Abstract
The neuroimmune system is significantly sexually dimorphic, with sex differences evident in the number and activation states of microglia, in the activation of astrocytes, and in cytokine release and function. Neuroimmune cells and signaling are now recognized as critical for many neural functions throughout the lifespan, including synaptic plasticity and memory function. Here we address the question of how cytokines, astrocytes, and microglia contribute to memory, and specifically how neuroimmune modulation of memory differentially affects males and females. Understanding sex differences in both normal memory processes and dysregulation of memory in psychiatric and neurological disorders is critical for developing treatment and preventive strategies for memory disorders that are effective for both men and women.
Keywords: Learning and memory, cytokines, females, microglial activation, neurogenesis, astrocytes, synaptic plasticity
Memory of places, events, and relationships between cues is critical for adapting to a rapidly changing world. The ability to vary memory strength as a function of importance—memories of dangerous or beneficial events, should be strengthened—is a critical feature of memory encoding. Modulation of memory by co-occurring emotion, stress, or immune state, can thereby increase or decrease memory strength as appropriate for current circumstances (Liang et al., 1986; Roozendaal et al., 2002). Given the fundamental nature of memory, it is somewhat surprising that memory function differs between males and females. Nevertheless, sex differences have been observed across a broad range of memory tasks including verbal memory (Kramer et al., 1988), context fear conditioning (Maren et al., 1994; Keiser et al., in review), extinction of fear conditioning (Lebron-Milad et al., 2012), reward-related learning (Quinn et al., 2007), and spatial memory (Voyer et al., 2016), among others (see also Andreano and Cahill, 2009; Galea et al., 2013). Sex differences are also evident across the cognitive strategies used to learn a task (Grissom et al., 2013; Shah et al., 2013), neural circuits recruited for memory (Gruene et al., 2014), the molecular mechanisms of memory (Keiser and Tronson, 2016), and the modulation of memory by stress (Waddell et al., 2008). Many disorders of memory, including Alzheimer’s disease (Seshadri et al., 1997) and post-traumatic stress disorder (Kessler et al., 2012), are more prevalent in women than in men (Sohrabji et al., 2016). Determining sex-specific mechanisms of memory formation and its dysregulation is of high importance for developing novel preventive and treatment strategies for both men and women.
There is growing evidence for a central role of immune cells and signaling in memory formation and its modulation (Donzis and Tronson, 2014; Haydon and Nedergaard, 2015; Wu et al., 2015). Immune proteins are required for normal synaptic plasticity (O'Connor and Coogan, 1999; del Rey et al., 2013) and stress may influence memory via interactions with immune signaling (Deak et al., 2015; Vecchiarelli et al., 2015). Furthermore, activation of the peripheral immune system during illness or injury, and the resulting neuroimmune response (Figure 1), dramatically alters memory processes. In patients of major surgery or illness, persistent cognitive deficits are common and correlate with levels of inflammatory signaling (Hovens:2014ib Petrovitch et al., 1998; Hudetz et al., 2009; Girard et al., 2010). Dysregulated immune activity contributes to neurological and psychiatric disorders of memory, including Alzheimer’s disease and post-traumatic stress disorder (Azizi et al., 2014; Wieck et al., 2014; Dzamba et al., 2016). Immune-related signaling is an important mechanism for synaptic plasticity and memory, as well as persistent cognitive dysregulation, after major illness and in psychiatric and neurological diseases.
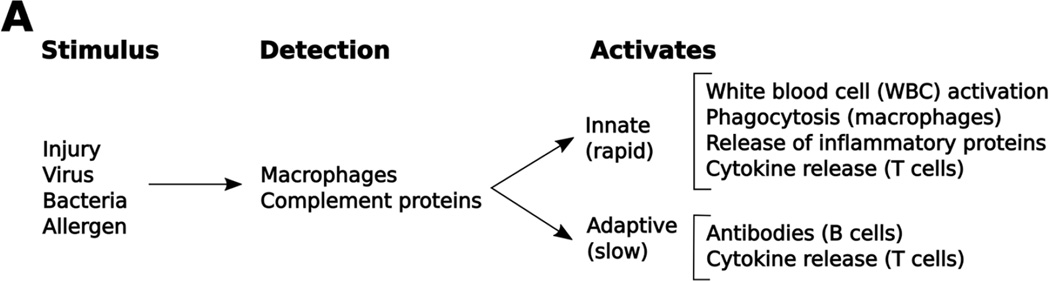
Figure 1.
A brief overview of innate and adaptive immune responses. Potentially dangerous “non-self” stimuli are detected by macrophage cells via toll-like receptors, and by complement via antibody binding. These, in turn, release cytokines that activate both innate and adaptive immune responses. The innate immune system acts via cytokine release and cell-based mechanisms to quickly eliminate these stimuli. The adaptive immune system works more slowly and produces antibodies to fight infections that cannot be removed by the innate system.
Given sex differences in mechanisms of memory, activation of immune signaling, and memory-related neurological and psychiatric disorders, it is likely that neuroimmune modulation of memory differs in males and females. If so, differential targeting of neuroimmune cells or immune signaling in males and females may provide novel, sex-specific pathways for intervening in disorders of memory. Here we will review the current literature on neuroimmune influences on memory with an emphasis on sex differences in memory modulation by cytokine signaling, activation of immune cells in the brain, and immune-related regulation of adult neurogenesis.
The neuroimmune system
The neuroimmune system and its role in neural processes is a relatively new area of study. In the 1980s, it was postulated that fever was caused by actions of “pyrogenic cytokines”, in particular IL-1, on the hypothalamus (Atkins, 1985). In contrast, other sickness behaviors including increased sleep, decreased grooming, and anorexic effects were believed to be caused by peripheral effects of inflammation (Hart, 1988). This discrepancy was clarified with the demonstration that IL-1 in the brain, and not peripheral immune signaling, directly mediates sickness behaviors (Nakamura et al., 1988), demonstrating for the first time a critical role for immune signaling in the brain on behavioral regulation. Subsequent evidence for immune-driven impairments in cognition (Gibertini et al., 1995), together with the correlation between immune changes and depression (Anisman et al., 2002; Dantzer et al., 2008; Capuron and Miller, 2011), additionally demonstrated a central role for neuroimmune signaling in complex neural functions.
In the brain, as in the periphery, cytokines are the major chemical cell-to-cell signaling proteins. The superfamily of cytokines includes interferons (e.g., IFNγ), interleukins (IL-1β, IL-4, IL-10), chemokines (e.g., CCL2, CXCL10, CX3CL1), tumor necrosis factors (e.g., TNFα) and some growth factors (insulin related growth factor (IGF), brain derived neurotrophic factor, (BDNF)), among others (Dinarello, 2007). Microglia, astrocytes, and neurons all secrete and express receptors for cytokines (Galic et al., 2012). Like other cell-to-cell signaling proteins, many cytokines act as a ligand, binding to their receptor and initiating second messengers, signal transduction, and transcription, often resulting in the production or suppression of additional cytokines (Greenhalgh and Hilton, 2001; Qiao et al., 2013). Others act as receptor antagonists (e.g., IL-1ra blocks the IL-1 receptor, preventing IL-1 binding) (Spulber et al., 2009), or adhesion proteins (e.g., CX3CL1 (fractalkine)) (Sheridan and Murphy, 2013). Cytokines are therefore well placed to mediate interactions between immune cells and neurons, acting as a major communication system in the brain (Adler et al., 2005).
The role of immune signaling in normal and dysregulated brain function is a growing focus of research. There are at least three distinct roles of the immune system in the brain. First, resident neuroimmune cells including microglia and astrocytes (Figure 2) have important functions in the absence of central or peripheral immune system activity. Without immune stimulation, glial-derived cytokines including IL-1 (Goshen et al., 2007) and CX3CL1 (Sheridan and Murphy, 2013), among others (Gadani et al., 2012), regulate normal neural functions and plasticity. Second, there is a classic immune role of increased neuroimmune signaling during inflammatory events such as stroke, brain infection, or traumatic brain injury, which mediates both neurotoxicity and neuroprotection (Pickering and O'Connor, 2007; Jha et al., 2015). Third, there is a strong role of signaling between the peripheral immune system and central nervous system (CNS) that influences behavioral states. Sickness behavior, in particular, has been well studied (Dantzer et al., 2008), which has led to a broader investigation of the role of immune modulation in emotional and behavioral states (and vice versa). Together, these findings suggest a critical role of neuroimmune signaling in normal neural functions, as well as modulation by peripheral or central immune activation.
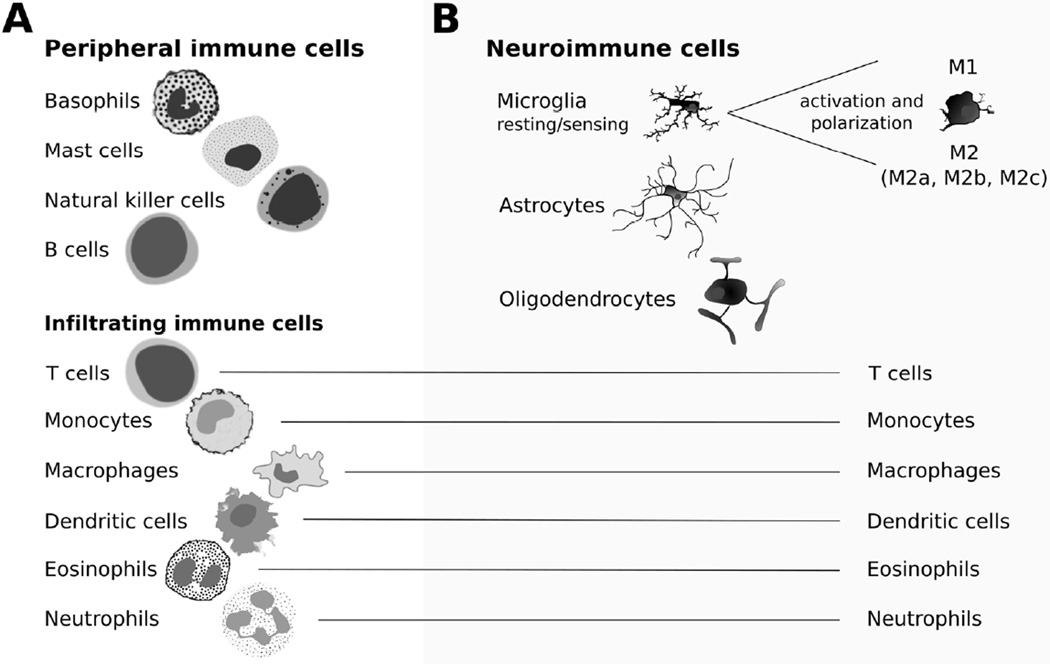
Figure 2.
