Abstract
Common brain mechanisms are thought to play a significant role across a multitude of chronic pain syndromes. In addition, there is strong evidence for the existence of sex differences in the prevalence of chronic pain and in the neurobiology of pain. Thus, it is important to consider sex when developing general principals of pain neurobiology. The goal of the current review is to evaluate what is known about sex-specific brain alterations across multiple chronic pain populations. A total of 15 sex difference and 143 single-sex manuscripts were identified out of 412 chronic pain neuroimaging manuscripts. Results from sex difference studies indicate more prominent primary sensorimotor structural and functional alterations in female chronic pain patients compared to male chronic pain patients; differences in the nature and degree of insula alterations, with greater insula reactivity in male patients; differences in the degree of anterior cingulate structural alterations; and differences in emotional-arousal reactivity. Qualitative comparisons of male-specific and female-specific studies appear to be consistent with the results from sex difference studies. Given these differences, mixed-sex studies of chronic pain risk creating biased data or missing important information and single-sex studies have limited generalizability. The advent of large scale neuroimaging databases will likely aid in building a more comprehensive understanding of sex differences and commonalities in brain mechanisms underlying chronic pain.
Keywords: Neuroimaging, pain, sex differences
INTRODUCTION
Chronic pain describes pain that persists beyond a specific amount of time, usually at least 3 months beyond the normal healing period (Tsay et al. 2015), and includes a group of disorders such as irritable bowel disease (IBS), migraine, chronic headache, chronic lower back pain, fibromyalgia, vulvodynia, dysmenorrhea, and chronic prostatitis/chronic pain syndrome (Smith et al. 2014). The prevalence of chronic pain is growing in the population with current estimates that up to 64% report a chronic pain condition over their lifetime in the United States (Johannes et al. 2010). According to the Institute of Medicine, the health burden and health care costs to the individual and to society are increasing dramatically with estimates as high as $600 million each year in the United States (https://nccih.nih.gov/). Often chronic pain conditions are associated with increased disability, increased depression and anxiety, loss of income, and social isolation (Smith et al. 2014).
Chronic pain is complex, the investigation of pathophysiological mechanisms are still in progress, and existing treatments are suboptimal (Kawi 2015). The majority of studies investigating the pathophysiology of chronic pain have emphasized the origination of nociceptive signals in peripheral nerve terminal followed by encoding and modulation of these signals in the central nervous system (Aronoff 2016). More recently, multimodal neuroimaging studies have revealed abnormalities in evoked brain responses, morphology, and functional and anatomical connectivity in regions associated with salience, emotional arousal, and sensorimotor networks in patients with chronic pain (Mayer et al. 2015). It remains to be determined if these changes are primary nervous system alterations contributing to chronic pain, or if they are secondary brain changes resulting from ongoing nociceptive input to the central nervous system from the periphery.
Many chronic pain conditions are associated with a lowered pressure pain threshold in body locations far from the affected area, a sign of central sensitization. In addition, patients with chronic pain frequently have comorbidity with other chronic pain and psychiatric conditions, the precise location of the pain and predominant symptoms frequently change over time, and patients with different conditions show similar responses to centrally targeted therapies. Thus, common brain mechanisms, such as central sensitization, are thought to exist across a multitude of chronic pain syndromes (Mayer et al. 2009; Phillips and Clauw 2011). Consequently, there is increasing interest in identifying general principles of dysfunctional neurocircuitry associated with chronic pain states regardless of the particular body location affected. Furthermore, chronic pain syndromes demonstrate well-known sex differences in prevalence, severity, and response to treatment, with women typically showing greater prevalence for many syndromes (Andersson et al. 1993; Barsky et al. 2001; Berkley 1997; Berkley et al. 2006). Sex is increasingly being understood as an important basic variable, influencing the quality and generalizability of biomedical research (Clayton 2016). Although men and women may be highly similar in the experience of pain, important sex differences exist at all levels in the signaling systems involved in pain processing and stress, suggesting major differences in the operation of pain mechanisms (Bale and Epperson 2015; Berkley 1997; Cahill 2006), as well as sex (biological) and gender (social) differences in behavioral responses to pain (Bartley and Fillingim 2013). Given these considerations, it is critical to incorporate potential sex differences in any development of general principles for understanding chronic pain. The goal of the current review is to lay the groundwork for this process by examining the current status of the empirical literature on sex-specific brain alterations across chronic pain populations.
METHODS
Manuscript identification
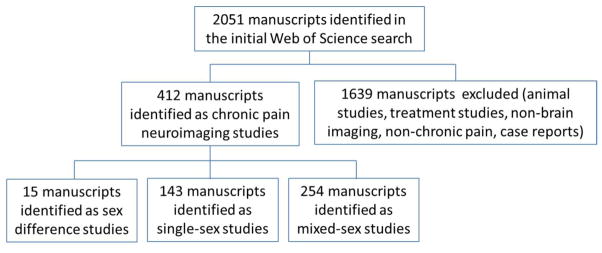
Literature searches were carried out in the Web of Science Core Collection (Thomson Reuters, New York) for neuroimaging studies of chronic pain populations published by December, 2015. The following chronic pain conditions impacting men and women were considered for this review: irritable bowel syndrome (IBS), functional dyspepsia, gastroesophageal reflux disease (GERD), migraine, cluster headache, tension type headache, temporomandibular disorder, burning mouth syndrome (BMS), atypical facial pain (AFP), chronic back/neck/shoulder/limb pain, osteoarthritis, whiplash, fibromyalgia, chronic widespread pain (CWP), chronic fatigue syndrome (CFS), urological chronic pelvic pain syndrome (UCPPS), and interstitial cystitis/painful bladder syndrome IC/PBS. In order to discern consistency in alterations across chronic pain disorders within sex, additional sex-specific chronic pain disorders (chronic prostatitis [CP], vulvodynia, vestibulitis, primary dysmenorrhea [PDM], premenstrual dysphoric disorder [PMDD], and endometriosis) were included in the review. These chronic pain conditions are all thought to have a strong central component (e.g., show pressure pain hypersensitivity) and are frequently co-morbid with other chronic pain conditions (Fernández-de-Las-Peñas et al. 2011; Herregods et al. 2015; Hsu et al. 2015; Li et al. 2013; Mayer et al. 2009; Phillips and Clauw 2011). Brain imaging techniques that were considered for the review included: structural magnetic resonance imaging (MRI), functional MRI (fMRI), positron emission tomography (PET), arterial spin labeling, diffusion tensor imaging (DTI), proton magnetic resonance spectroscopy (PMRS), and single-photon emission computed tomography (SPECT). Case reports, diagnostic neuroimaging, reviews, animal studies and treatment studies were excluded from consideration. Figure 1 depicts the manuscript selection process. A total of 412 potential manuscripts of interest were identified and further examined for their treatment of sex. Manuscripts with a focus on sex differences or sex hormones were identified by examination of the abstract and title. Single-sex studies were identified by examination of the title, abstract and methods section of the manuscript. Selected manuscripts were further classified in terms of evoked or non-evoked (or both) neuroimaging paradigms. Evoked paradigms use transient, experimental stimuli to identify changes in brain response (e.g. fMRI). Non-evoked paradigms focus on structural alterations in the brain (e.g. MRI, DTI), alterations in metabolism or neurotransmitter activity (e.g. PET), or intrinsic activity/connectivity alterations (e.g. resting-state fMRI). Resting-state fMRI may be used to identify state-based changes (e.g. interictal vs. ictal in migraine research) in activity/connectivity, which may differ from transient, evoked changes. Each type of neuroimaging modality produces different information about the role of the brain in chronic pain.
Figure 1.
The manuscript selection process is depicted.
RESULTS
The treatment of sex in the field of chronic pain neuroimaging
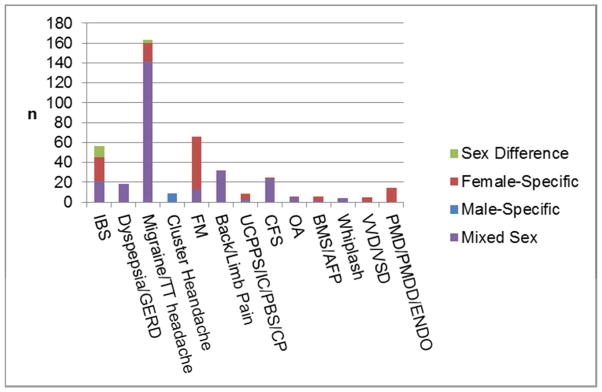
Out of 412 chronic pain neuroimaging manuscripts, a total of 15 sex difference (i.e., those comparing results for men and women), 143 single-sex, and 254 mixed-sex manuscripts were identified. Although some neuroimaging studies of menstrual-related pain disorders imaged across the menstrual cycle in order to compare a pain vs non-pain state, no manuscripts with a clear focus on sex hormones were identified. The proportion of sex-based studies for each of the pain conditions included in the literature search is shown in Figure 2. Sex difference manuscripts accounted for a small portion of the total literature (3.6%) and were concentrated in IBS and migraine research. A more substantial portion consisted of single-sex manuscripts (34.7%). These manuscripts tended to be segregated by pain diagnosis with studies in fibromyalgia, IBS, migraine, AFP, BMS, and IC/PBS mainly consisting of female-specific studies, while neuroimaging studies of cluster headache were exclusively male-specific. Furthermore, female-specific studies (n=129) vastly outnumbered male-specific studies (n=14).
