Abstract
Allergic contact dermatitis is an immune-mediated antigen-specific skin reaction to an allergenic chemical that corresponds to a delayed-type hypersensitivity response (type IV reaction). Allergic contact dermatitis should be suspected when skin lesions are localized to the site of previous applications of the culprit drug. Lesions appear after re-exposure in susceptible persons, with delayed onset (more than 24 h after exposure). The gold standard for diagnosis is patch (epicutaneous) testing; identification and removal of any potential causal agents is crucial. Diclofenac sodium 1% topical gel contains active (diclofenac sodium) and inactive ingredients. It is a widely used non-steroidal anti-inflammatory drug, known to cause allergic contact dermatitis, and especially photoallergic contact reactions. We present four cases of diclofenac-sodium-induced allergic contact dermatitis, diagnosed based on clinical grounds: intensively itchy eczematous lesions on the sites of drug application after several days of treatment. No allergic history and no other drug intake were reported by the patients. The application of diclofenac sodium 1% topical gel was strictly forbidden in all cases; potent topical steroids proved to be effective in all cases within 2 weeks of therapy. Patch tests were performed in all cases with European standard battery, with patients’ own diclofenac sodium 1% topical gels and with diclofenac sodium 1% in petrolatum 3 weeks after completion of local steroid therapy. Readings were done after 48 h (Day 2) and 72 h (Day 3) and proved to be positive only to patients’ diclofenac sodium 1% topical gel and diclofenac sodium 1% in petrolatum. No sun exposure was allowed during the testing, and any other treatments were forbidden.
Key Points
| Patients and physicians must be aware of the risk of cutaneous sensitization induced by topical diclofenac, a drug that is also extensively used as self-medication. |
| Discontinuation of topical application is the best therapeutic approach and patients should be informed about the allergic reaction to diclofenac and counseled about avoiding the culprit medication. |
Introduction
Contact dermatitis can be divided into irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) [1]. ACD is an immune-mediated antigen-specific skin reaction to an allergenic chemical that corresponds to a delayed-type hypersensitivity response (type IV reaction). The gold standard for diagnosis is patch (epicutaneous) testing [1]. This test has a sensitivity and specificity of between 70 and 80% [2]. Treatment of choice for ACD is topical corticosteroids, and a variety of symptomatic treatments can be used to relieve the itching. However, identification and removal of any potential causal agents is crucial [3]. Diclofenac is a widely used non-steroidal anti-inflammatory drug (NSAID) available in different formulations: tablets, ointments, plasters, suppositories, and lipid nanoemulsions for parenteral applications. Diclofenac sodium topical gel (DSTG) contains diclofenac sodium as the active ingredient, a white/yellow crystalline powder soluble in ethanol, moderately soluble in water and acetone, and partially insoluble in ether. Diclofenac sodium (C14H10Cl2NNaO2) is the sodium salt form of diclofenac, a benzene acetic acid derivate with analgesic, antipyretic, and anti-inflammatory activity. It inhibits cyclooxygenase (COX) non-selectively, reversibly, and competitively with subsequent inhibition of the formation of prostaglandins, thromboxanes and prostacylin, which are involved in mechanisms of pain, inflammation and fever. DSTG also contains other inactive ingredients: benzyl alcohol, hyaluronate sodium, polyethylene glycol (PEG), sodium metilparaben, carbomer 940 (polyacrylic acid), triethanolamine, and purified water. The use of topical agents containing PEG may cause ACD [4, 5]. DSTG is widely used to relieve the pain and reduce the inflammation and swelling of knees and joints of the hands; it is usually well tolerated and rarely associated with minor local skin irritation. The use of topical NSAIDs provides pain relief without associated severe systemic adverse events [6, 7]. The use of topical diclofenac is associated with fewer side effects than other topical NSAIDs and a lower rate of gastrointestinal complications than oral NSAIDs [6, 8]. Still, diclofenac may cause an increased risk of serious and fatal cardiovascular thrombotic events and it is contraindicated for the treatment of peri-operative pain in patients who have undergone coronary artery bypass graft (CABG) surgery [6, 8]. Photoallergic contact reactions have been reported with diclofenac sodium 3% topical gel used as a topical treatment for actinic keratoses [9]. Also, photoallergic contact dermatitis due to diclofenac gel used as a topical analgesic has been reported [10].