Immune cells in the periphery and in the brain. A) Cells of the peripheral immune system. Peripheral immune cells are predominantly white blood cells. Basophils and mast cells release cytokines and activate the innate immune system (Stone et al., 2010). Natural killer (NK) cells attack “non-self” invaders as part of the innate immune system (Mandal and Viswanathan, 2015). B-cells produces antibodies and are a critical part of the adaptive immune system (Haan et al., 2014). Many of these white blood cells also infiltrate into the central nervous system after immune challenge and contribute to the neuroimmune response (Weber et al., 2016). T cells (T helper, cytotoxic, and memory cells) are critical for the adaptive immune response, and sex-biased activation of T helper (Th) cells contribute to differences between males and females in the adaptive immune response. Monocytes rapidly release cytokines during inflammation and stress (Xiong and Pamer, 2015). Monocytes can differentiate into macrophages and dendritic cells. Macrophages have two activation states, M1 and M2, and are important for phagocytosis of invading cells and cytokine release (Italiani and Boraschi, 2014). Peripheral dendritic cells stimulate antibody production by T cells and B cells; (Bieber and Autenrieth, 2015). Eosinophils (Stone et al., 2010) and neutrophils (Kruger et al., 2015) are innate immune response cells activated during infections, and migrate to the brain during infection or injury. C) Cells of the neuroimmune system. Central nervous system-specific immune cells include microglia, astrocytes, and oligodendrocytes. In a resting state, characterized by highly branched processes, microglia are important for surveillance of the brain (Casano and Peri, 2015). Once activated by immune signaling, microglia form an amoeboid shape and release different cytokines based on their activation state: M1, M2a, M2b, or M2c (Crain et al., 2013). Astrocytes maintain the tripartite synapse, and release cytokines to modulate neuronal functions (Jensen et al., 2013). Oligodendrocytes release neurotrophins and repair myelin (Acosta et al., 2013).
The central role of immune cells and signaling proteins in complex neural functions, from behavioral regulation and affective processes to cognition and synaptic plasticity, makes neuroimmune function an important target for research. Given sex differences in memory processes, as well as in prevalence of psychiatric and neurological disorders (Seshadri et al., 1997; Kessler et al., 2012; Sohrabji et al., 2016), understanding the contribution of immune signaling in the brain is not complete without consideration of sex.
Sex differences in the peripheral immune system
It is well established that the peripheral immune system has differential activity in males and females. The sex differences are both quantitative, with greater immune activity in females compared with males, and qualitative, with differences in which cells and pathways are triggered after stimulation. Specifically, females have greater adaptive immune responses (i.e., stronger antibody production; Figure 1) than males (Klein et al., 2015). Innate immune responses also differ qualitatively between sexes with peripheral immune cells (Figure 2) producing different patterns of cytokines in males than in females (Moxley et al., 2002; Klein et al., 2015).
Sex differences in activation of immune cells and signaling start at the level of pathogen detection via toll-like receptors (TLRs) and their subsequent cytokine response (Scotland et al., 2011; Steeg and Klein, 2016). Whereas males show higher numbers of TLR4 (Roberts et al., 2013) receptors, females show stronger activation of intracellular signaling pathways (Zheng et al., 2006) and stronger cytokine response after TLR7 stimulation (Berghöfer et al., 2006), potentially mediating divergent responses of males and females. Strikingly, there are consistent sex differences in T-cell activation following an immune response. T-helper cells have multiple activation states, including “classic” Th1 and “alternative” Th2 states. These have different cytokine triggers, cytokine release profiles, and different functional consequences. Men show more Th1 cell activity, together with higher levels of Th1-associated cytokines including IFNγ and IL-2 after an immune challenge, and women show more “alternative” Th2 cells and associated cytokines IL-4 and IL-10 (Giron-Gonzalez et al., 2000; Engler et al., 2016). Mice also show this sex-dependent effect, with males showing stronger Th1 and females showing stronger Th2 responses (Huber and Pfaeffle, 1994). Macrophages can be similarly classified as M1 and M2 activation, based on cytokine responses and, like Th cells, there is a bias towards M1 macrophage polarization in males and M2 polarization in females during immune activation (Martinez and Gordon, 2014). Sex differences across all levels of the immune system demonstrate that males and females differ in both the strength as well as the type of immune response.
Differences in immune activity correlate with higher susceptibility to infection in men than women (Furman et al., 2014; Giefing-Kroll et al., 2015). Similarly, women show stronger immune responses to vaccines, with greater antibody production and more adverse effects compared with men (Klein et al., 2010; Furman, 2015). Autoimmune disorders are also more prevalent in women, who comprise more than 80% of affected patients (Fairweather et al., 2008). Specifically, women are more susceptible to autoimmune disorders with antibody-mediated pathology including the most common autoimmune disorders: multiple sclerosis and lupus, among others (Fairweather et al., 2008). In contrast, males are more likely to develop disorders with cell-mediated pathophysiology, such as acute myocarditis (Fairweather et al., 2008).
Both sex hormones (Lang, 2004; Lai et al., 2012; Furman et al., 2014) and chromosomes (Smith-Bouvier et al., 2008; Klein et al., 2010; Bianchi et al., 2012; Case et al., 2013) contribute to sex differences in immune activation. Circulating estrogens generally act to increase immune function in both male and female mice and may dose-dependently shift T cells toward a Th2 phenotype (Huber and Pfaeffle, 1994; Lang, 2004; Salem, 2004). Conversely, testosterone suppresses immune function in both sexes (Rantala et al., 2012; Furman et al., 2014). The chromosomal contribution is due to the high number of immune genes clustered on the X-chromosome (Libert et al., 2010), the X-complement (Smith-Bouvier et al., 2008), and the number of genes that escape from X-inactivation (XCI) (Bianchi et al., 2012). For example, several autoimmune disorders, including arthritis and thyroid diseases are correlated with “skewed” XCI and higher expression of immune-related genes in females (Uz et al., 2009; Ishido et al., 2015). The immune system is strongly influenced by sex hormones and sex chromosomes, resulting in sex differences across many measures of immune function. Whether—and how—sex differences in the immune system translate into differential influences on neural function is less well established.
Sex differences in the neuroimmune system
Sex differences in the peripheral immune system crossover to the neuroimmune system, which is also regulated by both sex hormones (Mor et al., 1999; Czlonkowska et al., 2005) and chromosomal factors (Stamova et al., 2012). In the brain, differential microglial migration and immune-related gene expression in males and females (Schwarz et al., 2012) emerge early in development. Differences in neuroimmune cells persist throughout the lifespan, with sex-dependent activation of astrocytes (Santos-Galindo et al., 2011; Acaz-Fonseca et al., 2015), microglia (Crain et al., 2013), and cytokine release (Speirs & Tronson, in review) in response to immune challenge. Individual cytokines have sex-specific functions in the brain. For example, IL-2 impairs adult neurogenesis only in males (Beck et al., 2005), and IL-13 has a female-specific role in symptoms of experimental autoimmune encephalitis (EAE) (Sinha et al., 2008). Sex differences in the neuroimmune system thus have important functional consequences.
A striking recent finding highlights qualitative sex differences in the neuroimmune system, namely that males and females have unique immune-neuron interactions in the spinal cord mediating persistent pain. In males, inflammatory pain models result in microglia-mediated persistent neuropathic pain. In contrast, T-cell-mediated mechanisms via peroxisome proliferator activated receptors (PPARɣ) mediate persistent pain in females (Sorge et al., 2015). These sex-specific mechanisms of pain, with different cell interactions and signaling pathways recruited in males and females, are important for development of effective treatments of pain in both sexes. Furthermore, these findings illustrate that divergent mechanisms in males and females are often different ways to achieve the same outcome (de Vries, 2004).
It is noteworthy that the male phenotype—TLR4 activation of microglia-to-neuron signaling (Sorge et al., 2011; 2015)—has long been considered the mechanism for neuropathic pain (Sorge et al., 2011). Due to the dominance of males in neuroscience research, whether females rely on different mechanisms than males is yet to be examined for most neuroimmune functions. Sex differences in cells, cytokines, and function of the neuroimmune system nevertheless suggest broader implications for fundamental neural functions.
Sex differences in behavioral effects of neuroimmune stimulation are also evident in fever and sickness behavior after peripheral immune activation. After vaccination, women show stronger febrile responses (Klein et al., 2015) suggesting differential activation of hypothalamic pathways (Spinedi et al., 2002). In rodent models, females also show greater behavioral and autonomic responses to immune challenges (Lipton and Ticknor, 1979; Yee and Prendergast, 2010; Engeland et al., 2003). Women show greater socio-emotional responses (Moieni et al., 2015) and depressed mood (Eisenberger et al., 2009) in response to lipopolysaccharide (LPS) injection compared with men, and rodent models show similar effects (Avitsur and Yirmiya, 1999; Yee and Prendergast, 2010). These findings suggest that not only does the peripheral immune system initiate neuroimmune signaling and modulate affective and physiological processes, it does so in sex-specific ways.
