Abstract
Background
IL-1α and IL-6 are associated with the prognosis of a wide range of cancers, but their value in cervical cancer remains controversial. The aim of this study was to investigate the expression of IL-1α and IL-6 in cervical cancer and their significance in clinical prognosis.
Material/Methods
The expression of IL-1α and IL-6 in 105 formalin-fixed, paraffin-embedded cervical cancer tissues and adjacent non-tumor tissues was examined by immunohistochemistry. The results were semi-quantitatively scored and analyzed by chi-square test. Patient overall survival (OS) data was collected by follow-up and analyzed by Kaplan-Meier analysis.
Results
The expression level of both IL-1α and IL-6 in cervical cancer tissue was higher than in adjacent non-tumor tissues (p<0.05). IL-1α expression was shown to be correlated with tumor size, FIGO histology grade, lymph node metastasis, stromal invasion, and tumor differentiation (p<0.05). IL-6 expression was shown to be correlated with tumor size, FIGO histology grade, and tumor differentiation (p<0.05). Patients with positive expression of IL-1α or IL-6 tended to have much shorter survival times than patients with negative expression. In addition, a multivariate Cox regression analysis demonstrated that IL-1α expression and lymph node metastasis were independent predictors of OS in cervical cancer patients.
Conclusions
The expression of IL-1α was significantly associated with tumor size, FIGO histology grade, lymph node metastasis, stromal invasion, and tumor differentiation. The expression of IL-6 was significantly associated with tumor size, FIGO histology grade, and tumor differentiation. Positive IL-1α and IL-6 expression was significantly correlated with poor prognosis. They may be considered valuable biomarkers for prognosis and potential therapeutic targets for cervical cancer.
MeSH Keywords: Interleukin-1alpha, Interleukin-6, Prognosis, Uterine Cervical Neoplasms
Background
Worldwide, cervical cancer is the second most common malignant tumor in women and is one of the leading causes of cancer-related death in developing countries [1,2]. Despite the general downward trend in incidence of cervical cancer, it is still a serious public health problem worldwide, especially in developing countries; in addition, cervical cancer is occurring in younger patients [3]. During the past decades, many distinct advances have been made in the prevention, surgical resection, radiotherapy, and chemotherapy of cervical cancer, but the prognosis of cervical cancer patients remains poor. The 5-year survival of patients with advanced cervical cancer is less than 40% [4]. Therefore, it is important that we better understand the molecular events involved in the invasion and metastasis of cervical cancer, and develop novel prognostic markers and therapeutic strategies for cervical cancer.
The infection of human papilloma virus (HPV) is considered the major cause of cervical cancer, but viral infection alone is not sufficient for the development of cervical cancer. The pathogenesis of cervical cancer is still unclear. Emerging evidence indicates that the chronic inflammatory response is significantly correlated with various types of cancers [5]. Interleukin 1(IL-1), as a pleiotropic pro-inflammatory cytokine, plays an important role in tumor growth and metastasis. The IL-1 family includes three main members that bind to the same receptor. Two of them are active, IL-1α and IL-1β, and the third one is an antagonist, IL-1Ra. IL-1α and IL-1β are coded by different genes, but their biological functions are identical. Interleukin 6 (IL-6), a 23- to 30-kDa pleiotropic cytokine, is produced by many types of cells like fibroblasts, immune cells, and some tumor cells. Increasing evidence reveals that IL-1α and IL-6 play crucial roles in cancer initiation, development, and metastasis [6,7]. However, to the best of our knowledge, the final role of IL-1α and IL-6 in cervical cancer remains unclear.
In the present study, we used tissue microarray and immunohistochemistry methods to detect the expression of IL-1α and IL-6 in 105 case-samples of human cervical cancer tissues and their paired adjacent tissues. The purpose of this study was to investigate the association of IL-1α and IL-6 expression with clinicopathological parameters of cervical cancer and evaluate the prognostic value of IL-1α and IL-6 expression in cervical cancer patients.
