Abstract
The use of tobacco products represents a major public health concern, especially among women. Epidemiological data have consistently demonstrated that women have less success quitting tobacco use and a higher risk for developing tobacco-related diseases. The deleterious effects of nicotine are not restricted to adulthood, as nicotinic acetylcholine receptors regulate critical aspects of neural development. However, the exact mechanisms underlying the particular sensitivity of women to develop tobacco dependence have not been well elucidated. In this review, we show that gonadal hormone-mediated sexual differentiation of the brain may be an important determinant of sex differences in the effects of nicotine. We highlight direct interactions between sex steroid hormones and ligand-gated ion channels critical for brain maturation, and discuss the extended and profound sexual differentiation of the brain. We emphasize that nicotine exposure during the perinatal and adolescent periods interferes with normal sexual differentiation and can induce long-lasting, sex-dependent alterations in neuronal structure, cognitive and executive function, learning and memory, and reward processing. We stress important age and sex differences in nicotine’s effects and emphasize the importance of including these factors in preclinical research that models tobacco dependence.
Keywords: Nicotinic acetylcholine receptors, hormones, tobacco, prenatal, neonatal, adolescent
Graphical Abstract
Sex differences in the effects of nicotine may be due to gonadal hormone-mediated sexual differentiation of the brain during the perinatal and adolescent periods. Exposure to nicotine during these developmental periods can produce long-lasting, sex-dependent changes in neuronal structure and function by inhibiting aromatase or inducing corticosterone release, for example.
Introduction
Nicotine exposure from tobacco products or electronic cigarettes (e-cigarettes) has numerous deleterious effects on overall health. Smoking remains one of the leading causes of preventable death in the United States despite heightened awareness of its dangers (Centers for Disease Control and Prevention 2011), and women seem to be particularly vulnerable. Women have lower success with cessation than men (Perkins 2001; Piper et al. 2010), and also have a higher risk of developing tobacco-related morbidity and mortality (Langhammer et al. 2003; Laviolette et al. 2007; Kiyohara and Ohno 2010; Allen et al. 2014). Preclinical research has also demonstrated that females are more sensitive than males to the rewarding effects of nicotine, as well as more sensitive to the aversive effects of nicotine withdrawal (O’Dell and Torres 2014).
In addition to negatively impacting overall health in adulthood, a large body of literature has indicated that nicotine can have detrimental effects on the brain throughout development, with important sex differences. Nicotine’s primary effects are mediated by activation and desensitization of neuronal nicotinic acetylcholine receptors (nAChRs), pentameric ligand-gated ion channels consisting of α2-α10 and β2-β4 subunits (Dwyer et al. 2009). Cholinergic activity at nAChRs is critical for neuronal path-finding, patterning and organization of sensory systems, and regulation of neurochemical systems involved in tobacco addiction (Lipton et al. 1988; Pugh and Berg 1994; Rossi et al. 2001; Feller 2002; Gotti and Clementi 2004; Dwyer et al. 2009). Exposure to nicotine during these critical developmental events can have profound and long-lasting effects. Approximately 10% of women continue to smoke during pregnancy (Tong et al. 2013), despite increased risk for sudden infant death syndrome (SIDS), attentional and cognitive deficits, and drug addiction in offspring (McCartney et al. 1994; Weissman et al. 1999; Buka et al. 2003; Dietz et al. 2010; Goldschmidt et al. 2012). Nicotine’s deleterious effects are not limited to the perinatal period, however, but extend to adolescence, a developmental period marked by continued maturation of brain regions critical for reward processing, learning and memory, and executive function (Yuan et al. 2015). Even brief exposure to nicotine during the adolescent period can have long-lasting effects on neurochemistry and behavior, including decreased cognitive function and enhanced drug reward (Jacobsen et al. 2005; Treur et al. 2015; Yuan et al. 2015).
The exact mechanisms underlying women’s enhanced vulnerability to tobacco dependence have not been fully elucidated. However, as gonadal steroids coordinate sexual differentiation of the brain and can interact directly with ligand-gated ion channel receptors, they are likely determinants of sex differences in nicotine reward and reinforcement. The classical organizational-activational hypothesis says that gonadal hormones differentiate the brain into male and female early in development, while activity of estrogens, progesterone, and androgens activate neural circuits involved in reproductive function after puberty (Arnold 2009). However, a large body of literature has emerged in recent years highlighting the important role of gonadal hormones in modulating a variety of behaviors, including learning, memory, and drug reward and reinforcement. Further, in contrast to the short span of sexual differentiation suggested in the original hypothesis, recent data describe continued sexual differentiation throughout puberty and adolescence (Sisk and Zehr 2005; McCarthy and Arnold 2011).
In this review, we discuss critical interactions between gonadal hormones and neurochemical systems mediating nicotine reward and reinforcement. We argue that sexual differentiation, beginning in the perinatal period and persisting into adolescence, is an important determinant of sex differences in brain structure and function that underlie females’ unique sensitivity to nicotine.
Sexual differentiation, gonadal steroids, and ligand-gated ion channels
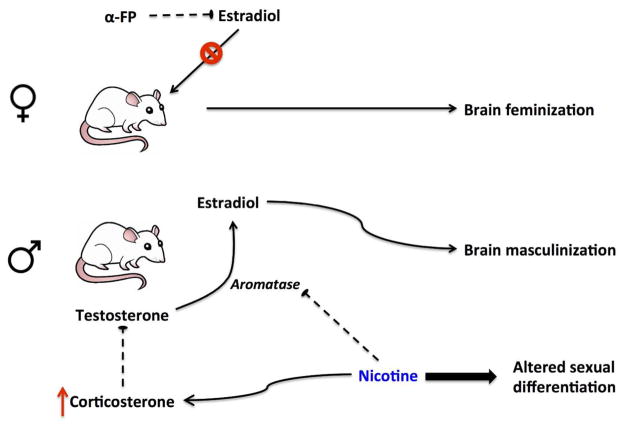
Sex differences in nicotine responses likely first emerge as a result of sexual differentiation of the brain. The earliest stages of sexual differentiation are determined by the SRY gene on the Y chromosome, which initiates testes development during the prenatal period. Absence of the SRY gene leads to the ‘default’ female phenotype, precluding masculinization and defeminization of the brain. Fetal masculinization then occurs under the control of gonadal hormones, with a surge of androgens that peaks at the end of the embryonic period in males before quickly falling after the first postnatal day (Konkle and McCarthy 2011). Testosterone is converted to estradiol via aromatase, an enzyme that is abundant and active during the perinatal androgen surge (George and Ojeda 1982; Roselli and Resko 1993) and is highly expressed in sexually dimorphic brain regions (Konkle and McCarthy 2011). In contrast, absence of the androgen surge and sequestration of excess estradiol by α-fetoprotein in the female results in early feminization of the brain.
Sex steroid hormones signal predominantly by binding to intracellular receptors, inducing dimerization and translocation to the nucleus where they bind to hormone response elements on DNA to regulate gene transcription (King and Greene 1984; O’Malley and Tsai 1992). However, recent data suggest that estrogens can induce more rapid signaling, likely via membrane-bound ERα receptors (Schultz et al. 2009), and that sex steroid hormones and their metabolites can interact directly with ligand-gated ion channels to influence excitatory and inhibitory neurotransmission (Hu et al. 2006). Ligand-gated ion channels coordinate multiple aspects of brain maturation and are influenced by gonadal hormones, providing one potential mechanism by which sex affects nicotine responses.
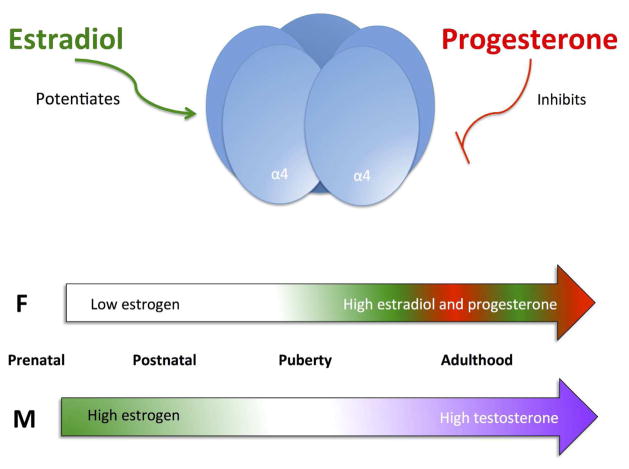
Estrogen and progesterone act as allosteric modulators of GABA, glutamate, and acetylcholine receptors, often with opposing effects. In particular, estrogen is a negative allosteric modulator of GABAA receptors (Murphy et al. 1998), whereas progesterone and its neuroactive metabolites (i.e., allopregnanalone and pregnanalone) promote inhibitory neurotransmission via enhancement of GABAA receptor function (Paul and Purdy 1992; Lan and Gee 1994; van Wingen et al. 2008; Gunn et al. 2011; Puia et al. 2012) and inhibition of glutamate-induced excitation and NMDA receptor binding (Smith et al. 1987; Cyr et al. 2000). The specific effects of circulating hormones or their neuroactive metabolites on ligand-gated ion channels may depend on the overall hormone milieu. During puberty, when the hypothalamic pituitary gonadal axis reawakens and cyclical surges in estrogens and androgens begin, progesterone actually acts as a negative allosteric modulator of GABAA receptors (Smith et al. 2009). This effect of progesterone is only evident during puberty, as pre- and post-pubertal rodents show positive allosteric modulation of GABAA receptors.
