Abstract
Background:
This study assessed the safety of the newly developed bridge-enhanced anterior cruciate ligament (ACL) repair (BEAR), which involves suture repair of the ligament combined with a bioactive scaffold to bridge the gap between the torn ligament ends. As the intra-articular environment is complex in its response to implanted materials, this study was designed to determine whether there would be a significant rate of adverse reaction to the implanted scaffold.
Hypothesis:
The primary hypothesis was that the implanted scaffold would not result in a deep joint infection (arthrocentesis with positive culture) or significant inflammation (clinical symptoms justifying arthrocentesis but negative culture). The secondary hypotheses were that patients treated with BEAR would have early postoperative outcomes that were similar to patients treated with ACL reconstruction with an autologous hamstring graft.
Study Design:
Cohort study; Level of evidence, 2.
Methods:
A total of 20 patients were enrolled in this nonrandomized, first-in-human study. Ten patients received BEAR treatment and 10 received a hamstring autograft ACL reconstruction. The BEAR procedure was performed by augmenting a suture repair with a proprietary scaffold, the BEAR scaffold, placed in between the torn ends of the ACL at the time of suture repair. The BEAR scaffold is to our knowledge the only device that fills the gap between the torn ligament ends to have current Investigational Device Exemption approval from the Food and Drug Administration. Ten milliliters of autologous whole blood were added to the scaffold prior to wound closure. Outcomes were assessed at 3 months postoperatively. The outcomes measures included postoperative pain, muscle atrophy, loss of joint range of motion, and implant failure (designated by an International Knee Documentation Committee grade C or D Lachman test and/or an absence of continuous ACL tissue on magnetic resonance images).
Results:
There were no joint infections or signs of significant inflammation in either group. There were no differences between groups in effusion or pain, and no failures by Lachman examination criteria (BEAR, 8 grade A and 2 grade B; ACL reconstruction, 10 grade A). Magnetic resonance images from all of the BEAR and ACL-reconstructed patients demonstrated a continuous ACL or intact graft. In addition, hamstring strength at 3 months was significantly better in the BEAR group than in the hamstring autograft group (mean ± SD: 77.9% ± 14.6% vs 55.9% ± 7.8% of the contralateral side; P < .001).
Conclusion:
The results of this study suggest that the BEAR procedure may have a rate of adverse reactions low enough to warrant a study of efficacy in a larger group of patients.
Keywords: anterior cruciate ligament, human, ACL reconstruction, ACL repair, bridge-enhanced ACL repair, BEAR
Primary suture repair of the anterior cruciate ligament (ACL) has been associated with a high rate of failure and has been largely replaced by ACL reconstruction for the past 4 decades.2,7 The reason for the high rate of failure has been thought to be related to a lack of vascularity in the ligament,3 and more recently, due to a premature loss of the blood clot that typically forms an early scaffold, or bridge, across the wound site.20,23 This bridge stimulates healing in ligaments (such as the medial collateral ligament [MCL]) that heal without surgical intervention. Preclinical work in large animal models has suggested that placement of a sponge capable of absorbing blood and stabilizing it in the ACL wound site can stimulate healing of the ligament.8,15,21 When this technique is combined with mechanical stabilization of the knee with a suture repair, the outcomes are similar between the bridge-enhanced ACL repair, or BEAR procedure, and ACL reconstruction in preclinical models.21,33
Prior studies using native polymers and tissues to enhance soft tissue repair within joints have been conducted, most commonly in the shoulder for rotator cuff surgery.13,17,29 Perhaps the most well-known of these implants is the RESTORE patch (DEPUY), a crosslinked, mechanically strong, construct made of porcine small intestine submucosa. The RESTORE patch was used to augment suture repair of the rotator cuff tendon in 2 studies: a randomized control trial of 30 patients13 and a second study of 25 patients who had the RESTORE patch implanted.17 In the first study, 3 (20%) of 15 RESTORE patients developed erythema, pain, and swelling at the shoulder within 6 weeks of implantation.13 In the second study, 4 (16%) of 25 patients had an overt inflammatory reaction at a mean of 13 days postoperatively. All 4 patients required surgical removal of the implant.17 Similarly, a porcine dermal patch used as a bridging construct (Permacol; Medtronic) for rotator cuff repair had 100% failure between 3 and 6 months after surgery.29 The RESTORE implant has a relatively high DNA content,4 and Permacol is crosslinked to enhance its durability. We hypothesized that use of an uncrosslinked scaffold with a low DNA content (the BEAR scaffold) would result in a low rate of adverse reaction when the scaffold was placed in the knee. The previously observed failure rates of 16% to 100% within the early postoperative period for RESTORE and Permacol implants justify the need for a short follow-up of 10 patients in the first-in-human study prior to proceeding to a larger controlled trial.