Material and Methods
Patients and tissues specimens
This study included a total of 105 formalin-fixed, paraffin-embedded cervical cancer tissues and their adjacent non-tumor tissues taken from patients at the time of resection surgery, from January 2002 to November 2013 at East Hospital, Tongji University. All cancers were confirmed as cervical cancer by the medical examination of hematoxylin and eosin after surgical resection. None of patients had received adjuvant chemotherapy, radiation therapy, or other anti-tumor therapies. Important related clinicopathological parameters of the patients, such as age, tumor size, FIGO stage, lymphatic metastasis, stromal invasion, differentiation, and survival time were obtained from each patient’s medical records and are shown in Tables 1 and 2. The survival time was calculated from the date of surgery to the date of death, or the last known follow-up. This study was approved by the ethics committee of East Hospital, Tongji University. All cervical cancer tissue samples include in this investigation were obtained with patients’ written informed consent.
Table 1.
Expression of IL-1α in relation to pathologic and clinical variables.
| Clinicopathological parameters | N2 | IL-1α xpression | χ2 | P value | |
|---|---|---|---|---|---|
| + | − | ||||
| All | 105 | 67 | 38 | ||
| Age (years) | 1.148 | 0.284 | |||
| <50 | 57 | 39 | 18 | ||
| ≥50 | 48 | 28 | 20 | ||
| Tumor size | 6.024 | 0.014 | |||
| <4 cm | 58 | 31 | 27 | ||
| ≥4 cm | 47 | 36 | 11 | ||
| FIGO stage | 19.661 | <0.0001 | |||
| <II A | 50 | 21 | 29 | ||
| >II B | 55 | 46 | 9 | ||
| Lymph node metastasis | 5.135 | 0.023 | |||
| Absence | 81 | 47 | 34 | ||
| Presence | 24 | 20 | 4 | ||
| Stromal invasion | 5.399 | 0.020 | |||
| <2/3 depth | 74 | 42 | 32 | ||
| ≥2/3 depth | 31 | 25 | 6 | ||
| Tumor differentiation | 7.513 | 0.006 | |||
| Well + moderate | 56 | 29 | 27 | ||
| Poor | 49 | 38 | 11 | ||
Table 2.
Expression of IL-6 in relation to pathologic and clinical variables.
| Clinicopathological parameters | N2 | IL-6 xpression | χ2 | P value | |
|---|---|---|---|---|---|
| + | − | ||||
| All | 105 | 63 | 42 | ||
| Age (years) | 1.199 | 0.274 | |||
| <50 | 57 | 38 | 19 | ||
| ≥50 | 48 | 27 | 21 | ||
| Tumor size | 5.695 | 0.017 | |||
| <4c m | 58 | 30 | 28 | ||
| ≥4 cm | 47 | 35 | 12 | ||
| FIGO stage | 10.239 | 0.001 | |||
| <IIA | 50 | 23 | 27 | ||
| >IIB | 55 | 42 | 13 | ||
| Lymph node metastasis | 0.005 | 0.945 | |||
| Absence | 81 | 50 | 31 | ||
| Presence | 24 | 15 | 9 | ||
| Stromal invasion | 2.817 | 0.093 | |||
| <2/3 depth | 74 | 42 | 32 | ||
| ≥2/3 depth | 31 | 23 | 8 | ||
| Tumor differentiation | 5.210 | 0.022 | |||
| Well + moderate | 56 | 29 | 27 | ||
| Poor | 49 | 36 | 13 | ||
Immunohistochemical staining
A standard immunohistochemistry method was used to inspect IL-1α and IL-6 expression in cervical cancer tissues and their adjacent tissues. Briefly, the tumor tissues and adjacent tissues were fixed in 10% formaldehyde and embedded in paraffin; then cut into 4-μm sections. All 4-μm tissue sections are dewaxed and rehydrated with xylene and graded alcohol, respectively. We washed the sections with buffer solution for 5 minutes and then added the primary antibody at 4°C overnight. After that, we washed the sections again and added the second antibody. Afterwards, we washed the sections with phosphate buffered saline (PBS) and stained them with 3, 3′-diaminobenzidine (DAB) for 5 minutes, then counterstained with hematoxylin. Finally, all the sections were assessed under an optical microscope by two independent investigators; any discrepancy in immunohistochemistry was resolved by consensus.