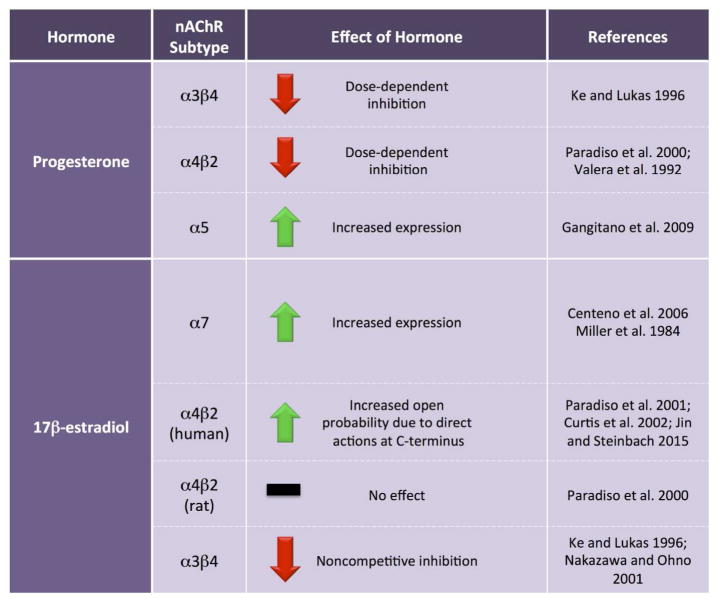
Sex steroids also modulate nicotinic acetylcholine receptor (nAChR) function (Figure 1). Progesterone and its A-ring metabolites dose-dependently inhibit function of α3β4 and α4β2 nAChRs (Valera et al. 1992; Ke and Lukas 1996; Paradiso et al. 2000), although physiological levels of progesterone may not be sufficient to inhibit α4β2 nAChRs (Paradiso et al. 2000). In contrast, progesterone may enhance the function of α5-containing nAChRs by binding to a progesterone response element in the promoter region to increase α5 mRNA levels in cultured cells and in the brains of ovariectomized female rats (Gangitano et al. 2009). 17β-estradiol increases α7 nAChR subunit expression in the dorsal raphe and locus coeruleus of macaques (Centeno et al. 2006) and potentiates human, but not rat, α4β2 nAChRs by direct actions at the C-terminus of the α4 subunit that result in an increase in the probability of open conformation (Figure 2; Paradiso et al. 2000; Paradiso et al. 2001; Curtis et al. 2002; Jin and Steinbach 2015). Both rodent and recombinant human α3β4 nAChRs are noncompetitively inhibited by estrogens, with more profound inhibition occurring after chronic exposure (Ke and Lukas 1996; Nakazawa and Ohno 2001). Alpha 7 nAChRs, as measured by α-bungarotoxin (α-BTX) binding, can be regulated by estradiol, as pre-pubertal gonadectomy in females, but not males, decreases α-BTX binding in the suprachiasmatic nucleus. Binding can be normalized by semi-chronic (3 week) or long-term (5 week) estradiol replacement, while short-term replacement with estradiol has no effect (Miller et al. 1984).
Figure 1. Ovarian hormones influence nicotinic acetylcholine receptors.
Progesterone and estradiol modulate both expression and function of multiple nAChR subtypes, often with opposing effects. nAChR = nicotinic acetylcholine receptor
Figure 2. Nicotinic acetylcholine receptor (nAChR) activity is modulated by different sex hormones throughout development.
Progesterone allosterically inhibits α4 nAChR subunits via a site outside the membrane pore (Valera et al., 1992), whereas estradiol potentiates the activity of human α4-containing nAChRs by increasing the opening probability (Curtis et al., 2002). The abundance of these steroids changes throughout development and across sexes. How this complex relationship between sex, nAChRs and development affects brain maturation and function is still being elucidated.
Early sexual differentiation, nAChRs, and perturbation by nicotine
The gestational period is a dynamic and critical developmental window when sexual differentiation is initiated and the nervous system begins to form under the control of the cholinergic system. Even brief exposure to nicotine during this period can produce long-lasting, sex-dependent alterations in neuronal structure and function.
Development of the cholinergic system
Cholinergic activity is observed as early as the gastrulation phase with the detection of choline acetyltransferase (ChAT) and acetylcholinesterase in the fetus (Mansvelder and Role 2006), and is implicated in morphogenic cell movements during early gestation (Lauder and Schambra 1999). Nicotine’s actions are mediated primarily by activation and desensitization of nAChRs, which appear during the first trimester in human brain and on the first day the rodent central nervous system forms, gestational day (G) 11. Each nAChR subtype has distinct and evolving patterns of distribution, implicating their differential roles in development and highlighting potential avenues for nicotine-associated perturbation (Dwyer et al. 2008).
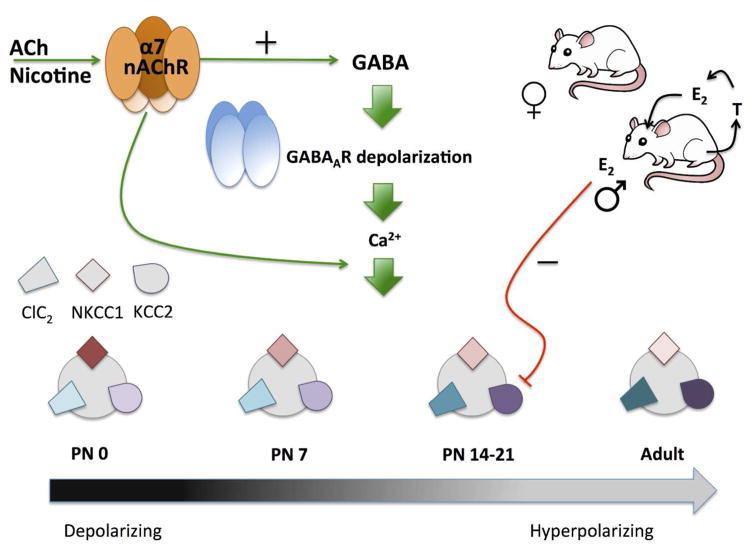
nAChR activity is important for a variety of developmental functions and, in many cases, serves a critical ontogenetic role (for review, please see (Dwyer et al. 2009). Although most of the ontogenetic studies of nAChR function do not differentiate between sexes, one early study did note that α-BTX binding to α7 nAChRs in the mouse corticomedial amygdala was higher in males than females at postnatal day (P) 14, a sex difference that was eliminated by neonatal castration of males (Arimatsu 1983). Furthermore, nAChR-mediated excitation of corticothalamic neurons in layer VI of prefrontal cortex is significantly larger in male rats during the first postnatal month when prefrontal circuitry underlying attention is actively maturing (Alves et al. 2010). Sex differences have also been noted in the role of nAChRs in the development of rodent hippocampus (Figure 3). During gestation and the early postnatal period, hippocampal GABA activity evokes an excitatory cell response as a result of a high internal Cl− concentration produced by predominance of the immature Na+/K+/Cl− cotransporter 1 (NKCC1) over the mature K+/Cl− cotransporter 2 (KCC2) (Rivera et al. 1999; Ben-Ari 2002). During the first two postnatal weeks, GABA switches to inhibitory signaling as levels of NKCC1 decline and KCC2 rise, a process that occurs significantly earlier in females than males (Nuñez and McCarthy 2007; Galanopoulou 2008). This switch in cotransporter expression is dependent on calcium influx into the cell, which is controlled by increased depolarization and activation of receptors with high calcium permeability, such as the α7 nAChR, which shows peak expression in hippocampus during this period (Galanopoulou et al. 2003; Galanopoulou 2008). Activation of presynaptic α7 nAChRs during this time also stimulates GABA release, as measured by giant depolarizing potentials (Maggi et al. 2001). Thus, the interaction of nAChR signaling with GABAergic transmission has a sex-dependent impact on early hippocampal development. Similar nAChR regulation of GABA signaling has been observed in other neural regions (Liu et al. 2006), some of which may exhibit sex-dependent transmitter regulation (McCarthy et al. 2002).
Figure 3. The role of α7 nAChRs in regulating GABAA receptor signaling in rodent neonatal hippocampus.
The GABAA receptor switches from depolarizing to hyperpolarizing during the early neonatal period as a result of changes in intracellular Cl−. This change, which is mediated by a shift in the expression of the Cl− cotransporters from NKCC1 to KCC2, occurs later in males than females as a result of high estradiol in males having an inhibitory effect on KCC2 expression. Levels of the Cl− channel, ClC2, and the Cl− cotransporters NKCC1 and KCC2, are pictured, with darker shades indicating higher expression levels. Activation of α7 nAChRs increases intracellular calcium levels both directly and through increased GABA release and GABAA receptor-induced depolarization, resulting in higher expression of KCC2. This regulates the switch of GABAA receptor signaling from depolarization to hyperpolarization. ACh = acetylcholine. T = testosterone. E2= estradiol.
Nicotine, sex, and nAChRs
Many studies have examined the long-term consequences of prenatal nicotine exposure (Dwyer et al. 2008), but few have examined potential sex differences in exposure outcomes (Table 1). However, the clinical literature on maternal smoking does suggest that such differences occur (Weissman et al. 1999). Although prenatal nicotine exposure induces complex alterations in brain structure and function, it has been shown to have more significant deleterious effects on cholinergic and serotonergic markers and β2-adrenergic function in males than females (Slotkin et al. 2007). Prenatal nicotine exposure also induces sex-dependent changes in myelin-associated gene and protein expression (Cao et al. 2013), and in dendritic complexity of the prefrontal cortex and nucleus accumbens (Mychasiuk et al. 2013). Subsequent drug reward is also sex-dependently altered by in utero nicotine exposure, with exposed males exhibiting increased preference for oral nicotine but no effect of maternal nicotine in females (Klein et al. 2003). Gestational nicotine may also alter normal sex-dependent nicotine responses by inhibiting aromatase activity (Barbieri et al. 1986). Prenatal nicotine also increases corticosterone levels via direct action on the adrenal gland, which deflates the pivotal testosterone surge during the perinatal period (Figure 4; von Ziegler et al. 1991; Sarasin et al. 2003). This nicotinic alteration of sex hormones levels leads to long-term changes in behavior, such as eliminating sex-specific sucrose preferences (Lichtensteiger and Schlumpf 1985).
