Abstract
Numerous studies have demonstrated differences between males and females in hippocampal structure, function and plasticity. There also are many studies about the different predisposition of males and females for disorders where the hippocampus plays an important role. Many of these reports focus on area CA1, but other subfields are very important also, and unlikely to be the same as area CA1 based on what is known. Here we review basic studies of male and female structure, function, and plasticity of area CA3 pyramidal cells of adult rats. The data suggest that the CA3 pyramidal cells of males and females are distinct in structure, function, and plasticity. These sex differences cannot be simply explained by the effects of circulating gonadal hormones. This view agrees with previous studies showing that there are substantial sex differences in the brain that cannot be normalized by removing the gonads and depleting peripheral gonadal hormones. Implications of these comparisons for understanding sex differences in hippocampal function and dysfunction are discussed.
Keywords: estradiol, testosterone, BDNF, androgen, gonadectomy, plasticity
I. Introduction: why consider sex differences in area CA3?
The hippocampus is a brain region that is classically considered to be critical to spatial memory, but has also been implicated in many other functions. Numerous sex differences in hippocampus have been described, with the majority of studies in area CA1 or the dentate gyrus (McLaughlin et al. 2009; McEwen 2010; Duarte-Guterman et al. 2015). There have also been a number of studies demonstrating differential effects of gonadal steroids on the various subfields of the hippocampus, also focusing primarily on area CA1 and the dentate gyrus (Hajszan et al. 2007; Woolley 2007; Duarte-Guterman et al. 2015). Although it is often assumed that all subfields of the hippocampus are similar to area CA1, this may not be true because of the differences in the circuitry (Andersen 1975) and divergent functional roles of the subfields (Kesner et al. 2004; Kesner 2007a; b).
Area CA3 is best known as the second station in the trisynaptic pathway that forms the major route of information flow in the hippocampus (Andersen 1975; Amaral and Witter 1995), with area CA1 being the final synapse (Figure 1A). The trisynaptic pathway is defined as the folllowing series of glutamatergic synapses: 1) from entorhinal cortical projection neurons to dentate gyrus granule cells, 2) from the dentate gyrus axons to area CA3 pyramidal cells, and 3) from area CA3 pyramidal cell axon collaterals to area CA1 (Figure 1A). As more information has accumulated about hippocampal circuitry, it has been shown that area CA3 may be more than just a relay station in the trisynaptic pathway. For example, the axons of area CA3 pyramidal cells project ‘back’ to the dentate gyrus, in the reverse direction to the trisynaptic pathway (Figure 1B; (Scharfman 2007)). Area CA3 also receives afferent input from other sources besides the dentate gyrus such as direct input from the entorhinal cortex and extrahippocampal pathways such as the septum (Figure 1C; (Witter 2007). It also projects to the contralateral CA1 and CA3 region (Witter 2007). Based on this connectivity, area CA3 could be considered as a “hub” or central station of the hippocampal circuit, rather than a second leg of the trisynaptic pathway (Figure 1D; (Scharfman 2007). This view underscores the importance of area CA3 and potential for it to be distinct from area CA1. The circuitry also suggests that sex differences in area CA3 could cause - or at the very least, influence - those in area CA1 and the dentate gyrus, placing even greater importance on understanding sex differences in area CA3.
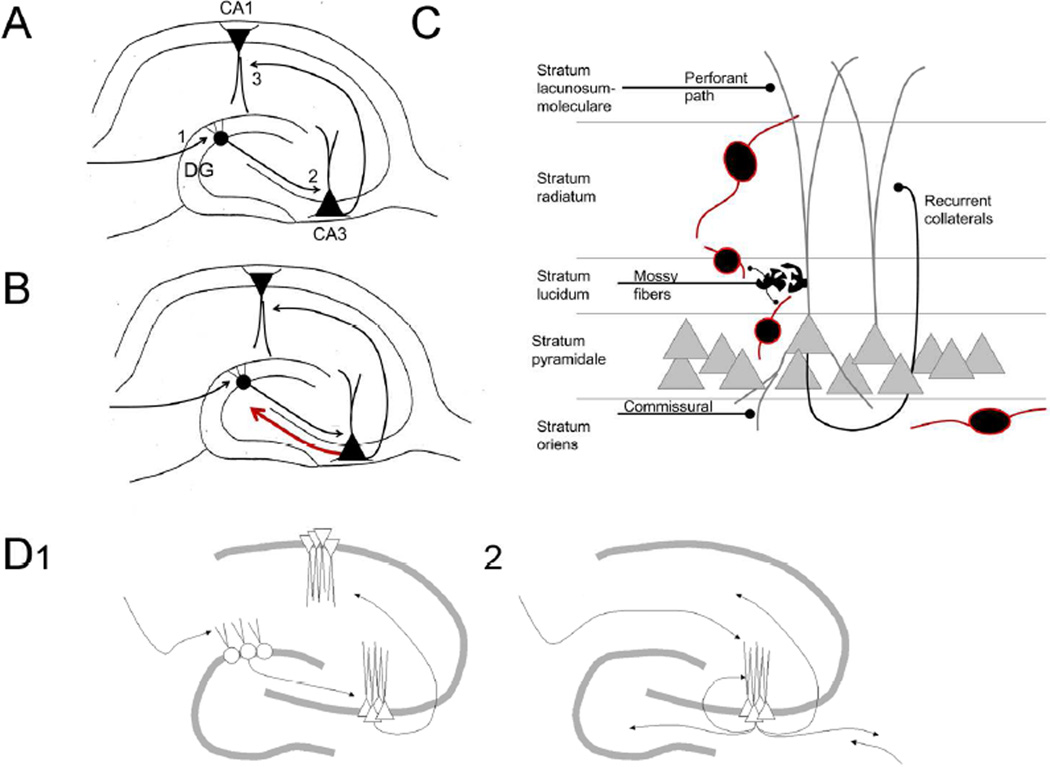
Figure 1.
Organization of area CA3.
A. A schematic illustrates the trisynaptic circuit. The first synapses is the entorhinal cortical projection called the perforant path which synapses on the outer two-thirds of the dentate gyrus granule cells. The second synapses is the mossy fiber axons of granule cells which innervate proximal dendrites of CA3 pyramidal cells. The third synapse is from area CA3 pyramidal cells which have Schaffer collateral axons that innervate the apical dendrites of CA1 pyramidal cells. DG= dentate gyrus.
B. There is a backprojection from area CA3 pyramidal cells to the DG (red). Axon collaterals of the pyramidal cells innervate neurons in the hilus as well as processes of cells with somata in other parts of the dentate gyrus. In ventral hippocampus there is a projection to granule cell proximal apical dendrites(Li et al. 1994).
C. The microcircuitry of area CA3 is shown in a simplified view. Pyramidal cells receive input from the perforant path in their distant apical dendrites, recurrent collaterals from other pyramidal cells in the central portion of the apical dendrite, and mossy fiber input proximally. Mossy fiber boutons have specialized ‘giant’ bouton with extension that innervate GABAergic neurons (black cells surrounded by red). The mossy fiber boutons contact complex spines on pyramidal cells. Additional input is from contralateral and cholinegic nuclei such as the septum; additional neuromodulatory input is also present. The GABAergic neurons are only a subset of those that are present (Freund and Buzsaki 1996).
The major inputs to area CA3 are shown in Figure 1C. They include the entorhinal cortical afferents from layer II which terminate on the distal dendrites of CA3 pyramidal cells in stratum lacunosum-moleculare. The granule cell axons (mossy fibers) from the dentate gyrus terminate on the proximal part of the apical dendrites of CA3 pyramidal cells in stratum lucidum (Figure 1C). These terminals and postsynaptic spines (mossy fiber synapses) are unique within the hippocampus because they are much larger, more complex, and contain numerous glutamatergic vesicles and postsynaptic densities (Blackstad and Kjaerheim 1961; Chicurel and Harris 1992). Axons from neurons in the contralateral hippocampus (commissural inputs) and cholinergic fibers innervate stratum oriens as well as other areas (Frotscher and Leranth 1985; Witter 2007); Figure 1C).
Functions of area CA3 are diverse and in some ways they are distinct from both area CA1 and other parts of the hippocampus (area CA2, the dentate gyrus). Some of these functions have been attributed to the recurrent collateral system of area CA3, which are the axon collaterals of CA3 pyramidal cells that form synapses in stratum radiatum on the apical dendrites of other area CA3 pyramidal cells (Figure 1C). These recurrent synapses are common in area CA3 (Witter 2007; Le Duigou et al. 2014) but sparse in area CA1 and the dentate gyrus, although there is some recurrent circuitry among the principal cells of area CA1 (pyramidal cells; (Thomson and Radpour 1991)) and the dentate gyrus (granule cells; (Molnar and Nadler 1999). This has led to the idea that area CA3 is an autoassociative network with the capacity to associate features of episodic memory such as objects and place, and store those memories so that they can be accurately retrieved at a later time (McNaughton 1991; Papp et al. 2007; Rolls 2013; Renno-Costa et al. 2014).