Case Presentation
Case 1
A 62-year-old female patient diagnosed many years ago with rheumatoid arthritis, treated orally with methotrexate 10 mg/week, applied DSTG 1% gel twice daily on her left wrist under occlusive dressing. She noticed erythema with slight edema on the site of drug application on the third day of use (Fig. 1). She also complained of pruritus. The patient had no history of atopy, no allergy history in the past, no other recent drug intake apart from methotrexate. Discontinuation of DSTG 1% gel was strongly advised. Potent steroid cream (mometasone) was applied once daily for the following 2 weeks resulting in full recovery of the skin. Patient was referred to the Allergy Department for patch testing 3 weeks after the cessation of topical corticosteroid therapy. No sun exposure was allowed during the testing, any other treatments were forbidden. Patch test readings were done after 48 h (Day 2) and 72 h (Day 3) and were positive only to DSTG 1% (+/++) and 1% diclofenac sodium in petrolatum (+/++); therefore, the ACD to diclofenac sodium was confirmed. The Naranjo Adverse Drug Reaction (ADR) Probability Scale was used and the score was calculated (patient’s total score was 6, indicating a probable ADR).
Fig. 1.
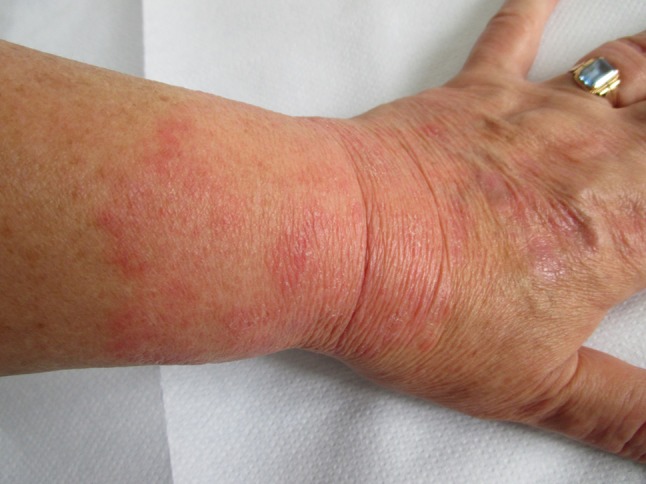
Clinical presentation of Case 1
Case 2
A 70-year-old female patient, suffering from right gonarthrosis, was examined at our Dermatology Unit for intense pruritus on inferior limbs, intense erythema and desquamation on the right knee, and secondary identical lesions on the left inferior limb. Dermatological examination revealed a large desquamating erythematous lesion, well delineated, associated with slight edema and scratch signs on the right knee. Scattered erythematous papules coved by scales and diffuse erythema were noticed on the left knee and left lower leg (Fig. 2). She described DSTG 1% gel application only on the right knee for 5 consecutive days. First complaints were noticed on the right knee, followed by occurrence of the same lesions on the left knee and left lower limb. The presumed mechanism of occurrence of the skin lesions on the other leg is transfer of DSTG 1% gel from right knee to other knee and id reaction. The application of DSTG was discontinued. The patient was treated with topical corticosteroid ointment (betamethasone) twice daily for 7 days resulting in complete regression of lesions. Clinical diagnosis of ACD to diclofenac sodium was confirmed by positive patch. A patch test was performed 5 weeks later and was positive only to the patient’s own DSTG 1% gel (++/+++) and 1% diclofenac sodium in petrolatum (++/+++). Readings were done at Day 2 and Day 3. The patient’s Naranjo ADR probability score was calculated (score was 6; ADR is probable).
Fig. 2.
