Abstract
Sex differences in adult brain function are frequently determined developmentally through the actions of steroid hormones during sensitive periods of prenatal and early postnatal life. In rodents, various cellular endpoints of the developing brain are affected by estradiol that is derived from the aromatization of circulating testosterone and/or synthesized within the brain. We have previously described a sex difference in neurogenesis in the hippocampus of neonatal rats that is modulated by estradiol. In this report, we examined a potential downstream regulator of the effects of estradiol on hippocampal cell proliferation by measuring gene expression of brain-derived neurotrophin (BDNF) in male and female neonatal rats in response to estradiol. Males had higher baseline Bdnf gene expression in dentate gyrus and CA1 regions of the hippocampus, compared to females. Neonatal administration of exogenous estradiol resulted in opposite effects on Bdnf expression in these areas of the neonatal hippocampus, such that Bdnf transcripts increased in CA1 but decreased in dentate. Blocking endogenous estradiol signaling by antagonizing estrogen receptors decreased Bdnf expression in the dentate of males, but not females, and had no effect in CA1. Interestingly, this sex difference and response to estradiol was not mirrored by translational output, as no differences in BDNF precursor peptide were observed. The sex- and region-specific effects of estradiol on Bdnf expression in the neonatal hippocampus suggest a complex functional relationship between these pleiotropic factors in regulating developmental neurogenesis.
Keywords: sex differences, estrogen, hippocampus, BDNF
Graphical abstract
In the hippocampus of neonatal rats, males have more Bdnf gene transcripts in the dentate gyrus and Ammon’s horn. Manipulation of estradiol signaling has region- and sex-specific effects on Bdnf gene expression during the early postnatal period.
Introduction
The hippocampus is critically involved in contextual learning and stress responding, and as such plays a vital role in both complex and innate behaviors (Fenselow and Dong, 2010; Sweatt, 2004). Aspects of learning and stress responding differ in males and females across the lifespan (Mahmoud et al., 2016; Schoenfeld and Gould, 2012) and disorders in which hippocampal dysfunction are implicated, such as depression, anxiety, or schizophrenia, differ between the sexes in terms of prevalence and/or presentation (McLean et al., 2011; Schoenfeld and Cameron, 2015). While evidence suggests many of these differences are secondary to the developmental organizational effects of gonadal steroids, the mechanisms by which this would occur are not known.
Previous studies in our laboratory have characterized a robust sex difference in cell genesis in the neonatal hippocampus of rats that is modulated by gonadal steroids (Bowers et al., 2010; Waddell et al., 2013; Zhang et al., 2008). During the first postnatal week, males have twice as many proliferating cells in the dentate gyrus and CA1 regions of the hippocampus, compared to females, and this difference is not due to altered rates of cell death. Although estradiol administration to neonatal females increases the number of proliferating cells in dentate and CA1 to levels seen in males, exogenous estradiol does not increase cell genesis in males. Conversely, disruption of estrogen signaling in neonatal males decreases cell proliferation, but has no effect in females (Bowers et al., 2010). The effects of estradiol on developmental cell genesis in the hippocampus are rapid, occurring within hours, and yet also persistent, as indicated by the number of proliferating cells that survive and differentiate (Bowers et al., 2010; Zhang et al., 2008). Up to 80% of new cells in the neonatal hippocampus of males or estradiol-treated females will differentiate into neurons, whereas only 40% will do so in untreated females (Bowers et al., 2010). Thus estradiol promotes both proliferation and differentiation of new neurons and thereby has an important role in organizing the hippocampal circuitry in a sex-specific manner.
The downstream cellular mechanisms that mediate the effects of estradiol on neurogenesis in the neonatal hippocampus are largely unknown, although a likely candidate is the neurotrophin BDNF. Many of the effects of estradiol in the hippocampus are mirrored by BDNF or have a demonstrated requirement for activation of the cognate BDNF receptor, TrKB (Scharfman and MacLusky, 2006). Numerous studies indicate a role for BDNF in regulating neurogenesis. For example, administration of BDNF to adult hippocampus increases cell proliferation (Scharfman et al., 2005), while knockdown of BDNF or conditional deletion of TrKB reduces proliferation (Lee et al., 2009; Taliaz et al., 2010). BDNF also negatively impacts cell survival via p75NTR receptor signaling (Catts et al., 2008).
Based on studies demonstrating a role for BDNF in regulating cell proliferation in the adult hippocampus, as well as the expression of BDNF and TrKB receptors in the neonatal hippocampus of rats (Solum and Handa, 2001), we hypothesized that estradiol modulates proliferation in the developing hippocampus by regulating BDNF expression. We report here a study in which BDNF transcript and peptide levels were quantified in hippocampal subregions of neonatal male and female rats following estradiol treatment. A baseline sex difference in Bdnf gene expression was observed which mirrors the sex difference in hippocampal cell genesis. However, the effects of estradiol on Bdnf differed among subregions of the hippocampus, and BDNF peptide levels did not change in parallel with Bdnf gene expression. Together, these observations indicate region-specific roles for BDNF in sculpting the developing hippocampus in males versus females.
Materials and Methods
Experimental animals, drug treatments and tissue processing
All procedures involving experimental animals were approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine. Sprague-Dawley rat pups were obtained from dams mated in the University of Maryland School of Medicine Animal Care Facility and housed in a 12-hour light/dark cycle. The day of birth was designated postnatal day 0 (PN0). On postnatal day 4 (PN4), subregions of the hippocampal formation were dissected, frozen on dry ice and stored at −80 °C until subsequent processing. For each hippocampal subregion, tissue from both hemispheres of the brain of an individual pup were combined.
To dissect individual subregions of the hippocampal formation, brains were bisected sagitally in ice-cold phosphate-buffered saline (pH 7.4; PBS) and thalamic tissue removed to expose the ventricular surface of the hippocampus. The entire dentate gyrus was removed as a discrete structure by inserting a fine-guage needle along the length of the hippocampal fissure. Fiber projections along the dentate axis proximal to CA3 were removed with fine foreceps. Ammon’s horn was dissected from the neocortical tissue and meninges removed using fine foreceps. CA1 and CA3 areas were separated along the length of the hippocampus.