Preclinical data suggest that the BEAR scaffold is 95% resorbed by 6 weeks and 100% resorbed by 8 weeks after surgery.25 Once the scaffold is fully resorbed, future adverse events related to infection, inflammation, or hypertrophic scar formation leading to loss of extension are less likely to be related to the scaffold material. In addition, failure to heal should be evident by the time the scaffold resorbs. Therefore, a 3-month endpoint was selected and approved by the US Food and Drug Administration (FDA) for this first-in-human study (IDE G140151). At 3-month follow-up, pain, effusion, range of motion, Lachman testing, and hamstring/hip abductor strength were measured. Outcomes for patients undergoing the BEAR procedure were compared with a control group undergoing ACL reconstruction with autologous hamstring tendon. Magnetic resonance imaging (MRI) was performed to assess the presence of healing tissue in the expected location of the ACL or ACL graft in both the BEAR and ACL reconstruction groups, respectively.
Methods
An Investigational Device Exemption (G140151) from the FDA and institutional review board approval from Boston Children’s Hospital (P0012985) were obtained prior to beginning the study. The trial also was registered on clinicaltrials.gov (NCT02292004). All patients granted their informed consent prior to participating in the study.
Study Design
This study was a first-in-human evaluation of the BEAR scaffold. It was designed as an interventional, parallel assignment, nonrandomized safety study. For all physical examination outcomes, the examiner was blinded to the group assignment and operative knee. All surgeries were performed at a single site (Boston Children’s Hospital) by a single surgeon (L.J.M.). Ten patients were enrolled in the interventional (BEAR) group and 10 in the control group (autograft hamstring ACL reconstruction). Enrollment began in February 2015 and was completed in October 2015. Patients were evaluated preoperatively, intraoperatively, and postoperatively at 2 weeks, 6 weeks, and 3 months for the purposes of this safety study, with longer follow-up planned for early efficacy measures should the results of the safety study justify continued follow-up.
Inclusion and Exclusion Criteria
Patients aged 18 to 35 years with a complete ACL tear who were less than 1 month from injury and who had at least 50% of the length of the ACL attached to the tibia on their preoperative MRI were eligible to enroll in the BEAR group. As the ACL remnant is commonly removed during ACL reconstruction, and thus resorption of the torn ACL over time was not as critical for the reconstruction group, patients with a complete ACL tear who were within 3 months of injury were eligible to enroll in the ACL reconstruction group. Patients with a partial ACL tear were not eligible for participation. Only patients determined to benefit from surgical intervention with autograft hamstring tendon graft were considered for this study. Patients were excluded from either group if they had a history of prior surgery on the knee, history of prior infection in the knee, or had risk factors that might adversely affect healing (nicotine/tobacco use, corticosteroids in the past 6 months, chemotherapy, diabetes, inflammatory arthritis). Patients were excluded if they had a displaced bucket-handle tear of the medial meniscus that required repair; all other meniscal injuries were included. Patients were also excluded at the time of surgery if they were found to have less than 50% of the length of the ACL still attached to the tibial footprint. Additionally, patients were excluded if they had a full-thickness chondral injury, a grade 3 MCL injury, a concurrent complete patellar dislocation, or an operative posterolateral corner injury.