Figure 2.
The number of manuscripts as classified in terms of the treatment of sex is shown for the chronic pain conditions included in the review. IBS, irritable bowel syndrome; GERD, gastroesophageal reflux disease; TT: tension-type; FM, fibromyalgia; UCPPS, urological chronic pelvic pain syndrome; IC/PBS, interstitial cystitis/painful bladder syndrome; CP, chronic prostatitis; CFS, chronic fatigue syndrome; OA, osteoarthritis; BMS, burning mouth syndrome; AFP, atypical facial pain; VVD, vulvodynia; VVS, vulvar vestibulitis syndrome; PDM, primary dysmenorrhea; PMDD, premenstrual dysphoric disorder; ENDO, endometriosis. No temporomandibular disorder manuscripts were identified.
Co-citation network analysis
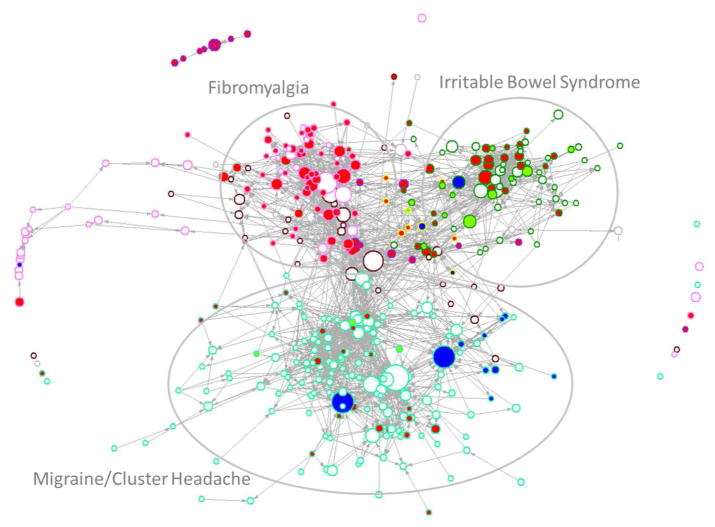
To gain a better understanding of the popularity and extent of influence of sex-based studies in the field of chronic pain neuroimaging, a co-citation network analysis was performed using Pajek visualization software. Co-citation network analyses assume that bibliographic elements act as concept surrogates, thus references included in a paper are considered to reflect some influence on its findings (Casillas and Acedo 2007). Co-citation network analyses identify groups of closely related documents that can be considered as belonging to the same ‘research front’ as well as provide information about how different fronts interrelate. All 412 identified chronic pain neuroimaging manuscripts were entered into the analysis visualized in Figure 3, which also incorporates information regarding the total number of citations for each manuscript. Visual examination of the overall structure in Figure 3 reveals that greater affinity exists among IBS, fibromyalgia, and urological/pelvic pain neuroimaging researchers, than between these groups and migraine/headache researchers. In addition, the popularity of sex-based studies (both sex difference and single-sex studies) varied with sex-based studies fairly well-cited and well-connected within the IBS subfield, while many sex-based studies were poorly cited and not well-connected within the migraine and headache subfield.
Figure 3.
A co-citation network analysis of chronic pain neuroimaging studies with a focus on the treatment of sex is depicted. Each manuscript of interest was coded according to its treatment of sex (represented by the color of the inside of the associated vertex: green, sex differences; red, female-specific; blue, male-specific; white, mixed-sex) and pain population (represented by the color of the vertex border: gastrointestinal, dark green; fibromyalgia and chronic fatigue syndrome, lavender; chronic back/limb pain, brown; urological pain, yellow; headache pain, teal; menstrual and vulvar pain, purple; other, gray). The size of each vertex was determined by the total number of citations the manuscript received by December 2015. Examination of Figure 3 indicates a fair degree of cross-citation among chronic pain neuroimaging researchers of different pain conditions, but less so for headache pain studies. The largest cluster in the figure (lower center) represents mainly migraine and cluster headache studies where despite the existence of sex difference manuscripts, sex appears to be generally not well-considered.
DISCUSSION
Sex difference neuroimaging studies of patients with chronic pain suggest that across pain conditions sex differences exist in the altered reactivity, morphology, and connectivity of major brain regions and networks involved in pain modulation (Table 1). Although additional regions/networks demonstrate sex differences, below we summarize the findings for three networks that, as of yet, comprise the majority of sex differences in chronic pain: salience, sensorimotor, and emotional-arousal networks. In addition to reviewing the findings from sex differences studies, the results from sex-specific studies (Supplementary Table 1) are also qualitatively evaluated for consistency with the sex difference studies.
Table 1.
Sex difference publications listed by year of publication.
| Sex Difference Publications | ||||
|---|---|---|---|---|
| Publication | Populations | Modality/Task | Main Results | Evoked vs. Non-evoked Paradigm |
| 2015 | ||||
| Faria et al 2015 | All Children: Female Migr = 14 Male Migr = 14 Female HC = 14 Male HC = 14 |
MRI (CT, GMV) |
Female Migr vs. Male Migr & HCs
|
Non-evoked paradigm |
| Irimia et al 2015 | Males IBS = 19 Females IBS = 14 Males HC = 23 Females HC= 33 |
DTI (FA) |
Male & Female IBS vs Male & Female HC
|
Non-evoked paradigm |
| Woodworth et al 2015 | Male UCPPS =26 Female UCPPS = 19 Male IBS =16 Female IBS = 23 Male HC= 30 Female HC = 26 |
DTI |
Female UCPPS vs. Male UCPPS
|
Non-evoked paradigm |
| 2014 | ||||
| Gupta et al 2014 | Female IBS = 28 Male IBS = 30 Female HC = 72 Male HC = 38 |
Resting fMRI (ICA) |
Male IBS vs. Male HC
|
Non-evoked paradigm |
| Hong et al 2014 | Female IBS = 24 Male IBS = 24 Female HC = 24 Male HC = 24 |
Resting fMRI (functional connectivity of the dorsal aINS) |
Female IBS vs. Female HC
|
Non-evoked paradigm |
| 2013 | ||||
| Ellingson et al 2013 | Female IBS = 21 Male IBS = 12 Female HC = 72 Male HC = 21 |
DTI (FA, MD) |
Female IBS vs. Male IBS
|
Non-evoked paradigm |
| Hong et al 2013 | Female IBS = 31 Male IBS = 29 Female HC = 76 Male HC = 42 |
Resting fMRI (fALFF) |
Female IBS vs. Female HC
|
Non-evoked paradigm |
| Jiang et al 2013 | Female IBS = 70 Male IBS = 20 Female HC = 155 Male HC = 21 |
MRI (CT) |
Female IBS vs. Female HC
|
Non-evoked paradigm |
| Labus et al 2013 | Female IBS = 27 Male IBS = 20 Female HC = 38 Male HC = 29 |
fMRI (non-painful emotional stimuli) |
(Male IBS - Female IBS) vs. (Male HC – Female HC)
|
Evoked non-pain paradigm |
| 2012 | ||||
| Maleki et al 2012 | Female Migr = 11 Male Migr = 11 Female HC = 11 Male HC = 11 |
MRI (CT, GMV); fMRI (thermal pain + 1°C stimuli) |
Female Migr vs. Female HC (Structural)
|
Both evoked pain paradigm and non-evoked paradigm |
| 2011 | ||||
| Liu et al 2011 | Female Migr = 20 Male Migr = 18 Female HC = 20 Male HC = 18 |
|
Female Migr vs. Male Migr
|
Non-evoked paradigm |
| 2008 | ||||
| Labus et al 2008 | Female IBS = 24 Male IBS = 22 |
PET (rectal balloon distension) |
Female IBS vs. Male IBS: Emotional-arousal network:
|
Evoked pain paradigm |
| 2003 | ||||
| Nakai et al 2003 | Female IBS = 6 Male IBS = 6 Female HC = 7 Male HC = 6 |
PET (5-HT synthesis) |
Female IBS vs. Female HC
|
Non-evoked paradigm |
| Naliboff et al 2003 | Female IBS = 23 Male IBS = 19 |
PET (rectal balloon distension) |
Female IBS vs. Male IBS
|
Evoked pain paradigm |
| 2000 | ||||
| Berman et al 2000 | Female IBS = 13 Male IBS = 17 |
PET (rectal distension) |
Female IBS vs. Male IBS
|
Evoked pain paradigm |
Abbreviations - Groups: HC, healthy controls; IBS, irritable bowel syndrome; MIGR, migraine; UCPPS, urological chronic pelvic pain
Abbreviations – Methods: CT, cortical thickness; DTI, diffusion tensor imaging; FA, fractional anisotropy; fMRI, functional magnetic resonance imaging; GMD, grey matter density; GMV, grey matter volume; MD, mean diffusivity; MRI, magnetic resonance imaging; PET, positron emission tomography
Abbreviations – Brain Regions: ACC, anterior cingulate cortex; aINS, anterior insula; AMYG, amygdala; BG, basal ganglia; dlPFC, dorsal lateral prefrontal cortex; HIPP, hippocampus; HYPO, hypothalamus; INS, insula; mPFC, medial prefrontal cortex; MTG; middle temporal gyrus; NAcc, nucleau accumbens; OFC, orbital frontal cortex; OFG, orbital frontal gyrus; PAG, periaqueductal gray; PCC, posterior cingulate cortex; PFC, prefrontal cortex; pgACC, pregenual anterior cingulate cortex; PHG, parahippocampal gyrus; pINS, posterior insula; PreCG, precentral gyrus; SI, primary somatosensory cortex; SFG, superior frontal gyrus; sgACC, subgenual anterior cingulate cortex; SMA, supplementary motor area; STG, superior temporal gyrus; THAL, thalamus
Salience Network
The anterior cingulate (ACC; involved in emotion-cognitive interactions) and anterior insula (involved in the integration of interoceptive, affective, and cognitive influences) form the salience network, which functions to identify the most salient, homeostatically-relevant events among all internal and external stimuli (Menon and Uddin 2010). The salience network, through the anterior insula, also mediates the ‘switching’ between activation of the default mode network (DMN) and other major networks in order to guide appropriate responses to the salient event (Menon and Uddin 2010). The insula and the ACC have long been recognized as important to pain and to be involved in pain-related perceptual, affective, and cognitive responses (Derbyshire et al. 1997).