Tissues from untreated animals were homogenized in lysis buffer consisting of 10 mM Tris•HCl pH 7.6, 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, and containing protease and phosphatase inhibitors (Sigma). A portion of the homogenate was frozen for subsequent analysis of BDNF peptide content. The remaining homogenate was used for isolation of total RNA. In this way, both protein and total RNA were obtained from the same cohort of untreated animals. Tissues were collected from 7 animals of each sex at PN4 and PN15.
For pups receiving drug treatments, estradiol benzoate (Sigma) or tamoxifen (Sigma) were administered at 100 μg in 0.1 ml sesame oil subcu. on PN0 and PN1. An equivalent volume of sesame oil was similarly administered as vehicle control. This dose of estradiol and tamoxifen is necessary to overcome circulating steroid hormone binding globulins present in the neonatal rat, and has previously been shown to elicit sexual differentiation of the rodent brain, including sex-specific effects in the developing hippocampus (Amateau et al., 2004; Bowers et al., 2010; Mong et al., 1999). Hippocampal subregions of treated animals were collected on PN4 and frozen for analyses of gene or protein expression(n = 5-7/group).
Quantitative PCR
Total RNA was isolated from tissue homogenates (untreated animals) or frozen tissues (animals treated in estradiol experiments) using a modified phenol extraction method, according to the manufacturer’s instructions (TriReagent RT, MRC Inc.). cDNA from hippocampal subregions was transcribed from 1 μg total RNA using a High Capacity cDNA Reverse Transcription Kit and random hexamers (LifeTechnologies). cDNA products were diluted 1:3 and 5 μl was used for fluorescence-based, real-time PCR in a reaction mixture containing SYBR© Green PCR Master Mix (LifeTechnologies) and 250nM primers. PCR reactions were performed for 40 cycles on a ViiA™7 Real-Time PCR System (ABI). PCR primers for Bdnf expression targeted the coding region of Bdnf Exon IX and were designed to quantify all transcripts. Bdnf transcripts were normalized to Gapdh as a proxy for total cellular RNA content, and expression among groups calculated using the ΔΔCt method (Pfaffl, 2001). Data were expressed as mean fold expression relative to females +/− standard error of mean fold expression. Mean Ct values for Gapdh differed by less than 0.2 Ct between males and females for all 3 hippocampal regions. Primer sequences were as follows: BDNFfwd: 5’GCGGCAGATAAAAAGACTGC-3’; BDNFrev: 5’-CAGTTGGCCTTTTGATACCG-3’. Gapdhfwd: 5’-TGGTGAAGGTCGGTGTGAACGG-3’, Gapdhrev: 5’-TCACAAGAGAAGGCAGCCCTGGT-3’.
BDNF ELISA
Frozen tissues were homogenized in lysis buffer containing protease and phosphatase inhibitors, as described above. Total free BDNF propeptide was measured using the BDNF Emax Immunoassay System (Promega), according to the manufacturer’s protocol. Tissue homogenates were diluted 1:5 in PBS (pH 7.4), acid treated at pH 3.5 for 15 minutes by the addition of 2N HCl, and neutralized with NaOH prior to dilution in Block and Sample Buffer. According to the manufacturer, this ELISA has a sensitivity of 15.6 pg/ml and exhibits less than 3% cross-reactivity to other neurotrophins.
Western immunoblotting
Protein (12 μg) from tissue homogenates was resolved on 10-20% gradient polyacrylamide gels (NuPage, LifeTechnologies) under reducing and denaturing conditions, and transferred to PVDF membrane. Membranes were blocked with Odyssey Blocking Buffer (LiCor Biosciences) diluted 1:1 in Tris-buffered saline (pH 7.4, TBS) prior to incubation overnight at 4°C in Blocking Buffer diluted 1:1 in TBS-Tween (0.05%) containing primary antibodies against BDNF (rabbit IgG against the middle portion of human BDNF, Aviva Systems Biology, Cat# ARP41970_P050; RRID:AB_10644597; diluted 1:800) and GAPDH (mouse monoclonal, Abcam, Cat# ab9484; RRID:AB_307274; diluted 1:5,000). Membranes were washed 3 times in TBS-Tween (0.05%) and incubated in Blocking Buffer diluted 1:1 in TBS-Tween/0.02% SDS containing secondary antibodies (IRDye©800 goat-anti-rabbit and IRDye©680 goat-anti-mouse IgG, LiCor Biosciences; both diluted 1:5000). After washing, membranes were imaged on an Odyssey CLx Infrared Imaging System (LiCor). Fluorescence intensity for each BDNF band was normalized to fluorescence intensity of the corresponding GAPDH band to obtain an integrated fluorescence value.
Antibody characterization
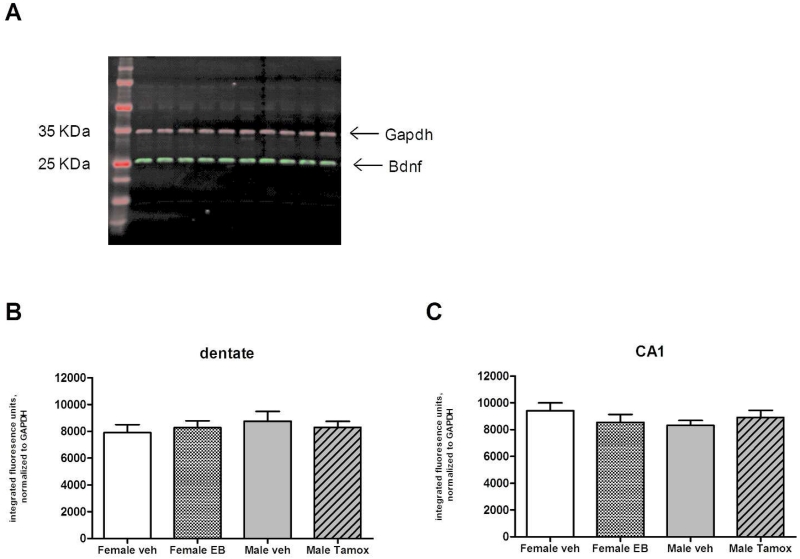
Table 1 lists the antibodies used in this study. The BDNF antiserum used (RRID:AB_10644597) recognized a single band of approximately 27.5 KDa on Western immunoblots of rat hippocampal tissue homogenate (see Figure 4). This band was eliminated when the antiserum was preabsorbed with a 4-fold molar excess of the immunizing peptide (1 ug antibody and 2 ug immunizing peptide) in TBS overnight at 4°C. The Gapdh monoclonal antibody recognized a single band of approximately 35 KDa on Western immunoblots of rat hippocampal tissue homogenate (Fig 4).