Sex differences in neuroimmune function span across cell types and cytokine responses (Santos-Galindo et al., 2011; Crain et al., 2013; Acaz-Fonseca et al., 2015), through development into adulthood (Schwarz et al., 2012; Tay et al., 2016), and from physiological responses to cognitive and affective processes (Lipton and Ticknor, 1979; Tonelli et al., 2008). These observations strongly implicate the neuroimmune system as a critical mediator of neural functions. Given the roles of microglia (Morris et al., 2013; Wu et al., 2015), astrocytes (Ben Achour and Pascual, 2010; Papa et al., 2014; Haydon and Nedergaard, 2015), cytokines (Goshen et al., 2007; Gadani et al., 2012; Donzis and Tronson, 2014), and growth factors (Peters et al., 2010; Chen et al., 2011) in learning and memory, we anticipate that differences in the neuroimmune system drive sex differences in memory and its modulation.
Neuroimmune modulation of memory: Avenues for investigating sex differences
Many aspects of neuroimmune signaling are required for neural plasticity during development (Williamson et al., 2011; Wu et al., 2015) and similar mechanisms are involved in synaptic plasticity and memory formation in adults (Donzis and Tronson, 2014). Given the known sex differences in neuroimmune function, it is likely that memory regulation differs between males and females. Few studies have directly examined this question, yet there is substantial indirect evidence supporting the likelihood of sex differences in immune modulation of memory.
1. Neuroimmune signaling and plasticity
Cytokines, including growth factors and chemokines, are critical for memory and synaptic plasticity (Donzis and Tronson, 2014). Induction of LTP induces expression of a number of cytokines including IL-1β, IL-6, and TNFα (del Rey et al., 2013), and several of these play important roles in plasticity and memory. IL-1β is required for synaptic plasticity under normal conditions, and either decreases or increases of this cytokine impair memory (Balschun et al., 2003; Goshen et al., 2007; Spulber et al., 2009). IL-6 plays a negative regulatory role in memory and LTP (Balschun et al., 2004; Braida et al., 2004; Hao et al., 2014) likely via JAK-STAT signaling (Hao et al., 2014). TNFα increases glutamatergic and decreases GABAergic receptor trafficking (Beattie et al., 2002; Stellwagen, 2005), thereby impairing plasticity. Furthermore, during aging, decreases of IL-4 contribute to memory impairments (Maher et al., 2005; Gadani et al., 2012). Chemokines also play important regulatory roles. For example, CCL2 and CCL3 impair memory and synaptic plasticity (Belarbi et al., 2013; Marciniak et al., 2015), whereas CX3CL1 is required for memory formation, possibly via glial- neuron interactions (Rogers et al., 2011; Sheridan and Murphy, 2013). Immune challenges such as LPS administration trigger cytokine elevations and impair memory consolidation (Pugh et al., 1998; Cunningham and Sanderson, 2008), memory retrieval (Czerniawski et al., 2015), and discriminative memory (Czerniawski and Guzowski, 2014). Cytokines are therefore important contributors to plasticity in normal conditions and in dysregulation of memory processes after an immune challenge.
Whether cytokines exert differential effects on memory processes in males and females remains unknown. There is good evidence, however, that males and females show different cytokine activation in the brain following an immune stimulus. After intranasal LPS, female rats show greater expression of IL-6 and TNFα in the brain than males (Tonelli et al., 2008). Furthermore, after systemic LPS injection males show later, prolonged activation of cytokines in the hippocampus (Speirs & Tronson, in review). Males also show a stronger hippocampal cytokine response after stress, with more TNFα and IL-1β compared with females (Pyter et al., 2013; Hudson et al., 2014). In addition, following ischemic stroke, female mice show increased microglial IL-4 but not IL-10 levels, whereas male mice show the opposite pattern: increased IL-10 but not IL-4 levels (Bodhankar et al., 2015). Given the roles of cytokines in synaptic plasticity and memory, such sex differences in patterns of neuroimmune activity suggest differential modulation of memory by cytokines in males and females.
Downstream of cytokine activation, second messenger signaling and transcription factors also show sex-specific activity. For example, p38MAPK mediates symptoms only in females in a mouse model of multiple sclerosis (Krementsov et al., 2014), and lipocalin-2 is required for increasing body temperature after LPS in females but not males (Hamzic et al., 2013). Furthermore, cox-2 is required for hippocampal memory only in females (Guzmán et al., 2009). Thus, cytokine-dependent signaling has different effects on neural function in males and females, suggesting the possibility of divergent effects of immune signaling on memory between the sexes.
Growth factors, including BDNF, IGF-1, and IGF-2, are important members of the cytokine superfamily. Inflammatory events can decrease neurotrophic factors in the brain, for example systemic inflammatory challenges decrease expression of bdnf (Lapchak et al., 1993) and other growth factors (Guan and Fang, 2006). Similarly, IGF-1 expression is regulated by cytokines (O'Connor et al., 2008) and conversely, IGF-1 acts to decrease expression of cytokines (Park et al., 2011). These interactions between cytokines and growth factors are critical mechanisms for mediating immune effects in the brain.
BDNF is strongly implicated as a necessary mediator in synaptic plasticity and memory (Andero et al., 2014), and cortical injections of BDNF are sufficient to induce plasticity (Peters et al., 2010). Sex hormones differentially regulate BDNF and its receptors in memory-related brain regions including the hippocampus and amygdala (Spencer-Segal et al., 2011; Luine and Frankfurt, 2013; Scharfman and MacLusky, 2014). In addition, regulation of bdnf gene expression during memory processes differs in males and females (Mizuno and Giese, 2012; Harris et al., 2016). Like BDNF, IGFs are important for normal memory processes. IGF-2 plays a critical role in memory consolidation and its enhancement (Chen et al., 2011; Lee et al., 2015) and both IGF-1 and IGF-2 can rescue memory deficits during aging (Deak and Sonntag, 2012; Steinmetz et al., 2016). There is also evidence for sex differences in IGF-1 and memory, where aging-related memory impairments are associated with decreased IGF-1 in men, but not women (Polimanti et al., 2016). Neurotrophins including BDNF and IGF may thereby contribute to sex differences in immune regulation of memory processes.
As the primary cell-to-cell signaling mechanisms in the neuroimmune system, cytokines play important roles in memory and its regulation. Cytokines and their downstream signaling pathways are differentially activated and exert different effects on neural function in males and females. In addition to the direct effects of cytokines and growth factors on receptor trafficking, synaptic regulation, and changes in gene expression, cytokines have a bidirectional relationship with neuroimmune cells. Cytokines are both secreted by and regulate the recruitment, activation, and function of neuroimmune cells that, in turn, are critical mediators of complex neural functions.
2. Neuroimmune cells and their activation
Cytokines, chemokines, and growth factors are instrumental in the activation of astrocytes and microglia (Figure 2). Astrocytes regulate synapse formation and stability (Zorec et al., 2015), and microglia act to continuously monitor or survey the brain and eliminate synapses via synaptic pruning (Wu et al., 2015). Dysregulation of these cells have been strongly implicated in disorders of neurodegenerative memory decline such as Alzheimer’s disease (Dzamba et al., 2016) and traumatic brain injury (Sajja et al., 2016). Astrocytes and microglia are critical for normal memory formation (Ben Achour and Pascual, 2010; Ota et al., 2013), however the precise contribution of glia in memory formation and its modulation, and sex-specific roles of glial cells, are not known.
Astrocyte processes wrap around neuronal synapses, form a “tripartite synapse”, and regulate neurotransmission via calcium-, glutamatergic-, and GABAergic-dependent mechanisms (Perea et al., 2009; Moraga-Amaro et al., 2014). For example, astrocytic TNFα mediates AMPA receptor trafficking in the post-synaptic membrane (Beattie et al., 2002) and GABA receptor trafficking (Pribiag and Stellwagen, 2013) at the synapse. In some models, astrocytic secretion of TNFα resulted in impaired memory (Habbas et al., 2015), demonstrating the importance of astrocytes in the modulation of memory by cytokines. During learning, there is also evidence that astrocytes retract from synapses (Ostroff et al., 2014), suggesting that astrocytes may oppose synaptic plasticity. This implies that mechanisms causing retraction of astroglial processes may be a critical mechanism of memory formation and modulation. In addition to synaptic signaling, astrocytes are required for energy production (Gold, 2014) which, given the high energy requirement of neural plasticity (Steinman et al., 2016), suggests multiple roles of astrocytes in the modulation of memory.
Understanding the role of astrocytes in cognitive processes is still in its infancy (Haydon and Nedergaard, 2015) and, with no studies directly comparing males and females, there is no direct evidence for sex differences in astrocytic contribution to memory. There are, however, several reasons to believe that such differences exist. First, astrocytes are sex-dependently activated as a consequence of ischemia (Chisholm and Sohrabji, 2016). Second, the release of cytokines by astrocytes differs markedly in males and females (Santos-Galindo et al., 2011; Loram et al., 2012; Acaz-Fonseca et al., 2015). Together these demonstrate suggest that sex differences in cytokines after immune challenge are mediated, in part, by astrocyte activation.
Astrocytes are unlikely to modulate memory in isolation. Rather, interactions with other immune cells, including microglia, are likely necessary for this process. For example, astrocyte-derived IL-10 causes both altered microglia activation and impaired LTP (Almolda et al., 2015). Conversely, microglial activation can result in astrocyte-mediated modulation of synaptic function, resulting in rapid increases of astrocytic glutamate release (Pascual et al., 2012). These findings suggest that astrocytes may mediate neural plasticity either by direct effects on synapses or via activation of microglial cells.
Microglia are also important regulators of synapses and memory. In a resting state, microglia extend and retract their processes to monitor synapse function (Nimmerjahn et al., 2005). These interactions can enhance or eliminate (prune) neuronal connections and circuits in an activity dependent manner (Tremblay et al., 2011). Bidirectional communication between neurons and microglia are critical for regulation of synapses and plasticity throughout the lifespan (see also Wu et al., 2015).