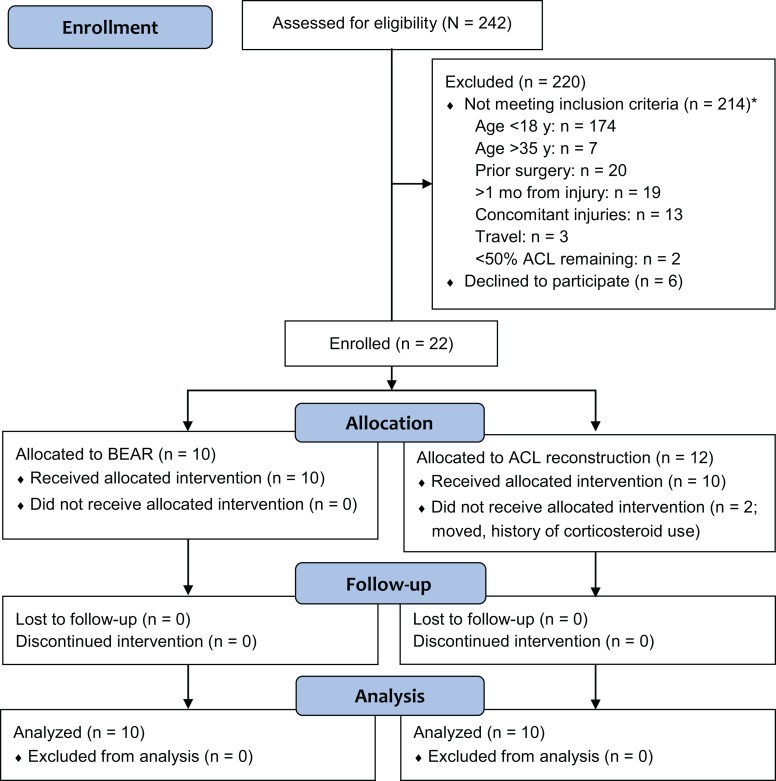
A total of 242 patients presenting with an ACL injury were screened for participation in this study (Figure 1). Patients were identified as possible candidates if they scheduled an appointment in our sports medicine division with a new knee injury and had an MRI confirming an ACL tear or if they contacted our research coordinator after hearing about the study. Of the 242 patients screened, 22 were enrolled (Figure 1), of which 2 were excluded before surgery: 1 due to a history of corticosteroid use not discovered in the initial enrollment meeting and the second patient elected to move to Florida for school. The primary reason for exclusion prior to enrollment was patient age (n = 181 patients).
Figure 1.
CONSORT 2010 flow diagram. *The total number of patients not meeting inclusion criteria totals to greater than 214, as some patients met more than 1 exclusion criterion. ACL, anterior cruciate ligament; BEAR, bridge-enhanced ACL repair.
