Abstract
Molecular imaging is playing an increasing role in the pre-treatment evaluation of low-grade gliomas. While glucose positron emission tomography (PET) can be helpful to differentiate low-grade from high-grade tumors, PET imaging with amino acid radiotracers has several advantages, such as better differentiation between tumors and non-tumorous lesions, optimized biopsy targeting and improved detection of tumor recurrence. This review provides a brief overview of single photon emission computed tomography (SPECT) studies followed by a more detailed review of clinical applications of glucose and amino acid PET imaging in low-grade hemispheric gliomas. We discuss key differences in the performance of the most commonly utilized PET radiotracers and highlight the advantage of PET/MRI fusion to obtain optimal information about tumor extent, heterogeneity and metabolism. Recent data also suggest that simultaneous acquisition of PET/MR images and the combination of advanced MRI techniques with quantitative PET can further improve the pre- and post-treatment evaluation of pediatric brain tumors.
Keywords: Pediatric glioma, low-grade, MRI, positron emission tomography, glucose, amino acid
1. Introduction
Neuroimaging is critical both as a first-line diagnostic modality and for detection of residual and recurrent tumors after initial treatment. Children with a suspected brain tumor often undergo an initial computerized tomography (CT) scan, which is followed by magnetic resonance imaging (MRI) in most cases. Most low-grade brain tumors are detected as hypointense hemispheric lesions with no or minimal mass effect, and variable enhancement on post-contrast MRI. Advanced imaging techniques, including perfusion MRI and MR spectroscopy (MRS) are increasingly used in pediatric brain tumor imaging to assist, among others, tumor grading and evaluation of tumor recurrence. Nuclear medicine techniques such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are not routine diagnostic modalities in pediatric brain tumor imaging; however, these techniques can provide clinically useful additional information in selected patient populations.
2. Principles of PET and SPECT imaging
PET scanning is a powerful technique to image and quantify various biological processes after injection of a radiolabeled compound. PET radiotracers are created by radiochemistry through labeling of molecules with a positron-emitting isotope generated by a cyclotron. Injected tracers undergo distribution and accumulation in various organs, including brain. Detection of the site and magnitude of this accumulation is based on the positron decay, resulting in generation of paired γ-photons that travel in opposite directions and are detected by the PET scanner. This is a key step that allows PET to have superior spatial resolution (typically around 5 mm on high-resolution clinical scanners), as opposed to SPECT, which uses radioisotopes with a longer half-life, but relies on detection of single photons; thus, its resolution is in the cm range.
A major advantage of PET over any other imaging modality is its versatility: by creating specific radiotracers, PET can image and measure virtually any biological functions and pathological processes, such as blood flow, transport, metabolic rate or receptor binding. While the number of potential PET tracers is almost infinite, 2-deoxy-2[18F]fluoro-D-glucose (FDG) remains the single most commonly utilized PET ligand in clinical practice, including tumor imaging. Accumulation of FDG is proportional with the transport and metabolism of glucose molecules in the imaged organ and tumor tissue. However, most low-grade brain tumors show low metabolism on FDG-PET and cannot be readily visualized by this technique [8]. An increasing number of non-FDG-PET tracers, mostly based on amino acid transport and metabolism, provide additional information regarding various tumor characteristics. Amino acid PET tracers, such as L-[methyl-11C]methionine (MET), O-(2-[18F]fluoroethyl)-L-tyrosine (FET), 18F-fluoro-l-dihydroxy-phenylalanine (FDOPA), or alpha[11C]methyl-L-tryptophan (AMT), are useful in assessing pediatric brain tumors, given the low uptake of these tracers in normal brain tissue, which results in a superior tumor-to–normal brain tissue contrast. 18F-labeled amino acid analogs have the advantage of longer half-life (110 min, as opposed to 20 min for 11C) thus making them more feasible for widespread clinical applications. All the commonly used amino acid PET tracers are transported to tumor tissue via the L-type amino acid transport system; however, their tumoral metabolism is different. Therefore, their performance in tumor imaging should be evaluated and compared to find the optimal tracer for the actual clinical application [reviewed by Juhasz et al. [16]; see advantages and limitations of each tracer in Table 1]. SPECT has been used primarily to provide an estimate of perfusion and cerebral blood flow, and its application in tumor imaging remained limited, particularly with the advent and development of advanced MRI techniques able to measure regional blood flow and blood volume by using clinical MRI sequences.
Table 1.
Comparison of amino acid PET tracers utilized for human brain tumor imaging (this table has been modified from Juhasz et al. [16]).