Sex-difference studies
Insula pain-related responses appear to be enhanced to a greater extent in male patients compared to female patients, at least in IBS and migraine pain populations, where direct comparisons have been made (Berman et al. 2000; Labus et al. 2013; Maleki et al. 2012a). Sex difference studies also indicate that sex influences the nature of insula alterations, at least in terms of intrinsic activity and connectivity (Gupta et al. 2014; Hong et al. 2013a; Hong et al. 2014; Jiang et al. 2013; Maleki et al. 2012b). In particular, altered connectivity between the insula and DMN may be more relevant to female pain patients than male patients, suggesting sex differences in internally-directed resources in response to stressful and salient events (Hong et al. 2014; Maleki et al. 2012b).
In contrast to the insula, ACC pain-related responses may be enhanced to a greater extent in female patients compared to male patients, at least in IBS (Naliboff et al. 2003). In addition, anatomical ACC alterations have been reported to be more prominent in female patients with IBS, with female patients demonstrating reduced subgenual ACC cortical thickness and increased mean diffusivity in cingulate white bundles compared to male patients (Ellingson et al. 2013; Jiang et al. 2013). However, male patients with IBS have demonstrated greater ACC reactivity to emotional stimuli previously shown to elicit greater behavioral and brain responses in healthy male subjects compared to healthy female subjects (Labus et al. 2013). Moreover, sex differences in altered ACC functional connectivity have been reported in both migraine and IBS studies, with enhanced ACC connectivity with emotional-arousal regions such as the amygdala and hippocampus in female patients with IBS compared with male patients (Gupta et al. 2014; Labus et al. 2013; Labus et al. 2008; Liu et al. 2011).
Single sex studies
Although pain-related insula reactivity may be enhanced to a greater degree in male patients than in female patients, single-sex studies demonstrate that both male and female patients in many chronic pain conditions show increased anterior/posterior insula responses to pain compared to same-sex healthy controls (Bannbers et al. 2012; Berman et al. 2008; Cook et al. 2004; Diers et al. 2011; Ellingson et al. 2012; Elsenbruch et al. 2010a; Elsenbruch et al. 2010b; Farmer et al. 2011; Hall et al. 2010; Hampson et al. 2013; Howard et al. 2012; Hubbard et al. 2015; Kim et al. 2011; Labus et al. 2013; Larsson et al. 2012; Lopez-Sola et al. 2014; Lowen et al. 2015; May et al. 1998b; McLoughlin et al. 2011; Pujol et al. 2009; Pukall et al. 2005; Rahm et al. 2015; Sprenger et al. 2007; Tu et al. 2010). Furthermore, female patients with FM show increased connectivity between insula and DMN regions, similar to female patients with IBS and migraine (Hong et al. 2014; Ichesco et al. 2014; Maleki et al. 2012b; Napadow et al. 2010), while altered insula connectivity of male patients with chronic prostatisis did not include DMN regions (Kutch et al. 2015). Additional male-specfic studies reporting altered insula connectivity were seed/region-specific and did not examine whether altered insula-DMN connectivity existed (Qiu et al. 2013; Qiu et al. 2012). Similar to the insula, both male and female patients have demonstrated increased ACC reactivity to pain relative to same-sex healthy controls (Hall et al. 2010; Howard et al. 2012; Larsson et al. 2012; Lowen et al. 2015; May et al. 1998a)Pujol 2009}(Albuquerque et al. 2006; Berman et al. 2008; Mayer et al. 2005). In addition, both male and female patients have demonstrated decreased ACC volume/density relative to same-sex healthy controls (As-Sanie et al. 2012; Burgmer et al. 2009; Jensen et al. 2013; Kuchinad et al. 2007; Mordasini et al. 2012; Robinson et al. 2011; Tu et al. 2013; Wood et al. 2009).
Interim summary
While single-sex studies demonstrate that insula and ACC pain reactivity is enhanced in both male and female patients with chronic pain relative to same-sex controls, sex difference studies show that important sex differences exist in the degree of of ACC structural alterations and the reactivity and functional organization of the insula and ACC in patients with chronic pain. Given the role of the insula and ACC in guiding the response to salient events, sex differences in altered reactivity and connectivity are likely to may impact vigilance, allocation of attentional resources and the mobilization of maladaptive processes in response to pain and stress in chronic pain patients.
Sensorimotor Network
The sensorimotor network includes the primary and secondary somatosensory and motor cortices as well as the basal ganglia. A recent neuroimaging study found a relationship between the cortical thickness of primary somatosensory cortex and pain thresholds in healthy controls, demonstrating an importance of primary somatosensory morphology in individual differences in pain sensitivity that may affect vulnerability to chronic pain (Erpelding et al. 2012).
Sex-difference studies
Sex difference studies suggest more prominent alterations exist in the primary sensorimotor cortex of female patients compared to male patients. In IBS, female but not male patients demonstrate increased cortical thickness of the sensorimotor cortex compared to same-sex controls (Jiang et al. 2013). Although a recent study did not find sex differences in the mean fractional anisotropy of all tracts innervating somatosensory cortex (Irimia et al. 2015), previous studies have shown reduced integrity of sensorimotor-related tracts in female IBS patients compared to male IBS patients (Ellingson et al. 2013; Woodworth et al. 2015). A potential reason for this discrepancy may be that sex differences occur within specific white matter tracts and this information is lost when averaging the fractional anisotropy across all tracts. In migraine patients, more dysfunctional connections and decreased centrality in networks including sensorimotor have been found in female patients compared to male patients (Liu et al. 2011). Furthermore, female children with migraines demonstrate greater cortical thickness increases in primary somatosensory cortex with age compared to male children with migraines (Faria et al. 2015).
Sex differences in sensorimotor alterations also extend beyond the cortex. Specifically, female patients with migraines demonstrate more dysfunctional connections involving the caudate and putamen (Liu et al. 2015b) and greater pain-related reactivity in the caudate, while male patients with migraines demonstrate greater pain-related reactivity in the putamen (Maleki et al. 2012b). Furthermore, female children with migraines demonstrate increased gray matter volume in the caudate and pallidum, suggesting an organizational/chromosomal rather than a sex hormone driven mechanism (Faria et al. 2015). Finally, female IBS patients demonstrate lower fractional anisotropy and mean diffusivity in the globus pallidus compared with male IBS patients (Ellingson et al. 2013).