Statistical analysis
The expression of IL-1α and IL-6 in cervical cancer and adjacent tissues was compared with paired Wilcoxon test. Chi-square test and Fisher’s exact test were used to assess the association between clinical characteristics of cervical cancer patients and IL-1α and IL-6 expression. The prognostic indicators of cervical cancer and IL-1α and IL-6 expression were determined using Kaplan-Meier survival analysis and log-rank test for univariate analysis; the significant variables resulting from univariate tests were included in the Cox multivariate regression analysis. A p value of <0.05 was considered statistically significant for all tests. Statistical analysis was performed using SPSS version 18.0 software (SPSS Inc., Chicago, USA).
Results
Expression of IL-1α and IL-6 significantly increased in cervical cancer tissues
Typical immunohistochemistry images of IL-1α and IL-6 in cervical cancer tissues and paired adjacent non-tumor tissues are shown in Figures 1 and 2. The positive percentages of IL-1α expression in cervical cancer and adjacent non-tumor tissues were 63.8% (67/105) and 8.6% (9/105), respectively. The positive percentages of IL-6 expression in cervical cancer and adjacent non-tumor tissues were 60.0% (63/105) and 10.5% (11/105), respectively. The chi-square test was used to confirm that the difference in the expression level of IL-1α and IL-6 between cervical cancer tissues and paired adjacent non-tumor tissues was statistically significant (p<0.05).
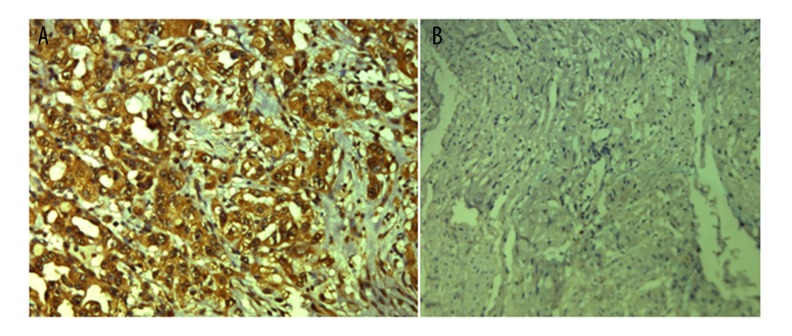
Figure 1.
Immunohistochemistry analysis of IL-1α expression. (A) Positive expression of IL-1α in cervical cancer tissues. (B) Negative expression of IL-1α in normal tissues. Original magnification: ×200.
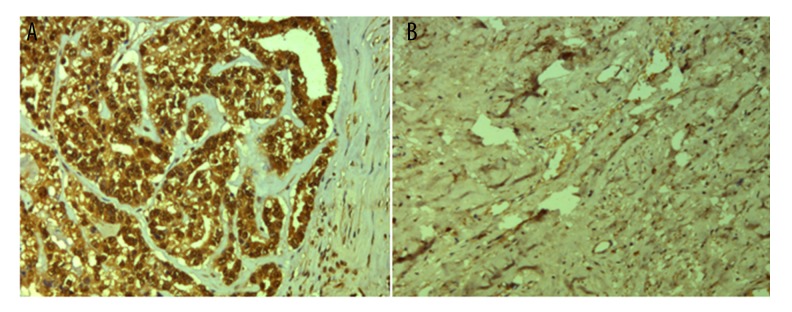
Figure 2.
Immunohistochemistry analysis of IL-6 expression. (A) Positive expression of IL-6 in cervical cancer tissues. (B) Negative expression of IL-6 in normal tissues. Original magnification: ×200.