Neuron-microglial interactions via chemokine CX3CL1 (Milior et al., 2016) play a critical role in memory processes (Sheridan and Murphy, 2013). CX3CL1 acts, in part, as an adhesion protein anchored to the neuronal membrane and interacting with its receptor (CX3CLR) on microglia (Chapman et al., 2000). Interaction of neurons and microglia via this pathway is required for synaptic plasticity and memory (Sheridan and Murphy, 2013), with memory impairments and blunted LTP evident in CX3CL1-deficient mice (Maggi et al., 2011; Rogers et al., 2011). Microglia-specific intracellular signaling and gene expression are also important for memory tasks. For example, IKKβ in microglia, but not neurons, is required for instrumental conditioning (Kyrargyri et al., 2014). Similarly, microglia-specific BDNF signaling is required for spine development and both motor learning and tone-dependent fear conditioning (Parkhurst et al., 2013). Therefore, resting microglia regulate synaptic plasticity and memory.
Microglia are activated by immune stimulation, resulting in amoeboid shaped cells that move to the site of damage or infection (Kettenmann et al., 2011). Activated microglia release cytokines and exert both enhancing (Delpech et al., 2015) and impairing (Tanaka et al., 2006; Vasek et al., 2016) effects on memory. Inhibition of microglia can shift LTP-inducing stimuli to result in long-term depression (LTD) via a TNFα-dependent mechanism (Zhong et al., 2010). Inflammatory signaling may modulate memory via microglia-dependent increases in AMPA and NMDA receptor currents and decreased LTP and LTD (Riazi et al., 2015). Immune challenges may thereby result in modification of memory via activation of microglia. Much of this work, however, has been done only with male animals. Given sex differences in microglia (Schwarz et al., 2012) and their function (Sorge et al., 2015) in the central nervous system, it remains unclear whether similar modulatory effects on memory would be observed in females.
Like macrophages and Th cells, active microglia have several phenotypes that influence their function, and sex differences in these active states have been described (Bodhankar et al., 2015; Xiong et al., 2015). During activation, microglia are polarized into several different activated phenotypes triggered by different cytokines and other factors. The “classical” activated microglia state is the M1 state, triggered by IFNγ and Th1 cytokines, and produces cytokines including TNFα, IL-1β, and IL-6. The M2 microglial activated states include M2a “alternative activation” triggered by IL-4 and IL-13; M2b “alternative activation type II”, and M2c “acquired deactivation” state triggered by IL-10 or glucocorticoids (Prinz and Priller, 2014; Walker and Lue, 2015; but see also Ransohoff, 2016). Whereas M1-microglia are important for the initiation of the inflammatory response, M2-microglia are predominantly neuroprotective (Xiong et al., 2016). More specifically, M2a produces neurotrophins (Bodhankar et al., 2015) and M2c is involved in remodeling after resolution of inflammation (Cherry et al., 2014). In this framework, females show a stronger predisposition for M2a polarization compared with males (Bodhankar et al., 2015; Xiong et al., 2015). Differential microglia polarization may explain—or be explained by—sex differences in cytokine levels in males and females.
Our understanding of the contribution of astrocytes and microglia to sex differences in the modulation of memory is constrained by both the newness of the study of these cells in synaptic plasticity, and within that, the lack of studies that include both males and females (See Table 1). A recent review by Hoogland and colleagues highlights how little is known about microglial activation after immune challenge. Of the studies examining microglial activation after systemic LPS administration, for example, only three examined both sexes, and only two more studied females (Hoogland et al., 2015). There are similarly few studies on sex differences in astrocyte function. Nevertheless, the cumulative evidence from other models, including pain (Sorge et al., 2015) and stroke (Bodhankar et al., 2015; Xiong et al., 2015; Chisholm and Sohrabji, 2016) suggests that the activation and function of glial cells are likely to differentially affect synaptic plasticity and memory processes in males and females.
Table 1.
Sex of animals or humans used in studies cited.
did not statistically compare males vs females;
humans.
3. Neuroimmune modulation of adult neurogenesis
In addition to acute effects on memory, immune signaling affects long-acting modulatory processes including neurogenesis (Monje et al., 2003; Borsini et al., 2015). Adult neurogenesis is the production of new neurons in the adult brain, created through asymmetric division of progenitor cells (proliferation). These cells then differentiate into neurons (50–60%, (Abrous et al., 2005)) or glia (gliogenesis, ~10%, (Steiner et al., 2004)) before becoming functionally integrated into the dentate gyrus. Integration of new neurons modifies the excitability of neuronal circuits (Song et al., 2012) and supports spatial (Snyder et al., 2005) and contextual fear (Drew et al., 2010) memory formation.
Both acute (Valero et al., 2014) and chronic (Zonis et al., 2015) inflammation, as well as chronic stressors (McKim et al., 2016), lead to dysregulation of adult neurogenesis. Studies of inflammatory signaling and neurogenesis show conflicting results: immune challenges can increase proliferation (Seguin et al., 2009), decrease proliferation (Islam et al., 2009), maintain neurogenesis by increasing survival of new cells (Ziv et al., 2006), decrease survival of newborn neurons (McKim et al., 2016), or impair integration of new cells into neural circuits (Jakubs et al., 2008).
Hippocampal neurogenesis is strongly influenced by cytokines. Specifically, IL-1 (Koo and Duman, 2008; Ben Menachem-Zidon et al., 2013), IL-2 (Beck et al., 2005), and TNFα (Chen and Palmer, 2013) reduce proliferation. IL-6 decreases multiple stages of neurogenesis including proliferation, differentiation of neural progenitor cells, and survival of new cells (Vallierès et al., 2002). These detrimental effects of cytokines on neurogenesis correlate with altered memory processes. For example, LPS-induced decreases in neurogenesis correlate with deficits in spatial memory (Valero et al., 2014). In addition to direct effects of cytokine-dependent signaling on neurogenesis, cytokines have indirect effects via decreased neurotrophic factors. For example, systemic LPS-induced immune signaling resulted in decreased BDNF and impaired differentiation of new cells into neurons. Here, BNDF levels and proliferation were rescued by wheel running despite persistent high levels of cytokines (Wu et al., 2007).
Immune regulation of neurogenesis also involves neuroimmune cells (Kuzumaki et al., 2010). Microglia, in particular, play a critical role in mediating neurogenesis (Ziv et al., 2006). CX3CL1 modulates activation of microglia in the neurogenic niche (Williams et al., 2014), increasing proliferation and contributing to survival of new cells (Bachstetter et al., 2011). In addition, microglia activated by INFɣ signaling increase survival (Butovsky et al., 2006). As in memory modulation, the polarization of microglia matters. LPS/TLR4 microglia activation (likely M1 microglia) results in reduced survival of new cells (Ekdahl et al., 2011), whereas IL-4-dependent microglia (possibly M2a) activation regulates a switch toward decreased neurogenesis and increased gliogenesis (Butovsky et al., 2006). Cytokine signaling also triggers differentiation of progenitor cells into non-neuronal cell types (Islam et al., 2009; Green et al., 2012), effectively decreasing neurogenesis by increasing gliogenesis.
Despite similar levels of neurogenesis in both sexes, this process is differentially susceptible to modulation in males and females. Estrogens and androgens have sex-specific effects on neurogenesis (Galea et al., 2013), likely through their receptors. Estrogen receptors are located on dividing cells in the neurogenic region of the hippocampus (Mitterling et al., 2010) and androgens mediate neurogenesis through receptors located in other regions of the hippocampus (Galea et al., 2013). Given the sex-biased roles of immune cells in the CNS (e.g., Sorge et al., 2015), sex hormones and neuroimmune signaling may interact during differentiation of precursor cells and thereby alter the proportions of new glial and neuronal cells produced. One of the few studies comparing males and females in immune regulation of neurogenesis (Table 1) demonstrated that IL-2 limits neurogenesis only in males (Beck et al., 2005). Thus, immune signaling may act as an intervening variable driving differential roles of new neurons in circuit dynamics of memory in males and females (Chow et al., 2013).
Implications of sex differences in immune modulation of memory
It is clear that the immune system plays a critical role in behavioral modification and regulation of neural plasticity throughout the lifespan. The complexity of interactions between cytokines and neuroimmune cells, between astrocytes, microglia, and neurons, and the roles of the neuroimmune system in normal and dysregulated memory processes, makes understanding the sex-specific roles of each process a daunting task. The task is made more difficult by the lack of studies that directly compare males and females (Table 1; Hoogland et al., 2015). Indeed, when males and females are directly compared, it becomes clear that different patterns of cytokines are triggered by an immune challenge (Speirs & Tronson, in review), and these differences are mediated in part by differential activation of astrocytes (Santos-Galindo et al., 2011; Acaz-Fonseca et al., 2015) and microglia (Schwarz et al., 2012; Bodhankar et al., 2015). The intricacies of neuroimmune signaling, together with the presence of sex differences at every level of analysis, suggests that the role of this system in plasticity is very different in males and females. Here, sex-specific cellular and signaling mechanisms likely results in differential modulation of neurons, spines, and synapses, as well as changes in long acting processes including neurogenesis.
Identifying similarities and differences in the mechanisms of neuroimmune modulation of memory in males and females is critical for developing targeted treatments for disorders of memory and cognition. Sex differences are particularly important as many of these disorders have sex differences in prevalence (Sohrabji et al., 2016). Women, for example, have higher rates of Alzheimer’s disease (Seshadri et al., 1997) and stress-related disorders including post-traumatic stress disorder (Kessler et al., 2012). Inflammatory signaling increases the risk of neurodegenerative disease (e.g., Tan et al., 2007; Azizi et al., 2014) or stress-related disorders (Wieck et al., 2014; Vecchiarelli et al., 2015; McKim et al., 2016). Thus understanding the similarities and differences in neuroimmune activation in males and females may help identify the specific components of immune signaling that confer higher risk and provide new, sex-specific avenues for prevention and treatment of memory disorders.