Table 1.
Primary antibodies used
| antigen | antibody source; host species; RRID |
immunogen | working concentration used |
|---|---|---|---|
| BDNF | Aviva Systems Biology, Cat# ARP41970_P050; rabbit polyclonal; RRID:AB_10644597 |
synthetic peptide homologous to the middle portion of human BDNF; EWVTAADKKTAVDMSGGTV TVLEKPVSKGQLKQFYETK CNPMGYTKEG |
1.25 μg/ml |
| GAPDH | Abcam, Cat# ab9484; mouse monoclonal; RRID:AB_307274 |
native human GAPDH | 0.2 μg/ml |
Figure 4.
Levels of BDNF propeptide in hippocampus of PN4 rats treated with estradiol or tamoxifen, as determined by Western immunoblotting. A) Representative blot demonstrating a single band at 28 KDa was detected with the BDNF antibody. No differences in BDNF levels were detected among vehicle, estradiol, or tamoxifen treated animals in the dentate gyrus (B) (ANOVA, p=0.7858) or CA1 (C) (ANOVA, p=0.4975). n= 5-6 animals per treatment for each sex.
Statistical analyses
qPCR and ELISA data from untreated animals were analyzed by 2-factor ANOVA with sex and region as independent variables, followed by pairwise post-hoc comparisons using Bonferroni correction. qPCR data from animals treated with estradiol and tamoxifen were analyzed using 2-factor ANOVA with sex and treatment as independent factors and Bonferroni post-hoc comparisons. ELISA data from animals treated with estradiol and tamoxifen were analysed using the Kruskal-Wallis test of ranks.Western immunoblotting data from treated and untreated animals were analyzed using single factor ANOVA across groups for each hippocampal region with Tukey’s multiple comparisons post test. All data were first analysed using Kolmogorov-Smirnov test for normalcy and Bartlett’s test for homogeneity of variances. Significance was set at p<0.05 for all analyses.
Results
Bdnf gene expression differs among hippocampal subregions and between males and females during the first postnatal week
Bdnf transcripts were quantitated in the CA1, CA3 and dentate gyrus regions of the hippocampal formation of developing rats using a real-time qPCR assay. Because the rat Bdnf gene is expressed from multiple promoters and alternatively spliced exons that result in 12 distinct transcripts (Aid et al., 2007), primers for this qPCR assay were designed to target the coding region of Exon IX, which is common to all transcripts.
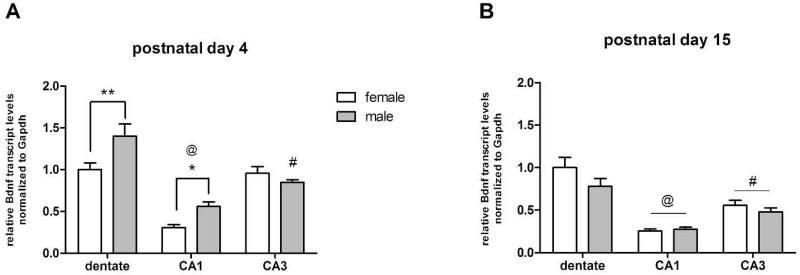
On postnatal day 4, 2-factor ANOVA indicated a significant main effect of sex on Bdnf gene expression [F(1,36)=7.664, n=7, p=0.0088]. Post-hoc analysis confirmed that males have more Bdnf transcripts in the dentate gyrus (P <0.01) and CA1 region of Ammon’s horn (P <0.05), compared to females (Figure 1A). A significant main effect of hippocampal subregion on Bdnf expression was also indicated [F(2,36)=39.49, n=7, p<0.0001], as well as an interaction of sex and region [F(2,36)=5.303, n=7, p=0.0096], driven by higher expression in the dentate and CA1 of males.
Figure 1.
Relative abundance of Bdnf transcripts in the dentate gyrus, CA1, and CA3 regions of the hippocampal formation of Sprague Dawley rats at postnatal day 4 (PN4) and postnatal day (PN15), as determined by real-time qPCR. Transcript levels are normalized to Gapdh transcripts and expressed as mean transcript abundance +/− s.e.m., relative to female dentate gyrus. A) On PN4, males have higher levels of Bdnf transcripts in dentate gyrus and CA1, compared to females (ANOVA, **p<0.01, *p<0.05). Regional differences in Bdnf expression were also seen at PN4, where more Bdnf transcripts were seen in dentate, compared to CA1 or CA3 (ANOVA, #p<0.01, males only compared to male dentate; @p<0.0001, both sexes, compared to dentate. B) No sex differences in Bdnf transcripts seen at PN15. Greatest regional expression in Bdnf was seen in dentate gyrus (ANOVA, #p<0.01, @p<0.0001, compared to dentate). n= 7 animals per sex.
A significant regional effect on Bdnf expression was also seen at postnatal day 15 [F(2,36)=40.65, n=7, p<0.0001], where mean relative expression levels were greatest in the dentate gyrus for both sexes (Figure 1B). No effect of sex on Bdnf expression was indicated among the 3 regions at this age [F(1,36)=2.619, n=7, p=0.1143].
