MP, Rosario ER. Androgen cell signaling pathways involved in neuroprotective actions. Horm Behav. 2008;53:693–705. doi: 10.1016/j.yhbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton JV, Stovall DW. Reproductive aging, menopause, and health outcomes. Ann N Y Acad Sci. 2010;1204:169–78. doi: 10.1111/j.1749-6632.2010.05526.x. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug and alcohol dependence. 1999;53:223–30. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS. Sex differences in hippocampal estradiol-induced N-methyl-D-aspartic acid binding and ultrastructural localization of estrogen receptor-alpha. Neuroendocrinology. 2005a;81:391–99. doi: 10.1159/000089557. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Staub D, Jasnow AM, Karatsoreos IN, Thornton JE, McEwen BS. Dihydrotestosterone increases hippocampal N-methyl-D-aspartate binding but does not affect choline acetyltransferase cell number in the forebrain or choline transporter levels in the CA1 region of adult male rats. Endocrinology. 2005b;146:2091–97. doi: 10.1210/en.2004-0886. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD. Multivariate revisit to “sex beyond the genitalia”. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1523961113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick CN, Gibbs RB, Woolley CS. A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J Neurosci. 2003;23:4479–90. doi: 10.1523/JNEUROSCI.23-11-04479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schally AV, Arimura A, Kastin AJ. Hypothalamic regulatory hormones. Science. 1973;179:341–50. doi: 10.1126/science.179.4071.341. [DOI] [PubMed] [Google Scholar]
- Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology. 2000;150:337–46. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 2010;20:2560–7. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–57. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Shors TJ. A trip down memory lane about sex differences in the brain. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2016:371. doi: 10.1098/rstb.2015.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–97. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30:16137–48. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS. Estrogen administration increases neuronal responses to excitatory amino acids as a long-term effect. Brain Res. 1989;503:354–57. doi: 10.1016/0006-8993(89)91691-0. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–3. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–27. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, Hamilton T, Colmers WF. Neuropeptide Y in the dentate gyrus. Progress in brain research. 2007;163:285–97. doi: 10.1016/S0079-6123(07)63017-9. [DOI] [PubMed] [Google Scholar]
- Stumpf WE. Estradiol-concentrating neurons: topography in the hypothalamus by dry-mount autoradiography. Science. 1968;162:1001–3. doi: 10.1126/science.162.3857.1001. [DOI] [PubMed] [Google Scholar]
- Stumpf WE, Sar M. Steroid hormone target sites in the brain: The differential distribution of estrogen, progestin, androgen and glucocorticosteroid. J Steroid Biochem. 1976;7:1163–70. doi: 10.1016/0022-4731(76)90050-9. [DOI] [PubMed] [Google Scholar]
- Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, et al. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130:151–63. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Timiras P. Electrical activity during the estrous cycle of the rat: cyclic changes in limbic structures. Endocrinology. 1968;83:207–16. doi: 10.1210/endo-83-2-207. [DOI] [PubMed] [Google Scholar]
- Thomas P. Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol. 2008;29:292–312. doi: 10.1016/j.yfrne.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S, Li L, Riazi S, Halagappa VK, Ecelbarger CM. Sex and age result in differential regulation of the renal thiazide-sensitive NaCl cotransporter and the epithelial sodium channel in angiotensin II-infused mice. Am J Nephrol. 2009;30:554–62. doi: 10.1159/000252776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft D, Gorski J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci. 1966;55:1574–81. doi: 10.1073/pnas.55.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Drake CT, et al. Ovarian steroids modulate leu-enkephalin levels and target leu-enkephalinergic profiles in the female hippocampal mossy fiber pathway. Brain Res. 2008;1232:70–84. doi: 10.1016/j.brainres.2008.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Jacome L, et al. Hippocampal dynorphin immunoreactivity increases in response to gonadal steroids and is positioned for direct modulation by ovarian steroid receptors. Neuroscience. 2009a;159:204–16. doi: 10.1016/j.neuroscience.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Williams TJ, Chapleau JD, Waters EM, McEwen BS, et al. Ovarian steroids alter mu opioid receptor trafficking in hippocampal parvalbumin GABAergic interneurons. Exp Neurol. 2009b doi: 10.1016/j.expneurol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. Subcellular relationships between cholinergic terminals and estrogen receptor-α in the dorsal hippocampus. J Comp Neurol. 2003;463:390–401. doi: 10.1002/cne.10753. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Bangasser D, Van Bockstaele EJ. Sex-biased stress signaling: the corticotropin-releasing factor receptor as a model. Mol Pharmacol. 2013;83:737–45. doi: 10.1124/mol.112.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hartesveldt C, Joyce J. Effects of estrogen on the basal ganglia. Neurosci Biobeha Rev. 1986;10:1–14. doi: 10.1016/0149-7634(86)90029-1. [DOI] [PubMed] [Google Scholar]
- van Kempen T, Marques-Lopes J, Glass MJ, Milner TA. Arterial hypertension and brain as an end organ. Scarborough, Ontario: Research Signpost/Transworld Research Network; 2016. pp. 195–221. [Google Scholar]