SIGNIFICANCE STATEMENT.
Inflammatory events such as illness or injury alter cognition, emotion, and memory via activation of the immune system in the brain. Recent studies have demonstrated that the immune system activates different mechanisms in male versus female brains. This suggests that the immune system may have different impacts on memory in males and females. In this review, we discuss ongoing research in the modification of memory by immune responses. We propose potential ways in which the neuroimmune activity can affect memory and contribute to differential prevalence of disorders of memory in men and women.
Acknowledgments
We would like to thank Ashley Keiser, Daria Tchessalova, Caitlin Posillico, and Brynne Raines for their very helpful comments on this manuscript. This work was supported by NIH NIMH R00MH093459 to NCT.
Supporting Grant information: NIH/NIMH R00MH093459
ROLE OF AUTHORS
NCT and KMC conceived, designed, wrote, and edited this review. KMC created all figures and illustrations.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare
REFERENCES
- Abrous DN, Koehl M, le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Acaz-Fonseca E, Duran JC, Carrero P, Garcia-Segura LM, Arevalo MA. Sex differences in glia reactivity after cortical brain injury. Glia. 2015;63:1966–1981. doi: 10.1002/glia.22867. [DOI] [PubMed] [Google Scholar]
- Acosta CMR, Cortes C, MacPhee H, Namaka MP. Exploring the role of nerve growth factor in multiple sclerosis: implications in myelin repair. CNSNDDT. 2013;12:1242–1256. doi: 10.2174/18715273113129990087. [DOI] [PubMed] [Google Scholar]
- Adler MW, Geller EB, Chen X, Rogers TJ. Viewing chemokines as a third major system of communication in the brain. AAPS J. 2005;7:E865–E870. doi: 10.1208/aapsj070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almolda B, de Labra C, Barrera I, Gruart A, Delgado-Garcia JM, Villacampa N, Vilella A, Hofer MJ, Hidalgo J, Campbell IL, Gonzalez B, Castellano B. Alterations in microglial phenotype and hippocampal neuronal function in transgenic mice with astrocyte-targeted production of interleukin-10. Brain Behav Immun. 2015;45:80–97. doi: 10.1016/j.bbi.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Andero R, Choi DC, Ressler KJ. BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. In: Shenoy SK, editor. Progress in Molecular Biology and Translational Science. Elsevier; 2014. pp. 169–192. Progress in Molecular Biology and Translational Science. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learning & Memory. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Anisman H, Kokkinidis L, Merali Z. Further evidence for the depressive effects of cytokines: Anhedonia and neurochemical changes. Brain Behav Immun. 2002;16:544–556. doi: 10.1016/s0889-1591(02)00011-9. [DOI] [PubMed] [Google Scholar]
- Atkins E. Fever: the old and new. J Infect Dis. 1985;149:339–348. doi: 10.1093/infdis/149.3.339. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Yirmiya R. The immunobiology of sexual behavior: gender differences in the suppression of sexual activity during illness. Pharmacol Biochem Behav. 1999;64:787–796. doi: 10.1016/s0091-3057(99)00165-3. [DOI] [PubMed] [Google Scholar]
- Azizi G, Khannazer N, Mirshafiey A. The potential role of chemokines in Alzheimer's disease Pathogenesis. Am J Alzheimers Dis Other Demen. 2014;29:415–425. doi: 10.1177/1533317513518651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32:2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Randolf A, Pitossi F, Schneider H, Rey A, Besedovsky HO. Hippocampal interleukin-1β gene expression during long-term potentiation decays with age. Ann NY Acad Sci. 2003;992:1–8. doi: 10.1111/j.1749-6632.2003.tb03132.x. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wetzel W, del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. FASEB J. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Zastrow Von M, Beattie MS, Malenka RC. Control of Synaptic Strength by Glial TNFα. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Beck RD, Jr, Wasserfall C, Ha GK, Cushman JD, Huang Z, Atkinson MA, Petitto JM. Changes in hippocampal IL-15, related cytokines, and neurogenesis in IL-2 deficient mice. Brain Res. 2005;1041:223–230. doi: 10.1016/j.brainres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Belarbi K, Jopson T, Arellano C, Fike JR, Rosi S. CCR2 deficiency prevents neuronal dysfunction and cognitive impairments induced by cranial irradiation. Cancer Res. 2013;73:1201–1210. doi: 10.1158/0008-5472.CAN-12-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Achour S, Pascual O. Glia: the many ways to modulate synaptic plasticity. Neurochemistry International. 2010;57:440–445. doi: 10.1016/j.neuint.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Ben Menachem-Zidon O, Menahem YB, Hur TB, Yirmiya R. Intra-Hippocampal Transplantation of Neural Precursor Cells with Transgenic Over-Expression of IL-1 Receptor Antagonist Rescues Memory and Neurogenesis Impairments in an Alzheimer’s Disease Model. Neuropsychopharmacology. 2013;39:401–414. doi: 10.1038/npp.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghöfer B, Frommer T, Haley G, Fink L. TLR7 ligands induce higher IFN-α production in females. J Immunol. 2006;177:2088–2096. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]
- Bianchi I, Lleo A, Gershwin ME, Invernizzi P. The X chromosome and immune associated genes. J Autoimmun. 2012;38:J187–J192. doi: 10.1016/j.jaut.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Bieber K, Autenrieth SE. Insights how monocytes and dendritic cells contribute and regulate immune defense against microbial pathogens. Immunobiology. 2015;220:215–226. doi: 10.1016/j.imbio.2014.10.025. [DOI] [PubMed] [Google Scholar]
- Bodhankar S, Lapato A, Chen Y, Vandenbark AA, Saugstad JA, Offner H. Role for microglia in sex differences after ischemic stroke: importance of M2. Metab Brain Dis. 2015;30:1515–1529. doi: 10.1007/s11011-015-9714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini A, Zunszain PA, Thuret S, Pariante CM. The role of inflammatory cytokines as key modulators of neurogenesis. TINS. 2015;38:145–157. doi: 10.1016/j.tins.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Braida D, Sacerdote P, Panerai AE, Bianchi M, Aloisi AM, Iosuè S, Sala M. Cognitive function in young and adult IL (interleukin)-6 deficient mice. Behav Brain Res. 2004;153:423–429. doi: 10.1016/j.bbr.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casano AM, Peri F. Microglia: multitasking specialists of the brain. Dev Cell. 2015;32:469–477. doi: 10.1016/j.devcel.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Case LK, Wall EH, Dragon JA, Saligrama N, Krementsov DN, Moussawi M, Zachary JF, Huber SA, Blankenhorn EP, Teuscher C. The Y chromosome as a regulatory element shaping immune cell transcriptomes and susceptibility to autoimmune disease. Genome Res. 2013;23:1474–1485. doi: 10.1101/gr.156703.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman GA, Moores K, Harrison D, Campbell CA, Stewart BR, Strijbos PJ. Fractalkine cleavage from neuronal membranes represents an acute event in the inflammatory response to excitotoxic brain damage. J Neurosci. 2000;20:RC87. doi: 10.1523/JNEUROSCI.20-15-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, Blitzer RD, Alberini CM. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469:491–497. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Palmer TD. Differential roles of TNFR1 and TNFR2 signaling in adult hippocampal neurogenesis. Brain Behav Immun. 2013;30:45–53. doi: 10.1016/j.bbi.2013.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm NC, Sohrabji F. Astrocytic response to cerebral ischemia is influenced by sex differences and impaired by aging. Neurobiol Dis. 2016;85:245–253. doi: 10.1016/j.nbd.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C, Epp JR, Lieblich SE, Barha CK, Galea LAM. Sex differences in neurogenesis and activation of new neurons in response to spatial learning and memory. Psychoneuroendocrinology. 2013;38:1236–1250. doi: 10.1016/j.psyneuen.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Crain JM, Nikodemova M, Watters JJ. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J Neurosci Res. 2013;91:1143–1151. doi: 10.1002/jnr.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Sanderson DJ. Malaise in the water maze: untangling the effects of LPS and IL-1beta on learning and memory. Brain Behav Immun. 2008;22:1117–1127. doi: 10.1016/j.bbi.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Guzowski JF. Acute neuroinflammation impairs context discrimination memory and disrupts pattern separation processes in hippocampus. J Neurosci. 2014;34:12470–12480. doi: 10.1523/JNEUROSCI.0542-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Miyashita T, Lewandowski G, Guzowski JF. Systemic lipopolysaccharide administration impairs retrieval of context-object discrimination, but not spatial, memory: Evidence for selective disruption of specific hippocampus-dependent memory functions during acute neuroinflammation. Brain Behav Immun. 2015;44:159–166. doi: 10.1016/j.bbi.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czlonkowska A, Ciesielska A, Gromadzka G, Kurkowska-Jastrzebska I. Estrogen and cytokines production - the possible cause of gender differences in neurological diseases. Curr Pharm Des. 2005;11:1017–1030. doi: 10.2174/1381612053381693. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J Gerontol A Biol Sci Med Sci. 2012;67:611–625. doi: 10.1093/gerona/gls118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak T, Quinn M, Cidlowski JA, Victoria NC, Murphy AZ, Sheridan JF. Neuroimmune mechanisms of stress: sex differences, developmental plasticity, and implications for pharmacotherapy of stress-related disease. Stress. 2015;18:367–380. doi: 10.3109/10253890.2015.1053451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rey A, Balschun D, Wetzel W, Randolf A, Besedovsky HO. A cytokine network involving brain-borne IL-1β, IL-1ra, IL-18, IL-6, and TNFα operates during long-term potentiation and learning. Brain Behav Immun. 2013;33:15–23. doi: 10.1016/j.bbi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Delpech JC, Saucisse N, Parkes SL, Lacabanne C, Aubert A, Casenave F, Coutureau E, Sans N, Layé S, Ferreira G, Nadjar A. Microglial Activation Enhances Associative Taste Memory through Purinergic Modulation of Glutamatergic Neurotransmission. J Neurosci. 2015;35:3022–3033. doi: 10.1523/JNEUROSCI.3028-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Historical Review of Cytokines. Eur J Immunol. 2007;37:S34–S45. doi: 10.1002/eji.200737772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzis EJ, Tronson NC. Modulation of learning and memory by cytokines: Signaling mechanisms and long term consequences. Neurobiol Learn Mem. 2014;115:68–77. doi: 10.1016/j.nlm.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single-but not multiple-trial contextual fear conditioning. Behav Neurosci. 2010;124:446–454. doi: 10.1037/a0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzamba D, Harantova L, Butenko O. Glial cells-the key elements of Alzheimer´s disease. Curr Alzheimer Res. 2016;13:894–911. doi: 10.2174/1567205013666160129095924. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine induced depressed mood and social pain: The role of sex differences. NeuroImage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2011;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeland CG, Kavaliers M, Ossenkopp K-P. Sex differences in the effects of muramyl dipeptide and lipopolysaccharide on locomotor activity and the development of behavioral tolerance in rats. Pharmacol Biochem Behav. 2003;74:433–447. doi: 10.1016/s0091-3057(02)01024-9. [DOI] [PubMed] [Google Scholar]