| PET tracer | MAIN ADVANTAGES | LIMITATIONS |
|---|---|---|
| MET | • Convenient synthesis • Widely tested in multiple centers • Better than FDG for low-grade tumors |
• 11C-labeled: 20 min half-life • Metabolized through multiple pathways • Studies mostly rely on SUVs, no kinetic analysis |
| FET | • 18F-labeled: 110 min half-life • Low uptake in inflammatory cells • Lack of metabolism, measures AA transport • Tracer kinetic analysis improves differentiation of glioma grades • Glioma recurrence vs. radiation injury differentiation excellent (≥90%) |
• Used in limited centers • Provides no information on intratumoral AA metabolism • Limited specificity for gliomas |
| FDOPA | • 18F-labeled: 110 min half-life • Measures AA transport • Better than FDG for differentiation of glioma recurrence vs. radiation injury |
• Brain tumor studies from a few centers • Most studies rely on SUVs, limited kinetic analysis • High physiologic uptake in striatum |
| AMT | • Measures tryptophan transport • Kinetic analysis can also estimate tryptophan metabolism via the immunosuppressive kynurenine pathway • Studies tested SUV, kinetic parameters • Can differentiate low-grade glioma types • Accurately differentiates recurrent glioma from radiation injury (90%) • Strong post-treatment prognostic value |
• 11C-labeled: 20 min half-life • Brain tumor studies from a single center |
Abbreviations:
AA = amino acid
AMT = 11C-alpha-methyl-L-tryptophan
FDG = 2-deoxy-2[18F]fluoro-D-glucose
FDOPA = 18F-fluoro-L-dihydroxy-phenylalanine
FET = O-(2-[18F]fluoroethyl)-L-tyrosine
MET = L-[methyl-11C]methionine
PET = positron emission tomography
SUV = standardized uptake value
3. SPECT studies of tumoral perfusion and amino acid transport
SPECT studies in pediatric oncology have been relatively scarce and most of them are dated in the late 80's and 90's, before the advent of advanced MRI techniques and widespread availability of PET imaging. Gamma-emitting isotopes used for evaluation of tumor-related perfusion changes include thallium-201 (201TI, half-life: 73 hours) and 99mTechnetium (99mTc, 6 hours half-life). Thallium uptake depends on cell membrane permeability, tumor type, regional blood flow and blood-brain barrier [4]. 99mTechnetium-labeled methoxyisobutylisonitrite (99mTc -MIBI) does not penetrate the intact blood-brain barrier and is taken up by the choroid plexus, nasopharyngeal tissue and scalp [2, 31]. In a comparative study of 19 children with brain tumor, 99mTc-MIBI could delineate the tumor borders better than 201TI based on the greater signal-to-noise ratio [31]. Sensitivity was 67% for both tracers with 91% specificity for thallium and 100% for MIBI.
In a prospective, comparative 201TI SPECT and MRI study in 24 pediatric brain tumors, 14 lesions showed thallium uptake [39]. In general, thallium SPECT underestimated tumor burden, as non-enhancing regions of the tumors were not thallium-avid. Thallium indices did not correlate with tumor type, tumor grade or biological aggressiveness. Based on this study, SPECT did not provide added clinical information to gadolinium-enhanced MRI. In a subsequent, comparative FDG-PET and 201TI-SPECT study of gangliogliomas, low-grade tumors showed hypo- or ametabolism on FDG-PET, and SPECT showed normal perfusion [19]. In contrast, high-grade gangliogliomas showed high activity on SPECT, while enhancement on CT and MRI showed a less tight relation to tumor grade suggesting that nuclear imaging may be more reliable for tumor grading than CT and MRI for presurgical treatment planning of gangliogliomas.
In a study of 20 children with brain tumors evaluated the clinical usefulness of 99mTc-MIBI SPECT [20]. Tumor type was verified by histopathology and progression of disease was determined clinically, when the patients showed clinical worsening. Only brain stem glioma, glioblastoma, pilocytic astrocytoma and choroid plexus carcinoma showed 99mTc-MIBI uptake, while other tumors, such as dysembryoplastic neuroepithelial tumor (DNET), ependymoma, craniopharyngeoma and medulloblastoma did not show any uptake on SPECT images. These findings suggested that variations of tracer uptake may be useful for differential diagnosis of certain tumor types.
L-3-[123I]iodo-α-methyl tyrosine (IMT)-SPECT is able to study amino acid transport in tumor tissue. In a study of children with pilocytic astrocytoma, IMT-SPECT suggested malignant features due to high uptake in 7 out of 16 cases, while FDG-PET showed high uptake in 7 of 10 patients [43]. These results indicated that both glucose and amino acid transport are often increased in these tumors despite the tumor's good prognosis. While IMT-SPECT has not been widely investigated for brain tumor imaging, the positron-labeled tyrosine analog FET became one of the most widely studied amino acid PET tracers for neuro-oncology applications in the last decade. These studies, along with studies using other PET radioligands, are summarized in the next sections.
4. PET imaging in pretreatment evaluation of pediatric gliomas
4.1. Differentiation of tumors vs. non-tumorous lesions
Due to low glucose metabolism, non-tumoral lesions that mimic brain tumors on MRI often cannot be accurately differentiated from low-grade gliomas by FDG-PET. PET imaging with amino acid derivative radiotracers are more useful in this respect due to the low background (normal brain) uptake. To test this, two recent pediatric studies evaluated the differentiating accuracy of amino acid PET in newly diagnosed brain lesions. In a study of 26 children (mean age: 13 years) FET-PET could differentiate neoplastic tissue (n=19, with a variety of histopathologic diagnosis, including 4 diffuse astrocytomas) from non-tumoral lesions with 77% accuracy (positive predictive value: 88%) when using a cutoff threshold of 1.7 maximum tumor-to-brain ratios [10]. A second study tested the value of FDOPA-PET combined with MRS in 27 children with suspected supratentorial infiltrative brain lesions [28]. Twenty-one of these lesions were gliomas and 6 non-neoplastic lesions. While specificity was similar between the two modalities (83%), MRS showed higher sensitivity (95%) than FDOPA-PET (76%), although the difference did not reach statistical significance. FDOPA uptake was evaluated by lesion-to-normal tissue ratios, and the overall accuracy of FDOPA was 78%, very similar to the accuracy reported in the FET-PET study above.
4.2. Assessment of tumor grade in newly diagnosed tumors
A common clinical application of PET imaging is the differentiation of low-grade from high-grade brain tumors, an important question with strong implications for treatment planning and prognosis. FDG-PET can be useful in this respect, as higher histological tumor grade is generally associated with higher FDG uptake. However, overlap of metabolic rates between tumor grades and hypermetabolic benign lesions such as pilocytic astrocytoma or choroid plexus papilloma, and, occasionally, ganglioglioma remain a significant pitfall of using FDG-PET for brain tumor grading [5, 25, 32]. Juvenile pilocytic astrocytoma is a highly vascularized tumor, which explains initial observations of both high FDG and methionine uptake in this tumor [11, 38]. Dynamic PET studies showed a steady tracer efflux after the initial uptake, suggesting leaky blood-brain barrier in these lesions [38].