Single sex studies
Compared to female healthy controls, increased primary somatosensory cortical thickness/gray matter density/volume has been observed in female patients with migraine (Kim et al. 2014), IBS (Labus et al. 2014; Labus et al. 2015), IC/PBS (Kairys et al. 2015), FM (Fallon et al. 2013; Lutz et al. 2008), and UCPPS (Bagarinao et al. 2014). In addition, patients with dysmenorrhea demonstrate greater menstrual-related increases in primary somatosensory cortex gray matter volume compared to female healthy controls (Tu et al. 2013). Furthermore, additional primary sensorimotor cortical alterations including connectivity, intrinsic activity, reactivity, and perfusion have been noted in female-specific chronic pain studies (Arkink et al. 2012; Burgmer et al. 2012; Ellingson et al. 2012; Guedj et al. 2008; Guedj et al. 2007; Gupta et al. 2015; Kamping et al. 2013; Kilpatrick et al. 2014; Kim et al. 2013; Lee et al. 2013; Liu et al. 2015a; Liu et al. 2015b; Pujol et al. 2014; Rahm et al. 2015; Tu et al. 2009).
Although male-specific studies are severely limited, none have reported gray matter changes within the primary sensorimotor (Farmer et al. 2011; Mordasini et al. 2012). However, increased functional connectivity between motor cortices and the posterior insular has been reported in male patients with CP relative to same-sex healthy controls (Kutch et al. 2015). In addition, alterations of the internal capsule have been reported in male patients with cluster headaches relative to same-sex healthy controls (Teepker et al. 2012). Thus, some important sensorimotor alterations may exist in male patients.
Interim summary
While single-sex studies suggest that some sensorimotor alterations may exist in male patients with chronic pain, sex difference studies show more prominent structural changes in primary sensorimotor cortices and a greater number of altered sensorimotor/basal ganglia functional connections in female chronic pain patients compared to male patients. Sex differences in primary sensorimotor cortical alterations may impact the development of effective transcranial magnetic stimulation-based therapies for chronic pain. In addition, sex differences in chronic pain-related basal ganglia alterations could impact vulnerability to drug abuse and obesity (Geha et al. 2014).
Emotional-Arousal Network
The emotional-arousal network includes the amygdala, hippocampus/parahippocampal gyrus, and ACC (Pezawas et al. 2005; Stein et al. 2007). The amygdala has long been considered to play a role in the affective modulation of pain (Carrasquillo and Gereau 2007; Neugebauer et al. 2004). In addition, similarities between pain and memory mechanisms in the hippocampus have been reported (Price and Inyang 2015). The ACC has already been discussed above as a region of the salience network involved in emotion-cognitive interactions and findings relating to the ACC have been presented; therefore, the following sections mainly focus on findings related to the amygdala and hippocampus.
Sex-difference studies
Early sex difference studies in IBS suggested that emotional-arousal responses (amygdala and ACC) and altered connectivity were greater in female patients compared to male patients (Labus et al. 2008; Naliboff et al. 2003). However, subsequent studies have demonstrated that male IBS patients may have greater reactivity (amygdala, hippocampus, and ACC) under specific experimental conditions, such as when viewing faces depicting emotions previously shown to elicit greater behavioral and brain responses in male subjects (fear and anger) (Labus et al. 2013). Thus, male and female IBS patients may have similar or analogous changes in emotional-arousal reactivity, with sex-specific triggers. Sex difference studies in migraine research also call into question the notion that emotional-arousal reactivity is greater in female patients as women with migraines have demonstrated greater deactivation of emotional-arousal regions (amygdala and hippocampus) compared to male patients, at least to thermal pain stimuli (Maleki et al. 2012b).
Single sex studies
Both female- and male-specific studies have demonstrated increased emotional-arousal reactivity (amygdala and hippocampus) and altered connectivity during pain in patients compared to same-sex controls (Gingnell et al. 2012; Howard et al. 2012; Jensen et al. 2012; Kamping et al. 2013; Khan et al. 2014; Kim et al. 2013; Liu et al. 2015b; Liu et al. 2012; Martucci et al. 2015; May et al. 1998a; Mayer et al. 2005; Qiu et al. 2013; Sprenger et al. 2006; Wilder-Smith et al. 2004).
Interim summary
Both sex-specific and sex difference studies suggest that male and female patients demonstrate increased emotional-arousal reactivity to pain with altered functional organization of the amygdala and hippocampus relative to same-sex healthy controls. However, the conditions eliciting the enhanced response may differ between male and female patients.
Barriers to progress
Sex differences clearly exist in brain responses to experimental evoked pain but most of these studies have been performed in healthy controls and not in patients with chronic pain (reviewed in (Fillingim et al. 2009)). However, the study of sex differences in the response to evoked pain within chronic pain populations is critical. As of yet, it is not clear how sex differences in the brain’s functional response to acute, experimental pain in healthy controls relates to the noted sex differences in chronic pain prevalence, symptoms, and response to treatment.
Furthermore, while the reported sex-specific structural brain changes are likely to contribute to the observed clinical and epidemiological sex differences in chronic pain, for the majority of chronic pain conditions, sex difference neuroimaging research is lacking. Rather, researchers either report mixed-sex studies or attempt to control for sex-based biology by focusing on a single sex (usually female). One result of this strategy is that male subjects are severely underrepresented. Although chronic pain is more prevalent in women, there are still substantial numbers of men suffering from chronic pain. The sex difference studies that have been conducted show that important sex differences exist in chronic pain. Thus, mixed- and single-sex studies of chronic pain have limited generalizability, risk creating biased data, and may miss important findings. For example, in Hong et al (Hong et al. 2013b), when sex was ignored, no differences in insula activity were found between IBS patients and healthy controls. When sex difference analyses were performed, it was found that insula low frequency power was increased in male patients with IBS and decreased in female patients compared to same-sex healthy controls. Controlling for sex would not have allowed this discovery. Another consequence of focusing on one sex within a particular pain condition is that only half the picture is being generated for that condition, which limits the generation of potentially fruitful scientific questions. The direct comparison of men and women offers a unique perspective, which can provide the critical spark that ignites fundamental breakthroughs (McCarthy et al. 2012).
Another potential barrier concerns bias in the diagnostic criteria for some chronic pain conditions. For example, the symptom criteria for fibromyalgia as defined by the American College of Rheumatology emphasizes the number of trigger points which may contribute to a female bias as women appear to innately have more tender points than men (Berkley 1997; Vincent et al. 2013a), thus subject recruitment for an adequately powered sex difference study is difficult. A more general view that focuses on the presence of chronic widespread pain (CWP) shows much less sex-bias (LeResche 1999). Given the demonstrated differences in men and women with other types of chronic pain, it appears likely that females with fibromyalgia should not represent all CWP patients. In another example, new thinking in pelvic pain has created a more inclusive diagnosis of urologic chronic pelvic pain syndrome (UCPPS), which incorporates what have previously been considered separate male and female syndromes. Previously, the female version was called interstitial cystitis/painful bladder syndrome (IC/PBS), and the male version was called chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). Thus sex difference studies in UCPPS would greatly add to the understanding of commonalities and differences in pelvic pain. Finally, chronic back pain is one of the most common pain conditions and affects both men and women, yet sex difference neuroimaging studies are lacking. Thus, neuroimaging studies of sex differences are feasible in a wide range of chronic pain conditions, but have not yet been realized. An important clinical consequence of identifying neurobiological sex differences in chronic pain patients is the concept that different brain mechanisms may play a role in the construction of the chronic pain experience, which in turn may require sex-specific treatment approaches to male and female patients.
An additional area in which a lack of research may be creating a barrier to progress concerns sex hormones. Sex hormones are well known to influence pain perception with estradiol having both pro-nociceptive and anti-nociceptive actions while testosterone appears to be mainly anti-nociceptive in both males and females (Aloisi and Bonifazi 2006; Craft 2007; Vincent and Tracey 2010). While the importance of studying sex hormone-related effects is most obvious in menstrual pain-related syndromes, many other chronic pain patients demonstrate cycle-related or menopausal-related changes in symptom severity (Heitkemper and Chang 2009; Martin 2009). Although a few neuroimaging studies have imaged across the menstrual cycle in menstrual-related pain populations, the focus was on high and low pain states, not the role of sex hormones in chronic pain (Tu et al. 2009; Tu et al. 2013; Vincent et al. 2011). Results from a recent neuroimaging study using healthy women suggest that in a low endogenous estradiol state, testosterone may be a key factor in modulating pain sensitivity via descending pathways of the brain with higher levels of endogenous testosterone associated with decreased thermal pain sensitivity (Vincent et al. 2013b). Thus, sex hormones affect pathways potentially important to chronic pain.