- Engler H, Benson S, Wegner A, Spreitzer I, Schedlowski M, Elsenbruch S. Men and women differ in inflammatory and neuroendocrine responses to endotoxin but not in the severity of sickness symptoms. Brain Behav Immun. 2016;52:18–26. doi: 10.1016/j.bbi.2015.08.013. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, Rose NR. Sex Differences in Autoimmune Disease from a Pathological Perspective. Am J Path. 2008;173:600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D. Sexual dimorphism in immunity: improving our understanding of vaccine immune responses in men. Expert Rev Vaccines. 2015;14:461–471. doi: 10.1586/14760584.2015.966694. [DOI] [PubMed] [Google Scholar]
- Furman D, Hejblum BP, Hejblum BP, Simon N, Simon N, Jojic V, Jojic V, Dekker CL, Dekker CL, Thiebaut R, Thiébaut R, Tibshirani RJ, Tibshirani RJ, Davis MM, Davis MM. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci USA. 2014;111:869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. J Immunol. 2012;189:4213–4219. doi: 10.4049/jimmunol.1202246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LAM, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK. Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol. 2013;25:1039–1061. doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- Galic MA, Riazi K, Pittman QJ. Cytokines and brain excitability. Front Neuroendocrinol. 2012;33:116–125. doi: 10.1016/j.yfrne.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibertini M, Newton C, Friedman H, Klein TW. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-beta. Brain Behav Immun. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14:309–321. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, Gordon SM, Canonico AE, Dittus RS, Bernard GR, Ely EW. Delirium as a Predictor of Long-Term Cognitive Impairment in Survivors of Critical Illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron-Gonzalez JA, Moral FJ, Elvira J, Garcia-Gil D, Guerrero F, Gavilan I, Escobar L. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur J Endocrinol. 2000;143:31–36. doi: 10.1530/eje.0.1430031. [DOI] [PubMed] [Google Scholar]
- Gold PE. Regulation of memory – From the adrenal medulla to liver to astrocytes to neurons. Brain Research Bulletin. 2014;105:25–35. doi: 10.1016/j.brainresbull.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Green HF, Treacy E, Keohane AK, Sullivan AM, O'Keeffe GW, Nolan YM. A role for interleukin-1β in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol Cell Neurosci. 2012;49:311–321. doi: 10.1016/j.mcn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Greenhalgh CJ, Hilton DJ. Negative regulation of cytokine signaling. J Leuk Biol. 2001;70:348–356. [PubMed] [Google Scholar]
- Grissom EM, Hawley WR, Hodges KS, Fawcett-Patel JM, Dohanich GP. Biological sex influences learning strategy preference and muscarinic receptor binding in specific brain regions of prepubertal rats. Hippocampus. 2013;23:313–322. doi: 10.1002/hipo.22085. [DOI] [PubMed] [Google Scholar]
- Gruene TM, Roberts E, Thomas V, Ronzio A, Shansky RM. Sex-Specific Neuroanatomical Correlates of Fear Expression in Prefrontal-Amygdala Circuits. Biol Psychiatry. 2014;78:186–193. doi: 10.1016/j.biopsych.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Fang J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav Immun. 2006;20:64–71. doi: 10.1016/j.bbi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Guzmán CB, Guzmán CB, Graham KA, Graham KA, Grace LA, Grace LA, Moore AH, Moore AH. Sex-dependent effect of cyclooxygenase-2 inhibition on mouse spatial memory. Behav Brain Res. 2009;199:355–359. doi: 10.1016/j.bbr.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan den JMM, Arens R, van Zelm MC. The activation of the adaptive immune system: cross-talk between antigen-presenting cells, T cells and B cells. Immunology Letters. 2014;162:103–112. doi: 10.1016/j.imlet.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Habbas S, Santello M, Becker D, Stubbe H, Zappia G, Liaudet N, Klaus FR, Kollias G, Fontana A, Pryce CR, Suter T, Volterra A. Neuroinflammatory TNFα Impairs Memory via Astrocyte Signaling. Cell. 2015;163:1730–1741. doi: 10.1016/j.cell.2015.11.023. [DOI] [PubMed] [Google Scholar]
- Hamzic N, Blomqvist A, Nilsberth C. Immune-Induced Expression of Lipocalin-2 in Brain Endothelial Cells: Relationship with Interleukin-6, Cyclooxygenase-2 and the Febrile Response. J Neuroendocrinol. 2013;25:271–280. doi: 10.1111/jne.12000. [DOI] [PubMed] [Google Scholar]
- Hao Y, Jing H, Bi Q, Zhang J, Qin L, Yang P. Intra-amygdala microinfusion of IL-6 impairs the auditory fear conditioning of rats via JAK/STAT activation. Behav Brain Res. 2014;275C:88–95. doi: 10.1016/j.bbr.2014.08.052. [DOI] [PubMed] [Google Scholar]
- Harris EP, Abel JM, Tejada LD, Rissman EF. Calbindin knockout alters sex-specific regulation of behavior and gene expression in amygdala and prefrontal cortex. Endocrinology. 2016 doi: 10.1210/en.2016-1055. en.2016–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Nedergaard M. How do astrocytes participate in neural plasticity? Cold Spring Harb Perspect Biol. 2015;7:a020438. doi: 10.1101/cshperspect.a020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogland ICM, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12:114. doi: 10.1186/s12974-015-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SA, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68:5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz JA, Patterson KM, Byrne AJ, Pagel PS, Warltier DC. Postoperative delirium is associated with postoperative cognitive dysfunction at one week after cardiac surgery with cardiopulmonary bypass. Psychol Rep. 2009;105:921–932. doi: 10.2466/PR0.105.3.921-932. [DOI] [PubMed] [Google Scholar]
- Hudson SP, Jacobson-Pick S, Anisman H. Sex differences in behavior and pro-inflammatory cytokine mRNA expression following stressor exposure and re-exposure. Neuroscience. 2014;277:239–249. doi: 10.1016/j.neuroscience.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Ishido N, Inoue N, Watanabe M, Hidaka Y, Iwatani Y. The relationship between skewed X chromosome inactivation and the prognosis of Graves“ and Hashimoto”s diseases. Thyroid. 2015;25:256–261. doi: 10.1089/thy.2014.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam O, Gong X, Rose-John S, Heese K. Interleukin-6 and neural stem cells: more than gliogenesis. Mol Biol Cell. 2009;20:188–199. doi: 10.1091/mbc.E08-05-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italiani P, Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubs K, Bonde S, Iosif RE, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Inflammation Regulates Functional Integration of Neurons Born in Adult Brain. J Neurosci. 2008;28:12477–12488. doi: 10.1523/JNEUROSCI.3240-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CJ, Massie A, De Keyser J. Immune players in the CNS: the astrocyte. J Neuroimmune Pharmacol. 2013;8:824–839. doi: 10.1007/s11481-013-9480-6. [DOI] [PubMed] [Google Scholar]
- Jha MK, Lee S, Park DH, Kook H, Park K-G, Lee I-K, Suk K. Diverse functional roles of lipocalin-2 in the central nervous system. Neurosci Biobehav Rev. 2015;49:135–156. doi: 10.1016/j.neubiorev.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Keiser AA, Tronson NC. Molecular Mechanisms of Memory in Males and Females. In: Shansky RM, editor. Sex Differences in the Central Nervous System. Elsevier; 2016. pp. 27–51. [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch U-K, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2015;109:9–15. doi: 10.1093/trstmh/tru167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1 is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Delis DC, Daniel M. Sex differences in verbal learning. J Clin Psychol. 1988;44:907–915. [Google Scholar]
- Krementsov DN, Noubade R, Dragon JA, Otsu K, Rincón M, Teuscher C. Sex-specific control of central nervous system autoimmunity by p38 mitogen-activated protein kinase signaling in myeloid cells. Ann Neurol. 2014;75:50–66. doi: 10.1002/ana.24020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger P, Saffarzadeh M, Weber ANR, Rieber N, Radsak M, Bernuth von H, Benarafa C, Roos D, Skokowa J, Hartl D. Neutrophils: Between host defence, immune modulation, and tissue injury. In: Dehio C, editor. PLoS Pathog. Vol. 11. 2015. p. e1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzumaki N, Ikegami D, Imai S, Narita M, Tamura R, Yajima M, Suzuki A, Miyashita K, Niikura K, Takeshima H, Ando T, Ushijima T, Suzuki T, Narita M. Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse. 2010;64:721–728. doi: 10.1002/syn.20800. [DOI] [PubMed] [Google Scholar]
- Kyrargyri V, Vega-Flores G, Gruart A, Delgado-García JM, Probert L. Differential contributions of microglial and neuronal IKKβ to synaptic plasticity and associative learning in alert behaving mice. Glia. 2014;63:549–566. doi: 10.1002/glia.22756. [DOI] [PubMed] [Google Scholar]