In one of the largest pediatric studies to assess PET-based tumor grading in pediatric brain tumors, the performance of FDG-PET and regional cerebral blood flow (rCBF) estimated by H215O-PET was evaluated in 38 children (mean age: 8 years) with a variety of primary, grade 1-4 CNS tumors [6]. The mean values for “hotspot”/brain index were the highest in grade 4 (4.3) and lowest in grade 1 and 2 (1.3) tumors; thus, FDG uptake correlated reasonably with malignancy grading (r=0.72, p<0.01). Outliers in the low-grade group included a choroid plexus papilloma and pilocytic astrocytoma with a high mean index (3.3, higher than grade 3 tumors). Overall, a hotspot/brain index below 1.83 provided the best cutoff threshold, with lower values indicating low-grade tumors. FDG-PET/MRI co-registration facilitated correct diagnosis and grading. Notably, rCBF values on H215O-PET did not correlate with malignancy grade.
A few studies evaluated the use of amino acid PET for tumor grading in pediatric tumors. Utriainen at al. [41] compared the performance of MET-PET with FDG-PET in 27 patients (mean age: 9 years), including 17 with supratentorial brain lesions. The majority of the 19 low-grade tumors were grade 1 pilocytic astrocytomas or glioneuronal tumors. On visual assessment, FDG-PET seemed better than MET-PET to differentiate low-grade from high-grade tumors, because all but one tumor showed increased MET uptake. Quantitative analysis (with tumor/cortex ratios), however, demonstrated a significantly higher uptake for both tracers in high-grade tumors, although with a considerable overlap between tumor grades. Tumor proliferative index (evaluated by Ki-67 labeling) showed no association with tumoral SUVs or ratios. Interestingly, the uptake of both tracers increased with age both in cortex and in the tumors. In Cox regression, both high FDG and MET tumor-to-frontal cortex SUV ratios were associated with disease progression.
FDOPA is an amino acid radiotracer whose tumoral uptake is proportional to amino acid transport (as the tracer does not undergo tumoral metabolism except in tumors with aromatic amino acid decarboxylase activity). In a prospective pilot study of 13 children with supratentorial astrocytomas (including 5 low-grade tumors), FDOPA uptake showed a significant correlation with tumor grade [27]. All tumors with high FDOPA uptake showed intratumoral heterogeneity, and PET/MRI imaging fusion was helpful for selecting the optimal biopsy site within the non-enhancing tumor mass in two PET-positive cases. Importantly, in 5 patients, PET/MRI fusion also influenced treatment strategy; in 4 of these patients, lack of FDOPA uptake (Figure 1) prompted conservative treatment (watchful waiting) thus avoiding unnecessary surgery or chemotherapy.
Figure 1.
Comparison of fluid-attenuated inversion recovery (FLAIR) MR (A) and FDOPA-PET/MR fusion images (B) in a patient with a WHO grade 2 diffuse astrocytoma. FDOPA did not show any tracer uptake in this low-grade glioma (from Morana et al. [27]).
A potential limitation of FDOPA compared to other amino acid PET tracers is its specific uptake in the putamen and caudate nucleus (containing dopaminergic terminals) that may affect the ability to assess striatal tumor infiltration. This has been addressed by a recent study of 28 pediatric patients with newly diagnosed or recurrent glioma (grade 1-4) [29]. The authors showed that fused PET/MRI images provided higher accuracy than PET/CT images, with a perfect prediction of involvement of the dorsal striatum by PET/MRI. False negative fused PET/MRI results included two low-grade gliomas where mild increases of PET uptake were confined to tumor portions outside the striatum.
Beyond measuring static PET tracer uptake, analysis of amino acid tracer kinetics has the ability to differentiate tumor grades and predict tumoral proliferative activity with a higher accuracy. Dynamic PET imaging provides information on the temporal characteristics of radiotracer distribution and allows PET tracer kinetic analysis for separation of tumoral tracer transport and trapping; for some tracers, the source of some of the trapped radioactivity is from metabolites. For example, in the case of the tryptophan derivative AMT, the tracer may undergo metabolism via the serotonin and also by the immunosuppressive kynurenine pathway [7, 9,16]; the latter is commonly upregulated in a variety of tumors, including gliomas, glioneuronal tumors and meningiomas [1,3,7,42]. AMT was shown to have a differential uptake in a variety of gliomas [13]. Using tracer kinetic analysis, net tryptophan transport rates were also found to show a strong correlation with tumor proliferative activity in grade 2–4 gliomas [15]. This tight relation between tryptophan transport and tumor cell proliferation is likely due to increased tumor vascularity as well as upregulation of the L-type amino transporter in highly proliferative tumors, both of which can increase amino acid transport from the blood to tumor tissue.
4.3. PET-guided tumor biopsy
Brain tumors are often heterogeneous, with the tumor mass containing components with variable biological behavior. For example, a low-grade glioma may harbor areas where the histologic characteristics are more consistent with a high-grade tumor. Accurate pre-treatment identification of such high-grade components is essential for biopsy targeting. In a report of two children with a thalamic lesion, fusion images of FET-PET and MRS were used for image-guided biopsy [24]. In the first case, FET-PET revealed high uptake in the thalami despite normal peaks on MRS, and also a metabolically active left cerebellar lesion. Stereotactic biopsy from this cerebellar lesion revealed an anaplastic astrocytoma. In the other case, with a bilateral thalamic MRI lesion, FET-PET showed a heterogeneous uptake in the MRI-defined tumor mass, with higher uptake (tumor-to-cortex ratio 1.6) in the midline thalamic region and the left pulvinar (Figure 2). Stereotactic biopsy showed a low-grade diffuse astrocytoma. The study provided proof-of-concept data that stereotactic biopsy using metabolic imaging and image fusion can enhance the diagnostic yield in cases of diffuse pediatric gliomas disclosing unexpected `hot spots'.