Estradiol has different effects on Bdnf gene expression among hippocampal subregions during the first postnatal week
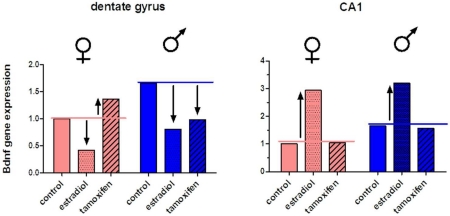
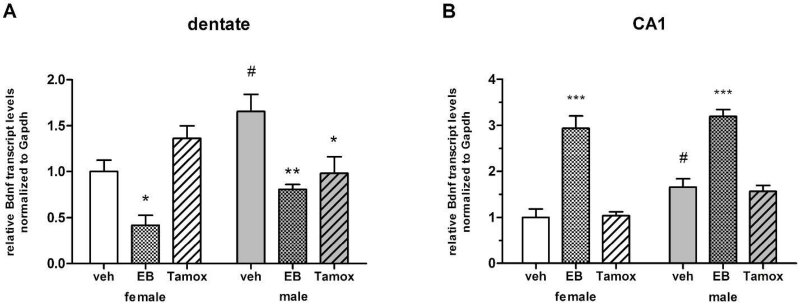
To determine if estrogen signaling influences Bdnf expression in the developing hippocampus, a second cohort of animals was treated systemically with estradiol benzoate, tamoxifen, or vehicle on the day of birth and 24 hours later, and Bdnf transcripts quantified in dentate and CA1 regions on PN4 via qPCR. Within the dentate, 2-factor ANOVA for sex and treatment indicated a significant main effect of treatment [F(2,31)=13.42, n=5-6, p<0.0001]. Post-hoc pairwise comparisons confirmed that Bdnf expression was significantly increased by estradiol treatment in both males (P<0.01) and females (P<0.05), compared to vehicle controls (Figure 2A). Surprisingly, tamoxifen treatment also decreased Bdnf expression in the dentate of males (P<0.05), to the same degree as estradiol, but had no effect in females (P>0.05). A significant interaction between sex and treatment in the dentate was indicated [F(2,31)=6.870, n=5-6, p=0040], however a main effect of sex was not [F(1,31)=3.478, n=5-6, p=0.0735], most likely because mean values for estradiol and tamoxifen treatments did not differ between males and females (P>0.05). Bdnf expression was significantly higher in vehicle treated males versus females (P<0.01), recapitulating the sex difference observed in untreated animals.
Figure 2.
Regional effects of estradiol and tamoxifen on Bdnf gene expression in postnatal hippocampus, as determined by real-time qPCR at PN4. Transcript levels were normalized to Gapdh and expressed relative to vehicle treated females within each region. A) In dentate, estradiol decreases Bdnf transcripts in both sexes, while tamoxifen decreases Bdnf transcripts in males only (ANOVA, *p<0.05, **p<0.01 compared to same sex vehicle control). B) In CA1, estradiol increases Bdnf gene expression in both sexes, while tamoxifen has no effect. (ANOVA, ***p<0.0001, compared to same sex vehicle control). Bdnf expression was higher in vehicle treated males compared to vehicle treated females in both regions (ANOVA, #p<0.05). n= 5-6 animals per treatment group for each sex.
In CA1, a significant main effect of treatment was also evident [F(2,30)=69.32, n=5-6, p<0.0001], although here estradiol treatment greatly increased Bdnf signaling in both males and females (P<0.0001 for both), while tamoxifen treatment had no effect in either sex (P>0.05). A main effect of sex was indicated [F(1,31)=11.79, n=5-6, p=0.0020], due to higher Bdnf expression in vehicle treated males compared to females (P<0.05). No interaction between sex and treatment was indicated [F(2,31)=0.7136, n=5-6, p=0.4992].
Estradiol does not alter BDNF propeptide content in the hippocampus during the first postnatal week
To examine the effect of estradiol on BDNF peptide levels, a third cohort of animals was treated on PN0 and PN1 with estradiol benzoate (female pups only), tamoxifen (males only), or vehicle. Because hippocampal cell genesis is not altered by estrogen treatment in males or tamoxifen treatment in females, these two groups were not included in this cohort in an effort to limit the number of experimental animals. BDNF peptide levels were determined in hippocampal subregions collected on PN4 using a commercially-available ELISA, and reported as pg of BDNF peptide per mg of total protein (Figure 3). Bartlett’s test indicated inhomogeneity of variance among treatment groups within the dentate, so means were compared within the three hippocampal subregions using non-parametric ANOVA. Nodifferences among groups within the dentate (p=0.0508; Figure 3A), CA1 (p=0.1463, Figure 3B) or CA3 (p=0.1598; Figure 3C) regions were indicated using the Kruskal-Wallis test by ranks.
Figure 3.
Levels of BDNF peptide in the hippocampus of PN4 animals treated with estradiol or tamoxifen, as determined by ELISA. No differences in BDNF content were detected among vehicle control or drug treated animals in the dentate gyrus (A) (non) parametric ANOVA, p=0.0508), CA1 (B) (non-parametric ANOVA, p=0.1463) or CA3 (C) (non-parametric ANOVA, p=0.1598). n= 5-6 animals per group for each sex.
Results from the ELISA assays were confirmed by comparing relative amounts of BDNF peptide in the same sample homogenates using Western immunoblotting. A single band at 28 KDa corresponding to the predicted molecular weight of the BDNF propeptide was obtained with the anti-BDNF antibody (Aviva Systems Biology, RRID:AB_10644597; Figure 4A). As with the ELISA results, one-way ANOVA indicated no differences among groups within the dentate or CA1 regions on PN4 (p=0.7858, p=0.4975; Figure 4B and C, respectively). CA3 was not assessed by Western immunoblotting.
The sex difference in Bdnf gene expression during the first postnatal week is not reflected in BDNF prohormone content
As shown in Figures 3 and 4, BDNF peptide levels did not differ in vehicle treated males and females on PN4. Western immunoblotting was used to test for baseline sex differences in BDNF peptide content in hippocampal subregions during the first postnatal week using the same cohort of untreated PN4 animals used to demonstrate a sex difference in Bdnf gene expression.As with the hippocampal homogenates from animals treated with estradiol and tamoxifen, a single band corresponding to the 28 KDa BDNF propeptide was detected. Two-factor ANOVA confirmed no effect of sex on BDNF propeptide content among the three hippocampal subregions at postnatal day 4 (p=0.9091), nor were there regional differences in propeptide content (p=0.9389; Figure 5A). No differences in BDNF propeptide content were seen between males and females (2-factor ANOVA, p=0.5086), or among the three hippocampal subregions (2-factor ANOVA, p=0.0551) from untreated animals at postnatal day 15 (Figure 5B).