Table 1.
Sex differences in nicotine effects across development in rodents
| Developmental period of exposure | Measure | Effect of nicotine | Citations |
|---|---|---|---|
| Gestation | Adrenergic function | Decreased β2-adrenergic-mediated adenylyl cyclase activity in males only | Slotkin et al. 2007 |
| Cholinergic function | In males, decreased ChAT activity and HC3
binding in the cerebral cortex; increased HC3 binding in the
hippocampus; decreased basal adenylyl cyclase signaling. In females, increased HC3 binding in the midbrain; decreased basal adenylyl cyclase signaling (less profound than males) |
Slotkin et al. 2007 | |
| Serotonergic function | Reduced 5-HT1A binding and increased 5-HT2 binding in males only | Slotkin et al. 2007 | |
| Myelin-associated gene and protein expression | In males, upregulation of myelin-associated
genes (e.g., Mbp, Plp1,
Gjc3, Mobp, Aspa) in the PFC, CPu,
and NAc during adolescence that generally normalized by
adulthood. In females, downregulation or normal expression of myelin-associated genes (e.g., Plp1, Gjc3, Mobp, Mbp, Cnp, Mal, Mog) in PFC, CPu, and NAc during adolescence. Downregulation in adult CPu of females |
Cao et al. 2013 | |
| Dendritic complexity | In males, increased complexity in AID and NAc
but decreased complexity in apical field of Cg3; increased spine density
everywhere but NAc; increased dendritic length in NAc and basilar Cg3
but decreased length in basilar PAR. In females, increased complexity in NAc but decreased complexity in AID; increased spine density in AID and PAR but decreased in Cg3 and NAc; reduced dendritic length in NAc, Cg3 and PAR |
Mychasiuk et al. 2013 | |
| Expression of KCC2 cotransporter | Greater in males | Damborsky and Winzer-Serhan 2012 | |
| Excitation of adult hippocampal CA1 | Increased excitation as measured by fEPSP slope at higher voltage inputs in males only | Damborsky et al. 2012 | |
| Sucrose preference | Eliminates normal sex difference (i.e., females have higher preference than males) | Lichtensteiger and Schlumpf 1985 | |
| Nicotine reward | Increased preference for oral nicotine in adolescent males only | Klein et al. 2003 | |
| Cognitive function | Mild spatial deficit in females only | Eppolito and Smith 2006 | |
| No change | Huang et al. 2007 | ||
| Adolescence | Nicotine self-administration | In females, a greater percentage acquire self-administration, acquisition is more rapid, and intake is higher compared to males | Lynch 2009; Li et al. 2014; Sanchez et al. 2014 |
| Adolescent rats of both sexes had higher nicotine self-administration than adults but the effect was more pronounced in adolescent males; responding in males decreased with age but did not in females | Levin et al. 2011 | ||
| Acetaldehyde enhances nicotine self-administration in both sexes, but only males reduce responding with age | Belluzzi et al. 2005; Park et al. 2007 | ||
| Females are more sensitive to yohimbine-induced increases in motivation for nicotine under progressive ratio responding | Li et al. 2014 | ||
| Nicotine reward | Adolescent males displayed enhanced nicotine conditioned place preference compared to adults, but adolescent females were more sensitive than adults to low dose nicotine without a difference in magnitude of preference | Torres et al. 2009 | |
| Adolescent males had higher nicotine-induced preference to a mid-range dose of nicotine than adolescent females | Lenoir et al. 2015 | ||
| Motivation for nicotine | Females display higher motivation under progressive ratio testing. Greater motivation is positively correlated with the ratio of estradiol:progesterone and negatively correlated with levels of progesterone alone; does not seem to vary across estrous cycle | Lynch 2009; Donny et al. 2000; Li et al. 2014 | |
| Nicotine withdrawal-related craving | Higher in females | Dickmann et al. 2009 | |
| Anxiolytic effects of acute nicotine | More pronounced in males | Damaj et al. 2001; Cao et al. 2010 | |
| Anxiety-like behavior during nicotine withdrawal | Late-emerging (P60) depression of locomotor activity in open-field testing in females with early adolescent nicotine exposure; no difference in males | Trauth et al. 2000 | |
| Decreased locomotor activity during short-term withdrawal from nicotine in females that normalized after 30 days; males had increased anxiety- and depressive-like activity that emerged after 30 days of withdrawal | Thanos et al. 2013 | ||
| Physical symptoms of nicotine withdrawal | No sex differences | Kota et al. 2007; Kota et al. 2008; Torres et al. 2013 | |
| Nicotine-induced changes in nAChR binding | Increased nAChR binding in striatum of adolescent females with no difference in adolescent males | Lenoir et al. 2015 | |
| Adulthood | Nicotine self-administration | Higher responding and faster acquisition in females | Donny et al. 2000; Lynch 2009 |
| Faster acquisition in males | Swalve et al. 2016 | ||
| No sex differences in acquisition rates | Feltenstein et al. 2012 | ||
| Nicotine intake | Higher intake during maintenance phase of self-administration | Donny et al. 2000; Chaudri et al. 2005; Rezvani et al. 2008; Grebenstein et al. 2013 | |
| No sex differences | Feltenstein et al. 2012; Swalve et al. 2016 | ||
| Nicotine metabolism | Repeated nicotine produces higher plasma levels in females than males | Harrod et al. 2007 | |
| Salience of non-drug conditioned cues | Higher in females during self-administration, extinction, and possibly withdrawal/craving | Perkins et al. 1999; Chaudri et al. 2005 | |
| Withdrawal | More robust in females than males, likely due to ovarian hormones | Torres et al. 2015; Torres and O’Dell 2016 | |
| Nicotine reward | Female rats are less sensitive than males; higher doses of nicotine are required to produce significant place preference | Lenoir et al. 2015 | |
| Females display place preference over a wider range of nicotine doses than males | Torres et al. 2009 | ||
| No significant preference at any tested dose | Yararbas et al. 2010 | ||
| Females had greater magnitude of nicotine place preference than males | Igiegas et al. 2009 | ||
| Anxiety-like behavior | Greater in females than males in open-field and elevated plus maze | Elliott et al. 2004; Caldarone et al. 2008; Cao et al. 2010 | |
| Corticosterone levels | Nicotine stimulates HPA axis activity and corticosterone release more in females than males | Cao et al. 2010; Gentile et al. 2011 |
Abbreviations: AID = agranular insular cortex, CPu = caudate putamen, ChAT = choline acetyltransferase, Cg3 = layer III of anterior cingulate cortex, fEPSP = field excitatory postsynaptic potential, HC3 = [3H]hemicholinium, HPA axis = hypothalamic pituitary adrenal axis, KCC2 = K+/Cl− cotransporter 2, nAChR = nicotinic acetylcholine receptor, NAc = nucleus accumbens, PAR = parietal cortex, P = postnatal day, PFC = prefrontal cortex
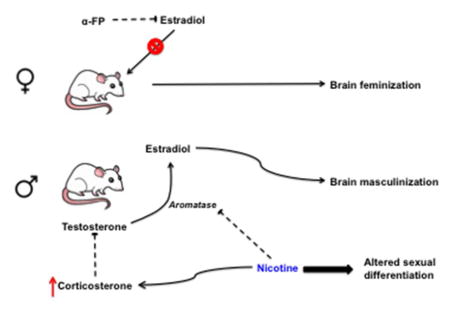
Figure 4. Schematic of fetal brain sexual differentiation and the effects of nicotine.
Male sexual differentiation is induced by estradiol, which is produced via aromatization of testosterone. Females are protected from circulating maternal estrogen by α-fetoprotein (α-FP). Nicotine inhibits aromatase activity, leading to a decrease in male brain estradiol. Nicotine also increases corticosterone levels during gestation, which then inhibits testosterone production.
Nicotine exposure during the early neonatal period of hippocampal maturation induces increased expression of the KCC2 cotransporter in male pups, a change that may influence the timing of the switch in GABA transmission from excitatory to inhibitory (Figure 3; Damborsky and Winzer-Serhan 2012). The long-term consequence of neonatal nicotine exposure is enhanced excitation in the adult hippocampal CA1, which is greater in males than females (Damborsky et al. 2012). Whereas this group has reported that neonatal nicotine treatment does not affect later cognitive function (Huang et al. 2007), others have noted that perinatal nicotine exposure produces a mild spatial learning deficit in in females (Eppolito and Smith 2006).
Adolescence: a second critical period of brain sexual differentiation and nicotine sensitivity
Continued sexual differentiation of the brain
Adolescence is a developmental period marked by dynamic maturation of limbic regions mediating reward and reinforcement, learning and memory, and executive function (Spear 2000; Yuan et al. 2015). It is also the time of peak onset of tobacco use, with the vast majority of smokers initiating use before the age of eighteen (Substance Abuse and Mental Health Services Administration 2011). The continued organizational effects of gonadal hormones during this period solidify the uniqueness of the male and female brain and are reflected by the emergence of many sex differences in nicotine reward and reinforcement. Although our emphasis in this review is on sex steroid hormones, it is important to note that puberty and adolescence are not synonymous. Puberty lasts approximately 5 years in humans (Sun et al. 2002) and 10–20 days in rodents (Sisk and Zehr 2005; Schneider 2013). Adolescence is a protracted and elaborate developmental period that extends beyond puberty (Spear 2000), when a significant portion of brain maturation occurs independently of pubertal influences (Yuan et al. 2015).
Adolescence is a period of increased responsiveness to both stress- and sex-hormones (Sinclair et al. 2014). Sex hormones continue to have organizational effects, while activational effects also emerge. Multiple brain sexual dimorphisms develop during adolescence, including regions beyond those involved in sexual behavior and reproduction. Both androgens and estrogens modulate white matter volume during adolescence, with estrogens having an inhibitory influence in both rats (Yates and Juraska 2008) and humans (Herting et al. 2012; Herting et al. 2014), whereas testosterone levels in adolescent boys are positively associated with white matter development (Herting et al. 2012). Both primary visual and medial prefrontal cortices show enhanced cell death in female but not male rodents during adolescence (Nuñez et al. 2001; Willing and Juraska 2015), and this divergence is prevented by pre-pubertal ovariectomy (Nuñez et al. 2001; Nuñez et al. 2002).