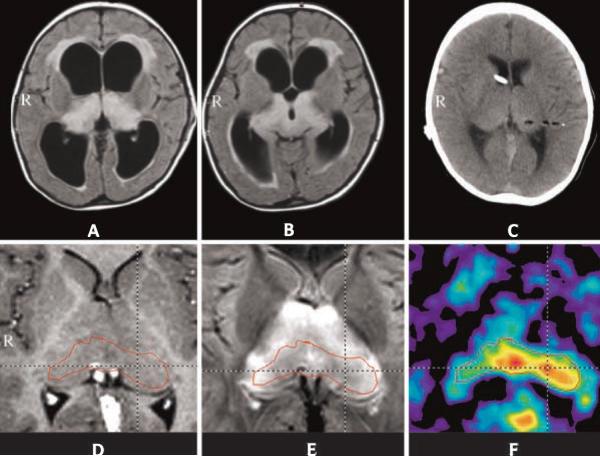
Figure 2.
MR and O-(2-[18F]fluoroethyl)-L-tyrosine (FET)-PET images of a 2-year old girl with bithalamic lesions, which appeared as hyperintense signals on FLAIR images (A, B). Postoperative CT scan (C) demonstrated the biopsy tract contrasting with air. MRI after shunting showed the thalamic region on the post-contrast T1-weighted image without pathologic enhancement (D) and symmetrical hyperintensity on the FLAIR sequence (E). The corresponding FET-PET demonstrated increased uptake (tumor/cortex ratio 1.6) in the dorsal midline of the bithalamic region as well as the left pulvinar. Histopathology indicated low-grade astrocytoma (from Messinger-Jünger et al. [24]).
In a subsequent, larger study of 37 pediatric brain tumors (including 11 low-grade tumors) inactive, active or highly active tumors were defined based on the maximum Choline/N-acetyl-aspartate (NAA) ratio on MR spectroscopic imaging and the highest tumoral glucose uptake on FDG-PET [12]. All tumors were metabolically heterogeneous, but high tumoral metabolic activity was observed more frequently on MRS compared to FDG-PET, although the agreement of tumor classification was weak between the two modalities. A voxel-wise comparison (to identify the area with the largest abnormal metabolism) showed an overlap in the majority of the studies (62%) (Figure 3). These findings suggested that FDG-PET and MRS detect similar, but not identical regions of tumor metabolic activity with limited agreement, thus, they provide complementary localizing information for the metabolically active tumor regions.
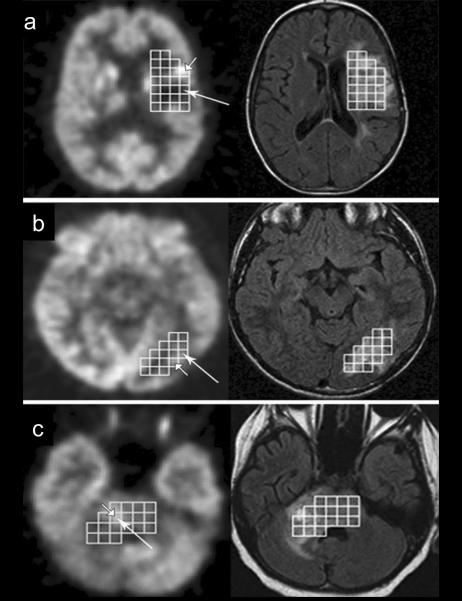
Figure 3.
Voxel-wise comparison of FDG-PET (left side) and FLAIR images (right side) with MR spectroscopic imaging voxels in 3 patients (a, b, c) with gliomas. Voxels with maximum choline/N-acetyl-aspartate (Cho/NAA) ratio are indicated by long arrows, while the brightest voxel with low Cho/NAA is shown by short arrow. a: No agreement between FDG-PET and MRS voxel selection. b and c: agreement between FDG-PET and MRS images. b: Selected voxels are adjacent to one another and c: the same voxel was chosen by both FDG-PET and MRS (from Hipp et al. [12]).
One of the largest pediatric brain tumor PET studies to date investigated the clinical impact of PET-guided stereotactic biopsy in children with a brain tumor using MET and FDG-PET in 85 children [35]. Fifty-five patients had a low-grade tumor and 35 children underwent PET-guided stereotactic biopsy. High MET uptake was found in 33 (focal uptake in 22 and extended in 11) of the 35 children (Figure 4). The biopsy included two trajectories in 23 of 35 cases. All PET-guided trajectories yielded tumor tissue. Non-diagnostic tissue samples were found in 7 cases, such as gliosis and necrosis, all yielded by MRI-guided trajectories. In addition, tumor grade was higher on the PET-guided trajectory than on the MR imaging–guided trajectory in 16 cases. Therefore, PET guidance reduced the number of non-contributive trajectories and significantly improved the diagnostic yield of the biopsy. PET-guided biopsy provided better delineation of the lesions than MRI and also guided resection of hypermetabolic area; it has also improved early postoperative detection of residual tumor.
Figure 4.