BEAR Scaffold
The BEAR scaffold was manufactured at Boston Children’s Hospital and completed all biocompatibility and sterility testing prior to use in the clinical study.25–27 The scaffold comprised extracellular matrix proteins, including collagen, that were obtained from bovine tissue. The DNA content of the scaffolds was less than 50 ng/mg of scaffold, and the scaffolds were not crosslinked. The scaffold measured 22 mm in diameter by 45 mm in length and was hydrophilic and able to absorb up to 5 times its weight in fluid. The BEAR scaffold softens when blood is added to it, making it conformable to the intra-articular notch and able to fill in the irregular contours of the gap between the torn ligament ends. The scaffold material has been tested in preclinical large animal studies15,16,22,38 in which the healing ACL was found to have similar mechanical properties to a bone–patellar tendon–bone allograft at 3, 6, and 12 months after surgery.21,33 In addition, pigs treated with the BEAR scaffold had a significantly lower rate of osteoarthritis than those treated with ACL reconstruction.21
Surgical Technique
The BEAR Procedure
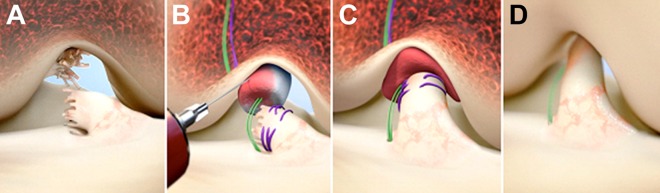
After the induction of general anesthesia, an examination was performed to verify the positive pivot shift on the injured side and to record the Lachman, range of motion, and pivot-shift examination results on both knees. Knee arthroscopy was performed, and meniscal injuries were treated if present. The tibial aimer (ACUFEX Director Drill Guide; Smith & Nephew) was used to place a 2.4-mm guide pin up through the tibia in the anterior 25% of the tibial ACL footprint, and the pin was overdrilled with a 4.5-mm reamer (4.5-mm Endoscopic Drill; Smith & Nephew). A guide pin was placed in the anterior 25% of the femoral ACL footprint, drilled up through the femur, and then overdrilled using the 4.5-mm reamer. A 2-inch arthrotomy was made at the medial border of the patellar tendon, and a whipstitch of No. 2 Vicryl was placed into the tibial stump of the torn ACL. Two No. 2 Ethibond sutures were looped through the 2 center holes of a cortical button (Endobutton; Smith & Nephew). The No. 2 Vicryl suture from the tibial stump had the free ends passed through the cortical button, and the button carrying the Ethibond and Vicryl sutures was passed through the femoral tunnel and engaged on the lateral femoral cortex. Both of the looped sutures of No. 2 Ethibond (4 matched ends) were passed through the scaffold and through the tibial tunnel. 10 mL of autologous blood was obtained from the antecubital vein and added to the scaffold. The scaffold was then passed up along the sutures into the femoral notch and the Ethibond sutures pulled distally and tied over a second cortical button on the anterior tibial cortex with the knee in full extension. The free ends of the No. 2 Vicryl suture from the ACL whipstitch coming through the femur were tightened and tied over the femoral cortical button to bring the ACL stump into the scaffold using an arthroscopic surgeon’s knot and knot pusher (Figure 2). The arthrotomy was closed in layers.
Figure 2.
Stepwise demonstration of the bridge-enhanced ACL repair (BEAR) technique using the BEAR scaffold. (A) In this technique, the torn ACL tissue is preserved. (B) A whipstitch using No. 2 Vicryl (purple) is placed into the tibial stump of the ACL. Small tunnels (4 mm) are drilled in the femur and tibia, and an Endobutton with two No. 2 Ethibond sutures (green) and the No. 2 Vicryl ACL sutures attached to it is passed through the femoral tunnel and engaged on the proximal femoral cortex. The Ethibond sutures are threaded through the BEAR scaffold, tibial tunnel, and secured in place with an extracortical button. The BEAR scaffold is then saturated with 10 mL of the patient’s blood, and (C) the tibial stump pulled up into the saturated scaffold. (D) The ends of the torn ACL then grow into the BEAR scaffold and the ligament reunites. ACL, anterior cruciate ligament.
ACL Reconstruction With Autologous Hamstring Tendon
After the induction of general anesthesia, an examination was performed to verify the positive pivot shift on the injured side and to record the Lachman, range of motion, and pivot-shift examination results on both knees. A standard hamstring autograft procedure was performed using a quadruple semitendinosus-gracilis graft looped over a continuous-loop cortical button (Endobutton) for proximal fixation and a bioabsorbable interference screw (BioRCI HA; Smith & Nephew) for tibial fixation. Concomitant treatment of meniscal injuries was performed.