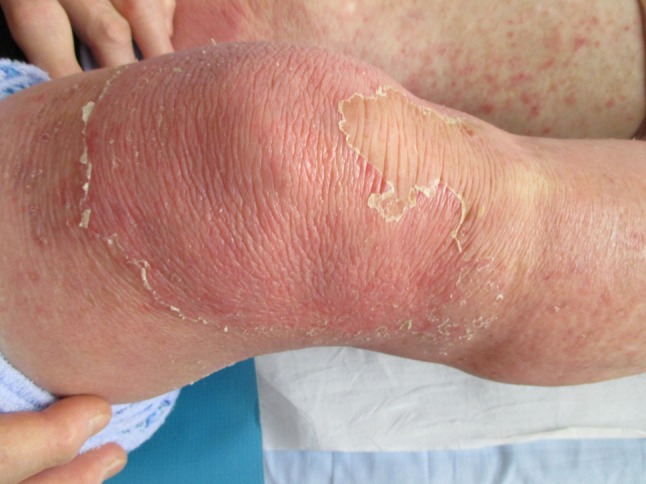
Clinical presentation of Case 2
Case 3
A healthy 51-year-old man was referred to the dermatology outpatient unit for intense erythema, edema, and pruritus on the lumbosacral area (Fig. 3) that had been noticed by the patient 4 days after self-application of DSTG 1% several times per day for 4 consecutive days for acute lumbago. He denied any drug intake and his medical history was unremarkable. The application of the culprit topical drug was stopped. Topical steroid (betamethasone) proved to be effective after 2 weeks of application. A patch test was performed 1 month later and proved to be positive at Day 2 and Day 3 to DSTG 1% (+/+) and 1% diclofenac sodium in petrolatum (+/+). The Naranjo ADR Probability Scale was used (total score was 6; ADR is probable).
Fig. 3.
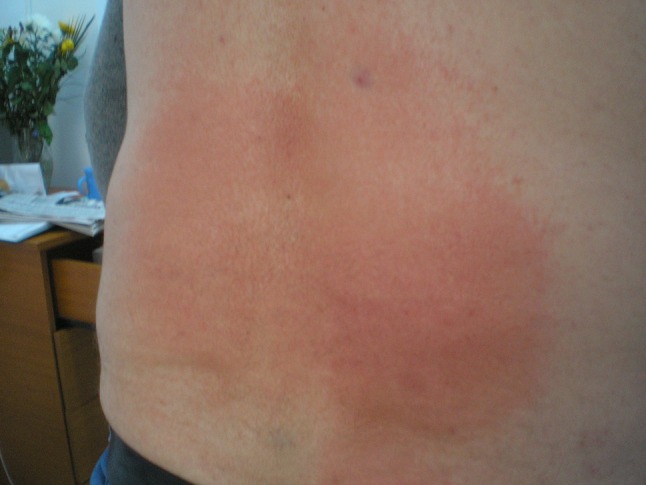
Clinical presentation of Case 3
Case 4
A young 23-year-old man, on treatment with adalimumab for psoriatic arthritis for the last 2 years, reported erythema, slight infiltration, and pruritus in the lumbar region. He had been applying DSTG 1% on the skin in the lumbosacral region twice daily for 7 days prior to the consultation (Fig. 4). Diclofenac was discontinued and local corticosteroid therapy (betamethasone) twice daily for 7 days was introduced, resulting in complete resolution of skin lesions. Three weeks later the ACD to diclofenac sodium was confirmed by patch test to DSTG 1% (+/++) and 1% diclofenac sodium in petrolatum (+/++). Readings were done after 48 and 96 h. The patient’s Naranjo ADR probability score was 6, indicating a probable ADR.
Fig. 4.