Stereotactic comparison of MET-PET (left) and contrast-enhanced MRI (right) of a metabolically heterogeneous, non-enhancing glioma. Based on MRI, biopsy was preferred to resection because of the deep tumor infiltration. MET-PET showed heterogeneous tracer uptake with a focus of maximum metabolism used as a biopsy target (arrow 1); the tissue obtained from this area showed anaplastic features, while biopsy from the area with low tracer uptake (and hypointensity on MRI, arrow 2) yielded non-tumoral tissue (modified from Pirotte et al. [35]).
4.4. Differentiation of low-grade tumor types
Histology of low-grade gliomas is an independent factor of survival: astrocytomas have worse prognosis than oligodendrogliomas, which have a better response to chemotherapy and better long-term prognosis [23, 26, 30]. DNETs and other glioneuronal tumors grow very slowly and they often present with medically refractory seizures. Therefore, differentiation of low-grade tumor types has clinical significance for designing optimal management. However, most imaging modalities provide no reliable markers to differentiate low-grade glioma types. Despite some distinctive features on MRI, nonspecific DNET is difficult to diagnose. In most cases, both DNETs and gangliogliomas are hypometabolic on FDG-PET [1, 17, 19, 22, 33]. Therefore, several investigators studied various amino acid PET tracers for differentiating DNETs from other tumor types and non-tumorous lesions.
In a study using AMT-PET, our group determined if kinetic parameters of AMT can differentiate among low-grade glioma types [14]. Sixteen of 23 patients had WHO grade 2 gliomas and 7 patients had grade 1 DNETs. While high static AMT uptake was found in most low-grade gliomas and also DNETs, including non-enhancing lesions on MRI (Figure 5), tracer kinetic analysis demonstrated significantly higher tumor/cortex ratios of tryptophan metabolic rates (≥1.3) in astrocytic tumors compared to oligodendrogliomas and DNETs (two tumors with very similar histologic characteristics). Thus, AMT-PET could be a useful technique in pretreatment differentiation of low-grade glial neoplasms.
Figure 5.
Comparison of FLAIR (A), post-contrast T1 (B), perfusion-weighted imaging (PWI) (C) and α-[11C]methyl-L-tryptophan (AMT)-PET images (D) of a low-grade glioma. FLAIR image showed a hyperintense mass in the right pre-central region, with no contrast enhancement and normal blood volume on PWI. AMT-PET showed high tryptophan uptake in the tumor mass, a common finding in low-grade gliomas.
In a subsequent study, our group also explored the specific use of AMT-PET in children with epileptogenic DNETs to evaluate the mechanism of tryptophan accumulation [1]. Increased AMT uptake (as compared to normal cortex) was present in 8 of the 11 tumors (despite low FDG uptake in all of them), and tracer kinetic analysis showed both high tumoral tryptophan transport and metabolic rates as a mechanism of AMT accumulation. As a unique finding, we also detected abnormal, increased cortical uptake beyond the MRI defined tumor region, which likely represented epileptic cortex. Non-resection of such cortical regions was associated with persistent seizures post-operatively. Therefore, AMT-PET could be helpful in surgical treatment planning in DNETs associated with medically refractory seizures.
Increased methionine uptake on PET was reported in both gangliogliomas and DNETs, with significantly higher tumor-to-gray matter ratios as compared to focal cortical dysplasia [33]. Other studies, however, reported no or minimal MET uptake in DNETs, suggesting that MET-PET may be helpful to differentiate such tumors from gliomas that typically show strong MET accumulation [17, 40]. In a study comparing various low-grade tumor types, both gliomas and gangliogliomas showed moderately or markedly increased MET uptake, while the majority (7 of 11) of DNETs showed no increased uptake. In contrast, a small FET-PET study demonstrated increased tracer uptake in most DNETs [18]. The reason for this discrepancy between FET and MET uptake in DNETs is unclear, although the differential effect of microvascular density and tumor blood flow may play a role.
4.5. Assessment of prognostic value
The prognostic value of FDOPA-PET in pediatric brain tumors was evaluated by two studies. In a study in 13 children FDOPA uptake showed a significant correlation with progression-free survival [27]. In a subsequent larger study of 27 children with brain tumors, including 10 low-grade gliomas, FDOPA uptake independently correlated with both overall survival (p=0.04) and progression-free survival (p<0.05), while MRS findings did not correlate with the clinical outcome [28].
4.6. Post-treatment evaluation
Imaging assessment of brain tumors following initial treatment is critical for an early diagnosis of residual/recurrent tumor, for differentiation of tumor recurrence from radiation injury, and for decisions regarding further treatment including reoperation.
Kruer et al. [21] reported one of the biggest pediatric studies to evaluate the value of post-operative FDG-PET to predict post-treatment progression. From 46 children with a low-grade astrocytoma, 18 patients had progressive disease during follow-up. PET hypermetabolism in the lesions was associated with shorter progression-free survival in both univariate and multivariate analyses. In contrast, age, tumor location, tumor size, mass effect, gadolinium enhancement, chemo- and/or radiation therapy, and size of total or subtotal resection did not show any difference between patients with and without progressive disease.
Among amino acid PET tracers, the clinical value of MET was investigated for the early postsurgical evaluation in 20 pediatric brain tumors [34]. PET scans were typically done between 2 to 7 days after surgery. Increased MET uptake (Figure 6) was found in 14 of the 20 patients and led to surgery in 11 of them thus confirming the tumor diagnosis. No MET uptake was detected in 6 patients, and follow-up MRI scans did not show tumor progression. In the 14 cases with high tumor tracer uptake, MRI scan was inconclusive in 11 cases. These findings suggested that early postoperative MET-PET could be useful for therapeutic decisions, including second-look surgery in pediatric brain tumors.
Figure 6.