The circuitry, taken together with ideas about an autoassociative function in CA3, has led to the idea that memories that are generated from entorhinal cortical and mossy fiber inputs, influenced by neuromodulators such as acetylcholine (Hasselmo 1995), are stored in the recurrent collateral network. Retrieval also occurs by activating this network. Synaptic mechanisms for storage are generally attributed to long-term potentiation (LTP) at recurrent synapses (Le Duigou et al. 2014), which is promoted by overlapping activity in the entorhinal and mossy fiber inputs (Song et al. 2000; Derrick 2007; Nolan et al. 2011).
One common phrase that captures the functions of the dentate gyrus and area CA3 is ‘pattern separation and completion.’ Pattern separation is thought to be a function of granule cells, which processes diverse patterns of entorhinal input and then presents the processed form to area CA3 by the mossy fibers (Leutgeb and Leutgeb 2007; Kesner and Rolls 2015; Knierim and Neunuebel 2016). In this way, the dentate gyrus is a so-called ‘pre-processor’ for area CA3 (Myers and Scharfman 2009; 2011). Pattern completion reflects the ability of area CA3 to retrieve memories even if a partial set of the original entorhinal and mossy fiber inputs occur (Leutgeb and Leutgeb 2007; Kesner and Rolls 2015; Knierim and Neunuebel 2016). This would be analogous to first memorizing a picture of a flower, and then recalling the flower later even by a part of the original picture. If these ideas are correct, area CA3 has very important functions in laying down episodic memories and retrieving them accurately.
Area CA3 is also the generator of hippocampal sharp waves (SPWs), large synchronous discharges of CA3 pyramidal cells (Buzsaki 1986; 1989). SPWs occur in rodents during behavioral arrest or sleep (Buzsaki 1986).. and are associated with high frequency oscillations called ripples (Buzsaki et al. 1992). SPWs and ripples (SPW-Rs) have been proposed to be critical to hippocampal memory because they lead to a high frequency burst of activity in CA1 leading to LTP, and ultimately LTP in cortical structures that are downstream of area CA1 (Buzsaki 1989). This is theoretically important because it allows short-term memory formed in hippocampal circuits to be stored long-term in downstream (cortical) structures (Buzsaki 1989). Thus, SPW-Rs are important to the consolidation and storage of memory. An important element of the idea that SPW and ripples contribute to memory consolidation is based on recordings in rodents showing that the ensembles of pyramidal cells that are active in the awake state repeat their activity patterns during SPWs in immobility and sleep (Foster and Wilson 2006; Karlsson and Frank 2009). Notably, almost all of the work to date about SPWs has been conducted in males.
II. Understanding sex differences in area CA3
A. Background
There is a good reason to consider sex differences in area CA3: the receptors for gonadal hormones are robust. In rodents, the receptor distribution has been characterized with immunocytochemical methods. Many of these receptors are located in the layer where mossy fiber synapses are located, or in the mossy fiber axons.
Androgen receptors are detectable immunocytochemically in an extraordinarily high proportion (67%) of axons in the stratum lucidum of CA3. This compares to 11–13% in the stratum oriens and stratum radiatum of CA3, and 10–12% in CA1 (Tabori et al. 2005). A smaller proportion (3–15%) of synapses, dendritic shafts and spines are also AR-immunoreactive in CA3, consistent with the view that androgens may be able to affect synaptic transmission in CA3 by acting at more than one component of the network.
With respect to estrogen, the mossy fiber pathway contains ERα and ERβ immunoreactivity (Torres-Reveron et al. 2009). ERβ immunoreactivity is found in some dynorphin-labeled mossy fiber terminals, in association with the plasmalemma and small synaptic vesicles. Although ERα immunoreactivity is not detectable in dynorphin-labeled mossy fiber terminals, some dynorphin-labeled terminals appear to synapse on ERa-labeled dendritic spines, suggesting that the pre- and post-synaptic elements of the mossy fiber-CA3 pathway may be regulated via different ER populations (Torres-Reveron et al. 2009).
Although we and others have focused on the potential for steroid effects on the pathways intrinsic to the hippocampus, it is also important to remember that these pathways are also regulated by afferent inputs from elsewhere in the brain – and these inputs may be sexually differentiated, as well as targets for the activational effects gonadal steroids. Stimulation of neurogenesis in the dentate gyrus of female rats appears to be mediated via afferent serotonergic input from the raphe nuclei (Banasr et al. 2001), possibly via activation of the estrogen receptors present in these nuclei (Leranth et al. 1999). The basal forebrain cholinergic system is also critical for the neurotrophic effects of gonadal steroids on the hippocampus. More than 40 years ago, Luine at al demonstrated significant differences between males and females in the regulation of choline acetyl transferase in the basal forebrain of rats (Luine et al. 1975; Luine et al. 1986). Selective destruction of forebrain cholinergic neurons using 192 IgG-saporin inhibits the effects of estradiol on both spine synapse density in area CA1 (Lam and Leranth 2003)) and neurogenesis in the dentate gyrus (Mohapel et al. 2005), consistent with the hypothesis that sub-cortical cholinergic afferents may provide a critical contribution to the estrogenic regulation of hippocampal plasticity. Intriguingly, the effects of androgen on hippocampal synaptic plasticity in the male appear to be less sensitive to transaction of sub-cortical afferents than is the case for estradiol in females (Kovacs et al. 2003; Mendell et al. 2013), consistent with the hypothesis that these afferents may be quantitatively less important in the hippocampal neurotrophic effects of androgens in males. Males may also differ from females in the mechanisms regulating neurogenesis: in males, survival of new neurons in the dentate gyrus appears to be androgen receptor-dependent (Hamson et al. 2013). Finally, recent work has demonstrated a sex difference in the effects of gonadal steroids on NeuN expression in cell bodies of the entorhinal cortex (Scharfman and MacLusky 2014c), raising the possibility that perforant path afferent input to the hippocampus may also be sexually differentiated.
B. Physiology
To understand how males and females might differ in area CA3, we began with a simple question: could one detect differences between females and males in intact adult rats using recordings made in hippocampal slices prepared acutely from these animals. These studies recorded responses of CA3 pyramidal cells to stimulation of the mossy fiber pathway because it is a projection to area CA3 that can be fairly well isolated (i.e., activated by a stimulating electrode without exciting other pathways) in hippocampal slices (Skucas et al. 2013). This is important because there are few pathways that can be activated selectively in area CA3, because axons and dendrites overlap extensively. Because of the lamination in the hippocampus, the mossy fiber synapses are in a lamina that is separate from the cell bodies, making it possible to use extracellular recordings to sample activity at the synapse and soma. Recordings at the mossy fiber synapses reflect the average EPSP generated in CA3 neurons (field EPSP), whereas recordings in the cell layer reflect the summed activity of the action potentials generated by the EPSPs, called population spikes. Current source density analysis of responses recorded along the CA3 somatodendritic axis can provide a verification of the location of synapses (current sinks) underlying the field potentials, as well as information about disynaptic pathways such as the recurrent collaterals activated after the mossy fiber excitation of pyramidal cells (Skucas et al. 2013).
With this approach we were intrigued to find that there were sex differences in the responses to mossy fiber stimulation and recurrent collaterals in area CA3 when adult male and female rats were studied (summarized below). This suggests that there are sex differences in the hippocampus even when isolated from the rest of the body and therefore the other sites of hormone synthesis and action. They are consistent with sex differences in structure from animals that were acutely perfused (also summarized below), supporting the idea that even at one point in time the sex differences are sufficiently robust that they can be documented.
The first study was done only in males and only looked at the population spike (Scharfman 1997). Later studies were conducted with similar animals, breeding, housing, experimental equipment and other conditions (Scharfman et al. 2003; Scharfman et al. 2007; Skucas et al. 2013), so they are compared here, although it is acknowledged that head-to-head comparisons of all experimental groups were not made and this is a limitation of the comparisons. Females were distinct from males, but the sex difference depended on the stage of the estrous cycle (Figure 2). The comparisons included males and females that were intact or gonadectomised (orchidectomy in males, ovariectomy in females) at 2–3 months of age, and females that were examined mid-morning of each day of the 4-day estrous cycle (proestrus, estrus, metestrus or diestrus 1, and diestrus 2; Figure 3). Differences were robust when males were compared to female rats that were used mid-morning of proestrus or estrus, stages of the cycle where circulating levels of estradiol are at their peak (proestrous morning) or 24 hrs later, after estradiol levels have returned to baseline and the serum levels of progesterone have just fallen from their peak (estrous morning; Figures 2, 3).