Increased expression of IL-1α and IL-6 correlates with clinicopathological parameters of patients with cervical cancer
The relationship between a patient’s clinical parameters and the expressions of IL-1α and IL-6 are shown in Tables 3 and 4. These results showed that the expression of IL-1α in cervical cancer tissues was significantly correlated with tumor size (χ2=6.024, p=0.014), FIGO histology grade (χ2=19.661, p<0.0001), lymph node metastasis (χ2=5.135, p=0.023), stromal invasion (χ2=5.399, p=0.020), and tumor differentiation (χ2=7.513, p=0.006). However, no statistical correlation was found between IL-1α expression and patient age (p>0.05). Similarly, the expression of IL-6 in cervical cancer tissues was significantly correlated with tumor size (χ2=5.695, p=0.017), FIGO histology grade (χ2=10.239, p=0.001), and tumor differentiation (χ2=5.210, p=0.022). However, there was no statistical correlation found between IL-6 expression and patient age, lymph node metastasis, or stromal invasion (p>0.05). Therefore, these results demonstrated that higher IL-1α and IL-6 expression in cervical cancer tissues was positively correlated with tumor metastasis and cancer progression, suggesting that IL-1α and IL-6 play important roles in tumor progression.
Table 3.
Analysis of independent correlation factors of colorectal cancer prognosis with Cox multivariate regression analysis.
| Factors | B | S.E. | Ward | Sig. | Exp(B) | 95% CI for Exp(B) | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| IL-1α expression | −0.736 | 0.252 | 8.562 | 0.003 | 0.479 | 0.293 | 0.784 |
| Age | 0.109 | 0.241 | 0.205 | 0.651 | 1.115 | 0.695 | 1.791 |
| Tumor size | −0.265 | 0.227 | 1.364 | 0.243 | 0.768 | 0.492 | 1.197 |
| FIGO stage | −0.113 | 0.296 | 0.146 | 0.703 | 0.893 | 0.500 | 1.595 |
| Lymph node metastasis | −1.393 | 0.313 | 19.810 | <0.0001 | 0.248 | 0.134 | 0.459 |
| Stromal invasion | −0.604 | 0.344 | 3.074 | 0.080 | 0.547 | 0.279 | 1.074 |
| Tumor differentiation | −0.112 | 0.314 | 0.126 | 0.722 | 0.894 | 0.483 | 1.656 |
Table 4.
Analysis of independent correlation factors of colorectal cancer prognosis with Cox multivariate regression analysis.
| Factors | B | S.E. | Ward | Sig. | Exp(B) | 95% CI for Exp(B) | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| IL-6 expression | −0.401 | 0.237 | 2.868 | 0.090 | 0.670 | 0.421 | 1.065 |
| Age | 0.222 | 0.238 | 0.874 | 0.350 | 1.249 | 0.784 | 1.989 |
| Tumor size | −0.455 | 0.222 | 4.201 | 0.060 | 0.635 | 0.391 | 1.083 |
| FIGO stage | −0.181 | 0.299 | 0.364 | 0.546 | 0.835 | 0.464 | 1.501 |
| Lymph node metastasis | −1.531 | 0.328 | 21.784 | <0.0001 | 0.216 | 0.114 | 0.411 |
| Stromal invasion | −0.369 | 0.337 | 1.195 | 0.274 | 0.692 | 0.357 | 1.340 |
| Tumor differentiation | −0.181 | 0.302 | 0.359 | 0.549 | 0.835 | 0.462 | 1.508 |
Correlation between IL-1α and IL-6 and prognosis for cervical cancer patients
In order to further evaluate the relationship between IL-1α and IL-6 expression and prognosis of cervical cancer, we performed log-rank survival analysis according to IL-1α and IL-6 expression level and patient survival data. The survival analysis demonstrated that the cervical cancer survival rate of patients with negative IL-1α or IL-6 expression was significantly better than that of patients with positive expression (p<0.05, Figures 3, 4). Furthermore, a multivariate Cox regression analysis demonstrated that IL-1α expression and lymph node metastasis were independent predictors of overall survival in cervical cancer patients.
Figure 3.