Postoperative MRI (a) and 11C-methionine (MET)-PET (b) of an ependymoma. MRI was performed 24 hours after surgery and MET-PET was done 5 days after resection. On MR images two linear signals were seen along the resection cavity (arrow), suggesting a questionable residual tumor. MET-PET showed high tracer uptake confirming a residual tumor mass (from Pirotte et al. [34]).
In a more recent study, Dunkl et al. [10] investigated 48 children and adolescents with brain tumors using FET-PET. Eighteen of them had recurrence or progression. In this post-treatment group, FET-PET detected metabolically active tumor (tumor-to-brain ratiomax≥1.7) in patients with a partial resection. An accuracy of 79% was achieved to identify tumor progression or recurrence based on static FET uptake values, and a slightly higher accuracy (82%) was obtained when the decision was based on tracer kinetic parameters. Based on these findings, FET-PET might be useful in the post-treatment follow-up and treatment planning in pediatric brain tumors.
5. Summary and discussion
Decisions regarding timing and extent of surgery for presumed low-grade hemispheric brain tumors largely rely on pretreatment imaging findings. Conventional imaging modalities such as CT and MRI provide little information regarding tumor biological behavior. Application of PET imaging in the pretreatment characterization of such tumors can enhance the diagnostic workup and inform the physician about tumor metabolic characteristics, which is strongly linked to tumor grade and prognosis. PET imaging of glucose metabolism has been used clinically and may be useful in selected cases, especially in differentiating low-grade from high-grade malignancies; however, FDG-PET is less useful to differentiate non-tumoral lesions from low-grade tumors because of low glucose uptake in most of these lesions. In addition, several low-grade tumor types (such as pilocytic astrocytomas) can present with unusually high glucose metabolic rates, thus giving the appearance of a high-grade tumor.
In order to overcome some of these limitations, a number of amino acid derivative PET radiotracers (used also in adult neuro-oncology) have been tested successfully in pre-treatment evaluation of pediatric tumors. Although most studies have been relatively small (typically including a few dozens of pediatric cases, at the most), they provided some compelling data demonstrating that amino acid PET can be useful to differentiate tumors from non-tumorous lesions, assess tumoral proliferative activity, and detect tumoral metabolic heterogeneity. A complete lack or paucity of amino acid uptake may prompt the clinician to opt for watchful waiting, as these tumors are usually indolent and may not need active treatment. Still, some low-grade tumors, such as glioneuronal tumors may show avid amino acid uptake due to increased vascularity and/or upregulation of amino acid transporters at the blood-tumor-barrier.
Assessment of PET images using MR image fusion is critical to enhance the accurate anatomical evaluation of the tumor mass and surrounding structures. PET/MRI fusion can assist optimization of stereotactic targeting for tumor biopsy. In addition, tumor characteristics can be further evaluated by advanced MRI, such as multivoxel spectroscopy or perfusion imaging to provide complementary information to PET metabolic images. Such multimodal imaging can yield a comprehensive assessment on tumor heterogeneity, cellular and metabolic characteristics. This approach should increasingly become the part of routine clinical radiological assessment as some of these PET radiotracers make a transition from research application to clinical practice.
6. Future directions
While the studies summarized above provided a wide array of proof-of-principle data regarding the potential clinical uses of PET imaging in low-grade pediatric gliomas, the next critical steps should include multicenter, multimodal imaging studies. These studies should employ standardized image acquisition methods and rely on quantitative assessment of structural and metabolic tumor characteristics for objective data analysis. The results could provide definitive data regarding the added clinical value of molecular imaging and highlight specific areas and subgroups where these modalities could deliver the strongest clinical impact. One of the unresolved issues is the question if any of the amino acid PET tracers provide superior information, and, therefore, could be preferred over the others. Tracers labeled with the longer half-life radioisotopes (such as F-18) should be preferred in large-scale, multicenter studies. In addition, testing of some additional PET tracers (such as those targeting tumoral hypoxia), which are under investigation in adult neuro-oncology studies, could be expanded for pediatric applications. The pediatric population could also greatly benefit from the application of emerging PET/MRI hybrid acquisition technology: these scanners deliver less radiation and can eliminate the need for multiple sedations for young children while providing a superior PET/MRI image fusion with the promise of improved metabolic quantification and direct, voxel-by-voxel comparison of quantitative MRI and PET image parameters in gliomas and other tumors [37]. Both advanced MRI and quantitative PET-derived metabolic maps can be incorporated into intraoperative stereotactic systems, thus providing optimal visualization of tumor heterogeneity and identification of high-risk tumor portions for biopsy and surgical treatment targeting. Multimodal tumor maps can also further improve assessment of prognosis and assist objective monitoring of treatment responses in future clinical studies. Thus, it can be expected that molecular imaging along with advanced MRI will play an increasing role in the management of pediatric brain tumors.