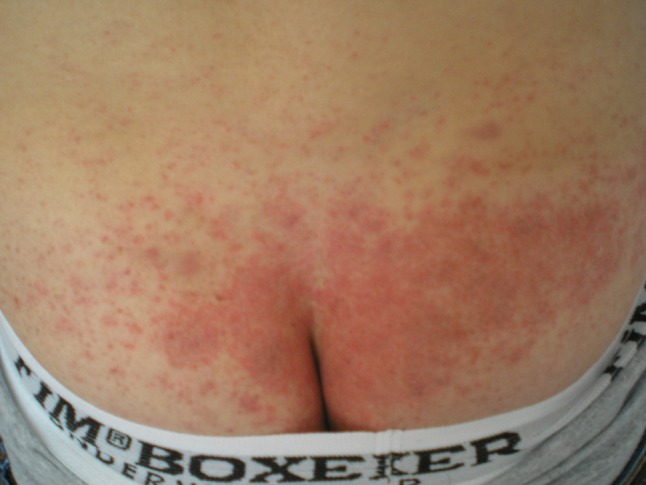
Clinical presentation of Case 4
Discussion
Our patients stated a clear history of the local administration of the drug during their examination. ACD should be suspected when skin lesions are localized to the site of previous applications of the culprit drug. We recommended drug-use cessation and treatment with potent local corticosteroids. The gold standard for the diagnosis of ACD (type IV hypersensitivity) is patch testing and identification and removal of any potential causal agents is of outmost importance [11–13]. The patients’ were using DSTG 1% gel, containing diclofenac sodium and other inactive ingredients including PEG. As PEG is a well-known allergen that can induce ACD [4, 5], we had to exclude that possibility by performing a patch test with the European standard battery, patients’ own gel and diclofenac sodium 1% in petrolatum. Readings were done after 48 and 96 h for every patient following the European Society of Contact Dermatitis guideline for diagnostic patch testing where (a) erythema, infiltration, and possibly papules were considered weak positive reaction (+); (b) erythema, infiltration, papules, and vesicles were considered strong positive reaction (++); and (c) intense erythema, infiltrate, and coalescing vesicles were considered extreme positive reaction (+++) [11–13]. The Naranjo ADR Probability Scale was used in all patients and the ADR was assigned to a probability category according to the score (score 0: ADR is doubtful, score 1–4: ADR is possible, score 5–8: ADR is probable, and score ≥9: ADR is definite [14]. In all four cases, we confirmed that the probable causative allergen in ACD was diclofenac sodium, a chemical substance with molecular weight 318.130 Da that is the active substance in the patients’ gels used for pain relief.
Conclusion
Clear distinction must be made between allergic and irritant reactions (Table 1) because of the different therapeutic measures and clinical outcomes. The present cases highlight ACD to topical diclofenac sodium diagnosed on clinical grounds and confirmed by patch testing. DSTG can cause serious skin adverse events, although application site reactions (application site dermatitis) are the most frequent reason for treatment discontinuation [8]. DSTG, when suspected as allergen, should be stopped immediately. The present cases of ACD to diclofenac sodium were diagnosed by clinical features and confirmed by patch testing. Discontinuation of topical application is the best therapeutic approach and patients should be informed about their allergic reaction to diclofenac. Avoidance of the medication in cases of a previously proven allergy to diclofenac is of great importance.
Table 1.
| Irritant contact dermatitis | Allergic contact dermatitis | |
|---|---|---|
| Prevalence | Very common | Less frequent |
| Symptoms | Burning, pruritus, pain | Pruritus |
| Clinical aspects | Erythema, swelling, blisters and pustules, desquamation, no distant spread | Erythema, edema, vesicles, bullae, distant spread |
| Sites | Site of direct contact | Site of contact and secondary lesions |
| Cause | Chemical irritants, dose-related response | Poison ivy, nickel, fragrances, neomycin, metals (jewelry), cosmetics, drugs |
| Prior exposure | Not necessary | Essential (lesions appear after re-exposure) |
| Susceptibility | Everyone | Susceptible persons |
| Onset | Rapid onset (4–12 h after contact) | Delayed onset (more than 24 h after exposure) |
| Mechanism | Direct cytotoxic effects (non-immune-modulated irritation) | Type IV T-cell mediated, delayed reaction, patch-test positive |
| Treatment | Avoidance of the substance | Antihistamines, topical steroids/oral desensitization |
Compliance with Ethical Standards
Written informed consent was obtained from each of the patients for publication of this case series and inclusion of the accompanying images. Copies of the written consents may be requested for review from the corresponding author.