Figure 2.
A summary of the physiological findings of rat pyramidal cells and their relationship to mossy fiber BDNF protein expression.
Hippocampal slice physiology and immunocytochemical observations are summarized for adult male rats, either intact or orchidectomized or females at different stages of the estrous cycle and after ovariectomy. MF= mossy fiber. Evoked-response: response recorded in stratum lucidum or pyramidale of area CA3 after a stimulus to the mossy fiber pathway. Experimental procedures were standardized as described previously (Skucas et al. 2013). Epileptiform = whether mossy fiber stimulation evoked repetitive field potentials (hyperexcitability) in response to a 10 second 1 Hz train of paired pulses (Scharfman et al. 2003; Scharfman et al. 2007). BDNF effect = the physiological effect on a mossy fiber evoked-response of superfused BDNF (Scharfman 1997; Scharfman et al. 2003). BDNF protein = Relative expression of BDNF protein by immunocytochemistry (Scharfman et al. 2003; Scharfman et al. 2007). nt = not tested. The micrographs at the bottom are from previous studies (Scharfman et al. 2003) or are unpublished.
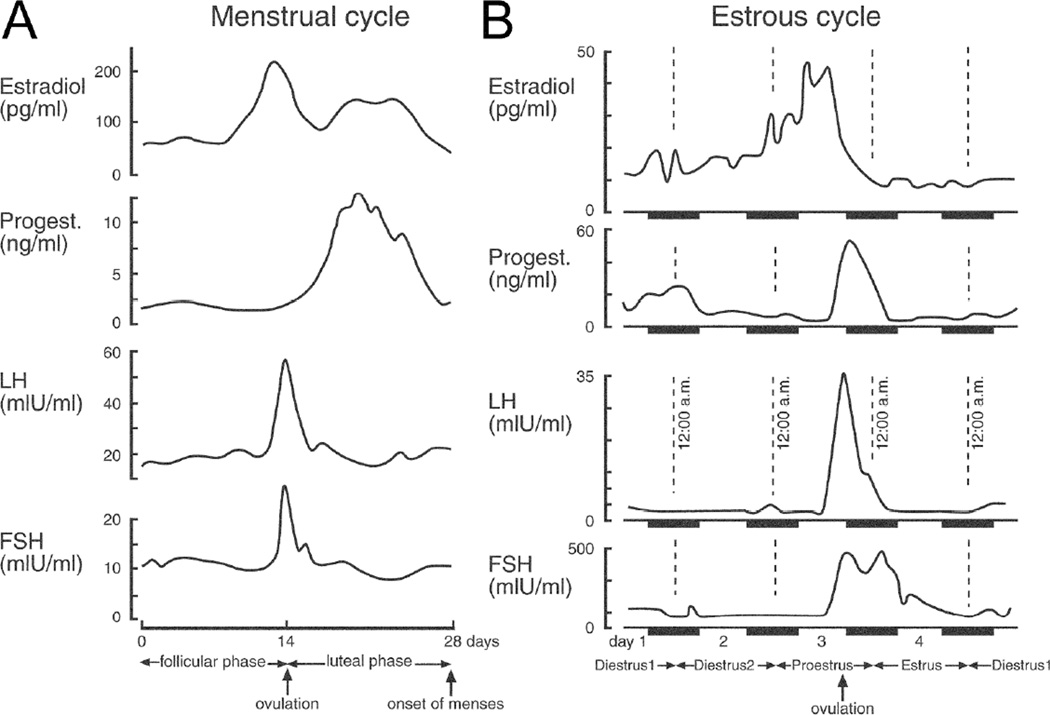
Figure 3.
The menstrual and estrous cycles.
A. The menstrual cycle of women is illustrated schematically. Progest. = progesterone. LH = luteinizing hormone. FSH = follicle stimulating hormone. From (Scharfman and MacLusky 2006).
In the first studies, females showed larger mossy-fiber evoked population spikes on the mornings of proestrous and estrous relative to males, metestrous females, and ovariectomized females (Scharfman 1997; Scharfman et al. 2003). (Scharfman 1997; Binder et al. 2001; Scharfman et al. 2003; Scharfman 2005). These studies suggested that there was a sex difference that biased transmission to be higher in females than males, if the cycle stage was proestrous or estrous morning. When studies of mossy fiber-evoked EPSPs (field EPSPs) were made, the largest field EPSPs were evoked in males compared to females that were evaluated on the mornings of proestrus, estrus, metestrus or diestrus 2 (Harte-Hargrove et al. 2015).
These data suggested that males have strong mossy fiber synapses relative to females, meaning the peak response evoked by mossy fiber stimulation in CA3 pyramidal cell was greater in males (Harte-Hargrove et al. 2015). However, females could show greater spike output by pyramidal cells on proestrous and estrous mornings, presumably because of factorssuch as disinhibition of the somata of pyramidal cells by reduced GABAergic inhibition at these times of the estrous cycle. For example, a disinhibition of the soma by weakened GABAergic input could allow a relatively small mossy fiber EPSP generated in a female to reach action potential threshold. In contrast, even if the EPSP was large as it could be in a male, an action potential might not be elicited if there was strong GABAergic inhibition of the soma. Because estradiol, progesterone, and testosterone, as well as their metabolites alter GABAergic inhibition in hippocampus (Smith et al. 2007; Maguire and Mody 2009; Huang and Woolley 2012; Reddy 2014; Tabatadze et al. 2015), this mechanism is an attractive explanation.
These experiments examined effects of single stimuli. In addition, paired stimuli were used to examine paired-pulse facilitation, a reflection of short-term plasticity, which is particularly large in the mossy fiber pathway (Nicoll and Schmitz 2005; McBain 2008). Post-tetanic potentiation (PTP) is another example of short-term plasticity and lasts minutes after a high frequency train (Zucker and Regehr 2002). Although no sex differences were revealed in paired-pulse facilitation, males tended to have greater facilitation, and males had significantly greater PTP (Harte-Hargrove et al. 2015). When LTP was compared, male LTP was weak compared to females, using a protocol that showed robust LTP in proestrous females (Harte-Hargrove et al. 2015). The robust LTP in proestrous females was in part due to trafficking of delta opioid receptors to the mossy fiber synapses on proestrous morning (Harte-Hargrove et al. 2015). Taken together, the data suggest that the mossy fiber synapse of males supports greater short-term plasticity than females, but females have a lower threshold for LTP than males. Some of these sex differences were due to the effects of estradiol on opioid receptor distribution in CA3 pyramidal cells (Harte-Hargrove et al., 2015).
The implications of these differences are interesting to consider in the context of theories about pattern separation and completion. One of the prevailing views is that mossy fibers are a “teaching input” to strengthen the ability of entorhinal input to be stored in area CA3 pyramidal cell synapses (for discussion of the ‘teaching input” see (Myers and Scharfman 2011)). This view rests on the evidence that the mossy fiber synapse is strong compared to other hippocampal synapses, meaning it evokes a very large excitatory event in the postsynaptic cell (Scharfman et al. 1990; von Kitzing et al. 1994; Henze et al. 2000; Jaffe and Gutierrez 2007), The strength of mossy fiber synapses is in part because of glutamatergic vesicles are densely concentration in the bouton, and the quantal size is large (Henze et al. 2000). As a result, a single presynaptic action potential can lead to a large EPSP in the postsynaptic pyramidal cell (von Kitzing et al. 1994; Henze et al. 2000; Jaffe and Gutierrez 2007). If mossy fibers in males have the greatest ability to excite CA3 pyramidal cells, the ability of mossy fibers to strengthen entorhinal synapses would be greater. However, if LTP of the mossy fibers has a lower threshold in females, the mossy fibers may have a stronger effect long-term compared to the male. Here the lower threshold in females is only on the proestrous and estrous mornings of the estrous cycle - diestrous females and ovariectomized females mossy fiber strength had weaker plasticity like males (Scharfman et al. 2003; Scharfman et al. 2007; Harte-Hargrove et al. 2015). The prediction is that female memory storage in area CA3 during proestrous and estrous mornings would be strong relativel to males and diestrous or ovariectomized females.
These views of area CA3 are based on a relatively focused group of studies with limitations. For example, inferences from gonadectomized rats about intact rats should be made with caution. The effects of hippocampal-derived steroids should be considered. Many other factors may make area CA3 more or less able to encode new memories. However, there is evidence to support some of the predictions. For example, the opioid antagonist naloxone was shown to decrease the preference of proestrous femals for the novel arm of the Y maze (Farhadinasab et al. 2009). Injection of the delta opioid receptor antagonist naltriben into the hippocampus of females impaired conditioned place preference (Billa et al. 2010).