Figure 5.
Levels of BDNF propeptide in dentate and CA1 regions of untreated animals at PN4 (A) and PN15 (B), as determined byWestern immunoblotting. No sex differences were noted among hippocampal subregions at PN4 (ANOVA, p=0.9091) or PN15 (ANOVA, p=0.5086). Regional differences in BDNF propeptide content were also not indicated at either PN4 (ANOVA, p=0.9689) or PN15 (ANOVA, p=0.0551).
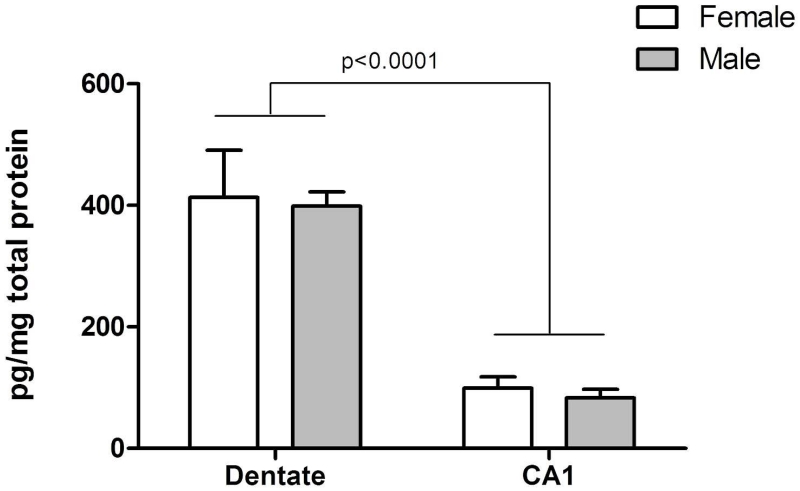
To confirm the results from Western immunoblotting, BDNF peptide content in dentate and CA1 was additionally assessed by ELISA in the same samples,. Two-factor ANOVA confirmed there was no effect of sex on BDNF peptide content in either region (p=0.7120; Figure 6). A significant main effect of hippocampal subregion on BDNF peptide levels was indicated [F(1,26)=58.45, n=7, p<0.0001]. BDNF peptide was higher in the dentate of both sexes, compared to CA1, recapitulating the relative regional abundances for Bdnf transcript (Figure 1A).
Figure 6.
Levels of BDNF propeptide in dentate and CA1 regions of untreated animals at PN4, as determined by ELISA. More BDNF was measured in dentate, compared to CA1. No difference between males and females was detected in either dentate or CA1 (ANOVA, p=0.7120). n= 7 animals per sex.
Discussion
In this study, we examined Bdnf expression in the dentate gyrus, CA1 and CA3 regions of the hippocampal formation in male and female rats during the first postnatal week. We used a qPCR assay targeted to the exon IX coding sequence of the Bdnf gene to quantify total levels of all BDNF transcripts, and found a baseline sex difference in Bdnf gene expression. Males had roughly 50% more Bdnf transcripts in the dentate gyrus and CA1, but not CA3, regions of the hippocampus during the first postnatal week. This sex difference was not detected at postnatal day 15. Intriguingly, the sex difference in Bdnf transcripts on postnatal day 4 mirrored cell proliferation in these regions of the hippocampus, where males produce roughly twice as many new cells in the dentate and CA1 at this time (Bowers et al., 2010; Lima et al., 2014; Zhang et al., 2008). However, the relationship between BDNF and cell genesis in the neonatal hippocampus is likely to be complex and region-specific, as estradiol positively regulates cell proliferation in both the dentate and CA1 (Bowers et al., 2010; Zhang et al., 2008), but had opposite effects on Bdnf expression between these two regions in the present study (Figure 2).
In contrast to long-standing interest in the role of BDNF in hippocampal plasticity and neurogenesis in adults, particularly with regard to effects of estrogen, far fewer studies have examined BDNF expression in the developing hippocampus. Damborsky and Winzer-Serhan (2012) reported neonatal male rats have higher hippocampal Bdnf gene expression in dentate, CA1 and CA3 subregions at PN5, but not PN8. This is largely consistent with our observations with the exception of CA3, where we did not detect sex differences. This discrepancy may be due to methodological differences, as Damborsky and Winzer-Serhan utilized in situ hybridization autoradiography to determine relative Bdnf transcript levels, and the present study measured transcripts via qPCR in dissected subregions of the hippocampus.
One of the first studies to examine the ontogeny of Bdnf expression in the developing hippocampus determined Bdnf transcript and peptide levels in neonatal male rats in relation to gonadal steroids. Castration on the day of birth decreases Bdnf gene expression for the first two postnatal weeks in CA1 and CA3, yet a single dose of estradiol on the day of birth in castrated animals restores Bdnf transcript levels (Solum and Handa 2002). This demonstrates that not only does estradiol upregulate Bdnf expression in Ammon’s horn of the developing hippocampus, but the duration of the effect of estradiol suggests that Bdnf expression during the first two weeks can be primed by the perinatal surge in gonadal steroids. In the present study, we manipulated estradiol signaling in neonatal rats using a paradigm of systemic administration of estradiol benzoate or the estrogen receptor antagonist tamoxifen. The main source of estrogen in the neonatal brain of rodents is the aromatization of circulating testosterone in males (Naftolin et al.,1971, 1975), although de novo synthesis of estradiol does occur in various brain areas of both sexes (Amateau et al., 2004), and in particular the hippocampus (Hojo et al., 2011; Mukai et al., 2006) The developing brain is shielded from the effects of maternal estradiol by α-fetoproteinpresent in the neonatal circulation (Bakker et al., 2006). The sequestering effects of this steroid binding globulin can be overcome with a relatively high dose of exogenous estradiol, and this has been titrated to achieve effects in females that mimic endogenous estradiol in males (Amateau et al., 2004)Using a masculinizing dose of estradiol, we observed an upregulation of Bdnf transcripts in CA1 in male and female neonates, similar to what was seen in males by Solum and Handa (2002). Interestingly, in the dentate of both sexes we observed the opposite effect of estradiol, where Bdnf gene expression was decreased. These opposing effects of estradiol between the two regions of the hippocampus may be explained by regional differences in estrogen receptor subtype and cellular localization. In rats, ERα mRNA and protein are upregulated in CA1 during the first two weeks postnatally, and immunocytochemistry localizes ERα to nuclei of pyramidal neurons (Ivanova and Beyer, 2000; O’Keefe and Handa, 1990; Solum and Handa, 2001). BDNF is expressed in pyramidal neurons of the CA1, its synthesis and secretion is promoted by estradiol in parallel with CREB activation (Zhou et al., 2005), and a functional estrogen response element is found in the Bdnf gene (Sohrabji et al.,1995). Together this suggests the observed upregulation of Bdnf gene expression in pyramidal neurons of neonatal CA1 occurs via classical genomic action of estrogen receptors. In the neonatal dentate gyrus, however, extranuclear ERβ is localized to the plasma membrane of immature granule neurons and also glia (Herrick et al., 2006), raising the possibility that estradiol may have nongenomic effects on Bdnf expression from these cell types in the dentate.