- Lai J-J, Lai K-P, Zeng W, Chuang K-H, Altuwaijri S, Chang C. Androgen Receptor Influences on Body Defense System via Modulation of Innate and Adaptive Immune Systems. Am J Path. 2012;181:1504–1512. doi: 10.1016/j.ajpath.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang TJ. Estrogen as an immunomodulator. Clin Immunol. 2004;113:224–230. doi: 10.1016/j.clim.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Hefti F. Systemic interleukin-1 β decreases brain-derived neurotrophic factor messenger RNA expression in the rat hippocampal formation. Neuroscience. 1993;53:297–301. doi: 10.1016/0306-4522(93)90196-m. [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K, Abbs B, Milad MR, Linnman C, Rougemount-Bucking A, Zeidan MA, Holt DJ, Goldstein JM. Sex differences in the neurobiology of fear conditioning and extinction: a preliminary fMRI study of shared sex differences with stress-arousal circuitry. Biol Mood Anxiety Disord. 2012;2:7. doi: 10.1186/2045-5380-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lee YW, Gao Q, Lee Y, Lee HE, Ryu JH. Exogenous insulin-like growth factor 2 administration enhances memory consolidation and persistence in a time-dependent manner. Brain Res. 2015;1622:466–473. doi: 10.1016/j.brainres.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Liang KC, Juler RG, McGaugh JL. Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res. 1986;368:125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010;10:594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- Lipton JM, Ticknor CB. Influence of sex and age on febrile responses to peripheral and central administration of pyrogens in the rabbit. J Physiol. 1979;295:263–272. doi: 10.1113/jphysiol.1979.sp012967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram LC, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HEW, Maier SF, Watkins LR. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology. 2012;37:1688–1699. doi: 10.1016/j.psyneuen.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience. 2013;239:34–45. doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Scianni M, Branchi I, D'Andrea I, D'Andrea I, Lauro C, Lauro C, Limatola C. CX(3)CR1 deficiency alters hippocampal-dependent plasticity phenomena blunting the effects of enriched environment. Front Cell Neurosci. 2011;5:22. doi: 10.3389/fncel.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher FO, Nolan Y, Lynch MA. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging. 2005;26:717–728. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Mandal A, Viswanathan C. Natural killer cells: In health and disease. Hematol Oncol Stem Cell Ther. 2015;8:47–55. doi: 10.1016/j.hemonc.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Marciniak E, Faivre E, Dutar P, Alves Pires C, Demeyer D, Caillierez R, Laloux C, Buée L, Blum D, Humez S. The chemokine MIP-1α/CCL3 impairs mouse hippocampal synaptic transmission, plasticity and memory. Sci Rep. 2015;5:15862. doi: 10.1038/srep15862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661:25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF, Godbout JP. Neuroinflammatory Dynamics Underlie Memory Impairments after Repeated Social Defeat. J Neurosci. 2016;36:2590–2604. doi: 10.1523/JNEUROSCI.2394-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milior G, Lecours C, Samson L, Bisht K, Poggini S, Pagani F, Deflorio C, Lauro C, Alboni S, Limatola C, Branchi I, Tremblay M-È, Maggi L. Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain Behav Immun. 2016;55:114–125. doi: 10.1016/j.bbi.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 2010;518:2729–2743. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Giese KP. Long-lasting regulation of hippocampal Bdnf gene transcription after contextual fear conditioning. Genes Brain Behav. 2012;11:651–659. doi: 10.1111/j.1601-183X.2012.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: Implications for sex differences in depression. Neuropsychopharmacology. 2015;40:1709–1716. doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory Blockade Restores Adult Hippocampal Neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Mor G, Nilsen J, Horvath T, Bechmann I, Brown S, Garcia-Segura LM, Naftolin F. Estrogen and microglia: A regulatory system that affects the brain. J Neurobiol. 1999;40:484–496. doi: 10.1002/(sici)1097-4695(19990915)40:4<484::aid-neu6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Moraga-Amaro R, Jerez-Baraona JM, Simon F, Stehberg J. Role of astrocytes in memory and psychiatric disorders. J Physiol Paris. 2014;108:240–251. doi: 10.1016/j.jphysparis.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Morris GP, Clark IA, Zinn R, Vissel B. Microglia: a new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiol Learn Mem. 2013;105:40–53. doi: 10.1016/j.nlm.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Moxley G, Posthuma D, Carlson P, Estrada E, Han J, Benson LL, Neale MC. Sexual dimorphism in innate immunity. Arthritis Rheum. 2002;46:250–258. doi: 10.1002/1529-0131(200201)46:1<250::AID-ART10064>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Nakanishi K, Kita A, Kadokawa T. Interleukin-1 induces analgesia in mice by a central action. Eur J Pharmacol. 1988;149:49–54. doi: 10.1016/0014-2999(88)90040-4. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, Kelley KW. Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol. 2008;252:91–110. doi: 10.1016/j.cellimm.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JJ, Coogan AN. Actions of the pro-inflammatory cytokine IL-1 beta on central synaptic transmission. Exp Physiol. 1999;84:601–614. [PubMed] [Google Scholar]
- Ostroff LE, Manzur MK, Cain CK, LeDoux JE. Synapses lacking astrocyte appear in the amygdala during consolidation of Pavlovian threat conditioning. J Comp Neurol. 2014;522:2152–2163. doi: 10.1002/cne.23523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota Y, Zanetti AT, Hallock RM. The role of astrocytes in the regulation of synaptic plasticity and memory formation. Neural Plast. 2013;2013:185463–185411. doi: 10.1155/2013/185463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa M, De Luca C, Petta F, Alberghina L, Cirillo G. Astrocyte-neuron interplay in maladaptive plasticity. Neurosci Biobehav Rev. 2014;42C:35–54. doi: 10.1016/j.neubiorev.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Park S-E, Dantzer R, Kelley KW, McCusker RH. Central administration of insulin-like growth factor-I decreases depressive-like behavior and brain cytokine expression in mice. J Neuroinflammation. 2011;8:12. doi: 10.1186/1742-2094-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, Lafaille JJ, Hempstead BL, Littman DR, Gan W-B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci USA. 2012;109:E197–E205. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. TINS. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovitch H, White L, Masaki KH, Ross GW, Abbott RD, Rodriguez BL, Lu G, Burchfiel CM, Blanchette PL, Curb JD. Influence of myocardial infarction, coronary artery bypass surgery, and stroke on cognitive impairment in late life. Am J Cardiol. 1998;81:1017–1021. doi: 10.1016/s0002-9149(98)00082-4. [DOI] [PubMed] [Google Scholar]
- Pickering M, O'Connor JJ. Pro-inflammatory cytokines and their effects in the dentate gyrus. Prog Brain Res. 2007;163:339–354. doi: 10.1016/S0079-6123(07)63020-9. [DOI] [PubMed] [Google Scholar]
- Polimanti R, Simonelli I, Zappasodi F, Ventriglia M, Pellicciari MC, Benussi L, Squitti R, Rossini PM, Tecchio F. Biological factors and age-dependence of primary motor cortex experimental plasticity. Neurol Sci. 2016;37:211–218. doi: 10.1007/s10072-015-2388-6. [DOI] [PubMed] [Google Scholar]
- Pribiag H, Stellwagen D. TNF-α downregulates inhibitory neurotransmission through protein phosphatase 1-dependent trafficking of GABA(A) receptors. J Neurosci. 2013;33:15879–15893. doi: 10.1523/JNEUROSCI.0530-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Kelly SD, Harrell CS, Neigh GN. Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain Behav Immun. 2013;30:88–94. doi: 10.1016/j.bbi.2013.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, Giannopoulou EG, Chan CH, Park S-H, Gong S, Chen J, Hu X, Elemento O, Ivashkiv LB. Synergistic activation of inflammatory cytokine genes by interferon-γ-induced chromatin remodeling and toll-like receptor signaling. Immunity. 2013;39:454–469. doi: 10.1016/j.immuni.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex chromosome complement regulates habit formation. Nat Neurosci. 2007;10:1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- Rantala MJ, Moore FR, Skrinda I, Krama T, Kivleniece I, Kecko S, Krams I. Evidence for the stress-linked immunocompetence handicap hypothesis in humans. Nat Comms. 2012;3:694. doi: 10.1038/ncomms1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazi K, Galic MA, Kentner AC, Reid AY, Sharkey KA, Pittman QJ. Microglia-Dependent Alteration of Glutamatergic Synaptic Transmission and Plasticity in the Hippocampus during Peripheral Inflammation. J Neurosci. 2015;35:4942–4952. doi: 10.1523/JNEUROSCI.4485-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BJ, Moussawi M, Huber SA. Sex differences in TLR2 and TLR4 expression and their effect on coxsackievirus-induced autoimmune myocarditis. Exp Mol Pathol. 2013;94:58–64. doi: 10.1016/j.yexmp.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, Weeber EJ, Bickford PC, Gemma C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci. 2011;31:16241–16250. doi: 10.1523/JNEUROSCI.3667-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. PNAS. 2002;99:13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajja VSSS, Hlavac N, VandeVord PJ. Role of Glia in Memory Deficits Following Traumatic Brain Injury: Biomarkers of Glia Dysfunction. Front Integr Neurosci. 2016;10:7. doi: 10.3389/fnint.2016.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem ML. Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr Drug Targets Inflamm Allergy. 2004;3:97–104. doi: 10.2174/1568010043483944. [DOI] [PubMed] [Google Scholar]