The relatively high threshold for mossy fiber LTP of males relative to proestrous females described above could be related to low levels of BDNF protein in the mossy fibers of males compared to females, because BDNF is normally expressed at relatively high levels in females on proestrous and estrous morning, and BDNF potentiates mossy fiber transmission (Gomez-Palacio-Schjetnan and Escobar 2008; Harte-Hargrove et al. 2013; Schildt et al. 2013; Scharfman and MacLusky 2014b); Figure 2). Furthermore, the relatively strong mossy fiber BDNF protein expression and mossy fiber-evoked responses of proestrous and estrous mornings of the estrous cycle are reduced by adding an inhibitor of TrkB (Scharfman et al. 2003), the principal receptor for BDNF in the adult brain (Figure 2). Although the main reason for high mossy fiber BDNF protein in females has been attributed to estradiol acting at an estrogen-response element on the BDNF gene, or actions that increase activity-dependent BDNF protein synthesis, progesterone may also enhance BDNF synthesis and androgen appears to keep mossy fiber BDNF protein relatively low (Scharfman et al. 2003; Scharfman et al. 2007; Harte-Hargrove et al. 2013; Skucas et al. 2013; Scharfman and MacLusky 2014b). The sensitivity of mossy fiber transmission to TrkB antagonism also increases after orchidectomy (Skucas et al. 2013).
These data suggested that while males and females differ in mossy fiber structure and function - they are not polar opposites. However, all the studies discussed above were based on experiments using gonadally intact animals, which meant that the observations could have been due to intrinsic sex differences or differential effects of circulating gonadal hormones present in each sex. To determine the effects of gonadal hormones, we performed further studies in gonadectomized animals to ask whether gonadectomized males (orchidectomized males) might be analagous to gonadectomize females (ovariectomized females).
Adult male rats were orchidectomized and examined at a several times after orchidectomy, like female rats that were ovariectomized. Housing, diet, time of day of the experiments, and other important factors (Scharfman and MacLusky 2014a; b) were similar. Orchidectomized male rats showed greater rather than reduced BDNF protein in the mossy fibers, higher not lower responses to mossy fiber stimulation, and other changes suggesting enhanced structure, function and plasticity after orchidectomy.
C. Structural comparisons
The studies described above used electrophysiology and immunocytochemistry of the mossy fiber pathway to understand sex differences and hormone action in area CA3. They suggested a large expansion of the mossy fibers after Gdx in males, but not females, and other aspects of neuronal structure were not investigated. To understand the structural changes in males and females better, a comparison was made of males and females using Golgi staining (Mendell et al. 2016). Many aspects of CA3 pyramidal cell structure were examined, including the mossy fiber pathway, to ask if Golgi analysis would confirm what had been previously shown with immunocytochemistry, that the mossy fiber pathway expanded after orchidectomy (described above). In addition, dendritic spine density, length and Sholl analysis of pyramidal cell dendrites were conducted. These experiments confirmed and extended the view that males and females are very different regardless of gonadectomy - they cannot be viewed as polar opposites regardless of the way the comparison is made (Mendell et al. 2016).
1. Comparing Intact males to orchidectomized males
Adult male rats were orchidectomized and compared to animals that experienced sham surgery. Golgi stained-sections were analyzed to address possible changes in the mossy fibers, as well as spine density and spine length of the apical dendrites, dividing the apical dendritic tree into three equally-spaced segments. Sholl analysis was used to examine the length of the apical dendrites in each of the divisions of the apical dendritic tree. Similar to the studies of the mossy fiber plexus with dynorphin immunostaining (Skucas et al. 2013), mossy fibers were significantly larger in the orchidectomized males compared to sham controls (Mendell et al. 2016). In addition, the dendritic lengths were significantly increased, especially in the middle third of the apical dendrites where the recurrent collateral synapses are located. These data support previous electrophysiological findings that this pathway was potentiated in orchidectomized males (Skucas et al. 2013). In addition, the total length of the apical dendritic tree was longer in orchidectomized males than sham rats (Mendell et al. 2016). In contrast, dendritic spine density and spine length did not differ extensively, but there was a significant reduction in spine density after orchidectomy (Mendell et al. 2016). These changes are summarized in Figure 4.
Figure 4.
Comparison of intact and gonadectomized male and female rats in area CA3. The structural changes that have been shown in the adult rat are highlighted (Mendell et al. 2016). Note that the recurrent collateral pathway and other aspects of structure also have not been analyzed.
A. In the female rat, there is a decrease in spines when intact females (1) are ovariectomized (2), but no detectable differences in mossy fiber axons or the dendritic length.
B. In the male rat, there are shorter dendrites than females in the intact condition (1), and after gonadectomy the dendrites lengthen (2). The mossy fiber projection expands, losing afferent specificity. The dendritic spine density declines but the total number of spines is similar, suggesting a redistribution.
2. Comparison of males to females
In parallel to the studies of males, ovariectomized females were compared to sham females (Mendell et al. 2016). The sham female groups included animals that were perfused mid-morning on either proestrus or metestrus. Interestingly, the differences in ovariectomized and sham females were mostly in spine density, not mossy fibers, nor dendritic length (Mendell et al. 2016); Figure 4). Ovariectomized females exhibited decreased spine density compared to sham females (Mendell et al. 2016) consistent with previous observations in females (Woolley 2007; Luine 2016). In contrast, dendritic spine length and Sholl analysis did not reveal differences in groups (Mendell et al. 2016). Therefore, the major differences in area CA3 pyramidal cells between intact and gonadectomized females were in pyramidal cell spine density, but the greatest changes between intact and gonadectomized males were in the mossy fiber axons and pyramidal cell dendritic length (Figure 4). The relatively small changes in spine density in males are consistent with previous studies in primates (Mendell et al. 2014).
III. Implications
These studies suggest that there are striking structural and functional sex differences in CA3 pyramidal cells of adult rats in their response to gonadectomy. These sex differences probably reflect fundamental differences between males and females, because orchidectomized males and ovariectomized females were distinct. This conclusion is supported by the striking effects of castration and testosterone replacement in early postnatal rats on adult CA3 pyramidal cell dendritic morphology (Isgor and Sengelaub 2003). In addition, there are also likely to be differential responses of the two sexes to circulating gonadal steroids. While effects of gonadal steroids in gonadectomized animals need to be tested more comprehensively, the available data suggest potential structure-function relationships that are intriguing and potentially important to hippocampal-dependent behavior.
A. Hormonal modulation of CA3 structure and neurotransmission
There were numerous differences in the mossy fibers between males and females. This was demonstrated most easily by the distinct effects of gonadectomy; loss of the testes in males led to facilitation and expansion of the mossy fiber pathway, whereas loss of the ovaries in the female led to weaker mossy fiber transmission.
These differences are consistent with a strong influence of estradiol and testosterone on mossy fiber physiology, with estradiol enhancing transmission and plasticity, in part because of an increase in BDNF protein in the mossy fibers, as discussed above. In contrast, testosterone appears to have a different effect, restricting the mossy fiber pathway to stratum lucidum and therefore confering greater afferent specificity. These effects appeared to be due to testicular synthesis of androgens acting at androgen receptors, because the testosterone metabolite dihydrotestosterone reversed effects (Skucas et al. 2013) but androstanediol, a metabolite acting at GABA receptors (Carver and Reddy 2013) did not.
Although in physiological studies, we have gone to great lengths to try to relate sex differences in hippocampal function to normal circulating hormone levels (Scharfman et al. 2007; Scharfman et al. 2003; Skucas et al. 2013), it remains to be determined whether replacement of the hormones removed by gonadectomy will reverse the observed effects. A specific question that has yet to be addressed is the extent to which local steroidogenesis may contribute to sex differences in hippocampal structure and function. This is a potentially very important factor. Studies from other laboratories have established that local steroidogenesis plays an essential role in the neuroendocrine regulation of reproductive physiology in females (Micevych and Sinchak 2008) as well as in sex differences in the control of hippocampal synapse formation (Fester et al. 2012; Fester and Rune 2015)). Moreover, testosterone is extensively metabolized to estradiol in hippocampus (Vierk et al. 2014). The data from gonadectomized rats nonetheless suggest that testosterone normally keeps the mossy fiber pathway restricted in space, weaker, and less plastic.