Sexual differentiation of major neurotransmitter systems also occurs during this period (Table 2). The noradrenergic locus coeruleus, for example, becomes a female-biased structure during late adolescence, with females exhibiting a more sustained increase in cell number as compared to males (Pinos et al. 2001). This sex difference is eliminated by neonatal androgen treatment of females, but not orchiectomy of males (Guillamón et al. 1988). The cholinergic system becomes sexually diergic almost entirely as a result of pubertal surges in gonadal hormones rather than perinatal hormone effects. Ovariectomy reduces ChAT mRNA and function, as well as choline uptake, in female rats, and this can be reversed by estradiol replacement (Gibbs et al. 1994; Yamamoto et al. 2007). Females also have greater basal acetylcholine release in the medial prefrontal cortex, premotor cortex, and supplementary motor cortex than males, likely due to sex differences in the number of ChAT-positive cells in the nucleus basalis of Meynert (Gibbs 1997; Takase et al. 2007; Takase et al. 2009). Furthermore, endogenous or supplemental estrogen enhances serotonin-mediated increases in acetylcholine release, likely through a 5-HT1A receptor mechanism (Matsuda et al. 2002).
Table 2.
Adolescent sexual differentiation of major neurotransmitter systems
| Neurotransmitter system | Sex difference | Measure | Mechanism | Citations |
|---|---|---|---|---|
| Norepinephrine | F > M | Size of locus coeruleus | More sustained increase in cell number. Eliminated by neonatal androgen treatment in females; unaffected by orchiectomy of males | Pinos et al. 2001; Guillamón et al. 1988 |
| Acetylcholine | F > M | Basal ACh release in mPFC, premotor, and supplementary motor cortices | Due to sex differences in the number of ChAT-positive cells in the nucleus basalis of Meynert | Gibbs 1997; Takase et al. 2007; Takase et al. 2009 |
| Dopamine | F > M | Number of dopamine neurons in the VTA and SN | Androgens suppress midbrain dopamine neuron number; exact mechanism unknown | Johnson et al. 2010a; Johnson et al. 2010b |
| M > F | Nucleus accumbens D1 receptor levels | Greater increase in receptor density. Not influenced by gonadal hormones | Andersen et al. 1997; Andersen and Teicher 2000; Andersen et al. 2002; Azam et al. 2007 | |
| F > M | Electrical or psychostimulant-evoked DA release | Faster release than uptake in females that is perturbed by electrical or psychostimulant stimulation | Walker et al. 2000; Kuhn et al. 2010 | |
| F > M | DA dynamics in striatum | Positively modulated by estrogens | Morissette and Di Paolo 1993; Becker 1999; Becker and Hu 2008 | |
| M > F | DA dynamics in mPFC | Positively modulated by androgens | Adler et al. 1999; Kritzer and Pugach 2001; Kritzer 2003 | |
|
M − F + |
NMDA receptor influence on mesocortical DA | Differential NMDAR-mediated activation of PFC pyramidal cells projecting to the VTA, possibly regulated by androgen levels | Locklear et al. 2016 | |
| M > F | Soma size of DA neurons | Determined by genetic sex; not influenced by gonadal hormones | Beyer et al. 1991; Kolbinger et al. 1991 | |
| M > F | nAChR-mediated ventral striatal DA release | Higher maximum nicotine-stimulated [3H]DA release at P30 and higher sensitivity at P40, as compared to adults. No age-related changes in nicotine efficacy or potency in females | Azam et al. 2007 |
Abbreviations: ACh = acetylcholine, ChAT = choline acetyltransferase, DA = dopamine, F = female, M = male mPFC = medial prefrontal cortex, NMDA/NMDAR = N-methyl-D-aspartate receptor, P = postnatal day, SN = substantia nigra, VTA = ventral tegmental area.
The dopaminergic system is critically involved in mediating the rewarding properties of drugs of abuse and exhibits prolonged maturation during adolescence (Yuan et al. 2015), as well as a variety of sex differences that are determined at varying times across development. Dopamine neurons in the ventral tegmental area and substantia nigra are more numerous in females than males, a sex difference that is eliminated by gonadectomy of males (Johnson et al. 2010a; Johnson et al. 2010b). Striatal dopamine receptors also exhibit major overproduction followed by pruning in adolescent males, with only a minor increase seen in females. By adulthood, dopamine receptor levels in the dorsal striatum are similar across sexes, whereas males have a higher density of D1 receptors in the nucleus accumbens. Gonadal steroid hormones do not contribute to the pruning of D2 receptors observed during adolescence in males (Andersen et al. 1997; Andersen and Teicher 2000; Andersen et al. 2002; Azam et al. 2007).
While basal levels of dopamine are comparable between males and females, electrical or psychostimulant evoked-dopamine release is greater in the dorsal striatum of females (Walker et al. 2000; Kuhn et al. 2010). Striatal dopamine dynamics are positively modulated by estrogens (Morissette and Di Paolo 1993; Becker 1999; Becker and Hu 2008), whereas androgens have a greater modulatory role over dopamine in the frontal cortex (Adler et al. 1999; Kritzer and Pugach 2001; Kritzer 2003). Interestingly, it seems that genetic sex, rather than gonadal hormones per se, determine sex differences in dopaminergic projections to the forebrain (Beyer et al. 1991; Kolbinger et al. 1991). In the prefrontal cortex, glutamatergic inputs have a unique, sex-dependent influence over dopamine function. In males, signaling at AMPA and NMDA receptors stimulate and inhibit, respectively, mesocortical dopamine release, suggesting that actions at these receptors oppose one another to balance dopamine system regulation. In contrast, both glutamate receptor subtypes appear to drive dopamine release in the female prefrontal cortex (Locklear et al. 2016).
We have shown alterations in the pharmacology of nAChR-mediated striatal dopamine release across the developmental spectrum, from the fetal period on (Azam et al. 2007). In particular, in the transition from adolescence to adulthood, there is a complex pattern of functional maturation of nAChRs in ventral, but not dorsal, striatum. In males, but not females, there are significant changes in both nicotine potency and efficacy during this developmental period. Chronic nicotine treatment during this period has also been shown to produce acute and long-term changes in dopamine systems that are more pronounced in males (Trauth et al. 2001).
The emergence of sex differences in nicotine reward and reinforcement
Continued divergence of the male and female brain during adolescence contributes to the sex differences in nicotine’s effects that typically emerge during this period. There is relatively little data directly examining interactions between age and sex on nicotine reward. However, female adolescents have been shown to exhibit greater withdrawal-related craving than males (Dickmann et al. 2009). A slowly growing body of literature in rodents also supports the view that female adolescents have a greater biological vulnerability to nicotine dependence.
Adolescent female rodents have been found to be more likely than males to acquire nicotine self-administration, to acquire the behavior more rapidly, and have higher nicotine intake (Lynch 2009; Li et al. 2014; Sanchez et al. 2014). Furthermore, female rodents display a late-emerging enhancement of motivation, as measured by progressive ratio, compared to males. The enhanced motivation for nicotine is positively correlated with the ratio of estradiol to progesterone and negatively correlated with levels of progesterone alone (Lynch 2009). Other groups reporting increased motivation in females have not found differences in responding across the estrous cycle (Donny et al. 2000; Li et al. 2014). Acetaldehyde, a non-nicotine constituent in tobacco smoke, enhances nicotine self-administration in adolescents of both sexes. However, males display a reduction in responding for the combination of nicotine and acetaldehyde as adolescence proceeds whereas females do not (Belluzzi et al. 2005; Park et al. 2007). A similar age- and sex-dependent interaction has been observed with nicotine alone (Levin et al. 2011).
Whether adolescent females are more sensitive than adult females to nicotine reward is unclear. Torres et al. (2009) did not find a significant age difference in females, whereas adolescent males displayed higher nicotine reward than adult males. A more recent study, using a conditioned place preference (CPP) paradigm, has found adolescent females to be more sensitive to the rewarding properties and less sensitive to the aversive properties of nicotine than adult females (Lenoir et al. 2015). Whereas adolescent females had a higher preference to a mid-range dose (0.4 mg/kg) of nicotine than adolescent males, this sex difference was not apparent in adults. In parallel to the behavioral results, only females displayed an increase in nAChR binding in the nucleus accumbens after 0.4 mg/kg nicotine and adolescent, but not adult, females had higher nAChR binding in the caudate putamen (Lenoir et al. 2015).
Stress is an important factor in the initiation and maintenance of smoking (Torres and O’Dell 2016), with female smokers showing heightened negative responses to stressful situations (Swan et al. 1993), and an association between smoking and depressive symptoms during adolescence (Jamner et al. 2003). Recent data also demonstrate that female college students are more likely than their male peers to report initiation of tobacco use as a way to alleviate negative mood states (Morrell et al. 2010). Furthermore, nicotine acutely activates the hypothalamic pituitary adrenal (HPA) axis, (al’Absi 2006; Cao et al. 2010), and the anxiolytic effects of acute nicotine are more pronounced in adolescent males than females (Damaj 2001; Cao et al. 2010). The pharmacological stressor, yohimbine, has been shown to stimulate nicotine self-administration and reinforcement efficacy in both male and female adolescent rats (Li et al. 2014). However, adolescent females are more sensitive to this effect, showing significantly increased progressive ratio responding at yohimbine and nicotine infusion doses to which males do not respond.