Although exogenous estradiol elicited opposite effects on Bdnf expression in dentate and CA1, these effects were nevertheless the same between neonatal males and females. In contrast, antagonizing endogenous estradiol signaling at ERα and ERβ with tamoxifen appeared to have a sex-specific effect in the dentate gyrus, but not CA1. Tamoxifen had no effect on Bdnf transcript levels in female dentate, but in males tamoxifen decreased Bdnf gene expression to the same level as in females. Because the 4-hydroxy metabolite of tamoxifen has significantly more affinity for the estrogen receptor than the drug itself (Fabian et al., 1981), the possibility is raised that the differential effects of tamoxifen in neonatal males and females may be due to sex differences in tamoxifen metabolism. However, the enzymes of the cytochrome P450 system which metabolize tamoxifen are present from birth in the rodent liver, and although low during the neonatal period, show a similar pattern of expression in males and females (Cui et al., 2012; Hart et al., 2009). In addition, the effects of exogenous estradiol administration in the brains of female neonatal rodents is blocked by systemic administration of tamoxifen (Gonzalez et al., Hilton et al., 2004). Together these indicate that tamoxifen is able to antagonize estrogen receptors similarly in neonatal males and females, and the effects observed in this study on Bdnf expression in the dentae are sex specific. At first glance this suggests that the baseline sex difference in Bdnf expression in dentate is promoted by estradiol. However, since estradiol and androgen content in the hippocampus at this age is equivalent in males and females (Amateau et al., 2004; Konkle and McCarthy, 2011), there must be sex-specific mechanisms downstream of estradiol signaling that mediate this difference.
One possible mechanism is the depolarizing action of GABA. In the adult brain GABA is the main inhibitory neurotransmitter, but immature neurons are depolarized in response to GABAA receptor activation. This results in numerous trophic effects in the developing brain, proximally mediated by an influx of Ca2+, which results in activation of the transcription factor CREB. Depolarizing GABA upregulates Bdnf gene expression and peptide content in developing neurons in a CREB-dependent manner (Berninger et al., 1995; Obrietan et al., 2002; Shieh et al., 1998). Sex differences in CREB content and the depolarizing actions of GABA are found in the neonatal hippocampus of rats. Males respond to GABAA receptor activation with a longer duration of calcium influx in a greater percentage of the neuronal population (Galanopoulou, 2008; Nuňez and McCarthy, 2007; 2009), and also have more activated CREB compared to females (Auger et al., 2001; Perrot-Sinal et al., 2003). Moreover, both the depolarizing actions of GABA and activated CREB content are positively regulated by estradiol (Nugent et al., 2012; Nuňez et al., 2005; Nuňez and McCarthy, 2009). The baseline sex differences in depolarizing GABA and CREB suggest a mechanism through which greater Bdnf expression is achieved in the neonatal hippocampus of males in spite of equivalent estradiol content between the sexes.
A surprising outcome of this study was the discordance between Bdnf transcript and peptide levels. Although the ELISA captured relative differences between dentate and CA1 regions that mirrored the results from qPCR, it did not detect any differences in peptide content between males and females, or in response to estradiol, and this was confirmed with Western immunoblotting. BDNF is synthesized and secreted as a glycosylated precursor propeptide of approximately 28 KDa, which is proteolytically cleaved to yield a mature peptide of 14 KDa (Mowla et al., 2001). Both the ELISA and the anti-BDNF antibody we used for Western immunoblotting are able to detect the 28 KDa propeptide and the mature form of BDNF, and were intended to measure total translational output, although we only detecteda band corresponding to the 28 KDa propeptide in our Western immunoblots, The abundance of BDNF precursor peptide relative to the mature form is greatest in the hippocampus of neonatal and juvenile animals, due to developmentally regulated expression of tissue plasminogen activator, which is essential for zymogen activation of the protease which cleaves proBDNF to the mature peptide (Pang et al., 2004; Yang et al., 2009). The amount of mature BDNF found in the hippocampus of mice during the first 15 days postnatally is extremely low (Yang et al., 2014), and therefore our inability to quantify mature BDNF is likely due to insufficient sensitivity in our Western immunoblots. Previous studies have noted differences between Bdnf transcript levels and peptide content. For example, Gibbs (1999) found that estradiol treatment of ovariectomized female rats increased Bdnf transcripts but had no effect on peptide levels in the hippocampus. In the neonatal male rat, castrationinduces a significant increase in BDNF mature peptide, in opposition to transcripts (Solum and Handa, 2002). More recently, Hill et al (2014) found that adolescent stressed male rats that had experienced maternal deprivation have lower levels of hippocampal Bdnf gene expression compared to unstressed animals, but higher levels of the mature BDNF peptide, while no changes are observed in the BDNF propeptide. Females undergoing maternal separation and adolescent stress also exhibit lower levels of mature BDNF peptide in the hippocampus, but no changes in propeptide or transcript levels. The BDNF propeptide and mature form can have opposing effects on several aspects of neuronal development, including cell proliferation (Hempstead, 2006). While BDNF increases cell genesis, as noted above, BDNF propeptide can promote apoptosis through preferential activation of p75NTR, rather than TrKB receptors, thereby decreasing cell genesis (Teng et al., 2005). We did not quantify mature BDNF in this study, but given the potential for sex-specific regulation of proBDNF cleavage, and the opposing effects of proBDNF and mature BDNF on cell genesis, this is worth future study.