These effects may not necessarily be detrimental, in functional terms, because under some circumstances restricted transmission may be beneficial. For example, it is important that the mossy fiber pathway not overlap with the recurrent collateral pathways in the adjacent cell layer, stratum radiatum. The segregation of these pathways and others is one of the hallmarks of hippocampal network organization, widely considered essential for normal function. In the pyramidal cell layer the mossy fiber synapses would potentially excite CA3 pyramidal cells too much. . Indeed, in orchidectomized rats there was susceptibility to spreading depression (Skucas et al. 2013). Different types of hyperexcitability have been shown to impair cognition in rodents (Kleen et al. 2010; Hernan et al. 2014), and humans (Bakker et al. 2012; Kleen et al. 2013). Drugs which reduce hyperactivity, such as anticonvulsants, improve cognition (Bakker et al 2012)..
Regarding neuronal structure, the data suggest that dendritic spine density in females is substantially reduced by ovariectomy, while in males the loss of testicular hormones induces relatively little change in spine density but a much greater degree of plasticity in axonal and dendritic structure (Figure 4). In CA3, the mossy fibers and recurrent collateral pathways seemed most affected in the male, because there were increased numbers of fibers or dendritic processes mainly where the recurrent collateral pathway is located (Mendell et al. 2016). Consistent with this idea, both the mossy fiber and recurrent collateral pathways were stronger in orchidectomized males (Skucas et al. 2013).
B. Implications for function: pattern separation and completion
How gonadal steroid regulation of hippocampal structure may contribute to pattern separation and pattern completion remains to be established. It seems likely that the effects of gonadal steroids are significant, in view of the evidence that estradiol and testosterone affect neurogenesis in the dentate gyrus (Galea et al. 2013). Several studies have suggested that adult neurogenesis contributes to pattern separation (Clelland et al. 2009; Sahay et al. 2011; Johnston et al. 2016). Interestingly, a very recent study has reported subtle sex differences in pattern separation, male spatial strategy users outperforming female spatial strategy users when separating similar, but not distinct, patterns (Yagi et al. 2016). That adult neurogenesis might play different roles in such tasks in males and females is suggested by the fact that adult neurogenesis was positively correlated with performance on similar pattern trials during pattern separation in female spatial strategy users, but negatively correlated with performance in male idiothetic strategy users (Yagi et al. 2016). Further work will be necessary to determine the specific effects of genetic sex, as opposed to effects of gonadal steroids, in regulating these endpoints.
It is interesting to consider the implications of the findings described above in CA3 in pattern separation and completion Two specific aspects of our observations are particularly relevant: the increased transmission and plasticity of the mossy fibers in proestrous and estrous females compared to males and the increased transmission and plasticity of the recurrent collateral system in orchidectomized males.
Regarding the female, one would predict that on proestrous and estrous morning, when the mossy fibers appear to synthesize the maximum BDNF protein and have both stronger transmission as well as LTP than other cycle stages, females would exhibit a greater ability to encode new episodic memories because of enhanced mossy fiber transmission and recurrent collateral synapses. The larger mossy fiber excitatory effect would lead to a greater probability of CA3 pyramidal cell spike generation, and the enhanced recurrent collateral transmission would make it more likely for storage to occur. However, there also would be a risk of hyperexcitability, which would potentially be detrimental by interfering with the network at the time memories are formed. Support for this idea comes from studies of animals which are epileptic. In between seizures there is hyperexcitability and events which reflect this hyperexcitability called interictal spikes interrupt cognition (Kleen et al. 2010; Kleen et al. 2013). Therefore, for both males and females, there are aspects of the effect of gonadal steroids that are likely to improve cognition and other aspect that are likely to decrease its efficacy.
In males, the effects of androgens seem to improve the peak mossy fiber response but restrict plasticity. These seemingly conflicting effects are consistent with the data available in the literature showing relatively modest effects of androgens on cognitive behavior in malesThese studies examinedboth androgen deprivation and testosterone treatment. Androgen deprivation therapy for treatment of prostate cancer results in only relatively slight alterations in cognitive function, characterized by some loss of function in terms of visuomotor performance, but non-significant effects on attention/working memory, executive functioning, language, verbal and visual memory (McGinty et al. 2014). Likewise, studies on the effects of testosterone supplementation in men with mild cognitive impairment and low testosterone levels have revealed only modest improvements in verbal memory and symptoms of depression (Cherrier et al. 2015). Thus the mixed effects of orchidectomy on hippocampal structure and function in animals mirror the relatively small changes in cognitive function observed in men in response to changing testosterone levels.
C. Implications for function: hippocampal SPWs
Taken together the results suggest that intact females would have robust CA3 SPWs during the proestrous and estrous stages and some evidence exists to support this idea (Pearce et al. 2008; Pearce et al. 2010). This prediction would potentially facilitate memory storage in females. The reason for the sex differences may not only be related to hippocampal mechanisms - sex differences in the extrahippocampal regulation of sleep could play a role. For example, there is a greater impairment in cognitive function affected by sleep in women than men (Santhi et al. 2016). There are also pronounced sex differences in rates of insomnia with women, who experience problems more frequently than men (Zhang and Wing 2006).
In males, there may be robust SPWs after orchidectomy because of the stronger recurrent collateral pathway. However, there is no clear distinction of this kind in the behavioral studies of hippocampal SPWs or behavior. Instead, there are diverse effects of sex on behaviors that involve hippocampus. When considering CA3-dependent tasks, no systematic studies of sex or the effects of gonadectomy and hormone replacement have been reported to our knowledge.
D. Other implications
The results also suggest potential mechanisms that could explain changes in behavior during puberty, given that there are rapid fluctuations in circulating gonadal steroid levels in both sexes at puberty. One of the hallmarks of puberty is fluctuations in mood. The ventral dentate gyrus has been suggested to be important for the stability of mood and normal affective function (Sahay et al. 2007). One of the prinicipal arguments is that damage to the dentate gyrus, particularly the granule cells of the dentate gyrus that are born in adulthood (adult-born granule cells), leads to depressive-like behavior (Sahay et al. 2007). In addition, drugs that are used to treat depression have proliferative effects on adult-born neurons, and the antidepressant effects of the drugs are blocked by reducing adult-born neurons (Manev et al. 2001; Malberg and Duman 2003; Santarelli et al. 2003; Jiang et al. 2005). Given the primary output of the dentate gyrus is the mossy fiber pathway, it is possible that the mood fluctuations in puberty are due to the waxing and waning in dentate gyrus output resulting from the changes in mossy fiber structure and function.
Puberty is also a time when neurological and psychiatric diseases are first manifested. For example, obsessive-compulsive disorder often occurs first during puberty (Zohar 1999). It is most common in women, where it first occurs during the same year as menarche in approximately 22% of cases (Pigott 1998; Zohar 1999; Labad et al. 2005). One potential reason is that the high levels of estrogen that initially occur at puberty leads to an increase in BDNF and consequent alterations in the structure and function of circuits that control behavior. It is interesting that hyperactivity has been described in OCD based on functional neuroimaging studies (Maia et al. 2008), and one of the hallmarks of the increase in BDNF in hippocampus after elevated estradiol is hyperactivity (Scharfman et al., 2003).
Schizophrenia is most common in pubertal males, with the sex difference resolving at older ages (Abel et al. 2010). Also, there is evidence that the mossy fiber pathway is structurally deficient or abnormal in schizophrenia. For example, defects in the normal formation of the mossy fibers result from deletion of DISC1 (Faulkner et al. 2008), a gene that is defective in a subset of individuals with schizophrenia. In chronic schizophrenia, it has been shown by anatomical studies that there are fewer mossy fiber synapses (Kolomeets et al. 2007). Therefore, when androgens fall during puberty, mossy fiber structure could become critically impaired in an individual who otherwise did not yet develop deficiencies that were substantial enough to cause changes in behavior. The changes in behavior could be reflected by the first episode of schizophrenia. Females would be relatively resistant because their hormonal fluctuations caused by ovariectomy do not appear to change mossy fiber structure, at least based on the work discussed above in rodents (Mendell et al. 2016). Notably, we have studied male rats before and after puberty to determine whether the effects we found in postpubertal males pertained to pubertal ages. In a comparison of orchidectomized and sham male rats, animals that were orchidectomized at 30 days of age showed a large increased in BDNF immunoreactivity in the mossy fibers 2 months later, compared to age-matched males that had sham surgery and were examined 2 months later. The increase in BDNF was similar to the increase in BDNF in orchidectomized rats that had surgery at 2 months of age, after puberty (Figure 5).