Postoperative Rehabilitation
For all patients, a locking hinged brace (TScope Premier Post-Op Knee Brace; Breg) was applied to limit joint range of motion to between 0° and 50° of knee flexion for the first 2 weeks postoperatively and then between 0° and 90° for the next 4 weeks unless they had a concomitant meniscal repair, in which case the brace range was restricted to between 0° and 40° for the first 4 weeks postoperatively before opening the brace up to between 0° and 90° of flexion. All patients were provided with a cold therapy unit (DonJoy Iceman; DJO Global) for postoperative use. Both groups followed the same standardized physical therapy protocol with no flexion greater than 90° for 6 weeks, partial weightbearing restricted for 2 weeks, then weightbearing as tolerated with crutches until 4 weeks postoperatively. Use of a functional ACL brace (CTi brace; OSSUR) was recommended from 6 to 12 weeks postoperatively.
Outcome Measures
Deep Joint Infection
All patients were monitored for signs of a possible deep joint infection (eg, fever >101°F, increasing pain in the knee, presence of an effusion, drainage from the knee). A protocol was established to perform a knee arthrocentesis if these symptoms arose, and if organisms were cultured from the joint fluid, the patient would have been classified as having a deep joint infection (according to the Centers for Disease Control’s National Healthcare Safety Network [CDC/NHSN] criteria12) and treated accordingly. Early stopping of the trial would have been considered if at any time during the study there had been 2 or more subjects in the intervention group who developed a deep joint infection or who experienced a serious adverse event of any type thought to be related to the BEAR scaffold.
Marked Inflammatory Reaction
All patients were monitored for signs of a swollen, warm knee. If a patient had presented with a swollen, warm knee and there was clinical suspicion of marked inflammation or a septic joint, an arthrocentesis would have been performed. If the synovial fluid culture was negative for organisms, the patient would have been classified as having a marked inflammatory reaction and treated accordingly.
Gait Impairment and Muscle Atrophy
If the patient could not ambulate independently and continued to require crutches for ambulation at the 6-week follow-up visit, the patient would have been classified as having muscle atrophy leading to gait impairment and treated accordingly. Thigh circumference was measured on each thigh at 5 and 10 cm above the patella for all patients at the 3-month time point. In addition, hamstring and hip abductor muscle strength were measured 3 months after surgery using a handheld dynamometer (Microfet 2 Manual Muscle Testing Handheld Dynamometer; Hoggan Scientific). Hamstring and hip abductor strength were measured on both sides, and all measures were performed and recorded in duplicate. The average of the 2 readings was used in the analysis. The hamstring strength was measured with the subject prone and the knee in 90° of flexion. The dynamometer was placed at the ankle and the patient instructed to pull the foot toward the hip with maximum effort. The hip abductor strength was tested with the patient lying on their side with the knee extended, placing the dynamometer over the midlateral thigh and instructing the patient to raise the leg.
Pain
If the patient needed to be readmitted to the hospital for parenteral pain medications, they would have been classified as having significant pain and treated accordingly. In addition, pain levels were queried at each postoperative visit using a visual analog scale (0-10).
Implant/Graft Failure
At 3 months, if the Lachman examination was rated as grade C (abnormal) or grade D (severely abnormal),1 the implant or graft would have been classified as a failure and the patient treated accordingly. The clinical Lachman examination grading was performed according to International Knee Documentation Committee (IKDC) recommendations as grade A (–1- to 2-mm side-to-side difference, firm endpoint), grade B (3- to 5-mm side-to-side difference, firm endpoint), grade C (either 3- to 5-mm side-to-side difference with a soft endpoint or a 6- to 10-mm side-to-side difference with a soft or firm endpoint), and grade D (>10-mm side-to-side difference).1 Knee sleeves were used to cover both knees for all patients, and the examiner was blinded as to surgical side and study group when performing the physical examination.