- Van Kempen TA, Dodos M, Woods C, Marques-Lopes J, Justice NJ, et al. Sex differences in NMDA GluN1 plasticity in rostral ventrolateral medulla neurons containing corticotropin-releasing factor type 1 receptor following slow-pressor angiotensin II hypertension. Neuroscience. 2015a;307:83–97. doi: 10.1016/j.neuroscience.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kempen TA, Gorecka J, Gonzalez AD, Soeda F, Milner TA, Waters EM. Characterization of neural estrogen signaling and neurotrophic changes in the accelerated ovarian failure mouse model of menopause. Endocrinology. 2014;155:3610–23. doi: 10.1210/en.2014-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kempen TA, Milner TA, Waters EM. Accelerated ovarian failure: a novel, chemically induced animal model of menopause. Brain Res. 2011;1379:176–87. doi: 10.1016/j.brainres.2010.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kempen TA, Narayan A, Waters EM, Marques-Lopes J, Iadecola C, et al. Alterations in the subcellular distribution of NADPH oxidase p47 in hypothalamic paraventricular neurons following slow pressor Angiotensin II hypertension in female mice with accelerated ovarian failure. J Comp Neurol. 2015b doi: 10.1002/cne.23944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–18. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Anrather J, Glass MJ, Tarsitano MJ, Zhou P, et al. Nox2, Ca2+, and protein skinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius. Hypertension. 2006;48:482–9. doi: 10.1161/01.HYP.0000236647.55200.07. [DOI] [PubMed] [Google Scholar]
- Wang G, Anrather J, Huang J, Speth RC, Pickel VM, Iadecola C. NADPH oxidase contributes to angiotensin II signaling in the nucleus tractus solitarius. J Neurosci. 2004;24:5516–24. doi: 10.1523/JNEUROSCI.1176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Coleman CG, Chan J, Faraco G, Marques-Lopes J, et al. Angiotensin II slowpressor hypertension enhances NMDA currents and NOX2-dependent superoxide production in hypothalamic paraventricular neurons. Am J Physiol Regul Integr Comp Physiol. 2013;304:R1096–106. doi: 10.1152/ajpregu.00367.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Torres-Revon AT, McEwen BS, Milner TA. Extranuclear progestin receptor immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2008;511:34–46. doi: 10.1002/cne.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling M, Christ M, Thiesen K. Membrane receptors for aldosterone: a novel pathway for mineralocorticoid action. Am J Physiol. 1992;263:E974. doi: 10.1152/ajpendo.1992.263.5.E974. [DOI] [PubMed] [Google Scholar]
- Weiland NG. Estradiol selectively regulates agonist binding sites on the N-methyl-D-aspartate receptor complex in the CA1 region of the hippocampus. Endocrinology. 1992;131:662–68. doi: 10.1210/endo.131.2.1353442. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Torres-Reveron A, Chapleau JD, Milner TA. Hormonal regulation of delta opioid receptor immunoreactivity in interneurons and pyramidal cells in the rat hippocampus. Neurobiol Learn Mem. 2011;95:206–20. doi: 10.1016/j.nlm.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witelson SF, Glezer II, Kigar DL. Women have greater density of neurons in posterior temporal cortex. J Neurosci. 1995;15:3418–28. doi: 10.1523/JNEUROSCI.15-05-03418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP. Organization of the Entorhinal-Hippocampal System: A Review of Current Anatomical Data. Hippocampus. 1993;3:33–44. [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA. 1998;95:4066–71. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley C, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–39. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley C, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–59. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Hormonal activation of the striatum and the nucleus accumbens modulates paced mating behavior in the female rat. Horm & Behav. 1997;32:114–24. doi: 10.1006/hbeh.1997.1412. [DOI] [PubMed] [Google Scholar]
- Xie CW, Lewis DV. Opioid-mediated facilitation of long-term potentiation at the lateral perforant path-dentate granule cell synapse. Journal of Pharmacology & Experimental Therapeutics. 1991;256:289. [PubMed] [Google Scholar]
- Xue B, Johnson AK, Hay M. Sex differences in angiotensin II- and aldosterone-induced hypertension: the central protective effects of estrogen. Am J Physiol Regul Integr Comp Physiol. 2013;305:R459–63. doi: 10.1152/ajpregu.00222.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin IIinduced hypertension in conscious mice. American journal of physiology Heart and circulatory physiology. 2005;288:H2177–84. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- Young W. The Hormones and Mating Behavior. In: Young W, editor. Sex and Internal Secretions. Baltimore: Williams and Wilkins; 1961. pp. 1173–239. [Google Scholar]
- Young WC, Goy RW, Phoenix CH. Hormones and Sexual Behavior. Science. 1964;143:212–8. doi: 10.1126/science.143.3603.212. [DOI] [PubMed] [Google Scholar]
- Yuen GS, McEwen BS, Akama KT. LIM kinase mediates estrogen action on the actin depolymerization factor Cofilin. Brain Res. 2010 doi: 10.1016/j.brainres.2010.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond R, McEwen BS. Selective retention of oestradiol by cell nuclei in specific brain regions of the ovariectomized rats. J Neurochem. 1970;17:889–99. doi: 10.1111/j.1471-4159.1970.tb02242.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circulation research. 2004;95:210–6. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal Ca1 dendrites. J Neurosci. 2003;23:2340–47. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]