Conflict of interest
Jerkovic Gulin Sandra and Chiriac Anca declare that there are no conflicts of interest concerning the content of this case report.
Funding
No financial support was received for the conduct of this case report or preparation of this manuscript.
References
- 1.Burkemper NM. Contact dermatitis, patch testing, and allergen avoidance. Mo Med. 2015;112(4):296–300. [PMC free article] [PubMed] [Google Scholar]
- 2.Nethercott JR, Holness DL. Validity of patch test screening trays in the evaluation of patients with allergic contact dermatitis. J Am Acad Dermatol. 1989;21(3):568. doi: 10.1016/S0190-9622(89)80228-2. [DOI] [PubMed] [Google Scholar]
- 3.Admani S, Jacob SE. Allergic contact dermatitis in children: review of the past decade. Curr Allergy Asthma Rep. 2014;14(4):421. doi: 10.1007/s11882-014-0421-0. [DOI] [PubMed] [Google Scholar]
- 4.Guijarro SC, Sánchez-Pérez J, García-Díez A. Allergic contact dermatitis to polyethylene glycol and nitrofurazone. Am J Contact Dermat. 1999;10(4):226–227. doi: 10.1053/AJCD01000226. [DOI] [PubMed] [Google Scholar]
- 5.Moreno Escobosa MC, Moya Quesada MC, Cruz Granados S, Amat López J. Contact dermatitis to antibiotic ointments. J Investig Allergol Clin Immunol. 2009;19(6):510–511. [PubMed] [Google Scholar]
- 6.Massey T, Derry S, Moore RA, McQuay HJ. Topical NSAIDs for acute pain in adults. Cochrane Database Syst Rev. 2010;6:CD007402. doi: 10.1002/14651858.CD007402.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derry S, Conaghan P, Da Silva JAP, Wiffen PJ, Moore RA. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane database Syst Rev. 2016;4:CD007400. doi: 10.1002/14651858.CD007400.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zacher J, Altman R, Bellamy N, Brühlmann P, Da Silva J, Huskisson E, et al. Topical diclofenac and its role in pain and inflammation: an evidence-based review. Curr Med Res Opin. 2008;24(4):925–950. doi: 10.1185/030079908X273066. [DOI] [PubMed] [Google Scholar]
- 9.Kowalzick L, Ziegler H. Photoallergic contact dermatitis from topical diclofenac in Solaraze gel. Contact Dermatitis. 2006;54(6):348–349. doi: 10.1111/j.0105-1873.2006.0645f.x. [DOI] [PubMed] [Google Scholar]
- 10.Akat P. Severe photosensitivity reaction induced by topical diclofenac. Indian J Pharmacol. 2013;45(4):408. doi: 10.4103/0253-7613.114999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansen JD, Aalto-Korte K, Agner T, Andersen KE, Bircher A, Bruze M, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—recommendations on best practice. Contact Dermatitis. 2015;73(4):195–221. doi: 10.1111/cod.12432. [DOI] [PubMed] [Google Scholar]
- 12.Brasch J, Becker D, Aberer W, Bircher A, Kränke B, Jung K, et al. Guideline contact dermatitis: S1-Guidelines of the German Contact Allergy Group (DKG) of the German Dermatology Society (DDG), the Information Network of Dermatological Clinics (IVDK), the German Society for Allergology and Clinical Immunology (DGAKI), th. Allergo J Int. 2014;23(4):126–138. doi: 10.1007/s40629-014-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nosbaum A, Vocanson M, Rozieres A, Hennino A, Nicolas J-F. Allergic and irritant contact dermatitis. Eur J Dermatol. 2009;19(4):325–332. doi: 10.1684/ejd.2009.0686. [DOI] [PubMed] [Google Scholar]
- 14.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]