Magnetic Resonance Imaging
MR images were obtained for all operated knees at 3 months. Using a 3-T scanner (Tim Trio; Siemens) and a 15-channel knee coil, the following sequences were performed of the surgical knee: 3-plane gradient echo localizer, axial and oblique sagittal fast spin echo T2 with fat saturation, coronal proton density with fat saturation, and volumetric 3D sagittal proton density SPACE with axial and coronal reformations. The region of the ACL repair or graft was assessed for integrity, continuity of fibers from femoral attachment/tunnel to tibial attachment/tunnel, as well as surrounding fluid and inflammatory change. Implant or graft failure would have been classified as the absence of intact, continuous fibers in the expected region of the ACL repair or graft.
Range of Motion
Passive and active range of motion were measured at each postoperative visit using a goniometer. Knee sleeves were used to cover both knees for all patients, and the examiner was blinded to the surgical side and study group when performing the physical examination.
Statistical Analysis
The study was not designed to provide high statistical power for comparing the treatment groups, and accordingly, the analysis is largely descriptive. We used formal statistical testing only to compare characteristics or outcomes that directly represent differences in the design of the 2 treatment approaches: time from injury to surgery (2-sample t test), length of ACL tibial remnant (recorded in 4 ordered categories, Cochran-Armitage trend test), and hamstring strength (2-sample t test). All P values are 2-sided.
Results
Baseline Characteristics
The baseline characteristics of both groups are shown in Table 1. In summary, the 2 groups were similar with regard to age, sex, race, and body mass index (BMI). The mean age of the patients was 24 years in both groups. The majority of the injuries in both groups was noncontact and occurred during sports participation. The time from injury to surgery was significantly longer in the ACL reconstruction group (mean, 21 vs 53 days). One patient in the ACL reconstruction group was noted to have an MCL tear on the preoperative MRI.
TABLE 1.
Baseline Characteristics of the 2 Study Groupsa
| Characteristic | BEAR Group (n = 10) | ACLR Group (n = 10) |
|---|---|---|
| Demographics | ||
| Male, n | 4 | 2 |
| White, non-Hispanic, n | 7 | 8 |
| Age, y, mean ± SD (range) | 24.1 ± 4.9 (18.1-34.6) | 24.6 ± 5.5 (18.6-33.8) |
| BMI, mean ± SD | 24.2 ± 2.0 | 25.1 ± 2.9 |
| Injury to surgery, d, mean ± SD (range)b | 20.8 ± 4.8 (11-28) | 52.9 ± 16.7 (24-80) |
| Injury and symptoms, n | ||
| Left knee injured | 5 | 6 |
| Mechanism: sports | 10 | 9 |
| Noncontact injury | 9 | 9 |
| MRI findings, n | ||
| Torn PCL | 0 | 0 |
| Torn MCL | 0 | 1 |
aACLR, anterior cruciate ligament reconstruction; BEAR, bridge-enhanced ACL repair; BMI, body mass index; MCL, medial collateral ligament; PCL, posterior cruciate ligament.
bStatistically significant difference between groups (P < .001).
Preoperative MRI Appearance of the ACL Tear
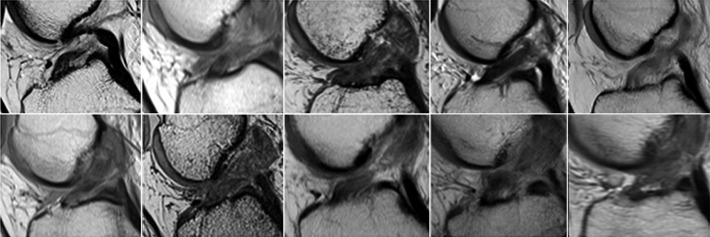
The preoperative MRI examinations were not included as part of the study and were performed at several imaging centers using varied scanners and protocols. All preoperative MR examinations included at least oblique sagittal fluid sensitive (T2 with fat saturation or inversion recovery) and proton density sequences as well as 1 sequence in the coronal and axial planes. The preoperative MRI demonstrated a complete ACL tear in the proximal half of the ligament for all patients who underwent the BEAR procedure (Figure 3). On the oblique sagittal images, the ACL fibers were incompletely visualized and edema was seen throughout the region of the injured ligament, indicating a large zone of injury at the time of rupture. Similar findings were found in the group undergoing ACL reconstruction (Figure 4).