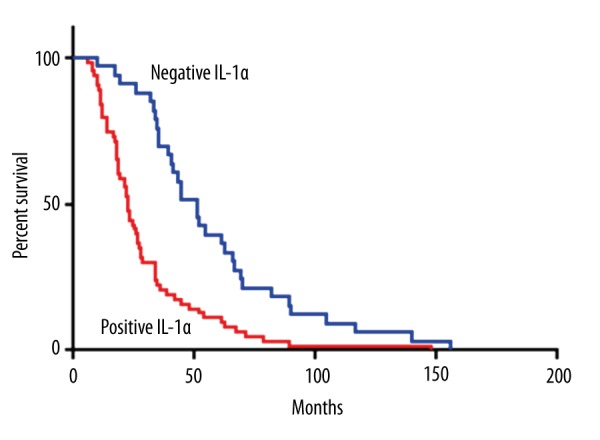
Kaplan-Meier survival curves stratified by IL-1α.
Figure 4.
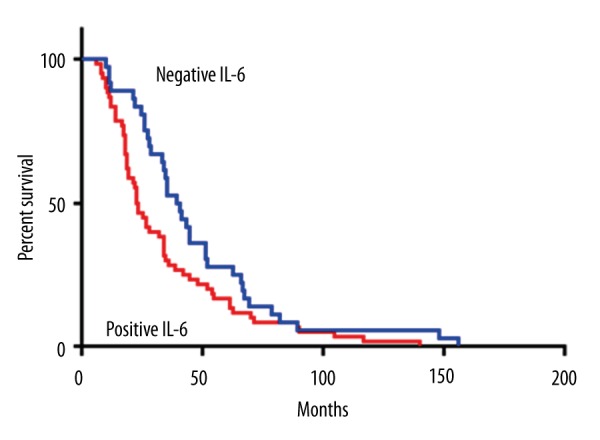
Kaplan-Meier survival curves stratified by IL-6.
Discussion
In the past decade, various studies have provided substantial evidence to support the role of inflammation and inflammation-related pathways in the pathogenesis of numerous human cancers, including cervical cancer [8–10]. It has been shown that the inflammatory microenvironment consists of many important components such as tumor cells, stromal cells, and immune and inflammatory cells. All of these components interact intimately and produce chemokines, growth factors, and adhesion molecules, and further promote the initiation and progression of many cancers [11,12].
IL-1α, a key inflammatory signaling cytokine, is secreted by various types of cells such as monocytes, macrophages, neutrophils, smooth muscle cells, fibroblasts, and cervical epithelium [13]. Increasing evidence suggests that IL-1α within the cancer microenvironment can induce the expression of growth factors, such as IL-6, IL-8, tumor necrosis factor-α (TNF-α), vascular endothelial growth factor (VEGF); metastatic genes such as the matrix metalloproteinases (MMPs); transforming growth factor-β (TGFβ), and stimulate the production of angiogenic proteins [14–16]. IL-1α plays an important role in the development, invasion, and metastasis of cancer. Previous studies have found elevated expression of IL-1α in a variety of cancers such as breast cancer [17], pancreatic cancer [18], and head and neck cancer [19]. Moreover, the enhanced expression of IL-1α has been correlated with poorer prognosis of cancer.
IL-6, a 23- to 30-kDa multifunctional cytokine, is produced by numerous kinds of immune and non-immune cells, including fibroblasts, immune cells, and some cancer cells [20]. IL-6 production can be induced by a wide range of stimuli, including IL-1 [21]. Emerging evidence revealed that IL-6 is a master regulator of cell proliferation, immune defense, and hematopoiesis, and plays a crucial role in the development and progression of various cancers [22–24]. IL-6 can regulate cancer cell survival, proliferation, and metastasis by activating JAK-STAT3 signaling pathway and the Ras-MAPK signaling pathway [25,26]. The activation of STAT3 has been shown to maintain NF-κB activity and enhance the expression of angiogenesis-related genes such as VEGF [27,28]. Therefore, angiogenesis oncology signaling pathways within the inflammatory microenvironment maybe a crucial mechanism of IL-6 induced cervical cancer. Furthermore, IL-6 can promote B cell differentiation and proliferation and then induce antibody secretion in B cells. Moreover, IL-6 can enhance, directly or indirectly, NK cell cytotoxicity and T cell tumoricidal activity [29]. This evidence further demonstrates the important role of IL-6 in an inflammatory microenvironment.