Conclusions and Future Directions
This review demonstrated that, when examined, significant sex differences exist in the altered morphology, connectivity, and response to pain in the brain of chronic pain patients. Sex difference studies indicate more prominent primary sensorimotor structural and functional alterations in female chronic pain patients compared to male chronic pain patients; differences in the nature and degree of insula alterations, with greater insula reactivity in male patients; differences in the degree of anterior cingulate structural alterations; and differences in emotional-arousal reactivity that be driven mainly by sex differences in the effectiveness of emotional-arousal triggers. Qualitative comparisons of male-specific and female-specific studies appear to be consistent with the results from sex difference studies.
Sex difference research has been concentrated in few pain disorders, and the large number of single-sex studies are severely imbalanced, favoring female chronic pain patients. In addition, the co-citation analysis revealed areas in which sex-based research and collaboration is needed. Much work is still needed to advance a neurobiological understanding of the differences between male and female chronic pain patients with the goal of improving chronic pain for both men and women. Big data efforts, such as the recently developed PAIN neuroimaging database (PainRepository.org), which contains neuroimaging scans from a wide variety of pain patients and healthy controls, can provide the sample sizes needed to facilitate analyses leading to a more comprehensive understanding of the commonalities and differences in brain alterations of male and female patients across chronic pain conditions, particularly with the use of standardized imaging protocols (Labus et al. 2016).
Supplementary Material
Significance.
Chronic pain disorders are associated with a great burden to the individual and to society. Brain alterations are involved in chronic pain and recent developments suggest that common mechanisms may exist across a multitude of chronic pain conditions. In addition, sex differences exist at all levels in the signaling systems involved in pain processing and stress. This review summarizes what is known about sex-specific brain alterations found across chronic pain populations and where additional research is needed. These findings have implications for the development of effective treatments for both male and female patients with chronic pain.
Acknowledgments
Funding Support: Supported by NIH grants DK048351, DK064539, K01 DK085133
Footnotes
Conflict of Interest: None
- Arpana Gupta: Acquisition of data, analysis, partial drafting of the manuscript, editing
- Emeran A Mayer: Concept design, interpretation, editing
- Connor Fling: Acquisition of data, editing
- Jennifer S Labus: Concept design, editing
- Bruce D Naliboff: Interpretation, editing
- Jui-Yang Hong: Acquisition of data, analysis, editing
- Lisa A Kilpatrick: Concept design, acquisition, analysis, partial drafting of the manuscript, interpretation, editing
References
- Albuquerque RJC, de Leeuw R, Carlson CR, Okeson JP, Miller CS, Andersen AH. Cerebral activation during thermal stimulation of patients who have burning mouth disorder: An fMRI study. Pain. 2006;122(3):223–234. doi: 10.1016/j.pain.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Bonifazi M. Sex hormones, central nervous system and pain. Horm Behav. 2006;50(1):1–7. doi: 10.1016/j.yhbeh.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Andersson HI, Ejlertsson G, Leden I, Rosenberg C. Chronic pain in a geographically defined general population: studies of differences in age, gender, social class, and pain localization. Clin J Pain. 1993;9(3):174–182. doi: 10.1097/00002508-199309000-00004. [DOI] [PubMed] [Google Scholar]
- Arkink EB, Bleeker EJW, Schmitz N, Schoonman GG, Wu O, Ferrari MD, van Buchem MA, van Osch MJP, Kruit MC. Cerebral perfusion changes in migraineurs: a voxelwise comparison of interictal dynamic susceptibility contrast MRI measurements. Cephalalgia. 2012;32(4):279–288. doi: 10.1177/0333102411435985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff GM. What Do We Know About the Pathophysiology of Chronic Pain? Implications for Treatment Considerations. Med Clin North Am. 2016;100(1):31–42. doi: 10.1016/j.mcna.2015.08.004. [DOI] [PubMed] [Google Scholar]
- As-Sanie S, Harris RE, Napadow V, Kim J, Neshewat G, Kairys A, Williams D, Clauw DJ, Schmidt-Wilcke T. Changes in regional gray matter volume in women with chronic pelvic pain: A voxel-based morphometry study. Pain. 2012;153(5):1006–1014. doi: 10.1016/j.pain.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagarinao E, Johnson KA, Martucci KT, Ichesco E, Farmer MA, Labus J, Ness TJ, Harris R, Deutsch G, Apkarian AV, Mayer EA, Clauw DJ, Mackey S. Preliminary structural MRI based brain classification of chronic pelvic pain: A MAPP network study. Pain. 2014;155(12):2502–2509. doi: 10.1016/j.pain.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nat Neurosci. 2015;18(10):1413–1420. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannbers E, Gingnell M, Engman J, Morell A, Comasco E, Kask K, Garavan H, Wikstrom J, Poromaa IS. The effect of premenstrual dysphoric disorder and menstrual cycle phase on brain activity during response inhibition. Journal of Affective Disorders. 2012;142(1–3):347–350. doi: 10.1016/j.jad.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Barsky AJ, Peekna HM, Borus JF. Somatic symptom reporting in women and men. J Gen Intern Med. 2001;16(4):266–275. doi: 10.1046/j.1525-1497.2001.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52–58. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20(3):371–380. doi: 10.1017/s0140525x97221485. discussion 435–513. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Zalcman SS, Simon VR. Sex and gender differences in pain and inflammation: a rapidly maturing field. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R241–244. doi: 10.1152/ajpregu.00287.2006. [DOI] [PubMed] [Google Scholar]
- Berman S, Munakata J, Naliboff BD, Chang L, Mandelkern M, Silverman D, Kovalik E, Mayer EA. Gender differences in regional brain response to visceral pressure in IBS patients. Eur J Pain. 2000;4(2):157–172. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller JA, Ruby K, Jarcho J, Mayer EA. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28(2):349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgmer M, Gaubitz M, Konrad C, Wrenger M, Hilgart S, Heuft G, Pfleiderer B. Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosom Med. 2009;71(5):566–573. doi: 10.1097/PSY.0b013e3181a32da0. [DOI] [PubMed] [Google Scholar]
- Burgmer M, Pfleiderer B, Maihofner C, Gaubitz M, Wessolleck E, Heuft G, Pogatzki-Zahn E. Cerebral mechanisms of experimental hyperalgesia in fibromyalgia. Eur J Pain. 2012;16(5):636–647. doi: 10.1002/j.1532-2149.2011.00058.x. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7(6):477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Carrasquillo Y, Gereau RW. Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. J Neurosci. 2007;27(7):1543–1551. doi: 10.1523/JNEUROSCI.3536-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casillas J, Acedo F. Evolution of the Intellectual Structure of Family Business Literature: A Bibliometric Study of FBR. Family Business Review. 2007;20(2):141–162. [Google Scholar]
- Clayton JA. Studying both sexes: a guiding principle for biomedicine. FASEB J. 2016;30(2):519–524. doi: 10.1096/fj.15-279554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DB, Lange G, Ciccone DS, Liu WC, Steffener J, Natelson BH. Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol. 2004;31(2):364–378. [PubMed] [Google Scholar]
- Craft RM. Modulation of pain by estrogens. Pain. 2007;132(Suppl 1):S3–12. doi: 10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73(3):431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- Diers M, Schley MT, Rance M, Yilmaz P, Lauer L, Rukwied R, Schmelz M, Flor H. Differential central pain processing following repetitive intramuscular proton/prostaglandin E(2) injections in female fibromyalgia patients and healthy controls. Eur J Pain. 2011;15(7):716–723. doi: 10.1016/j.ejpain.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Ellingson BM, Mayer E, Harris RJ, Ashe-McNally C, Naliboff BD, Labus JS, Tillisch K. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain. 2013;154(9):1528–1541. doi: 10.1016/j.pain.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson LD, Shields MR, Stegner AJ, Cook DB. Physical activity, sustained sedentary behavior, and pain modulation in women with fibromyalgia. J Pain. 2012;13(2):195–206. doi: 10.1016/j.jpain.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. 2010a;139(4):1310–1319. doi: 10.1053/j.gastro.2010.06.054. [DOI] [PubMed] [Google Scholar]
- Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. 2010b;59(4):489–495. doi: 10.1136/gut.2008.175000. [DOI] [PubMed] [Google Scholar]
- Erpelding N, Moayedi M, Davis KD. Cortical thickness correlates of pain and temperature sensitivity. Pain. 2012;153(8):1602–1609. doi: 10.1016/j.pain.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Fallon N, Chiu YH, Li XY, Nurmikko TJ, Stancak A. Ipsilateral cortical activation in fibromyalgia patients during brushing correlates with symptom severity. Clinical Neurophysiology. 2013;124(1):154–163. doi: 10.1016/j.clinph.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Faria V, Erpelding N, Lebel A, Johnson A, Wolff R, Fair D, Burstein R, Becerra L, Borsook D. The migraine brain in transition: girls vs boys. Pain. 2015;156(11):2212–2221. doi: 10.1097/j.pain.0000000000000292. [DOI] [PubMed] [Google Scholar]
- Farmer MA, Chanda ML, Parks EL, Baliki MN, Apkarian AV, Schaeffer AJ. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2011;186(1):117–124. doi: 10.1016/j.juro.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-de-Las-Peñas C, Ortega-Santiago R, Cuadrado ML, López-de-Silanes C, Pareja JA. Bilateral widespread mechanical pain hypersensitivity as sign of central sensitization in patients with cluster headache. Headache. 2011;51(3):384–391. doi: 10.1111/j.1526-4610.2010.01791.x. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha P, Dearaujo I, Green B, Small DM. Decreased food pleasure and disrupted satiety signals in chronic low back pain. Pain. 2014;155(4):712–722. doi: 10.1016/j.pain.2013.12.027. [DOI] [PubMed] [Google Scholar]
- Gingnell M, Morell A, Bannbers E, Wikstrom J, Poromaa IS. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Hormones and Behavior. 2012;62(4):400–406. doi: 10.1016/j.yhbeh.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Guedj E, Cammilleri S, Niboyet J, Dupont P, Vidal E, Dropinski JP, Mundler O. Clinical correlate of brain SPECT perfusion abnormalities in fibromyalgia. J Nucl Med. 2008;49(11):1798–1803. doi: 10.2967/jnumed.108.053264. [DOI] [PubMed] [Google Scholar]
- Guedj E, Taieb D, Cammilleri S, Lussato D, de Laforte C, Niboyet J, Mundler O. Voxel-by-voxel analysis of brain SPECT perfusion in Fibromyalgia. Nuclear Instruments & Methods in Physics Research Section a-Accelerators Spectrometers Detectors and Associated Equipment. 2007;571(1–2):85–88. [Google Scholar]
- Gupta A, Kilpatrick L, Labus J, Tillisch K, Braun A, Hong JY, Ashe-McNalley C, Naliboff B, Mayer EA. Early Adverse Life Events and Resting State Neural Networks in Patients With Chronic Abdominal Pain: Evidence for Sex Differences. Psychosomatic Medicine. 2014;76(6):404–412. doi: 10.1097/PSY.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Rapkin A, Gill Z, Kilpatrick L, Fling C, Stains J, Masghati S, Tillisch K, Mayer E, Labus JS. Disease-Related Differences in Resting State Networks: A Comparison between Localized Provoked Vulvodynia, Irritable Bowel Syndrome, and Healthy Control Subjects. Pain. 2015 doi: 10.1097/01.j.pain.0000461289.65571.54. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GBC, Kamath MV, Collins S, Ganguli S, Spaziani R, Miranda KL, Bayati A, Bienenstock J. Heightened central affective response to visceral sensations of pain and discomfort in IBS. Neurogastroenterology and Motility. 2010;22(3) doi: 10.1111/j.1365-2982.2009.01436.x. [DOI] [PubMed] [Google Scholar]
- Hampson JP, Reed BD, Clauw DJ, Bhavsar R, Gracely RH, Haefner HK, Harris RE. Augmented central pain processing in vulvodynia. J Pain. 2013;14(6):579–589. doi: 10.1016/j.jpain.2013.01.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkemper MM, Chang L. Do fluctuations in ovarian hormones affect gastrointestinal symptoms in women with irritable bowel syndrome? Gend Med. 2009;6(Suppl 2):152–167. doi: 10.1016/j.genm.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herregods TV, Bredenoord AJ, Smout AJ. Pathophysiology of gastroesophageal reflux disease: new understanding in a new era. Neurogastroenterol Motil. 2015;27(9):1202–1213. doi: 10.1111/nmo.12611. [DOI] [PubMed] [Google Scholar]
- Hong JY, Kilpatrick LA, Labus J, Gupta A, Jiang ZG, Ashe-McNalley C, Stains J, Heendeniya N, Ebrat B, Smith S, Tillisch K, Naliboff B, Mayer EA. Patients with Chronic Visceral Pain Show Sex-Related Alterations in Intrinsic Oscillations of the Resting Brain. Journal of Neuroscience. 2013a;33(29):11994–12002. doi: 10.1523/JNEUROSCI.5733-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JY, Kilpatrick LA, Labus JS, Gupta A, Katibian D, Ashe-McNalley C, Stains J, Heendeniya N, Smith SR, Tillisch K, Naliboff B, Mayer EA. Sex and Disease-Related Alterations of Anterior Insula Functional Connectivity in Chronic Abdominal Pain. Journal of Neuroscience. 2014;34(43):14252–14259. doi: 10.1523/JNEUROSCI.1683-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JY, Kilpatrick LA, Labus JS, Jiang ZG, Gupta A, Ashe-McNalley C, Stains J, Ebrat B, Tillisch K, Naliboff BD, Mayer EA. Differences in Resting-State Bold Oscillation Signals Between Healthy and IBS Subjects. Gastroenterology. 2013b;144(5):S559–S559. [Google Scholar]
- Howard MA, Sanders D, Krause K, O’Muircheartaigh J, Fotopoulou A, Zelaya F, Thacker M, Massat N, Huggins JP, Vennart W, Choy E, Daniels M, Williams SCR. Alterations in Resting-State Regional Cerebral Blood Flow Demonstrate Ongoing Pain in Osteoarthritis An Arterial Spin-Labeled Magnetic Resonance Imaging Study. Arthritis and Rheumatism. 2012;64(12):3936–3946. doi: 10.1002/art.37685. [DOI] [PubMed] [Google Scholar]
- Hsu CS, Liu TT, Wen SH, Wang CC, Yi CH, Chen JH, Lei WY, Orr WC, Fabio P, Chen CL. Clinical, metabolic, and psychological characteristics in patients with gastroesophageal reflux disease overlap with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2015;27(5):516–522. doi: 10.1097/MEG.0000000000000334. [DOI] [PubMed] [Google Scholar]
- Hubbard CS, Hong J, Jiang Z, Ebrat B, Suyenobu B, Smith S, Heendeniya N, Naliboff BD, Tillisch K, Mayer EA, Labus JS. Increased attentional network functioning related to symptom severity measures in females with irritable bowel syndrome. Neurogastroenterology and Motility. 2015;27(9):1282–1294. doi: 10.1111/nmo.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichesco E, Schmidt-Wilcke T, Bhavsar R, Clauw DJ, Peltier SJ, Kim J, Napadow V, Hampson JP, Kairys AE, Williams DA, Harris RE. Altered Resting State Connectivity of the Insular Cortex in Individuals With Fibromyalgia. Journal of Pain. 2014;15(8):815–826. doi: 10.1016/j.jpain.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia A, Labus JS, Torgerson CM, Van Horn JD, Mayer EA. Altered viscerotopic cortical innervation in patients with irritable bowel syndrome. Neurogastroenterology and Motility. 2015;27(8):1075–1081. doi: 10.1111/nmo.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SCR, Choy E, Mainguy Y, Vitton O, Gracely RH, Gollub R, Ingvar M, Kong J. Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Molecular Pain. 2012;8 doi: 10.1186/1744-8069-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Srinivasan P, Spaeth R, Tan Y, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SCR, Choy E, Vitton O, Gracely R, Ingvar M, Kong J. Overlapping Structural and Functional Brain Changes in Patients With Long-Term Exposure to Fibromyalgia Pain. Arthritis and Rheumatism. 2013;65(12):3293–3303. doi: 10.1002/art.38170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, Dinov ID, Labus J, Shi YG, Zamanyan A, Gupta A, Ashe-McNalley C, Hong JY, Tillisch K, Toga AW, Mayer EA. Sex-Related Differences of Cortical Thickness in Patients with Chronic Abdominal Pain. Plos One. 2013;8(9) doi: 10.1371/journal.pone.0073932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11(11):1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Kairys AE, Schmidt-Wilcke T, Puiu T, Ichesco E, Labus JS, Martucci K, Farmer MA, Ness TJ, Deutsch G, Mayer EA, Mackey S, Apkarian AV, Maravilla K, Clauw DJ, Harris RE. Increased Brain Gray Matter in the Primary Somatosensory Cortex is Associated with Increased Pain and Mood Disturbance in Patients with Interstitial Cystitis/Painful Bladder Syndrome. Journal of Urology. 2015;193(1):131–137. doi: 10.1016/j.juro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamping S, Bomba IC, Kanske P, Diesch E, Flor H. Deficient modulation of pain by a positive emotional context in fibromyalgia patients. Pain. 2013;154(9):1846–1855. doi: 10.1016/j.pain.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Kawi J. Managing chronic pain in primary care. The Nurse practitioner. 2015 doi: 10.1097/01.NPR.0000460854.37363.37. [DOI] [PubMed] [Google Scholar]