Figure 3.
Preoperative magnetic resonance appearance of the injured anterior cruciate ligament (ACL) in the 10 patients in the bridge-enhanced ACL repair (BEAR) cohort.
Figure 4.
Preoperative magnetic resonance appearance of the injured anterior cruciate ligament (ACL) in the 10 patients in the ACL reconstruction cohort.
Intraoperative Findings
All patients had at least 50% of the length of the ACL preserved as a tibial remnant on diagnostic arthroscopy, and the maximum distance from the tibial stump to the femoral origin of the ACL was 50% of the length of the native ACL (Table 2). The numbers of patients with a concomitant meniscal tear were similar in the 2 groups, as was the degree of effusion at the time of surgery. Side-to-side differences in Lachman testing were similar in the 2 groups, and all patients had either a pivot glide or “clunk” with pivot-shift testing under anesthesia.
TABLE 2.
Intraoperative Findingsa
| Characteristic | BEAR Group (n = 10) | ACLR Group (n = 10) | P Value |
|---|---|---|---|
| Length of ACL tibial remnant, n | 0.13 | ||
| 0%-24% | 0 | 0 | |
| 25%-49% | 0 | 0 | |
| 50%-74% | 9 | 6 | |
| ≥75% | 1 | 4 | |
| 1 or more meniscal tears, nb | 4 | 5 | |
| Effusion grade (0-3)c | |||
| Mean ± SD | 1.3 ± 0.7 | 0.9 ± 0.8 | |
| Highest score, n | 2 | 2 | |
| Soft endpoint on Lachman test, nc | 10 | 9 | |
| Pivot shift, n | |||
| Glide | 2 | 3 | |
| Clunk | 8 | 7 |
aACLR, anterior cruciate ligament reconstruction; BEAR, bridge-enhanced ACL repair.
bBEAR group: 1 lateral tear in 1 patient, 2 lateral tears in 1 patient, 1 medial tear in 2 patients; ACLR group: 1 lateral tear in 3 patients, 2 lateral tears in 1 patient, 1 medial tear in 1 patient.
cn = 9 in the ACLR group.
Outcome Measures
Adverse Events
Postoperatively, no patient in either group had a superficial or deep infection involving the operative knee, a marked inflammatory reaction, pain requiring hospital readmission, a soft endpoint or a grade C or D on Lachman examination (suggestive of repair or graft failure), or absence of tissue in the notch on MRI at 3 months postoperatively. Adverse events that were observed in both groups were nausea (6 patients in the BEAR group, 2 in the ACL reconstruction group), pain requiring oral medications (all 20 patients), joint effusion (all 20 patients), localized area of temporary paresthesia on the lower leg (1 patient in each group), and continuing crutch use at 6 weeks (2 patients in each group). Additional adverse events in the BEAR group included 1 patient with postoperative vomiting, 1 with a skin infection involving the nonoperative lower extremity, 1 patient with a 4-cm2 area of frostbite that resolved by the 3-month time point, 1 patient with a fall from a stage at a concert, 1 patient with a Baker cyst, and 1 who had a 1-day urticarial event noted 6 weeks postoperatively that resolved spontaneously. Additional adverse events in the ACL reconstruction group included 1 patient with a postoperative fever that resolved spontaneously, 1 patient who developed noncardiac chest pain postoperatively that also resolved, 1 patient with decreased joint range of motion postoperatively treated with a course of anti-inflammatory drugs, 1 with an episode of dizziness, and 2 who developed a deep venous thrombosis in the postoperative period and were treated with anticoagulation.