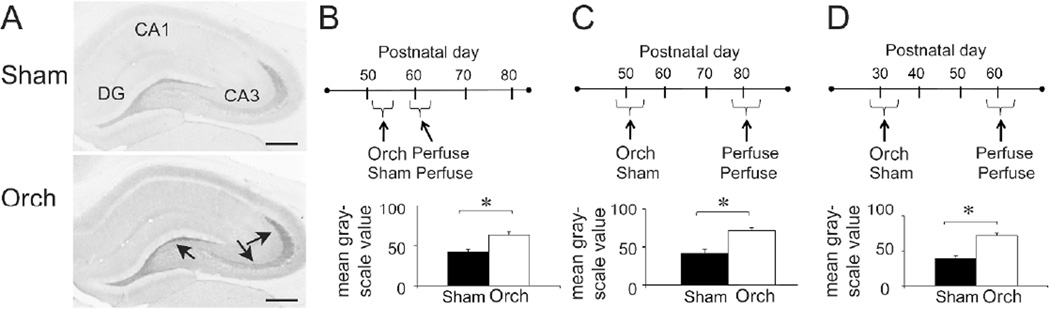
Figure 5.
Orchidectomized male rats increased BDNF immunoreactivity in the mossy fibers.
A-B. BDNF-immunoreactivity in a sham (A) and orchidectomized rat (B) using a polyclonal antibody to BDNF (Amgen-Regeneron partners). DG, Dentate gyrus. Arrows point to the mossy fibers. Calibration, 500 µm.
C-E. The experimental protocols and quantifications of BDNF immunoreactivity show that BDNF protein was relatively high in orchidectomized rat mossy fibers if the surgery was postpubertal and rats were examined 2 weeks later (C), 2 months after surgery (D) or rats were orchidectomized before puberty with a 2 month interval between surgery and BDNF immunocytochemistry (E). Asterisks denote statistical signficance (p<0.05).
Sprouted mossy fibers in area CA3 have been found in rodent models of temporal lobe epilepsy where there can also be robust sprouting in the dentate gyrus itself, in the inner molecular layer (Holmes et al. 1998; Cross and Cavazos 2007; Sutula and Dudek 2007; Buckmaster 2012). Therefore it is interesting that epilepsy often develops in puberty, especially the type of epilepsy where the hippocampus plays a role, temporal lobe epilepsy (IOM 2012). In acquired temporal lobe epilepsy, an early life infection or traumatic injury is thought to be a precipitating factor, but the epilepsy does not appear for decades later, either during puberty or afterwards (Scharfman and Pedley 2006). For those males who develop epilepsy in puberty, it may be that the fluctuations in androgens precipitate the first seizure because the changes in mossy fibers may cause the underlying pathology to reach a critical threshold of hyperexcitability. Orchidectomized rats not only showed increased mossy fibers but also increased predisposition to seizure-like events called spreading depression (Skucas et al. 2013), supporting the idea that falling androgens could precipitate seizures in a predisposed individual
An obvious caveat to this discussion is that the studies we have performed to date have not looked directly at the changes occurring during puberty, so the relationships discussed above must still be regarded as speculative. Additional work needs to be done to determine whether similar structural and functional relationships to circulating steroid levels exist in developing as well as aged animals. Nonetheless, the data suggest that studies about the actions of steroid hormones on the mossy fiber-CA3 system in experimental animals have the potential to improve our understanding of the fundamental neurobiology and mechanisms underlying neurological and psychiatric disorders, in both men and women.
IV. Summary and Conclusions
We have summarized several studies about area CA3 of the adult rat where we have compared the influence of sex and gonadal steroids on structure and function of the network. Regarding physiology, mossy fiber synapses appear to be more plastic in the female and able to elicit more firing of pyramidal cells at proestrous and estrous cycle stages, but the peak amplitude of the synaptic response to mossy fiber stimulation and paired-pulse facilitation is greater in males. These differences may help explain the basis for differences in behavior in vivo or disease.
We also found that gonadectomy of the female leads to alterations that are very different from gonadectomy of the male. In females, dendritic spines of CA3 pyramidal cells are remarkably altered by cycle stage and ovariectomy but in males, the mossy fiber and recurrent collateral synapses, and dendritic arbors were greatly affected by gonadectomy. These differences support the view that the male and female brain are differentiated at a very early stage of brain development and gonadal steroids that are active after puberty are not the only factor in shaping structure in area CA3.
Signfiicance.
Sex differences in the brain have been known for a long time, but there has been increased emphasis on their importance in recent years because of new mandates to include both sexes in research studies. Although welcome, studying sex differences in the brain is complex. One reason is that characteristics of the male and female brain are often not the same even if one removes the sex organs or sex hormones. A brain region where this point is well made is the CA3 subregion of the hippocampus, the focus of this review. We point out the ways that this subregion is influenced by sex and the potential implications for hippocampal function.
Acknowledgments
Supported by NIH (HES) and the NSERC (NJM).
Footnotes
Conflict of Interest statement:
The authors have no conflict of interests.
Author contributions:
Both authors wrote the manuscript.
References
- Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. International review of psychiatry. 2010;22:417–428. doi: 10.3109/09540261.2010.515205. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. San Diego: Academic Press; 1995. [Google Scholar]
- Andersen P. Organization of hippocampal neurons and their interconnections. In: Isaacson RL, Pribram KH, editors. The hippocampus. New York: Plenum; 1975. [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. European journal of neuroscience. 2001;14:1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- Billa SK, Xia Y, Moron JA. Disruption of morphine-conditioned place preference by a delta2-opioid receptor antagonist: Study of mu-opioid and delta-opioid receptor expression at the synapse. European journal of neuroscience. 2010;32:625–631. doi: 10.1111/j.1460-9568.2010.07314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Croll SD, Gall CM, Scharfman HE. BDNF and epilepsy: Too much of a good thing? Trends in neurosciences. 2001;24:47–53. doi: 10.1016/s0166-2236(00)01682-9. [DOI] [PubMed] [Google Scholar]
- Blackstad TW, Kjaerheim A. Special axo-dendritic synapses in the hippocampal cortex: Electron and light microscopic studies on the layer of mossy fibers. Journal of comparative neurology. 1961;117:133–159. doi: 10.1002/cne.901170202. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS. Mossy fiber sprouting in the dentate gyrus. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's basic mechanisms of the epilepsies. Bethesda (MD): 2012. [PubMed] [Google Scholar]
- Buzsaki G. Hippocampal sharp waves: Their origin and significance. Brain research. 1986;398:242–252. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Two-stage model of memory trace formation: A role for "noisy" brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: Regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology. 2013;230:151–188. doi: 10.1007/s00213-013-3276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier MM, Anderson K, Shofer J, Millard S, Matsumoto AM. Testosterone treatment of men with mild cognitive impairment and low testosterone levels. American journal of Alzheimer's disease and other dementias. 2015;30:421–430. doi: 10.1177/1533317514556874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicurel ME, Harris KM. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. Journal of comparative neurology. 1992;325:169–182. doi: 10.1002/cne.903250204. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DJ, Cavazos JE. Synaptic reorganization in subiculum and CA3 after early-life status epilepticus in the kainic acid rat model. Epilepsy research. 2007;73:156–165. doi: 10.1016/j.eplepsyres.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrick BE. Plastic processes in the dentate gyrus: A computational perspective. Progress in brain research. 2007;163:417–451. doi: 10.1016/S0079-6123(07)63024-6. [DOI] [PubMed] [Google Scholar]
- Duarte-Guterman P, Yagi S, Chow C, Galea LA. Hippocampal learning, memory, and neurogenesis: Effects of sex and estrogens across the lifespan in adults. Hormones and behavior. 2015;74:37–52. doi: 10.1016/j.yhbeh.2015.05.024. [DOI] [PubMed] [Google Scholar]
- Farhadinasab A, Shahidi S, Najafi A, Komaki A. Role of naloxone as an exogenous opioid receptor antagonist in spatial learning and memory of female rats during the estrous cycle. Brain research. 2009;1257:65–74. doi: 10.1016/j.brainres.2008.12.043. [DOI] [PubMed] [Google Scholar]