- Santos-Galindo M, Acaz-Fonseca E, Bellini MJ, Garcia-Segura LM. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol Sex Dif. 2011;2:7. doi: 10.1186/2042-6410-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Differential regulation of BDNF, synaptic plasticity and sprouting in the hippocampal mossy fiber pathway of male and female rats. Neuropharmacology. 2014;76(Pt C):696–708. doi: 10.1016/j.neuropharm.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012;120:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011;118:5918–5927. doi: 10.1182/blood-2011-03-340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin JA, Brennan J, Mangano E, Hayley S. Proinflammatory cytokines differentially influence adult hippocampal cell proliferation depending upon the route and chronicity of administration. Neuropsychiatr Dis Treat. 2009;5:5–14. [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, D'Agostino RB. Lifetime risk of dementia and Alzheimer's disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- Shah DS, Prados J, Gamble J, De Lillo C, Gibson CL. Sex differences in spatial memory using serial and search tasks. Behav Brain Res. 2013;257:90–99. doi: 10.1016/j.bbr.2013.09.027. [DOI] [PubMed] [Google Scholar]
- Sheridan GK, Murphy KJ. Neuron-glia crosstalk in health and disease: fractalkine and CX3CR1 take centre stage. Open Biol. 2013;3:130181–130181. doi: 10.1098/rsob.130181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Kaler LJ, Proctor TM, Teuscher C, Vandenbark AA, Offner H. IL-13-mediated gender difference in susceptibility to autoimmune encephalomyelitis. J Immunol. 2008;180:2679–2685. doi: 10.4049/jimmunol.180.4.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, Arnold AP, Singh RR, Voskuhl RR. A role for sex chromosome complement in the female bias in autoimmune disease. Journal of Experimental Medicine. 2008;205:1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Welsh CJ, Reddy DS. Sex Differences in Neurological Diseases. In: Shansky RM, editor. Sex Differences in the Central Nervous System. Elsevier; 2016. pp. 297–323. [Google Scholar]
- Song J, Christian KM, Ming G-L, Song H. Modification of hippocampal circuitry by adult neurogenesis. Dev Neurobiol. 2012;72:1032–1043. doi: 10.1002/dneu.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin J-S, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011;31:15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal JL, Waters EM, Bath KG, Chao MV, McEwen BS, Milner TA. Distribution of phosphorylated TrkB receptor in the mouse hippocampal formation depends on sex and estrous cycle stage. J Neurosci. 2011;31:6780–6790. doi: 10.1523/JNEUROSCI.0910-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinedi E, Gaillard RC, Chisari A. Sexual dimorphism of neuroendocrine-immune interactions. Front Horm Res. 2002;29:91–107. doi: 10.1159/000061059. [DOI] [PubMed] [Google Scholar]
- Spulber S, Mateos L, Oprica M, Cedazo-Minguez A, Winblad B, Schultzberg M. Impaired long term memory consolidation in transgenic mice overexpressing the human soluble form of IL-1ra in the brain. J Neuroimmunol. 2009;208:46–53. doi: 10.1016/j.jneuroim.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Stamova B, Tian Y, Jickling G, Bushnell C, Zhan X, Liu D, Ander BP, Verro P, Patel V, Pevec WC, Hedayati N, Dawson DL, Jauch EC, Pancioli A, Broderick JP, Sharp FR. The X-chromosome has a different pattern of gene expression in women compared with men with ischemic stroke. Stroke. 2012;43:326–334. doi: 10.1161/STROKEAHA.111.629337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg vom LG, Klein SL. SeXX Matters in Infectious Disease Pathogenesis. In: Heitman J, editor. PLoS Pathog. Vol. 12. 2016. p. e1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B, Kronenberg G, Jessberger S, Brandt MD, Reuter K, Kempermann G. Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia. 2004;46:41–52. doi: 10.1002/glia.10337. [DOI] [PubMed] [Google Scholar]
- Steinman MQ, Gao V, Alberini CM. The Role of Lactate-Mediated Metabolic Coupling between Astrocytes and Neurons in Long-Term Memory Formation. Front Integr Neurosci. 2016;10:10. doi: 10.3389/fnint.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz AB, Johnson SA, Iannitelli DE, Pollonini G, Alberini CM. Insulin-like growth factor 2 rescues aging-related memory loss in rats. Neurobiol Aging. 2016;44:9–21. doi: 10.1016/j.neurobiolaging.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D. Differential Regulation of AMPA Receptor and GABA Receptor Trafficking by Tumor Necrosis Factor. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, Benjamin EJ, Au R, Kiel DP, Wolf PA, Seshadri S. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Ide M, Shibutani T, Ohtaki H, Numazawa S, Shioda S, Yoshida T. Lipopolysaccharide-induced microglial activation induces learning and memory deficits without neuronal cell death in rats. J Neurosci Res. 2006;83:557–566. doi: 10.1002/jnr.20752. [DOI] [PubMed] [Google Scholar]
- Tay TL, Savage J, Hui CW, Bisht K, Tremblay M-E. Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J Physiol. 2016 doi: 10.1113/JP272134. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli LH, Holmes A, Postolache TT. Intranasal immune challenge induces sex-dependent depressive-like behavior and cytokine expression in the brain. Neuropsychopharmacology. 2008;33:1038–1048. doi: 10.1038/sj.npp.1301488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay MÈ, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The Role of Microglia in the Healthy Brain. J Neurosci. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uz E, Mustafa C, Topaloglu R, Bilginer Y, Dursun A, Kasapcopur O, Ozen S, Bakkaloglu A, Ozcelik T. Increased frequency of extremely skewed X chromosome inactivation in juvenile idiopathic arthritis. Arthritis Rheum. 2009;60:3410–3412. doi: 10.1002/art.24956. [DOI] [PubMed] [Google Scholar]
- Valero J, Mastrella G, Neiva I, Sánchez S, Malva JO. Long-term effects of an acute and systemic administration of LPS on adult neurogenesis and spatial memory. Front Neurosci. 2014;8:83. doi: 10.3389/fnins.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallières L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasek MJ, et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534:538–543. doi: 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli HA, Gandhi CP, Gray JM, Morena M, Hassan KI, Hill MN. Divergent responses of inflammatory mediators within the amygdala and medial prefrontal cortex to acute psychological stress. Brain Behav Immun. 2015;51:70–91. doi: 10.1016/j.bbi.2015.07.026. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer SD, Saint-Aubin J. Sex differences in visual-spatial working memory: A meta-analysis. Psychon Bull Rev. 2016 doi: 10.3758/s13423-016-1085-7. [Epub before print] [DOI] [PubMed] [Google Scholar]
- Waddell J, Bangasser DA, Shors TJ. The basolateral nucleus of the amygdala is necessary to induce the opposing effects of stressful experience on learning in males and females. J Neurosci. 2008;28:5290–5294. doi: 10.1523/JNEUROSCI.1129-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DG, Lue L-F. Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res Ther. 2015;7:56. doi: 10.1186/s13195-015-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MD, Godbout JP, Sheridan JF. Repeated Social Defeat, Neuroinflammation, and Behavior: Monocytes Carry the Signal. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.102. [Epub before print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieck A, Grassi-Oliveira R, Hartmann do Prado C, Teixeira AL, Bauer ME. Neuroimmunoendocrine interactions in post-traumatic stress disorder: focus on long-term implications of childhood maltreatment. Neuroimmunomodulation. 2014;21:145–151. doi: 10.1159/000356552. [DOI] [PubMed] [Google Scholar]
- Williams JL, Holman DW, Klein RS. Chemokines in the balance: maintenance of homeostasis and protection at CNS barriers. Front Cell Neurosci. 2014;8:154. doi: 10.3389/fncel.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LL, Sholar PW, Mistry RS, Smith SH, Bilbo SD. Microglia and memory: Modulation by early-life infection. J Neurosci. 2011;31:15511–15521. doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-W, Chen Y-C, Yu L, Chen H-I, Jen CJ, Huang A-M, Tsai H-J, Chang Y-T, Kuo Y-M. Treadmill exercise counteracts the suppressive effects of peripheral lipopolysaccharide on hippocampal neurogenesis and learning and memory. J Neurochem. 2007;103:2471–2481. doi: 10.1111/j.1471-4159.2007.04987.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 2015;36:605–613. doi: 10.1016/j.it.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Pamer EG. Monocytes and infection: modulator, messenger and effector. Immunobiology. 2015;220:210–214. doi: 10.1016/j.imbio.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Xu L, Wei L, White RE, Ouyang Y-B, Giffard RG. IL-4 Is Required for Sex Differences in Vulnerability to Focal Ischemia in Mice. Stroke. 2015;46:2271–2276. doi: 10.1161/STROKEAHA.115.008897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X-Y, Liu L, Yang Q-W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016;142:23–44. doi: 10.1016/j.pneurobio.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Yee JR, Prendergast BJ. Sex-specific social regulation of inflammatory responses and sickness behaviors. Brain Behav Immun. 2010;24:942–951. doi: 10.1016/j.bbi.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R, Pan G, Thobe BM, Choudhry MA, Matsutani T, Samy TSA, Kang S-C, Bland KI, Chaudry IH. MyD88 and Src are differentially regulated in Kupffer cells of males and proestrus females following hypoxia. Mol Med. 2006;12:65–73. doi: 10.2119/2006-00030.Zheng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Zhou L-J, Ren W-J, Xin W-J, Li Y-Y, Zhang T, Liu X-G. The direction of synaptic plasticity mediated by C-fibers in spinal dorsal horn is decided by Src-family kinases in microglia: The role of tumor necrosis factor-α. Brain Behav Immun. 2010;24:874–880. doi: 10.1016/j.bbi.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- Zonis S, Pechnick RN, Ljubimov VA, Mahgerefteh M, Wawrowsky K, Michelsen KS, Chesnokova V. Chronic intestinal inflammation alters hippocampal neurogenesis. J Neuroinflammation. 2015;12:65. doi: 10.1186/s12974-015-0281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorec R, Horvat A, Vardjan N, Verkhratsky A. Memory Formation Shaped by Astroglia. Front Integr Neurosci. 2015;9:56. doi: 10.3389/fnint.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]