Anxiety-like and depressive-like behaviors during withdrawal from chronic nicotine are also influenced by age of exposure and sex, although data are equivocal. In an open-field test of anxiety-like behavior, one group reported a late-emerging depression of locomotor activity (P60) in females with nicotine exposure from P30–44, without any change in behavior during drug exposure or in early withdrawal. Exposed males were not different from controls at any time point (Trauth et al. 2000). In contrast, Thanos et al. (2013) observed decreased locomotor activity in females during short-term withdrawal from adolescent nicotine. This effect was normalized after a protracted withdrawal, whereas males displayed enhanced anxiety- and depressive-like behaviors after a 30-day withdrawal. Physical symptoms of nicotine withdrawal are similar in male and female adolescents (Kota et al. 2007; Kota et al. 2008; Torres et al. 2013).
Sustained sex differences in nicotine’s effects in adulthood
Sex differences in nicotine responses persist in adulthood. The effects of gonadal hormones on these responses are not always directly assessed, but increasing data emphasize their role. Women have less success with cessation than men (Perkins 2001; Piper et al. 2010), experiencing more intense craving, higher cortisol levels during tobacco abstinence, and higher rates of tobacco withdrawal-associated depression and anxiety (al’Absi 2006; Schnoll et al. 2007; Xu et al. 2008). There is some evidence for interactions between circulating hormones or menstrual cycle phase with smoking behavior and cessation success. In women, estrogen seems to promote smoking behavior while progesterone discourages smoking (Sofuoglu et al. 2009; Lynch and Sofuoglu 2010). As a result, drug reward is greatest during times of high estradiol and low progesterone, such as the follicular phase. Indeed, women report reduced craving and decreased enjoyment or feelings of pleasure from smoking during the luteal phase (Allen et al. 2015; Goletiani et al. 2015). This relationship is mirrored in rodents, where midbrain dopamine neuron firing rate and burst firing is higher during estrous and diestrous compared to proestrous (Zhang et al. 2008), and dopamine release is inhibited by progesterone but promoted by estradiol (Becker and Beer 1986; Walker et al. 2012). Interestingly, a recent meta-analysis reported greater and more intense withdrawal symptoms during the luteal phase (Weinberger et al. 2015), suggesting that progesterone might contribute to decreased dopamine levels during tobacco withdrawal that are associated with craving.
In rodent models, the influence of sex on nicotine reward and reinforcement in adults is less consistent. Some groups report higher or more rapid acquisition in females (Donny et al. 2000; Lynch 2009), faster acquisition in males (Swalve et al. 2016), or no sex differences (Feltenstein et al. 2012). In the maintenance phase of self-administration, females tend to have higher drug intake than males (Donny et al. 2000; Chaudhri et al. 2005; Rezvani et al. 2008; Grebenstein et al. 2013), although some studies report no effect of sex (Feltenstein et al. 2012; Swalve et al. 2016). Some sex differences are potentially mediated by ovarian hormone-induced changes in nicotine metabolism. Repeated intravenous nicotine produces higher plasma levels of nicotine in females than males, which may contribute to enhanced nicotine reinforcement. This sex difference is eliminated by ovariectomy (Harrod et al. 2007). Non-drug conditioned cues are also more salient to females than males during self-administration, extinction, and potentially withdrawal/craving (Perkins et al. 1999; Chaudhri et al. 2005). In addition, females experience more robust withdrawal from chronic nicotine compared to males (Torres and O’Dell 2016), an effect that seems to be due to ovarian hormones (Torres et al. 2015).
Research examining the rewarding properties of nicotine, frequently measured by CPP, is conflicting. Whereas one group reported that higher doses of nicotine are necessary for the development of CPP in females compared to males (Lenoir et al. 2015), another group reported no CPP in females at any dose tested (Yararbas et al. 2010). These studies are in contrast to Isiegas et al. (2009) who showed that female mice exhibited a greater magnitude of nicotine preference than males, and Torres et al. (2009) who reported that adult females display place preference over a wider range of nicotine doses than males. Interestingly, ovarian hormones seem to be necessary for nicotine reward, as ovariectomy precludes nicotine CPP (Torres et al. 2009). Similarly, ovarian hormones may also protect against the aversive properties of nicotine, as a high dose of nicotine (1.2 mg/kg) induced a significant aversion in intact females, but not ovariectomized females or intact males (Torres et al. 2009).
Nicotine enhances anxiety-like behavior in female rodents, but not males, as measured by elevated plus maze (Elliott et al. 2004; Caldarone et al. 2008) and open-field behavior (Cao et al. 2010). Furthermore, chronic nicotine treatment is anxiogenic in females, but not males (Caldarone et al. 2008). These sex differences may reflect differential sensitivity of the stress system that increases females’ vulnerability to tobacco use (Torres and O’Dell 2016). Females have greater expression than males of CRF-1 receptors and hypersecretion of CRF in the locus coeruleus (Curtis et al. 2006; Bangasser et al. 2010; Bangasser et al. 2013), as well as lower levels of β-arrestin2, an intracellular protein that internalizes CRF-1 receptors to inactivate them (Bangasser et al. 2010; Bangasser and Valentino 2012). Furthermore, acute nicotine stimulates HPA axis activity and peripheral corticosterone secretion to a greater extent in adult females than in males (Cao et al. 2010; Gentile et al. 2011).
Although clinical data suggest important interactions between circulating hormones and tobacco smoking, they are correlational. Additionally, the majority of preclinical reports of sex differences in nicotine reward and reinforcement do not see an effect of circulating gonadal hormones (i.e., no variation across the estrous cycle). Instead, pre-pubertal gonadectomy can attenuate or even completely prevent these normal sex differences (Harrod et al. 2007; Torres et al. 2009). Ultimately, this highlights the critical organizational influence of gonadal hormones during perinatal and adolescent development as the primary determinants of sex differences in nicotine’s effects.
Conclusion
Tobacco use, and increasingly e-cigarette use, are a major public health concern. Data at epidemiological, clinical, and preclinical levels consistently demonstrate that females are especially sensitive to the aversive effects of nicotine withdrawal. Age interacts strongly with sex in determining responses to nicotine as well, which is evidenced by recent findings that the rate of decline in smoking over the last ten years is slowing in adolescent girls (Allen et al. 2014).
Despite this, preclinical research has predominantly focused on adult males, failing to model a significant portion of the population. As a result, the exact mechanisms underlying females’ unique sensitivity to the effects of nicotine are poorly understood. However, sex steroid hormones represent an important potential mediator of nicotine’s differential effects in females. Early in development, gonadal hormones begin the extended process of sexual differentiation of the brain, which is predominantly mediated by masculinization and defeminization of the male brain via aromatization of testosterone into estradiol. The process of sexual differentiation is not completed until the end of adolescence, a period also marked by major reorganization of brain regions critical for executive function, reward processing, and motivated behavior. Ultimately, it may be the extended and profound organizational effects of gonadal hormones on neuronal structure, function, and neurochemistry, rather than the acute effects of circulating steroids, that primarily underlie female sensitivity to the effects of nicotine and tobacco.
Significance Statement.
There are substantial sex differences in the effects and outcomes of nicotine exposure, but the exact mechanism underlying these sex differences is unclear. This review highlights the developmental impacts of nicotine on sexual differentiation, and describes how nicotine exposure during the perinatal and adolescent periods can have gender-specific effects on neuronal structure and function. It further emphasizes the importance of including age and sex as factors in preclinical research that models tobacco use.
Acknowledgments
Financial support: This work was supported by NIH grant DA 040440 (FL) and Tobacco-Related Disease Research Program project grant 21RT-0136 (FL).
Footnotes
Conflict of Interest Statement
The authors do not have any known or potential conflicts of interest, including any financial, personal, or other relationships within three years of beginning the submitted work, with people or organizations that could inappropriately influence or be perceived to influence their work. No conflicts have been identified.
Role of Authors
SJC, KEL, and FML conceived the work, reviewed and discussed the manuscript, contributed to writing the article, and approved the article in its final form.