- Faulkner RL, Jang MH, Liu XB, Duan X, Sailor KA, Kim JY, Ge S, Jones EG, Ming GL, Song H, Cheng HJ. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proceedings of the National Academy of Sciences (USA) 2008;105:14157–14162. doi: 10.1073/pnas.0806658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fester L, Prange-Kiel J, Zhou L, Blittersdorf BV, Bohm J, Jarry H, Schumacher M, Rune GM. Estrogen-regulated synaptogenesis in the hippocampus: Sexual dimorphism in vivo but not in vitro. Journal of steroid biochemistry and molecular biology. 2012;131:24–29. doi: 10.1016/j.jsbmb.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Fester L, Rune GM. Sexual neurosteroids and synaptic plasticity in the hippocampus. Brain research. 2015;1621:162–169. doi: 10.1016/j.brainres.2014.10.033. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: A combined light and electron microscopic study. Journal of comparative neurology. 1985;239:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Gomez-Palacio-Schjetnan A, Escobar ML. In vivo BDNF modulation of adult functional and morphological synaptic plasticity at hippocampal mossy fibers. Neuroscience letters. 2008;445:62–67. doi: 10.1016/j.neulet.2008.08.069. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Milner TA, Leranth C. Sex steroids and the dentate gyrus. Progress in brain research. 2007;163:399–415. doi: 10.1016/S0079-6123(07)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamson DK, Wainwright SR, Taylor JR, Jones BA, Watson NV, Galea LA. Androgens increase survival of adult-born neurons in the dentate gyrus by an androgen receptor-dependent mechanism in male rats. Endocrinology. 2013;154:3294–3304. doi: 10.1210/en.2013-1129. [DOI] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Maclusky NJ, Scharfman HE. Brain-derived neurotrophic factor-estrogen interactions in the hippocampal mossy fiber pathway: Implications for normal brain function and disease. Neuroscience. 2013;239:46–66. doi: 10.1016/j.neuroscience.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Varga-Wesson A, Duffy AM, Milner TA, Scharfman HE. Opioid receptor-dependent sex differences in synaptic plasticity in the hippocampal mossy fiber pathway of the adult rat. Journal of neuroscience. 2015;35:1723–1738. doi: 10.1523/JNEUROSCI.0820-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation and cortical function: Modeling the physiological basis of behavior. Behavioural brain research. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: A review. Neuroscience. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Hernan AE, Alexander A, Jenks KR, Barry J, Lenck-Santini PP, Isaeva E, Holmes GL, Scott RC. Focal epileptiform activity in the prefrontal cortex is associated with long-term attention and sociability deficits. Neurobiology of disease. 2014;63:25–34. doi: 10.1016/j.nbd.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: Morphological and behavioral effects. Annals of neurology. 1998;44:845–857. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGlur-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instittutes of Medicine. Epilepsy across the spectrum: Promoting health and understanding. Washington DC: National Academies Press; 2012. [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. Journal of neurobiology. 2003;55:179–190. doi: 10.1002/neu.10200. [DOI] [PubMed] [Google Scholar]
- Jaffe DB, Gutierrez R. Mossy fiber synaptic transmission: Communication from the dentate gyrus to area CA3. Progress in brain research. 2007;163:109–132. doi: 10.1016/S0079-6123(07)63006-4. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, Zhang X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. Journal of clinical investigation. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston ST, Shtrahman M, Parylak S, Goncalves JT, Gage FH. Paradox of pattern separation and adult neurogenesis: A dual role for new neurons balancing memory resolution and robustness. Neurobiology of learning and memory. 2016;129:60–68. doi: 10.1016/j.nlm.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nature neuroscience. 2009;12:913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Reviews in the neurosciences. 2004;15:333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- Kesner RP. A behavioral analysis of dentate gyrus function. Progress in brain research. 2007a;163:567–576. doi: 10.1016/S0079-6123(07)63030-1. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learning & memory. 2007b;14:771–781. doi: 10.1101/lm.688207. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Rolls ET. A computational theory of hippocampal function, and tests of the theory: New developments. Neuroscience and biobehavioral reviews. 2015;48:92–147. doi: 10.1016/j.neubiorev.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Kleen JK, Scott RC, Holmes GL, Lenck-Santini PP. Hippocampal interictal spikes disrupt cognition in rats. Annals of neurology. 2010;67:250–257. doi: 10.1002/ana.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleen JK, Scott RC, Holmes GL, Roberts DW, Rundle MM, Testorf M, Lenck-Santini PP, Jobst BC. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology. 2013;81:18–24. doi: 10.1212/WNL.0b013e318297ee50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Neunuebel JP. Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics. Neurobiology of learning and memory. 2016;129:38–49. doi: 10.1016/j.nlm.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolomeets NS, Orlovskaya DD, Uranova NA. Decreased numerical density of CA3 hippocampal mossy fiber synapses in schizophrenia. Synapse. 2007;61:615–621. doi: 10.1002/syn.20405. [DOI] [PubMed] [Google Scholar]
- Kovacs EG, MacLusky NJ, Leranth C. Effects of testosterone on hippocampal CA1 spine synaptic density in the male rat are inhibited by fimbria/fornix transection. Neuroscience. 2003;122:807–810. doi: 10.1016/j.neuroscience.2003.08.046. [DOI] [PubMed] [Google Scholar]
- Labad J, Menchon JM, Alonso P, Segalas C, Jimenez S, Vallejo J. Female reproductive cycle and obsessive-compulsive disorder. Journal of clinical psychiatry. 2005;66:428–435. doi: 10.4088/jcp.v66n0404. quiz 546. [DOI] [PubMed] [Google Scholar]
- Lam TT, Leranth C. Role of the medial septum diagonal band of broca cholinergic neurons in oestrogen-induced spine synapse formation on hippocampal CA1 pyramidal cells of female rats. European journal of neuroscience. 2003;17:1997–2005. doi: 10.1046/j.1460-9568.2003.02637.x. [DOI] [PubMed] [Google Scholar]
- Le Duigou C, Simonnet J, Telenczuk MT, Fricker D, Miles R. Recurrent synapses and circuits in the CA3 region of the hippocampus: An associative network. Frontiers in cellular neuroscience. 2014;7:262. doi: 10.3389/fncel.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Horvath TL. Estrogen receptor-alpha in the raphe serotonergic and supramammillary area calretinin-containing neurons of the female rat. Experimental brain research. 1999;128:417–420. doi: 10.1007/s002210050863. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learning & memory. 2007;14:745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- Li XG, Somogyi P, Ylinen A, Buzsaki G. The hippocampal CA3 network: An in vivo intracellular labeling study. Journal of comparative neurology. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- Luine V. Estradiol: Mediator of memories, spine density and cognitive resilience to stress in female rodents. Journal of steroid biochemistry and molecular biology. 2016;160:189–195. doi: 10.1016/j.jsbmb.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Khylchevskaya RI, McEwen BS. Effect of gonadal steroids on activities of monoamine oxidase and choline acetylase in rat brain. Brain research. 1975;86:293–306. doi: 10.1016/0006-8993(75)90704-0. [DOI] [PubMed] [Google Scholar]
- Luine VN, Renner KJ, McEwen BS. Sex-dependent differences in estrogen regulation of choline acetyltransferase are altered by neonatal treatments. Endocrinology. 1986;119:874–878. doi: 10.1210/endo-119-2-874. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinology. 2009;34(Suppl 1):S84–S90. doi: 10.1016/j.psyneuen.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia TV, Cooney RE, Peterson BS. The neural bases of obsessive-compulsive disorder in children and adults. Development and psychopathology. 2008;20:1251–1283. doi: 10.1017/S0954579408000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: Reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Manev H, Uz T, Smalheiser NR, Manev R. Antidepressants alter cell proliferation in the adult brain in vivo and in neural cultures in vitro. European journal of pharmacology. 2001;411:67–70. doi: 10.1016/s0014-2999(00)00904-3. [DOI] [PubMed] [Google Scholar]
- McBain CJ. Differential mechanisms of transmission and plasticity at mossy fiber synapses. Progress in brain research. 2008;169:225–240. doi: 10.1016/S0079-6123(07)00013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: Mechanisms of neuronal remodeling. Annals of the New York Academy of Sciences. 2010;1204(Suppl):E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty HL, Phillips KM, Jim HS, Cessna JM, Asvat Y, Cases MG, Small BJ, Jacobsen PB. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: A systematic review and meta-analysis. Supportive care in cancer. 2014;22:2271–2280. doi: 10.1007/s00520-014-2285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Molecular neurobiology. 2009;40:166–182. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- McNaughton BL. Associative pattern completion in hippocampal circuits: New evidence and new questions. Brain research reviews. 1991;16:193–220. [Google Scholar]
- Mendell AL, MacLusky NJ, Leranth C. Unilateral fimbria/fornix transection prevents the synaptoplastic effect of dehydroepiandrosterone in the hippocampus of female, but not male, rats. Neuroscience & Medicine. 2013;4:134. [Google Scholar]
- Mendell AL, Szigeti-Buck K, MacLusky NJ, Leranth C. Orchidectomy does not significantly affect spine synapse density in the CA3 hippocampal subfield in st. Kitts vervet monkeys (chlorocebus aethiops sabaeus) Neuroscience letters. 2014;559:189–192. doi: 10.1016/j.neulet.2013.10.061. [DOI] [PubMed] [Google Scholar]