In summary, we have found sex and region-specific differences in the relationship between Bdnf gene expression and estradiol in the neonatal hippocampus. Further studies which examine how this relationship is differentially mediated among the hippocampal subregions, as well as a more detailed analysis of the effects of sex and estradiol on BDNF propeptide processing, may identify BDNF as a key factor in sexual differentiation of the developing hippocampus.
Significance Statement.
Estrogen and BDNF have critical roles in sculpting the developing brain. This study examined the relationship between estrogen and BDNF in neonatal male and female rats in an area of the brain responsible for learning and stress responding, the hippocampus. Manipulation of estradiol signaling had differing and sex-specific effects on Bdnf gene expression in different subregions of the hippocampal formation, raising the possiblity of cell-type specific effects of estrogen on the developing hippocampus. In contrast, estradiol had no effect on levels of BDNF precursor peptide, adding to the already complicated picture of BDNF synthesis in response to developmental factors.
Acknowledgements
This work was supported by NINDS award 040726B1 to MMM.
Supported by NINDS award 2R56 NS050525-09 to MMM.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85(3):525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145(6):2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Auger AP, Perrot-Sinal TS, McCarthy MM. Excitatory versus inhibitory GABA as a divergence point in steroid-mediated sexual differentiation of the brain. Proc Natl Acad Sci U S A. 2001;98(14):8059–8064. doi: 10.1073/pnas.131016298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- Berninger B, Marty S, Zafra F, da Penha Berzaghi M, Thoenen H, Lindholm D. GABAergic stimulation switches from enhancing to repressing BDNF expression in rat hippocampal neurons during maturation in vitro. Development. 1995;121(8):2327–2335. doi: 10.1242/dev.121.8.2327. [DOI] [PubMed] [Google Scholar]
- Bowers JM, Waddell J, McCarthy MM. A developmental sex difference in hippocampal neurogenesis is mediated by endogenous oestradiol. Biol Sex Differ. 2010;1(1):8. doi: 10.1186/2042-6410-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catts VS, Al-Menhali N, Burne TH, Colditz MJ, Coulson EJ. The p75 neurotrophin receptor regulates hippocampal neurogenesis and related behaviours. Eur J Neurosci. 2008;28(5):883–892. doi: 10.1111/j.1460-9568.2008.06390.x. [DOI] [PubMed] [Google Scholar]
- Cui JY, Renaud HJ, Klaassen CD. Ontogeny of Novel Cytochrome P450 Gene Isoforms during Postnatal Liver Maturation in Mice. Drug Metabolism and Disposition. 2012;40(6):1226–1237. doi: 10.1124/dmd.111.042697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damborsky JC, Winzer-Serhan UH. Effects of sex and chronic neonatal nicotine treatment on Na(2)(+)/K(+)/Cl(−) co-transporter 1, K(+)/Cl(−) co-transporter 2, brain-derived neurotrophic factor, NMDA receptor subunit 2A and NMDA receptor subunit 2B mRNA expression in the postnatal rat hippocampus. Neuroscience. 2012;225:105–117. doi: 10.1016/j.neuroscience.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian C, Tilzer L, Sternson L. Comparative binding affinities of tamoxifen, 4-hydroxytamoxifen, and desmethyltamoxifen for estrogen receptors isolated from human breast carcinoma: correlation with blood levels in patients with metastatic breast cancer. Biopharm Drug Dispos. 1981;2:381–390. doi: 10.1002/bdd.2510020407. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou A. Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABA(A) receptors. Journal of Neuroscience. 2008;28:1557–1567. doi: 10.1523/JNEUROSCI.5180-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions o the adult brain. Brain Research. 1999;844:20–27. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- Gonzales KL, Quadros-Mennella P, Tetel MJ, Wagner CK. Anatomically-Specific Actions of Oestrogen Receptor in the Developing Female Rat Brain: Effects of Oestradiol and Selective Oestrogen Receptor Modulators on Progestin Receptor Expression. Journal of Neuroendocrinology. 2012;24(2):285–291. doi: 10.1111/j.1365-2826.2011.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SN, Cui Y, Klaassen CD, Zhong X-b. Three Patterns of Cytochrome P450 Gene Expression during Liver Maturation in Mice. Drug Metabolism and Disposition. 2009;37(1):116–121. doi: 10.1124/dmd.108.023812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead B. Dissecting the Diverse Actions of Pro- and Mature Neurotrophins. Current Alzheimer Research. 2006;3(1):19–24. doi: 10.2174/156720506775697061. [DOI] [PubMed] [Google Scholar]
- Herrick SP, Waters EM, Drake CT, McEwen BS, Milner TA. Extranuclear estrogen receptor beta immunoreactivity is on doublecortin-containing cells in the adult and neonatal rat dentate gyrus. Brain Res. 2006;1121(1):46–58. doi: 10.1016/j.brainres.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Hill RA, Klug M, Kiss Von Soly S, Binder MD, Hannan AJ, van den Buuse M. Sex-specific disruptions in spatial memory and anhedonia in a “two hit” rat model correspond with alterations in hippocampal brain-derived neurotrophic factor expression and signaling. Hippocampus. 2014;24(10):1197–1211. doi: 10.1002/hipo.22302. [DOI] [PubMed] [Google Scholar]
- Hilton GD, Ndubuizu AN, McCarthy MM. Neuroprotective effects of estradiol in newborn female rat hippocampus. Developmental Brain Research. 2004;150(2):191–198. doi: 10.1016/j.devbrainres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Higo S, Kawato S, Hatanaka Y, Ooishi Y, Murakami G, Ishii H, Komatsuzaki Y, Ogiue-Ikeda M, Mukai H, Kimoto T. Hippocampal Synthesis of Sex Steroids and Corticosteroids: Essential for Modulation of Synaptic Plasticity. Frontiers in Endocrinology. 2011;2:43. doi: 10.3389/fendo.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova T, Beyer C. Ontogenetic expression and sex differences of aromatase and estrogen receptor-alpha/beta mRNA in the mouse hippocampus. Cell Tissue Res. 2000;300(2):231–237. doi: 10.1007/s004410000199. [DOI] [PubMed] [Google Scholar]
- Konkle AT, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152(1):223–235. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Son H. Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep. 2009;42(5):239–244. doi: 10.5483/bmbrep.2009.42.5.239. [DOI] [PubMed] [Google Scholar]