Acknowledgment
The studies with AMT-PET have been supported by a grant (R01 CA123451) from the National Cancer Institute
References
- 1.Alkonyi B, Mittal S, Zitron I, et al. Increased tryptophan transport in epileptogenic dysembryoplastic neuroepithelial tumors. J Neurooncol. 2012;107:365–72. doi: 10.1007/s11060-011-0750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagni B, Pinna L, Tamarozzi R, et al. SPET imaging of intracranial tumours with 99Tcm-sestamibi. Nucl Med Commun. 1995;16:258–64. doi: 10.1097/00006231-199504000-00157. [DOI] [PubMed] [Google Scholar]
- 3.Batista CE, Juhasz C, Muzik O, et al. Imaging correlates of differential expression of indoleamine 2,3-dioxygenase in human brain tumors. Mol Imaging Biol. 2009;11:460–6. doi: 10.1007/s11307-009-0225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black KL, Hawkins RA, Kim KT, Becker DP, Lerner C, Marciano D. Use of thallium-201 SPECT to quantitate malignancy grade of gliomas. J Neurosurg. 1989;71:342–6. doi: 10.3171/jns.1989.71.3.0342. [DOI] [PubMed] [Google Scholar]
- 5.Borgwardt L, Larsen HJ, Pedersen K, Højgaard L. Practical use and implementation of PET in children in a hospital PET centre. Eur J Nucl Med Mol Imaging. 2003;30:1389–1397. doi: 10.1007/s00259-003-1263-5. [DOI] [PubMed] [Google Scholar]
- 6.Borgwardt L, Højgaard L, Carstensen H, et al. Increased fluorine-18 2-fluoro-2-deoxy-D-glucose (FDG) uptake in childhood CNS tumors is correlated with malignancy grade: a study with FDG positron emission tomography/magnetic resonance imaging coregistration and image fusion. J Clin Oncol. 2005;1(23):3030–7. doi: 10.1200/JCO.2005.02.074. [DOI] [PubMed] [Google Scholar]
- 7.Bosnyák E, Kamson DO, Guastella AR, et al. Molecular imaging correlates of tryptophan metabolism via the kynurenine pathway in human meningiomas. Neuro Oncol. 2015;17:1284–92. doi: 10.1093/neuonc/nov098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W. Clinical applications of PET in brain tumors. J Nucl Med. 2007;48:1468–1481. doi: 10.2967/jnumed.106.037689. [DOI] [PubMed] [Google Scholar]
- 9.Chugani DC, Muzik O. Alpha[C-11]methyl-L-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. J Cereb Blood Flow Metab. 2000;20:2–9. doi: 10.1097/00004647-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Dunkl V, Cleff C, Stoffels G, et al. The usefulness of dynamic O-(2-18F-fluoroethyl)-L-tyrosine PET in the clinical evaluation of brain tumors in children and adolescents. J Nucl Med. 2015;56:88–92. doi: 10.2967/jnumed.114.148734. [DOI] [PubMed] [Google Scholar]
- 11.Fulham MJ, Melisi JW, Nishimiya J, Dwyer AJ, Di Chiro G. Neuroimaging of juvenile pilocytic astrocytomas: an enigma. Radiology. 1993;189:221–5. doi: 10.1148/radiology.189.1.8372197. [DOI] [PubMed] [Google Scholar]
- 12.Hipp SJ, Steffen-Smith EA, Patronas N, et al. Molecular imaging of pediatric brain tumors: comparison of tumor metabolism using 18F-FDG-PET and MRSI. J Neurooncol. 2012;109:521–7. doi: 10.1007/s11060-012-0918-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juhász C, Chugani DC, Muzik O, et al. In vivo uptake and metabolism of alpha-[11C]methyl-L-tryptophan in human brain tumors. J Cereb Blood Flow Metab. 2006;26:345–57. doi: 10.1038/sj.jcbfm.9600199. [DOI] [PubMed] [Google Scholar]
- 14.Juhász C, Muzik O, Chugani DC, et al. Differential kinetics of α-[11C]methyl-L-tryptophan on PET in low-grade brain tumors. J Neurooncol. 2011;102:409–15. doi: 10.1007/s11060-010-0327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juhász C, Chugani DC, Barger GR, et al. Quantitative PET imaging of tryptophan accumulation in gliomas and remote cortex: correlation with tumor proliferative activity. Clin Nucl Med. 2012;37:838–42. doi: 10.1097/RLU.0b013e318251e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juhász C, Dwivedi S, Kamson DO, Michelhaugh SK, Mittal S. Comparison of amino acid positron emission tomographic radiotracers for molecular imaging of primary and metastatic brain tumors. Mol Imaging. 2014;13 doi: 10.2310/7290.2014.00015. doi:10.2310/7290.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan AM, Lawson MA, Spataro J, et al. Positron emission tomography using [18F] fluorodeoxyglucose and [11C] l-methionine to metabolically characterize dysembryoplastic neuroepithelial tumors. J Child Neurol. 1999;14:673–7. doi: 10.1177/088307389901401009. [DOI] [PubMed] [Google Scholar]
- 18.Kasper BS, Struffert T, Kasper EM, et al. 18Fluoroethyl-L-tyrosine-PET in long-term epilepsy associated glioneuronal tumors. Epilepsia. 2011;52:35–44. doi: 10.1111/j.1528-1167.2010.02754.x. [DOI] [PubMed] [Google Scholar]
- 19.Kincaid PK, El-Saden SM, Park SH, Goy BW. Cerebral gangliogliomas: preoperative grading using FDG-PET and 201Tl-SPECT. AJNR Am J Neuroradiol. 1998;19:801–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Kirton A, Kloiber R, Rigel J, Wolff J. Evaluation of pediatric CNS malignancies with (99m)Tc-methoxyisobutylisonitrile SPECT. J Nucl Med. 2002;43:1438–43. [PubMed] [Google Scholar]
- 21.Kruer MC, Kaplan AM, Etzl MM, Jr, et al. The value of positron emission tomography and proliferation index in predicting progression in low-grade astrocytomas of childhood. J Neurooncol. 2009;95:239–45. doi: 10.1007/s11060-009-9922-4. [DOI] [PubMed] [Google Scholar]