- Khan SA, Keaser ML, Meiller TF, Seminowicz DA. Altered structure and function in the hippocampus and medial prefrontal cortex in patients with burning mouth syndrome. Pain. 2014;155(8):1472–1480. doi: 10.1016/j.pain.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Kutch JJ, Tillisch K, Naliboff BD, Labus JS, Jiang Z, Farmer MA, Apkarian AV, Mackey S, Martucci KT, Clauw DJ, Harris RE, Deutsch G, Ness TJ, Yang CC, Maravilla K, Mullins C, Mayer EA. Alterations in Resting State Oscillations and Connectivity in Sensory and Motor Networks in Women with Interstitial Cystitis/Painful Bladder Syndrome. Journal of Urology. 2014;192(3):947–955. doi: 10.1016/j.juro.2014.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim JB, Suh SI, Seo WK, Oh K, Koh SB. Thickening of the somatosensory cortex in migraine without aura. Cephalalgia. 2014;34(14):1125–1133. doi: 10.1177/0333102414531155. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim SH, Seo J, Han SW, Nam EJ, Kim SK, Lee HJ, Lee SJ, Kim YT, Chang Y. Increased power spectral density in resting-state pain-related brain networks in fibromyalgia. Pain. 2013;154(9):1792–1797. doi: 10.1016/j.pain.2013.05.040. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chang Y, Kim JH, Song HJ, Seo J, Han SW, Nam EJ, Choi TY, Lee SJ, Kim SK. Insular cortex is a trait marker for pain processing in fibromyalgia syndrome - blood oxygenation level-dependent functional magnetic resonance imaging study in Korea. Clinical and Experimental Rheumatology. 2011;29(6):S19–S27. [PubMed] [Google Scholar]
- Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27(15):4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutch JJ, Yani MS, Asavasopon S, Kirages DJ, Rana M, Cosand L, Labus JS, Kilpatrick LA, Ashe-McNalley C, Farmer MA, Johnson KA, Ness TJ, Deutsch G, Harris RE, Apkarian AV, Clauw DJ, Mackey SC, Mullins C, Mayer EA. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: A MAPP: Research Network Neuroimaging Study. Neuroimage Clin. 2015;8:493–502. doi: 10.1016/j.nicl.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labus JS, Dinov ID, Jiang Z, Ashe-McNalley C, Zamanyan A, Shi Y, Hong JY, Gupta A, Tillisch K, Ebrat B, Hobel S, Gutman BA, Joshi S, Thompson PM, Toga AW, Mayer EA. Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain. 2014;155(1):137–149. doi: 10.1016/j.pain.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labus JS, Gupta A, Coveleskie K, Tillisch K, Kilpatrick L, Jarcho J, Feier N, Bueller J, Stains J, Smith S, Suyenobu B, Naliboff B, Mayer EA. Sex differences in emotion-related cognitive processes in irritable bowel syndrome and healthy control subjects. Pain. 2013;154(10):2088–2099. doi: 10.1016/j.pain.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labus JS, Naliboff B, Kilpatrick L, Liu C, Ashe-McNalley C, dos Santos IR, Alaverdyan M, Woodworth D, Gupta A, Ellingson BM, Tillisch K, Mayer EA. Pain and Interoception Imaging Network (PAIN): A multimodal, multisite, brain-imaging repository for chronic somatic and visceral pain disorders. Neuroimage. 2016;124(Pt B):1232–1237. doi: 10.1016/j.neuroimage.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41(3):1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labus JS, Van Horn JD, Gupta A, Alayerdyan M, Torgerson C, Ashe-McNalley C, Irimia A, Hong JY, Naliboff B, Tillisch K, Mayer EA. Multivariate morphological brain signatures predict patients with chronic abdominal pain from healthy control subjects. Pain. 2015;156(8):1545–1554. doi: 10.1097/j.pain.0000000000000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MBO, Tillisch K, Craig AD, Engstrom M, Labus J, Naliboff B, Lundberg P, Strom M, Mayer EA, Walter SA. Brain Responses to Visceral Stimuli Reflect Visceral Sensitivity Thresholds in Patients With Irritable Bowel Syndrome. Gastroenterology. 2012;142(3):463–U111. doi: 10.1053/j.gastro.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Song HJ, Decety J, Seo J, Kim SH, Nam EJ, Kim SK, Han SW, Lee HJ, Do Y, Chang Y. Do patients with fibromyalgia show abnormal neural responses to the observation of pain in others? Neurosci Res. 2013;75(4):305–315. doi: 10.1016/j.neures.2013.01.013. [DOI] [PubMed] [Google Scholar]
- LeResche L. Gender considerations in the epidemiology of chronic pain. In: Crombie I, Croft P, Linton S, LeResche L, Von Korff M, editors. Epidemiology of Pain. Seattle: IASP Press; 1999. pp. 43–52. [Google Scholar]
- Li X, Cao Y, Wong RK, Ho KY, Wilder-Smith CH. Visceral and somatic sensory function in functional dyspepsia. Neurogastroenterol Motil. 2013;25(3):246–253. e165. doi: 10.1111/nmo.12044. [DOI] [PubMed] [Google Scholar]
- Liu JX, Lan L, Mu JY, Zhao L, Yuan K, Zhang Y, Huang LY, Liang FR, Tian J. Genetic Contribution of Catechol-O-Methyltransferase in Hippocampal Structural and Functional Changes of Female Migraine Sufferers. Human Brain Mapping. 2015a;36(5):1782–1795. doi: 10.1002/hbm.22737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JX, Qin W, Nan JF, Li J, Yuan K, Zhao L, Zeng F, Sun JB, Yu DH, Dong MH, Liu P, von Deneen KM, Gong QY, Liang FR, Tian J. Gender-Related Differences in the Dysfunctional Resting Networks of Migraine Suffers. Plos One. 2011;6(11) doi: 10.1371/journal.pone.0027049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JX, Zhao L, Lei FF, Zhang Y, Yuan K, Gong QY, Liang FR, Tian J. Disrupted Resting-State Functional Connectivity and Its Changing Trend in Migraine Suffers. Human Brain Mapping. 2015b;36(5):1892–1907. doi: 10.1002/hbm.22744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JX, Zhao L, Li GY, Xiong SW, Nan JF, Li J, Yuan K, von Deneen KM, Liang FR, Qin W, Tian J. Hierarchical Alteration of Brain Structural and Functional Networks in Female Migraine Sufferers. Plos One. 2012;7(12) doi: 10.1371/journal.pone.0051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Sola M, Pujol J, Wager TD, Garcia-Fontanals A, Blanco-Hinojo L, Garcia-Blanco S, Poca-Dias V, Harrison BJ, Contreras-Rodriguez O, Monfort J, Garcia-Fructuoso F, Deus J. Altered Functional Magnetic Resonance Imaging Responses to Nonpainful Sensory Stimulation in Fibromyalgia Patients. Arthritis & Rheumatology. 2014;66(11):3200–3209. doi: 10.1002/art.38781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen MBO, Mayer E, Tillisch K, Labus J, Naliboff B, Lundberg P, Thorell LH, Strom M, Engstrom M, Walter S. Deficient habituation to repeated rectal distensions in irritable bowel syndrome patients with visceral hypersensitivity. Neurogastroenterology and Motility. 2015;27(5):646–655. doi: 10.1111/nmo.12537. [DOI] [PubMed] [Google Scholar]
- Lutz J, Jager L, de Quervain D, Krauseneck T, Padberg F, Wichnalek M, Beyer A, Stahl R, Zirngibl B, Morhard D, Reiser M, Schelling G. White and Gray Matter Abnormalities in the Brain of Patients With Fibromyalgia A Diffusion-Tensor and Volumetric Imaging Study. Arthritis and Rheumatism. 2008;58(12):3960–3969. doi: 10.1002/art.24070. [DOI] [PubMed] [Google Scholar]
- Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia. 2012a;32(8):607–620. doi: 10.1177/0333102412445622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N, Linnman C, Brawn J, Burstein R, Becerra L, Borsook D. Her versus his migraine: multiple sex differences in brain function and structure. Brain. 2012b;135(Pt 8):2546–2559. doi: 10.1093/brain/aws175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin VT. Ovarian hormones and pain response: a review of clinical and basic science studies. Gend Med. 2009;6(Suppl 2):168–192. doi: 10.1016/j.genm.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Martucci KT, Shirer WR, Bagarinao E, Johnson KA, Farmer MA, Labus JS, Apkarian AV, Deutsch G, Harris RE, Mayer EA, Clauw DJ, Greicius MD, Mackey SC. The posterior medial cortex in urologic chronic pelvic pain syndrome: detachment from default mode network-a resting-state study from the MAPP Research Network. Pain. 2015;156(9):1755–1764. doi: 10.1097/j.pain.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A, Bahra A, Buchel C, Frackowiak RSJ, Goadsby PJ. Hypothalamic activation in cluster headache attacks. Lancet. 1998a;352(9124):275–278. doi: 10.1016/S0140-6736(98)02470-2. [DOI] [PubMed] [Google Scholar]