The results of our study demonstrated that the expression of IL-1α and IL-6 was correlated with invasion and progression of cervical cancer. Meanwhile, the cervical cancer survival rate of patients with negative IL-1α or IL-6 expression was significantly better than that of patients with positive expression. IL-1α and IL-6 are implicated as important to the cytokine network in the development of cervical cancer through several complicated mechanisms that involve cell proliferation, apoptosis, angiogenesis, inflammatory microenvironment, and numerous complex signaling pathways. It is well known that cancer pathogenesis is a multi-factor, multi-step, complicated process that involves gene-gene and gene-environment interactions. Large and well-designed studies are still needed to elucidate the pathogenesis of cervical cancer.
Conclusions
Our study showed that the expression of IL-1α and IL-6 was significantly elevated in cervical cancer tissues. Kaplan-Meier and Cox regression analysis demonstrated that IL-1α and IL-6 was associated with cervical cancer progression and metastasis and they might act as important molecular markers for prognosis, and be potential therapeutic targets for cervical cancer.
Footnotes
Conflict of interest
None.
Source of support: This research was supported in part by the National Nature Science Foundation of China (81573008), the Fund of Pudong Health Bureau of Shanghai (PWRd2014-01), and the Project of Key Disciplines Group Construction of Pudong Health Bureau of Shanghai (PWZxq2014-04)
References
- 1.Dorton BJ, Elias KM, Growdon W, Horowitz NS. Biomarkers in Gynecologic Cancers Red cell distribution width (RDW) as a novel marker for predicting recurrence of high-grade cervical dysplasia or carcinoma in active smokers. Gynecologic Oncology. 2015;136:400. [Google Scholar]
- 2.Colombo N, Carinelli S, Colombo A, et al. ESMO Guidelines Working Group. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:27–32. doi: 10.1093/annonc/mds268. [DOI] [PubMed] [Google Scholar]
- 3.Kawano M, Mabuchi S, Matsumoto Y, et al. Prognostic significance of pretreatment thrombocytosis in cervical cancer patients treated with definitive radiotherapy. Int J Gynecol Cancer. 2015;25:1656–62. doi: 10.1097/IGC.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 4.Oei AL, van Leeuwen CM, Ten Cate R, et al. Hyperthermia selectively targets human papillomavirus in cervical tumors via p53-dependent apoptosis. Cancer Res. 2015;75:5120–29. doi: 10.1158/0008-5472.CAN-15-0816. [DOI] [PubMed] [Google Scholar]
- 5.Chen MF, Chen PT, Chen WC, et al. The role of PD-L1 in the radiation response and prognosis for esophageal squamous cell carcinoma related to IL-6 and T-cell immunosuppression. Oncotarget. 2016;7(7):7913–24. doi: 10.18632/oncotarget.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo Y, Xu F, Lu T, et al. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38:904–10. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, Russell MR, Shahriari K, et al. Interleukin-1beta promotes skeletal colonization and progression of metastatic prostate cancer cells with neuroendocrine features. Cancer Res. 2013;73:3297–305. doi: 10.1158/0008-5472.CAN-12-3970. [DOI] [PubMed] [Google Scholar]
- 8.Prueitt RL, Wallace TA, Glynn SA, et al. An immune-inflammation gene expression signature in prostate tumors of smokers. Cancer Res. 2016;76:1055–65. doi: 10.1158/0008-5472.CAN-14-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyengar NM, Zhou XK, Gucalp A, et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-2239. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prevete N, Liotti F, Marone G, et al. Formyl peptide receptors at the interface of inflammation, angiogenesis and tumor growth. Pharmacol Res. 2015;102:184–91. doi: 10.1016/j.phrs.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Santhanam S, Alvarado DM, Ciorba MA. Therapeutic targeting of inflammation and tryptophan metabolism in colon and gastrointestinal cancer. Transl Res. 2016;167:67–79. doi: 10.1016/j.trsl.