References
- Adler A, Vescovo P, Robinson JK, Kritzer MF. Gonadectomy in adult life increases tyrosine hydroxylase immunoreactivity in the prefrontal cortex and decreases open field activity in male rats. Neuroscience. 1999;89(3):939–954. doi: 10.1016/s0306-4522(98)00341-8. [DOI] [PubMed] [Google Scholar]
- al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59(3):218–227. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Allen AM, Lunos S, Heishman SJ, al’Absi M, Hatsukami D, Allen SS. Subjective response to nicotine by menstrual phase. Addict Behav. 2015;43:50–53. doi: 10.1016/j.addbeh.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AM, Oncken C, Hatsukami D. Women and Smoking: The Effect of Gender on the Epidemiology, Health Effects, and Cessation of Smoking. Curr Addict Rep. 2014;1(1):53–60. doi: 10.1007/s40429-013-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves NC, Bailey CDC, Nashmi R, Lambe EK. Developmental sex differences in nicotinic currents of prefrontal layer VI neurons in mice and rats. PLoS ONE. 2010;5(2):e9261. doi: 10.1371/journal.pone.0009261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8(6):1495–1498. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev. 2000;24(1):137–141. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27(6):683–691. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- Arimatsu Y. Short- and long-term influences of neonatal sex steroids on alphabungarotoxin binding capacity in the mouse amygdala. Neuroscience. 1983;9(4):873–877. doi: 10.1016/0306-4522(83)90275-0. [DOI] [PubMed] [Google Scholar]
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55(5):570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Chen Y, Leslie FM. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience. 2007;144(4):1347–1360. doi: 10.1016/j.neuroscience.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BAS, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15(9):877, 896–877, 904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Reyes BAS, Piel D, Garachh V, Zhang XY, Plona ZM, Van Bockstaele EJ, Beck SG, Valentino RJ. Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol Psychiatry. 2013;18(2):166–173. doi: 10.1038/mp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol. 2012;32(5):709–723. doi: 10.1007/s10571-012-9824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri RL, Gochberg J, Ryan KJ. Nicotine, cotinine, and anabasine inhibit aromatase in human trophoblast in vitro. J Clin Invest. 1986;77(6):1727–1733. doi: 10.1172/JCI112494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64(4):803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Becker JB, Beer ME. The influence of estrogen on nigrostriatal dopamine activity. Behav Brain Res. 1986;19(1):27–33. doi: 10.1016/0166-4328(86)90044-6. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30(4):705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3(9):728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Beyer C, Pilgrim C, Reisert I. Dopamine content and metabolism in mesencephalic and diencephalic cell cultures: sex differences and effects of sex steroids. J Neurosci. 1991;11(5):1325–1333. doi: 10.1523/JNEUROSCI.11-05-01325.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study. Am J Psychiatry. 2003;160(11):1978–1984. doi: 10.1176/appi.ajp.160.11.1978. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, King SL, Picciotto MR. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neurosci Lett. 2008;439(2):187–191. doi: 10.1016/j.neulet.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Belluzzi JD, Loughlin SE, Dao JM, Chen Y, Leslie FM. Locomotor and stress responses to nicotine differ in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96(1):82–90. doi: 10.1016/j.pbb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Dwyer JB, Gautier NM, Leslie FM, Li MD. Central myelin gene expression during postnatal development in rats exposed to nicotine gestationally. Neurosci Lett. 2013;553:115–120. doi: 10.1016/j.neulet.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno ML, Henderson JA, Pau KYF, Bethea CL. Estradiol increases alpha7 nicotinic receptor in serotonergic dorsal raphe and noradrenergic locus coeruleus neurons of macaques. J Comp Neurol. 2006;497(3):489–501. doi: 10.1002/cne.21026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital signs: current cigarette smoking among adults aged ≥18 Years—United states, 2005–2010. Morbidity and Mortality Weekly Report. 2011 [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 2005;180(2):258–266. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31(3):544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Curtis L, Buisson B, Bertrand S, Bertrand D. Potentiation of human alpha4beta2 neuronal nicotinic acetylcholine receptor by estradiol. Mol Pharmacol. 2002;61(1):127–135. doi: 10.1124/mol.61.1.127. [DOI] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Di Paolo T. Regional and selective effects of oestradiol and progesterone on NMDA and AMPA receptors in the rat brain. J Neuroendocrinol. 2000;12(5):445–452. doi: 10.1046/j.1365-2826.2000.00471.x. [DOI] [PubMed] [Google Scholar]
- Damaj MI. Influence of gender and sex hormones on nicotine acute pharmacological effects in mice. J Pharmacol Exp Ther. 2001;296(1):132–140. [PubMed] [Google Scholar]
- Damborsky JC, Griffith WH, Winzer-Serhan UH. Chronic neonatal nicotine exposure increases excitation in the young adult rat hippocampus in a sex-dependent manner. Brain Res. 2012;1430:8–17. doi: 10.1016/j.brainres.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damborsky JC, Winzer-Serhan UH. Effects of sex and chronic neonatal nicotine treatment on Na2+/K+/Cl− co-transporter 1, K+/Cl− co-transporter 2, brain-derived neurotrophic factor, NMDA receptor subunit 2A and NMDA receptor subunit 2B mRNA expression in the postnatal rat hippocampus. Neuroscience. 2012;225:105–117. doi: 10.1016/j.neuroscience.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickmann PJ, Mooney ME, Allen SS, Hanson K, Hatsukami DK. Nicotine withdrawal and craving in adolescents: effects of sex and hormonal contraceptive use. Addict Behav. 2009;34(6–7):620–623. doi: 10.1016/j.addbeh.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM. Infant morbidity and mortality attributable to prenatal smoking in the U.S. Am J Prev Med. 2010;39(1):45–52. doi: 10.1016/j.amepre.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 2000;151(4):392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today. 2008;84(1):30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122(2):125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott BM, Faraday MM, Phillips JM, Grunberg NE. Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2004;77(1):21–28. doi: 10.1016/j.pbb.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Eppolito AK, Smith RF. Long-term behavioral and developmental consequences of pre- and perinatal nicotine. Pharmacol Biochem Behav. 2006;85(4):835–841. doi: 10.1016/j.pbb.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Feller MB. The role of nAChR-mediated spontaneous retinal activity in visual system development. J Neurobiol. 2002;53(4):556–567. doi: 10.1002/neu.10140. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121(3):240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. Sexually dimorphic expression of KCC2 and GABA function. Epilepsy Res. 2008;80(2–3):99–113. doi: 10.1016/j.eplepsyres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS, Kyrozis A, Claudio OI, Stanton PK, Moshé SL. Sex-specific KCC2 expression and GABA(A) receptor function in rat substantia nigra. Exp Neurol. 2003;183(2):628–637. doi: 10.1016/s0014-4886(03)00213-9. [DOI] [PubMed] [Google Scholar]
- Gangitano D, Salas R, Teng Y, Perez E, De Biasi M. Progesterone modulation of alpha5 nAChR subunits influences anxiety-related behavior during estrus cycle. Genes Brain Behav. 2009;8(4):398–406. doi: 10.1111/j.1601-183X.2009.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile NE, Andrekanic JD, Karwoski TE, Czambel RK, Rubin RT, Rhodes ME. Sexually diergic hypothalamic-pituitary-adrenal (HPA) responses to single-dose nicotine, continuous nicotine infusion, and nicotine withdrawal by mecamylamine in rats. Brain Res Bull. 2011;85(3–4):145–152. doi: 10.1016/j.brainresbull.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George FW, Ojeda SR. Changes in aromatase activity in the rat brain during embryonic, neonatal, and infantile development. Endocrinology. 1982;111(2):522–529. doi: 10.1210/endo-111-2-522. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of estrogen on basal forebrain cholinergic neurons vary as a function of dose and duration of treatment. Brain Res. 1997;757(1):10–16. doi: 10.1016/s0006-8993(96)01432-1. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Wu D, Hersh LB, Pfaff DW. Effects of estrogen replacement on the relative levels of choline acetyltransferase, trkA, and nerve growth factor messenger RNAs in the basal forebrain and hippocampal formation of adult rats. Exp Neurol. 1994;129(1):70–80. doi: 10.1006/exnr.1994.1148. [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Cornelius MD, Day NL. Prenatal cigarette smoke exposure and early initiation of multiple substance use. Nicotine Tob Res. 2012;14(6):694–702. doi: 10.1093/ntr/ntr280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goletiani NV, Siegel AJ, Lukas SE, Hudson JI. The effects of smoked nicotine on measures of subjective States and hypothalamic-pituitary-adrenal axis hormones in women during the follicular and luteal phases of the menstrual cycle. J Addict Med. 2015;9(3):195–203. doi: 10.1097/ADM.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74(6):363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Grebenstein P, Burroughs D, Zhang Y, LeSage MG. Sex differences in nicotine self-administration in rats during progressive unit dose reduction: implications for nicotine regulation policy. Pharmacol Biochem Behav. 2013;114–115:70–81. doi: 10.1016/j.pbb.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamón A, de Blas MR, Segovia S. Effects of sex steroids on the development of the locus coeruleus in the rat. Brain Res. 1988;468(2):306–310. doi: 10.1016/0165-3806(88)90143-5. [DOI] [PubMed] [Google Scholar]
- Gunn BG, Brown AR, Lambert JJ, Belelli D. Neurosteroids and GABA(A) Receptor Interactions: A Focus on Stress. Front Neurosci. 2011;5:131. doi: 10.3389/fnins.2011.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod SB, Booze RM, Mactutus CF. Sex differences in nicotine levels following repeated intravenous injection in rats are attenuated by gonadectomy. Pharmacol Biochem Behav. 2007;86(1):32–36. doi: 10.1016/j.pbb.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Kan E, Dahl RE, Sowell ER. The role of testosterone and estradiol in brain volume changes across adolescence: a longitudinal structural MRI study. Hum Brain Mapp. 2014;35(11):5633–5645. doi: 10.1002/hbm.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex. 2012;22(9):1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Watson CJ, Kennedy RT, Becker JB. Estradiol attenuates the K+-induced increase in extracellular GABA in rat striatum. Synapse. 2006;59(2):122–124. doi: 10.1002/syn.20221. [DOI] [PubMed] [Google Scholar]