- May A, Kaube H, Buchel C, Eichten C, Rijntjes M, Juptner M, Weiller C, Diener HC. Experimental cranial pain elicited by capsaicin: a PET study. Pain. 1998b;74(1):61–66. doi: 10.1016/S0304-3959(97)00144-9. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115(3):398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Bushnell MC International Association for the Study of Pain. Functional pain syndromes : presentation and pathophysiology. Seattle: IASP Press; 2009. p. xviii.p. 580. [Google Scholar]
- Mayer EA, Gupta A, Kilpatrick LA, Hong JY. Imaging brain mechanisms in chronic visceral pain. Pain. 2015;156(Suppl 1):S50–63. doi: 10.1097/j.pain.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 2012;32(7):2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin MJ, Stegner AJ, Cook DB. The relationship between physical activity and brain responses to pain in fibromyalgia. J Pain. 2011;12(6):640–651. doi: 10.1016/j.jpain.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordasini L, Weisstanner C, Rummel C, Thalmann GN, Verma RK, Wiest R, Kessler TM. Chronic pelvic pain syndrome in men is associated with reduction of relative gray matter volume in the anterior cingulate cortex compared to healthy controls. J Urol. 2012;188(6):2233–2237. doi: 10.1016/j.juro.2012.08.043. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Berman S, Chang L, Derbyshire SWG, Suyenobu B, Vogt BA, Mandelkern M, Mayer EA. Sex-related differences in IBS patients: Central processing of visceral stimuli. Gastroenterology. 2003;124(7):1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62(8):2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10(3):221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25(2):141–154. doi: 10.1016/j.berh.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Inyang KE. Commonalities between pain and memory mechanisms and their meaning for understanding chronic pain. Prog Mol Biol Transl Sci. 2015;131:409–434. doi: 10.1016/bs.pmbts.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Lopez-Sola M, Ortiz H, Vilanova JC, Harrison BJ, Yucel M, Soriano-Mas C, Cardoner N, Deus J. Mapping brain response to pain in fibromyalgia patients using temporal analysis of FMRI. PLoS One. 2009;4(4):e5224. doi: 10.1371/journal.pone.0005224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Macia D, Garcia-Fontanals A, Blanco-Hinojo L, Lopez-Sola M, Garcia-Blanco S, Poca-Dias V, Harrison BJ, Contreras-Rodriguez O, Monfort J, Garcia-Fructuoso F, Deus J. The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia. Pain. 2014;155(8):1492–1503. doi: 10.1016/j.pain.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Pukall CF, Strigo IA, Binik YM, Amsel R, Khalife S, Bushnell MC. Neural correlates of painful genital touch in women with vulvar vestibulitis syndrome. Pain. 2005;115(1–2):118–127. doi: 10.1016/j.pain.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Qiu EC, Wang Y, Ma L, Tian LX, Liu RZ, Dong Z, Xu X, Zou ZT, Yu SY. Abnormal Brain Functional Connectivity of the Hypothalamus in Cluster Headaches. Plos One. 2013;8(2) doi: 10.1371/journal.pone.0057896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu EC, Yu SY, Liu RZ, Wang Y, Ma L, Tian LX. Altered regional homogeneity in spontaneous cluster headache attacks: a resting-state functional magnetic resonance imaging study. Chin Med J (Engl) 2012;125(4):705–709. [PubMed] [Google Scholar]
- Rahm B, Lacour M, Decety J, Muller J, Scheidt CE, Bauer J, Konig R, Wirsching M, Glauche V, Ohlendorf S, Unterbrink T, Hartmann A, Joos AA. Self-perspective leads to increased activation of pain processing brain regions in fibromyalgia. Comprehensive Psychiatry. 2015;59:80–90. doi: 10.1016/j.comppsych.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Robinson ME, Craggs JG, Price DD, Perlstein WM, Staud R. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. J Pain. 2011;12(4):436–443. doi: 10.1016/j.jpain.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Wilkie R, Uthman O, Jordan JL, McBeth J. Chronic pain and mortality: a systematic review. Plos One. 2014;9(6):e99048. doi: 10.1371/journal.pone.0099048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger T, Ruether KV, Boecker H, Valet M, Berthele A, Pfaffenrath V, Woller A, Tolle TR. Altered metabolism in frontal brain circuits in cluster headache. Cephalalgia. 2007;27(9):1033–1042. doi: 10.1111/j.1468-2982.2007.01386.x. [DOI] [PubMed] [Google Scholar]
- Sprenger T, Willoch F, Miederer M, Schindler F, Valet M, Berthele A, Spilker ME, Forderreuther S, Straube A, Stangier I, Wester HJ, Tolle TR. Opioidergic changes in the pineal gland and hypothalamus in cluster headache: a ligand PET study. Neurology. 2006;66(7):1108–1110. doi: 10.1212/01.wnl.0000204225.15947.f8. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. Neuroimage. 2007;36(3):736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Teepker M, Menzler K, Belke M, Heverhagen JT, Voelker M, Mylius V, Oertel WH, Rosenow F, Knake S. Diffusion tensor imaging in episodic cluster headache. Headache. 2012;52(2):274–282. doi: 10.1111/j.1526-4610.2011.02000.x. [DOI] [PubMed] [Google Scholar]
- Tsay A, Allen TJ, Proske U, Giummarra MJ. Sensing the body in chronic pain: a review of psychophysical studies implicating altered body representation. Neurosci Biobehav Rev. 2015;52:221–232. doi: 10.1016/j.neubiorev.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Tu CH, Niddam DM, Chao HT, Chen LF, Chen YS, Wu YT, Yeh TC, Lirng JF, Hsieh JC. Brain morphological changes associated with cyclic menstrual pain. Pain. 2010;150(3):462–468. doi: 10.1016/j.pain.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Tu CH, Niddam DM, Chao HT, Liu RS, Hwang RJ, Yeh TC, Hsieh JC. Abnormal cerebral metabolism during menstrual pain in primary dysmenorrhea. Neuroimage. 2009;47(1):28–35. doi: 10.1016/j.neuroimage.2009.03.080. [DOI] [PubMed] [Google Scholar]
- Tu CH, Niddam DM, Yeh TC, Lirng JF, Cheng CM, Chou CC, Chao HT, Hsieh JC. Menstrual pain is associated with rapid structural alterations in the brain. Pain. 2013;154(9):1718–1724. doi: 10.1016/j.pain.2013.05.022. [DOI] [PubMed] [Google Scholar]
- Vincent A, Lahr BD, Wolfe F, Clauw DJ, Whipple MO, Oh TH, Barton DL, St Sauver J. Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res (Hoboken) 2013a;65(5):786–792. doi: 10.1002/acr.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent K, Tracey I. Sex hormones and pain: the evidence from functional imaging. Curr Pain Headache Rep. 2010;14(5):396–403. doi: 10.1007/s11916-010-0139-1. [DOI] [PubMed] [Google Scholar]
- Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I. Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain. 2011;152(9):1966–1975. doi: 10.1016/j.pain.2011.03.029. [DOI] [PubMed] [Google Scholar]
- Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I. Brain imaging reveals that engagement of descending inhibitory pain pathways in healthy women in a low endogenous estradiol state varies with testosterone. Pain. 2013b;154(4):515–524. doi: 10.1016/j.pain.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53(11):1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PB, Glabus MF, Simpson R, Patterson JC. Changes in Gray Matter Density in Fibromyalgia: Correlation With Dopamine Metabolism. Journal of Pain. 2009;10(6):609–618. doi: 10.1016/j.jpain.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Woodworth D, Mayer E, Leu K, Ashe-McNalley C, Naliboff BD, Labus JS, Tillisch K, Kutch JJ, Farmer MA, Apkarian AV, Johnson KA, Mackey SC, Ness TJ, Landis JR, Deutsch G, Harris RE, Clauw DJ, Mullins C, Ellingson BM, Network MR. Unique Microstructural Changes in the Brain Associated with Urological Chronic Pelvic Pain Syndrome (UCPPS) Revealed by Diffusion Tensor MRI, Super-Resolution Track Density Imaging, and Statistical Parameter Mapping: A MAPP Network Neuroimaging Study. Plos One. 2015;10(10) doi: 10.1371/journal.pone.0140250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.