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes JV, Fernandes TAAD, De Azevedo JCV, et al. Link between chronic inflammation and human papillomavirus-induced carcinogenesis. Oncol Lett. 2015;9:1015–26. doi: 10.3892/ol.2015.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly PN, Romero DL, Yang Y, et al. Selective interleukin-1 receptor-associated kinase 4 inhibitors for the treatment of autoimmune disorders and lymphoid malignancy. J Exp Med. 2015;212:2189–201. doi: 10.1084/jem.20151074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mar AC, Chu CH, Lee HJ, et al. Interleukin-1 receptor type 2 acts with c-Fos to enhance the expression of interleukin-6 and vascular endothelial growth factor A in colon cancer cells and induce angiogenesis. J Biol Chem. 2015;290:22212–24. doi: 10.1074/jbc.M115.644823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon MJ, Hong E, Choi Y, et al. Interleukin-1 alpha, promotes extracellular shedding of syndecan-2 via induction of matrix metalloproteinase-7 expression. Biochem Biophys Res Commun. 2014;446:487–92. doi: 10.1016/j.bbrc.2014.02.142. [DOI] [PubMed] [Google Scholar]
- 16.Inoue A, Obayashi K, Sonoda Y, et al. Regulation of matrix metalloproteinase-1 and alpha-smooth muscle actin expression by interleukin-1 alpha and tumour necrosis factor alpha in hepatic stellate cells. Cytotechnology. 2016 doi: 10.1007/s10616-016-9948-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtzman SH, Anderson KH, Wang Y, et al. Cytokines in human breast cancer: IL-1alpha and IL-1beta expression. Oncol Rep. 1999;6:65–70. doi: 10.3892/or.6.1.65. [DOI] [PubMed] [Google Scholar]
- 18.Tang RF, Wang SX, Zhang FR, et al. Interleukin-1alpha, 6 regulate the secretion of vascular endothelial growth factor A, C in pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2005;4:460–63. [PubMed] [Google Scholar]
- 19.Wolf JS, Chen Z, Dong G, et al. IL (interleukin)-1alpha promotes nuclear factor-kappaB and AP-1-induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas. Clin Cancer Res. 2001;7:1812–20. [PubMed] [Google Scholar]
- 20.Yao X, Huang J, Zhong H, et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141:125–39. doi: 10.1016/j.pharmthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Ataie-Kachoie P, Pourgholami MH, Richardson DR, Morris DL. Gene of the month: Interleukin 6 (IL-6) J Clin Pathol. 2014;67:932–37. doi: 10.1136/jclinpath-2014-202493. [DOI] [PubMed] [Google Scholar]
- 22.Horn F, Henze C, Heidrich K. Interleukin-6 signal transduction and lymphocyte function. Immunobiology. 2000;202:151–67. doi: 10.1016/S0171-2985(00)80061-3. [DOI] [PubMed] [Google Scholar]
- 23.Hao W, Zhu Y, Zhou H. Prognostic value of interleukin-6 and interleukin-8 in laryngeal squamous cell cancer. Med Oncol. 2013;30:333. doi: 10.1007/s12032-012-0333-6. [DOI] [PubMed] [Google Scholar]
- 24.Ho LJ, Luo SF, Lai JH. Biological effects of interleukin-6: Clinical applications in autoimmune diseases and cancers. Biochem Pharmacol. 2015;97:16–26. doi: 10.1016/j.bcp.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Lee H, Herrmann A, et al. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nature Reviews Cancer. 2014;14:736–46. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Ye Y, Zhang H, et al. Diagnostic and prognostic value of serum Interleukin-6 in colorectal cancer. Medicine (Baltimore) 2016;95:e2502. doi: 10.1097/MD.0000000000002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen MF, Lin PY, Wu CF, et al. IL-6 expression regulates tumorigenicity and correlates with prognosis in bladder cancer. Plos One. 2013;8:e61901. doi: 10.1371/journal.pone.0061901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aggarwal BB, Kunnumakkara AB, Harikumar KB, et al. Signal transducer and activator of transcription-3, inflammation, and cancer how intimate is the relationship? Ann NY Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, Xue Y. Characterization of solid tumors induced by polycyclic aromatic hydrocarbons in mice. Med Sci Monit Basic Res. 2015;21:81–85. doi: 10.12659/MSMBR.893945. [DOI] [PMC free article] [PubMed] [Google Scholar]