- Huang LZ, Liu X, Griffith WH, Winzer-Serhan UH. Chronic neonatal nicotine increases anxiety but does not impair cognition in adult rats. Behav Neurosci. 2007;121(6):1342–1352. doi: 10.1037/0735-7044.121.6.1342. [DOI] [PubMed] [Google Scholar]
- Isiegas C, Mague SD, Blendy JA. Sex differences in response to nicotine in C57Bl/6:129SvEv mice. Nicotine Tob Res. 2009;11(7):851–858. doi: 10.1093/ntr/ntp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57(1):56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jamner LD, Whalen CK, Loughlin SE, Mermelstein R, Audrain-McGovern J, Krishnan-Sarin S, Worden JK, Leslie FM. Tobacco use across the formative years: a road map to developmental vulnerabilities. Nicotine Tob Res. 2003;5(Suppl 1):S71–S87. doi: 10.1080/14622200310001625573. [DOI] [PubMed] [Google Scholar]
- Jin X, Steinbach JH. Potentiation of Neuronal Nicotinic Receptors by 17β-Estradiol: Roles of the Carboxy-Terminal and the Amino-Terminal Extracellular Domains. PLoS ONE. 2015;10(12):e0144631. doi: 10.1371/journal.pone.0144631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Day AE, Ho CC, Walker QD, Francis R, Kuhn CM. Androgen decreases dopamine neurone survival in rat midbrain. J Neuroendocrinol. 2010a;22(4):238–247. doi: 10.1111/j.1365-2826.2010.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Ho CC, Day AE, Walker QD, Francis R, Kuhn CM. Oestrogen receptors enhance dopamine neurone survival in rat midbrain. J Neuroendocrinol. 2010b;22(4):226–237. doi: 10.1111/j.1365-2826.2010.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke L, Lukas R. Effects of Steroid Exposure on Ligand Binding and Functional Activities of Diverse Nicotinic Acetyicholine Receptor Subtypes. 1996 doi: 10.1046/j.1471-4159.1996.67031100.x. [DOI] [PubMed] [Google Scholar]
- King WJ, Greene GL. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Kiyohara C, Ohno Y. Sex differences in lung cancer susceptibility: a review. Gend Med. 2010;7(5):381–401. doi: 10.1016/j.genm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Klein LC, Stine MM, Pfaff DW, Vandenbergh DJ. Laternal nicotine exposure increases nicotine preference in periadolescent male but not female C57B1/6J mice. Nicotine Tob Res. 2003;5(1):117–124. doi: 10.1080/14622200307257. [DOI] [PubMed] [Google Scholar]
- Kolbinger W, Trepel M, Beyer C, Pilgrim C, Reisert I. The influence of genetic sex on sexual differentiation of diencephalic dopaminergic neurons in vitro and in vivo. Brain Res. 1991;544(2):349–352. doi: 10.1016/0006-8993(91)90079-b. [DOI] [PubMed] [Google Scholar]
- Konkle ATM, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152(1):223–235. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Damaj MI. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology (Berl) 2008;198(2):201–210. doi: 10.1007/s00213-008-1117-8. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322(1):399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Long-term gonadectomy affects the density of tyrosine hydroxylase-but not dopamine-beta-hydroxylase-, choline acetyltransferase- or serotonin-immunoreactive axons in the medial prefrontal cortices of adult male rats. Cereb Cortex. 2003;13(3):282–296. doi: 10.1093/cercor/13.3.282. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Pugach I. Administration of tamoxifen but not flutamide to hormonally intact, adult male rats mimics the effects of short-term gonadectomy on the catecholamine innervation of the cerebral cortex. J Comp Neurol. 2001;431(4):444–459. doi: 10.1002/1096-9861(20010319)431:4<444::aid-cne1082>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Johnson M, Thomae A, Luo B, Simon SA, Zhou G, Walker QD. The emergence of gonadal hormone influences on dopaminergic function during puberty. Horm Behav. 2010;58(1):122–137. doi: 10.1016/j.yhbeh.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan NC, Gee KW. Neuroactive steroid actions at the GABAA receptor. Horm Behav. 1994;28(4):537–544. doi: 10.1006/hbeh.1994.1052. [DOI] [PubMed] [Google Scholar]
- Langhammer A, Johnsen R, Gulsvik A, Holmen TL, Bjermer L. Sex differences in lung vulnerability to tobacco smoking. Eur Respir J. 2003;21(6):1017–1023. doi: 10.1183/09031936.03.00053202. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Schambra UB. Morphogenetic roles of acetylcholine. Environ Health Perspect. 1999;107(Suppl 1):65–69. doi: 10.1289/ehp.99107s165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette L, Lacasse Y, Doucet M, Lacasse M, Marquis K, Saey D, Leblanc P, Maltais F. Chronic obstructive pulmonary disease in women. Can Respir J. 2007;14(2):93–98. doi: 10.1155/2007/463435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Starosciak AK, Ledon J, Booth C, Zakharova E, Wade D, Vignoli B, Izenwasser S. Sex differences in conditioned nicotine reward are age-specific. Pharmacol Biochem Behav. 2015;132:56–62. doi: 10.1016/j.pbb.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Slade S, Wells C, Cauley M, Petro A, Vendittelli A, Johnson M, Williams P, Horton K, Rezvani AH. Threshold of adulthood for the onset of nicotine self-administration in male and female rats. Behav Brain Res. 2011;225(2):473–481. doi: 10.1016/j.bbr.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zou S, Coen K, Funk D, Shram MJ, Lê AD. Sex differences in yohimbine-induced increases in the reinforcing efficacy of nicotine in adolescent rats. Addict Biol. 2014;19(2):156–164. doi: 10.1111/j.1369-1600.2012.00473.x. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W, Schlumpf M. Prenatal nicotine affects fetal testosterone and sexual dimorphism of saccharin preference. Pharmacol Biochem Behav. 1985;23(3):439–444. doi: 10.1016/0091-3057(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Frosch MP, Phillips MD, Tauck DL, Aizenman E. Nicotinic antagonists enhance process outgrowth by rat retinal ganglion cells in culture. Science. 1988;239(4845):1293–1296. doi: 10.1126/science.3344435. [DOI] [PubMed] [Google Scholar]
- Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314(5805):1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- Locklear MN, Cohen AB, Jone A, Kritzer MF. Sex Differences Distinguish Intracortical Glutamate Receptor-Mediated Regulation of Extracellular Dopamine Levels in the Prefrontal Cortex of Adult Rats. Cereb Cortex. 2016;26(2):599–610. doi: 10.1093/cercor/bhu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94(1):43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Sofuoglu M. Role of progesterone in nicotine addiction: evidence from initiation to relapse. Exp Clin Psychopharmacol. 2010;18(6):451–461. doi: 10.1037/a0021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Sher E, Cherubini E. Regulation of GABA release by nicotinic acetylcholine receptors in the neonatal rat hippocampus. J Physiol (Lond) 2001;536(Pt 1):89–100. doi: 10.1111/j.1469-7793.2001.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, Role LW. Brain Development. Oxford University Press; 2006. Neuronal Receptors for Nicotine: Functional Diversity and Developmental Changes; pp. 341–362. [Google Scholar]
- Matsuda Y, Hirano H, Watanabe Y. Effects of estrogen on acetylcholine release in frontal cortex of female rats: involvement of serotonergic neuronal systems. Brain Res. 2002;937(1–2):58–65. doi: 10.1016/s0006-8993(02)02465-4. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14(6):677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Perrot-Sinal TS. Getting excited about GABA and sex differences in the brain. Trends Neurosci. 2002;25(6):307–312. doi: 10.1016/s0166-2236(02)02182-3. [DOI] [PubMed] [Google Scholar]
- McCartney JS, Fried PA, Watkinson B. Central auditory processing in school-age children prenatally exposed to cigarette smoke. Neurotoxicol Teratol. 1994;16(3):269–276. doi: 10.1016/0892-0362(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Miller MM, Silver J, Billiar RB. Effects of gonadal steroids on the in vivo binding of [125I]alpha-bungarotoxin to the suprachiasmatic nucleus. Brain Res. 1984;290(1):67–75. doi: 10.1016/0006-8993(84)90736-4. [DOI] [PubMed] [Google Scholar]
- Morissette M, Di Paolo T. Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology. 1993;58(1):16–22. doi: 10.1159/000126507. [DOI] [PubMed] [Google Scholar]
- Morrell HER, Cohen LM, McChargue DE. Depression vulnerability predicts cigarette smoking among college students: Gender and negative reinforcement expectancies as contributing factors. Addict Behav. 2010;35(6):607–611. doi: 10.1016/j.addbeh.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18(7):2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R, Muhammad A, Gibb R, Kolb B. Long-term alterations to dendritic morphology and spine density associated with prenatal exposure to nicotine. Brain Res. 2013;1499:53–60. doi: 10.1016/j.brainres.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Ohno Y. Modulation by estrogens and xenoestrogens of recombinant human neuronal nicotinic receptors. Eur J Pharmacol. 2001;430(2–3):175–183. doi: 10.1016/s0014-2999(01)01389-9. [DOI] [PubMed] [Google Scholar]
- Nuñez JL, Lauschke DM, Juraska JM. Cell death in the development of the posterior cortex in male and female rats. J Comp Neurol. 2001;436(1):32–41. [PubMed] [Google Scholar]
- Nuñez JL, McCarthy MM. Evidence for an extended duration of GABA-mediated excitation in the developing male versus female hippocampus. Dev Neurobiol. 2007;67(14):1879–1890. doi: 10.1002/dneu.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez JL, Sodhi J, Juraska JM. Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. J Neurobiol. 2002;52(4):312–321. doi: 10.1002/neu.10092. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV. A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology. 2014;76(Pt B):566–580. doi: 10.1016/j.neuropharm.2013.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley BW, Tsai MJ. Molecular pathways of steroid receptor action. Biol Reprod. 1992;46(2):163–167. doi: 10.1095/biolreprod46.2.163. [DOI] [PubMed] [Google Scholar]
- Paradiso K, Sabey K, Evers AS, Zorumski CF, Covey DF, Steinbach JH. Steroid inhibition of rat neuronal nicotinic alpha4beta2 receptors expressed in HEK 293 cells. Mol Pharmacol. 2000;58(2):341–351. doi: 10.1124/mol.58.2.341. [DOI] [PubMed] [Google Scholar]
- Paradiso K, Zhang J, Steinbach JH. The C terminus of the human nicotinic alpha4beta2 receptor forms a binding site required for potentiation by an estrogenic steroid. J Neurosci. 2001;21(17):6561–6568. doi: 10.1523/JNEUROSCI.21-17-06561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Belluzzi JD, Han S-H, Cao J, Leslie FM. Age, sex and early environment contribute to individual differences in nicotine/acetaldehyde-induced behavioral and endocrine responses in rats. Pharmacol Biochem Behav. 2007;86(2):297–305. doi: 10.1016/j.pbb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6(6):2311–2322. [PubMed] [Google Scholar]