- Lima M, Malheiros J, Negrigo A, Tescarollo F, Medeiros M, Suchecki D, Tannus A, Guinsburg R, Covolan L. Sex-related long-term behavioral and hippocampal cellular alterations after nociceptive stimulation throughout postnatal development in rats. Neuropharmacology. 2014;77:268–276. doi: 10.1016/j.neuropharm.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Mahmoud R, Wainwright SR, Galea LAM. Sex hormones and adult hippocampal neurogenesis: Regulation, implications, and potential mechanisms. Frontiers in Neuroendocrinology. doi: 10.1016/j.yfrne.2016.03.002. [DOI] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. Journal of Psychiatric Research. 2011;45(8):1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19(4):1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem. 2001;276(16):12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- Mukai H, Tsurugizawa T, Ogiue-Ikeda M, Murakami G, Hojo Y, Ishii H, Kimoto T, Kawato S. Local Neurosteroid Production in the Hippocampus: Influence on Synaptic Plasticity of Memory. Neuroendocrinology. 2006;84(4):255–263. doi: 10.1159/000097747. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan K, Davies I, Reddy V, Flores F, Petro Z, Kuhn M, White R, Takaoka Y, Wolin L. The formation of estrogens by central neuroendocrine tissues. Recent Progress in Hormone Research. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan K, Petro Z. Aromatization of androstenedione by limbic system tissue from human foetuses. Journal of Endocrinology. 1971;51:795–796. doi: 10.1677/joe.0.0510795. [DOI] [PubMed] [Google Scholar]
- Nugent BM, Valenzuela CV, Simons TJ, McCarthy MM. Kinases SPAK and OSR1 are upregulated by estradiol and activate NKCC1 in the developing hypothalamus. J Neurosci. 2012;32(2):593–598. doi: 10.1523/JNEUROSCI.5415-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, Bambrick LL, Krueger BK, McCarthy MM. Prolongation and enhancement of gamma-aminobutyric acid receptor mediated excitation by chronic treatment with estradiol in developing rat hippocampal neurons. Eur J Neurosci. 2005;21(12):3251–3261. doi: 10.1111/j.1460-9568.2005.04175.x. [DOI] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Evidence for an extended duration of GABA-mediated excitation in the developing male versus female hippocampus. Dev Neurobiol. 2007;67(14):1879–1890. doi: 10.1002/dneu.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Resting intracellular calcium concentration, depolarizing Gamma-Aminobutyric Acid and possible role of local estradiol synthesis in the developing male and female hippocampus. Neuroscience. 2009;158(2):623–634. doi: 10.1016/j.neuroscience.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrietan K, Gao XB, Van Den Pol AN. Excitatory actions of GABA increase BDNF expression via a MAPK-CREB-dependent mechanism--a positive feedback circuit in developing neurons. J Neurophysiol. 2002;88(2):1005–1015. doi: 10.1152/jn.2002.88.2.1005. [DOI] [PubMed] [Google Scholar]
- O’Keefe JA, Handa RJ. Transient elevation of estrogen receptors in the neonatal rat hippocampus. Brain Res Dev Brain Res. 1990;57(1):119–127. doi: 10.1016/0165-3806(90)90191-z. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306(5695):487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Auger AP, McCarthy MM. Excitatory actions of GABA in developing brain are mediated by l-type Ca2+ channels and dependent on age, sex, and brain region. Neuroscience. 2003;116(4):995–1003. doi: 10.1016/s0306-4522(02)00794-7. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28(6):278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192(2):348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 2006;27(4):415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, Cameron HA. Adult Neurogenesis and Mental Illness. Neuropsychopharmacology. 2015;40(1):113–128. doi: 10.1038/npp.2014.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Experimental Neurology. 2012;233(1):12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20(4):727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92(24):11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Localization of estrogen receptor alpha (ER alpha) in pyramidal neurons of the developing rat hippocampus. Brain Res Dev Brain Res. 2001;128(2):165–175. doi: 10.1016/s0165-3806(01)00171-7. [DOI] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22(7):2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Hippocampal function in cognition. Psychopharmacology (Berl) 2004;174(1):99–110. doi: 10.1007/s00213-004-1795-9. [DOI] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry. 2010;15(1):80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25(22):5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Bowers JM, Edwards NS, Jordan CL, McCarthy MM. Dysregulation of neonatal hippocampal cell genesis in the androgen insensitive Tfm rat. Horm Behav. 2013;64(1):144–152. doi: 10.1016/j.yhbeh.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M. Gender Differences in the Effects of Prenatal Stress on Brain Development and Behaviour. Neurochemical Research. 2007;32(10):1730–1740. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- Yang J, Harte-Hargrove Lauren C, Siao C-J, Marinic T, Clarke R, Ma Q, Jing D, LaFrancois John J, Bath Kevin G, Mark W, Ballon D, Lee Francis S, Scharfman Helen E, Hempstead Barbara L. proBDNF Negatively Regulates Neuronal Remodeling, Synaptic Transmission, and Synaptic Plasticity in Hippocampus. Cell Reports. 2014;7(3):796–806. doi: 10.1016/j.celrep.2014.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, Chen ZY, Mark W, Tessarollo L, Lee FS, Lu B, Hempstead BL. Neuronal release of proBDNF. Nat Neurosci. 2009;12(2):113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Konkle AT, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27(4):791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhang H, Cohen RS, Pandey SC. Effects of estrogen treatment on expression of brain-derived neurotrophic factor and cAMP response element-binding protein expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinology. 2005;81(5):294–310. doi: 10.1159/000088448. [DOI] [PMC free article] [PubMed] [Google Scholar]