- 22.Lee DY, Chung CK, et al. Dysembryoplastic neuroepithelial tumor: radiological findings (including PET, SPECT, and MRS) and surgical strategy. J Neurooncol. 2000;47:167–174. doi: 10.1023/a:1006401305247. [DOI] [PubMed] [Google Scholar]
- 23.Mason WP, Krol GS, DeAngelis LM. Low-grade oligodendroglioma responds to chemotherapy. Neurology. 1996;46:203–7. doi: 10.1212/wnl.46.1.203. [DOI] [PubMed] [Google Scholar]
- 24.Messing-Jünger AM, Floeth FW, Pauleit D, et al. Multimodal target point assessment for stereotactic biopsy in children with diffuse bithalamic astrocytomas. Childs Nerv Syst. 2002;18:445–449. doi: 10.1007/s00381-002-0644-6. [DOI] [PubMed] [Google Scholar]
- 25.Meyer PT, Spetzger U, Mueller HD, Zeggel T, Sabri O, Schreckenberger M. High F-18 FDG uptake in a low-grade supratentorial ganglioma: a positron emission tomography case report. Clin Nucl Med. 2000;25:694–7. doi: 10.1097/00003072-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Mittal S, Szlaczky MC, Barger GR. Low-grade gliomas in adults. Curr Treat Options Neurol. 2008;10:271–84. doi: 10.1007/s11940-008-0030-0. [DOI] [PubMed] [Google Scholar]
- 27.Morana G, Piccardo A, Milanaccio C, et al. Value of 18F-3,4-dihydroxyphenylalanine PET/MR image fusion in pediatric supratentorial infiltrative astrocytomas: a prospective pilot study. J Nucl Med. 2014;55:718–23. doi: 10.2967/jnumed.113.125500. [DOI] [PubMed] [Google Scholar]
- 28.Morana G, Piccardo A, Puntoni M, et al. Diagnostic and prognostic value of 18F-DOPA PET and 1H-MR spectroscopy in pediatric supratentorial infiltrative gliomas: a comparative study. Neuro Oncol. 2015;17:1637–47. doi: 10.1093/neuonc/nov099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morana G, Puntoni M, Garrè ML, et al. Ability of 18F-DOPA PET/CT and fused 18F-DOPA PET/MRI to assess striatal involvement in paediatric glioma. Eur J Nucl Med Mol Imaging. 2016 Feb 25;2016 doi: 10.1007/s00259-016-3333-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Olson JD, Riedel E, DeAngelis LM. Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology. 2000;54:1442–8. doi: 10.1212/wnl.54.7.1442. [DOI] [PubMed] [Google Scholar]
- 31.O'Tuama LA, Treves ST, Larar JN, et al. Thallium-201 versus technetium-99m-MIBI SPECT in evaluation of childhood brain tumors: a within-subject comparison. J Nucl Med. 1993;34:1045–51. [PubMed] [Google Scholar]
- 32.Patil S, Biassoni L, Borgwardt L. Nuclear medicine in pediatric neurology and neurosurgery: epilepsy and brain tumors. Semin Nucl Med. 2007;37:357–381. doi: 10.1053/j.semnuclmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Phi JH, Paeng JC, Lee HS, et al. Evaluation of focal cortical dysplasia and mixed neuronal and glial tumors in pediatric epilepsy patients using 18F-FDG and 11C-methionine PET. J Nucl Med. 2010;51:728–34. doi: 10.2967/jnumed.109.070920. [DOI] [PubMed] [Google Scholar]
- 34.Pirotte B, Levivier M, Morelli D, et al. Positron emission tomography for the early postsurgical evaluation of pediatric brain tumors. Childs Nerv Syst. 2005;21:294–300. doi: 10.1007/s00381-004-1071-7. [DOI] [PubMed] [Google Scholar]
- 35.Pirotte BJ, Lubansu A, Massager N, et al. Clinical impact of integrating positron emission tomography during surgery in 85 children with brain tumors. J Neurosurg Pediatr. 2010;5:486–99. doi: 10.3171/2010.1.PEDS09481. [DOI] [PubMed] [Google Scholar]
- 36.Pollack IF. Brain tumors in children. N Engl J Med. 1994;331:1500–1507. doi: 10.1056/NEJM199412013312207. [DOI] [PubMed] [Google Scholar]
- 37.Puttick S, Bell C, Dowson N, Rose S, Fay M. PET, MRI, and simultaneous PET/MRI in the development of diagnostic and therapeutic strategies for glioma. Drug Discov Today. 2015;20:306–17. doi: 10.1016/j.drudis.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Roelcke U, Radü EW, Hausmann O, Vontobel P, Maguire RP, Leenders KL. Tracer transport and metabolism in a patient with juvenile pilocytic astrocytoma. A PET study. J Neurooncol. 1998;36:279–83. doi: 10.1023/a:1005898109520. [DOI] [PubMed] [Google Scholar]
- 39.Rollins NK, Lowry PA, Shapiro KN. Comparison of gadolinium-enhanced MR and thallium-201 single photon emission computed tomography in pediatric brain tumors. Pediatr Neurosurg. 1995;22:8–14. doi: 10.1159/000121293. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg DS, Demarquay G, Jouvet A, et al. [11C]-Methionine PET: dysembryoplastic neuroepithelial tumours compared with other epileptogenic brain neoplasms. J Neurol Neurosurg Psychiatry. 2005;76:1686–1692. doi: 10.1136/jnnp.2004.051607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Utriainen M, Metsähonkala L, Salmi TT, et al. Metabolic characterization of childhood brain tumors: comparison of 18F-fluorodeoxyglucose and 11C-methionine positron emission tomography. Cancer. 2002;95:1376–86. doi: 10.1002/cncr.10798. [DOI] [PubMed] [Google Scholar]
- 42.Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 43.Weckesser M, Matheja P, Rickert CH, et al. High uptake of L-3-[123I]iodo-alpha-methyl tyrosine in pilocytic astrocytomas. Eur J Nucl Med. 2001;28:273–81. doi: 10.1007/s002590000462. [DOI] [PubMed] [Google Scholar]