- Perkins KA. Smoking Cessation in Women. CNS Drugs. 2001;15(5):391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and selfadministration: review of human and animal evidence. Nicotine Tob Res. 1999;1(4):301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Pinos H, Collado P, Rodríguez-Zafra M, Rodríguez C, Segovia S, Guillamón A. The development of sex differences in the locus coeruleus of the rat. Brain Res Bull. 2001;56(1):73–78. doi: 10.1016/s0361-9230(01)00540-8. [DOI] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, Loh W-Y. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010;12(6):647–657. doi: 10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh PC, Berg DK. Neuronal acetylcholine receptors that bind alpha-bungarotoxin mediate neurite retraction in a calcium-dependent manner. J Neurosci. 1994;14(2):889–896. doi: 10.1523/JNEUROSCI.14-02-00889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G, Gullo F, Dossi E, Lecchi M, Wanke E. Novel modulatory effects of neurosteroids and benzodiazepines on excitatory and inhibitory neurons excitability: a multi-electrode array recording study. Frontiers in Neural Circuits. 2012;6:94. doi: 10.3389/fncir.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Eddins D, Slade S, Hampton DS, Christopher NC, Petro A, Horton K, Johnson M, Levin ED. Neonatal 6-hydroxydopamine lesions of the frontal cortex in rats: persisting effects on locomotor activity, learning and nicotine self-administration. Neuroscience. 2008;154(3):885–897. doi: 10.1016/j.neuroscience.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397(6716):251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA. Aromatase activity in the rat brain: hormonal regulation and sex differences. J Steroid Biochem Mol Biol. 1993;44(4–6):499–508. doi: 10.1016/0960-0760(93)90254-t. [DOI] [PubMed] [Google Scholar]
- Rossi FM, Pizzorusso T, Porciatti V, Marubio LM, Maffei L, Changeux JP. Requirement of the nicotinic acetylcholine receptor beta 2 subunit for the anatomical and functional development of the visual system. Proc Natl Acad Sci U S A. 2001;98(11):6453–6458. doi: 10.1073/pnas.101120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH, Lynch WJ. Sex differences in the effect of wheel running on subsequent nicotine-seeking in a rat adolescent-onset selfadministration model. Psychopharmacology (Berl) 2014;231(8):1753–1762. doi: 10.1007/s00213-013-3359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasin A, Schlumpf M, Müller M, Fleischmann I, Lauber ME, Lichtensteiger W. Adrenal-mediated rather than direct effects of nicotine as a basis of altered sex steroid synthesis in fetal and neonatal rat. Reprod Toxicol. 2003;17(2):153–162. doi: 10.1016/s0890-6238(02)00119-3. [DOI] [PubMed] [Google Scholar]
- Schneider M. Adolescence as a vulnerable period to alter rodent behavior. Cell Tissue Res. 2013;354(1):99–106. doi: 10.1007/s00441-013-1581-2. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Lerman C. Treating tobacco dependence in women. J Womens Health (Larchmt) 2007;16(8):1211–1218. doi: 10.1089/jwh.2006.0281. [DOI] [PubMed] [Google Scholar]
- Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB. Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci. 2009;29(6):1897–1903. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair D, Purves-Tyson TD, Allen KM, Weickert CS. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology (Berl) 2014;231(8):1581–1599. doi: 10.1007/s00213-013-3415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26(3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Rudder CL, Ryde IT, Tate CA, Seidler FJ. Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at 6 months of age. Neuropsychopharmacology. 2007;32(5):1082–1097. doi: 10.1038/sj.npp.1301231. [DOI] [PubMed] [Google Scholar]
- Smith SS, Aoki C, Shen H. Puberty, steroids and GABA(A) receptor plasticity. Psychoneuroendocrinology. 2009;34(Suppl 1):S91–S103. doi: 10.1016/j.psyneuen.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Chapin JK, Woodward DJ. Progesterone alters GABA and glutamate responsiveness: a possible mechanism for its anxiolytic action. Brain Res. 1987;400(2):353–359. doi: 10.1016/0006-8993(87)90634-2. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Mooney M. Progesterone effects on subjective and physiological responses to intravenous nicotine in male and female smokers. Hum Psychopharmacol. 2009;24(7):559–564. doi: 10.1002/hup.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD, USA: Substance Abuse & Mental Health Services Administration; 2011. pp. 11–4658. Report nr HHS Publication No. (SMA) [Google Scholar]
- Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, Himes JH, Ryan AS. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110(5):911–919. doi: 10.1542/peds.110.5.911. [DOI] [PubMed] [Google Scholar]
- Swalve N, Smethells JR, Carroll ME. Sex differences in the acquisition and maintenance of cocaine and nicotine self-administration in rats. Psychopharmacology (Berl) 2016;233(6):1005–1013. doi: 10.1007/s00213-015-4183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Ward MM, Carmelli D, Jack LM. Differential rates of relapse in subgroups of male and female smokers. J Clin Epidemiol. 1993;46(9):1041–1053. doi: 10.1016/0895-4356(93)90172-w. [DOI] [PubMed] [Google Scholar]
- Takase K, Kimura F, Yagami T, Mitsushima D. Sex-specific 24-h acetylcholine release profile in the medial prefrontal cortex: simultaneous measurement of spontaneous locomotor activity in behaving rats. Neuroscience. 2009;159(1):7–15. doi: 10.1016/j.neuroscience.2008.12.039. [DOI] [PubMed] [Google Scholar]
- Takase K, Mitsushima D, Funabashi T, Kimura F. Sex difference in the 24-h acetylcholine release profile in the premotor/supplementary motor area of behaving rats. Brain Res. 2007;1154:105–115. doi: 10.1016/j.brainres.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Thanos P, Delis F, Rosko L, Volkow ND. Passive Response to Stress in Adolescent Female and Adult Male Mice after Intermittent Nicotine Exposure in Adolescence. J Addict Res Ther Suppl. 2013;6:007. doi: 10.4172/2155-6105.S6-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong VT, Dietz PM, Morrow B, D’Angelo DV, Farr SL, Rockhill KM, England LJ Centers for Disease C, Prevention. Trends in smoking before, during, and after pregnancy--Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000–2010. MMWR Surveill Summ. 2013;62(6):1–19. [PubMed] [Google Scholar]
- Torres OV, Gentil LG, Natividad LA, Carcoba LM, O’Dell LE. Behavioral, Biochemical, and Molecular Indices of Stress are Enhanced in Female Versus Male Rats Experiencing Nicotine Withdrawal. Front Psychiatry. 2013;4:38. doi: 10.3389/fpsyt.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology (Berl) 2009;206(2):303–312. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, O’Dell LE. Stress is a principal factor that promotes tobacco use in females. Prog Neuropsychopharmacol Biol Psychiatry. 2016 doi: 10.1016/j.pnpbp.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Pipkin JA, Ferree P, Carcoba LM, O’Dell LE. Nicotine withdrawal increases stress-associated genes in the nucleus accumbens of female rats in a hormone-dependent manner. Nicotine Tob Res. 2015;17(4):422–430. doi: 10.1093/ntr/ntu278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Ali SF, Slotkin TA. Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Res. 2001;892(2):269–280. doi: 10.1016/s0006-8993(00)03227-3. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. Persistent and delayed behavioral changes after nicotine treatment in adolescent rats. Brain Res. 2000;880(1–2):167–172. doi: 10.1016/s0006-8993(00)02823-7. [DOI] [PubMed] [Google Scholar]
- Treur JL, Willemsen G, Bartels M, Geels LM, van Beek JHDA, Huppertz C, van Beijsterveldt CEM, Boomsma DI, Vink JM. Smoking During Adolescence as a Risk Factor for Attention Problems. Biol Psychiatry. 2015;78(9):656–663. doi: 10.1016/j.biopsych.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Valera S, Ballivet M, Bertrand D. Progesterone modulates a neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1992;89(20):9949–9953. doi: 10.1073/pnas.89.20.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Bäckström T, Buitelaar JK, Fernández G. Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry. 2008;13(3):325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- von Ziegler NI, Schlumpf M, Lichtensteiger W. Prenatal nicotine exposure selectively affects perinatal forebrain aromatase activity and fetal adrenal function in male rats. Brain Res Dev Brain Res. 1991;62(1):23–31. doi: 10.1016/0165-3806(91)90186-m. [DOI] [PubMed] [Google Scholar]
- Walker QD, Johnson ML, Van Swearingen AED, Arrant AE, Caster JM, Kuhn CM. Individual differences in psychostimulant responses of female rats are associated with ovarian hormones and dopamine neuroanatomy. Neuropharmacology. 2012;62(7):2267–2277. doi: 10.1016/j.neuropharm.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95(4):1061–1070. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Smith PH, Allen SS, Cosgrove KP, Saladin ME, Gray KM, Mazure CM, Wetherington CL, McKee SA. Systematic and meta-analytic review of research examining the impact of menstrual cycle phase and ovarian hormones on smoking and cessation. Nicotine Tob Res. 2015;17(4):407–421. doi: 10.1093/ntr/ntu249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry. 1999;38(7):892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- Willing J, Juraska JM. The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience. 2015;301:268–275. doi: 10.1016/j.neuroscience.2015.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Azizian A, Monterosso J, Domier CP, Brody AL, Fong TW, London ED. Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob Res. 2008;10(11):1653–1661. doi: 10.1080/14622200802412929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kitawaki J, Kikuchi N, Okubo T, Iwasa K, Kawata M, Honjo H. Effects of estrogens on cholinergic neurons in the rat basal nucleus. J Steroid Biochem Mol Biol. 2007;107(1–2):70–79. doi: 10.1016/j.jsbmb.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Yararbas G, Keser A, Kanit L, Pogun S. Nicotine-induced conditioned place preference in rats: sex differences and the role of mGluR5 receptors. Neuropharmacology. 2010;58(2):374–382. doi: 10.1016/j.neuropharm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Yates MA, Juraska JM. Pubertal ovarian hormone exposure reduces the number of myelinated axons in the splenium of the rat corpus callosum. Exp Neurol. 2008;209(1):284–287. doi: 10.1016/j.expneurol.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Cross SJ, Loughlin SE, Leslie FM. Nicotine and the adolescent brain. J Physiol (Lond) 2015;593(16):3397–3412. doi: 10.1113/JP270492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Yang S, Yang C, Jin G, Zhen X. Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology (Berl) 2008;199(4):625–635. doi: 10.1007/s00213-008-1188-6. [DOI] [PubMed] [Google Scholar]