- Mendell AL, Atwi SM, Bailey CD, McCloseky DP, Scharfman HE, MacLusky NJ. Expansion of mossy fibers and CA3 apical dendritic length accompanies the fall in dendritic spine density after gonadectomy in male, but not female, rats. Brain structure and function. 2016 doi: 10.1007/s00429-016-1237-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Sinchak K. Synthesis and function of hypothalamic neuroprogesterone in reproduction. Endocrinology. 2008;149:2739–2742. doi: 10.1210/en.2008-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapel P, Leanza G, Kokaia M, Lindvall O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiology of aging. 2005;26:939–946. doi: 10.1016/j.neurobiolaging.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Molnar P, Nadler JV. Mossy fiber-granule cell synapses in the normal and epileptic rat dentate gyrus studied with minimal laser photostimulation. Journal of neurophysiology. 1999;82:1883–1894. doi: 10.1152/jn.1999.82.4.1883. [DOI] [PubMed] [Google Scholar]
- Myers CE, Scharfman HE. A role for hilar cells in pattern separation in the dentate gyrus: A computational approach. Hippocampus. 2009;19:321–337. doi: 10.1002/hipo.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CE, Scharfman HE. Pattern separation in the dentate gyrus: A role for the CA3 backprojection. Hippocampus. 2011;21:1190–1215. doi: 10.1002/hipo.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nature reviews neuroscience. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- Nolan CR, Wyeth G, Milford M, Wiles J. The race to learn: Spike timing and stdp can coordinate learning and recall in CA3. Hippocampus. 2011;21:647–660. doi: 10.1002/hipo.20777. [DOI] [PubMed] [Google Scholar]
- Papp G, Witter MP, Treves A. The CA3 network as a memory store for spatial representations. Learning & memory. 2007;14:732–744. doi: 10.1101/lm.687407. [DOI] [PubMed] [Google Scholar]
- Pearce P, Friedman D, Scharfman HE. Hippocampal sharp wave frequency (spw) fluctuates across the estrous cycle in the adult female rat. Soc for Neurosci Online Meeting Planner. 2008 [Google Scholar]
- Pearce P, Skucas VA, Scharfman HE. Hormonal status influences hippocampal eeg in the adult rat. Soc for Neurosci Online Meeting Planner. 2010 [Google Scholar]
- Pigott TA. Obsessive-compulsive disorder: Symptom overview and epidemiology. Bulletin of the Menninger Clinic. 1998;62:A4–A32. [PubMed] [Google Scholar]
- Reddy DS. Neurosteroids and their role in sex-specific epilepsies. Neurobiology of disease. 2014;72(Pt B):198–209. doi: 10.1016/j.nbd.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renno-Costa C, Lisman JE, Verschure PF. A signature of attractor dynamics in the CA3 region of the hippocampus. PLoS computational biology. 2014;10:e1003641. doi: 10.1371/journal.pcbi.1003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. A quantitative theory of the functions of the hippocampal CA3 network in memory. Frontiers in cellular neuroscience. 2013;7:98. doi: 10.3389/fncel.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Drew MR, Hen R. Dentate gyrus neurogenesis and depression. Progress in brain research. 2007;163:697–722. doi: 10.1016/S0079-6123(07)63038-6. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Santhi N, Lazar AS, McCabe PJ, Lo JC, Groeger JA, Dijk DJ. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proceedings of the National Academy of Sciences (USA) 2016;113:E2730–E2739. doi: 10.1073/pnas.1521637113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Kunkel DD, Schwartzkroin PA. Synaptic connections of dentate granule cells and hilar neurons: Results of paired intracellular recordings and intracellular horseradish peroxidase injections. Neuroscience. 1990;37:693–707. doi: 10.1016/0306-4522(90)90100-i. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Hyperexcitability in combined entorhinal/hippocampal slices of adult rat after exposure to brain-derived neurotrophic factor. Journal of neurophysiology. 1997;78:1082–1095. doi: 10.1152/jn.1997.78.2.1082. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: A potential role for brain-derived neurotrophic factor. The Journal of neuroscience. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Brain-derived neurotrophic factor (BDNF) and the dentate gyrus mossy fibers: Implications for epilepsy. In: Stanton PK, Bramham CR, Scharfman HE, editors. Synaptic plasticity and transynaptic signaling. New York: Springer; 2005. pp. 201–220. [Google Scholar]
- Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia. 2006;47:1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Pedley TA. Temporal lobe epilepsy. In: Gilman S, editor. The neurobiology of disease. New York: Academic Press; 2006. pp. 349–368. [Google Scholar]
- Scharfman HE. The CA3 "backprojection" to the dentate gyrus. Progress in brain research. 2007;163:627–637. doi: 10.1016/S0079-6123(07)63034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Hintz TM, Gomez J, Stormes KA, Barouk S, Malthankar-Phatak GH, McCloskey DP, Luine VN, Maclusky NJ. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. European journal of neuroscience. 2007;26:2595–2612. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Sex differences in the neurobiology of epilepsy: A preclinical perspective. Neurobiology of disease. 2014a;72(Pt B):180–192. doi: 10.1016/j.nbd.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Differential regulation of BDNF, synaptic plasticity and sprouting in the hippocampal mossy fiber pathway of male and female rats. Neuropharmacology. 2014b;76(Pt C):696–708. doi: 10.1016/j.neuropharm.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Sex differences in the neurobiology of epilepsy: A preclinical perspective. Neurobiology of disease. 2014c doi: 10.1016/j.nbd.2014.07.004. S0969-9961:00198-00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildt S, Endres T, Lessmann V, Edelmann E. Acute and chronic interference with BDNF/trkb-signaling impair LTP selectively at mossy fiber synapses in the CA3 region of mouse hippocampus. Neuropharmacology. 2013;71:247–254. doi: 10.1016/j.neuropharm.2013.03.041. [DOI] [PubMed] [Google Scholar]
- Skucas VA, Duffy AM, Harte-Hargrove LC, Magagna-Poveda A, Radman T, Chakraborty G, Schroeder CE, MacLusky NJ, Scharfman HE. Testosterone depletion in adult male rats increases mossy fiber transmission, LTP, sprouting in area CA3 of hippocampus. The Journal of neuroscience. 2013;33:2338–2355. doi: 10.1523/JNEUROSCI.3857-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA(a) receptors: Focus on the alpha4 and delta subunits. Pharmacology & therapeutics. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Miller KD, Abbott LF. Competitive hebbian learning through spike-timing-dependent synaptic plasticity. Nature neuroscience. 2000;3:919–926. doi: 10.1038/78829. [DOI] [PubMed] [Google Scholar]
- Sutula TP, Dudek FE. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: An emergent property of a complex system. Progress in brain research. 2007;163:541–563. doi: 10.1016/S0079-6123(07)63029-5. [DOI] [PubMed] [Google Scholar]
- Tabatadze N, Huang G, May RM, Jain A, Woolley CS. Sex differences in molecular signaling at inhibitory synapses in the hippocampus. The Journal of neuroscience. 2015;35:11252–11265. doi: 10.1523/JNEUROSCI.1067-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, Milner TA. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130:151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Radpour S. Excitatory connections between CA1 pyramidal cells revealed by spike triggered averaging in slices of rat hippocampus are partially NMDA receptor mediated. European journal of neuroscience. 1991;3:587–601. doi: 10.1111/j.1460-9568.1991.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Jacome L, Luine VN, Drake CT, McEwen BS, Milner TA. Hippocampal dynorphin immunoreactivity increases in response to gonadal steroids and is positioned for direct modulation by ovarian steroid receptors. Neuroscience. 2009;159:204–216. doi: 10.1016/j.neuroscience.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierk R, Brandt N, Rune GM. Hippocampal estradiol synthesis and its significance for hippocampal synaptic stability in male and female animals. Neuroscience. 2014;274:24–32. doi: 10.1016/j.neuroscience.2014.05.003. [DOI] [PubMed] [Google Scholar]
- von Kitzing E, Jonas P, Sakmann B. Quantal analysis of excitatory postsynaptic currents at the hippocampal mossy fiber-CA3 pyramidal cell synapse. Advances in second messenger and phosphoprotein research. 1994;29:235–260. doi: 10.1016/s1040-7952(06)80019-4. [DOI] [PubMed] [Google Scholar]
- Witter MP. Intrinsic and extrinsic wiring of CA3: Indications for connectional heterogeneity. Learning & memory. 2007;14:705–713. doi: 10.1101/lm.725207. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annual review of pharmacology and toxicology. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Yagi S, Chow C, Lieblich SE, Galea LA. Sex and strategy use matters for pattern separation, adult neurogenesis, and immediate early gene expression in the hippocampus. Hippocampus. 2016;26:87–101. doi: 10.1002/hipo.22493. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wing YK. Sex differences in insomnia: A meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- Zohar AH. The epidemiology of obsessive-compulsive disorder in children and adolescents. Child and adolescent psychiatric clinics of North America. 1999;8:445–460. [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annual review of physiology. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]