Outcome Measures Recorded Longitudinally
The outcome measures recorded longitudinally are presented in Table 3. In brief, there were no differences in the reported level of pain postoperatively, active flexion or extension of the operated knee, or the effusion grade.
TABLE 3.
Outcomes Measured Longitudinallya
| Outcome | Baseline | 1-2 Weeks | 6 Weeks | 3 Months |
|---|---|---|---|---|
| Pain score (0-10) | ||||
| BEAR | 5.89 ± 2.83 | 2.66 ± 2.35 | 1.91 ± 1.50 | |
| ACLR | 7.16 ± 2.28 | 1.67 ± 1.61 | 1.37 ± 2.34 | |
| Active flexion differenceb | ||||
| BEAR | –31.3 ± 31.0 | –78.8 ± 18.7 | –42.1 ± 20.5 | –14.0 ± 15.4 |
| ACLR | –21.3 ± 16.3 | –77.8 ± 19.5 | –35.6 ± 15.9 | –7.1 ± 11.6 |
| Active extension differenceb | ||||
| BEAR | 8.8 ± 10.7 | 5.0 ± 2.8 | 4.1 ± 2.3 | 2.2 ± 1.7 |
| ACLR | 6.9 ± 7.2 | 8.0 ± 5.5 | 4.0 ± 3.6 | 2.5 ± 2.6 |
| Effusion grade | ||||
| BEAR | 1.5 ± 0.7 | 1.8 ± 0.4 | 1.4 ± 0.5 | 0.5 ± 0.5 |
| ACLR | 1.9 ± 1.0 | 1.9 ± 1.0 | 1.3 ± 0.5 | 0.9 ± 0.6 |
aData are reported as mean ± SD; n = 10 per group at every time point. ACLR, anterior cruciate ligament reconstruction; BEAR, bridge-enhanced ACL repair.
bDifferences between injured and contralateral knee.
Outcome Measures Recorded at 3 Months Postoperatively
The secondary outcome measures recorded at 3 months are listed in Table 4. The Lachman examination, hip abduction strength, IKDC, duration of crutch use, and time to return to school/work were similar in the 2 groups. The percent recovery of hamstring strength was greater at 3 months for the BEAR group than the autograft hamstring reconstruction group (78% ± 14% vs 56% ± 8%; P < .001).
TABLE 4.
Outcomes Measured Only at 3 Months or Measured as Time Durationa
| Outcome | Mean ± SD or n |
|---|---|
| Lachman laxity difference, mmb | |
| BEAR | 1.10 ± 1.45 |
| Grade A, n | 8 |
| Grade B, n | 2 |
| ACLR | 0.60 ± 0.97 |
| Grade A, n | 10 |
| Grade B, n | 0 |
| Hamstring strength, % contralateralc | |
| BEAR | 77.9 ± 14.6 |
| ACLR | 55.9 ± 7.8 |
| Hip abduction, % contralateral | |
| BEAR | 95.4 ± 10.9 |
| ACLR | 96.8 ± 10.3 |
| IKDC score (0-100) | |
| BEAR | 54.3 ± 6.4 |
| ACLR | 60.7 ± 10.2 |
| Return to school/work, wk | |
| BEAR | 3.1 ± 3.3 |
| ACLR | 4.0 ± 4.2 |
| Time using crutches, wk | |
| BEAR | 4.7 ± 1.3 |
| ACLR | 4.8 ± 1.7 |
| Thigh circumference 5 cm above patella, % contralateral | |
| BEAR | 98.3 ± 1.7 |
| ACLR | 98.7 ± 2.5 |
| Thigh circumference 10 cm above patella, % contralateral | |
| BEAR | 94.1 ± 2.8 |
| ACLR | 95.4 ± 3.1 |
aACLR, anterior cruciate ligament reconstruction; BEAR, bridge-enhanced ACL repair.
bDifference between injured and contralateral knee; firm Lachman endpoint in all cases.
cStatistically significant difference between groups (P < .001).
Magnetic Resonance Imaging
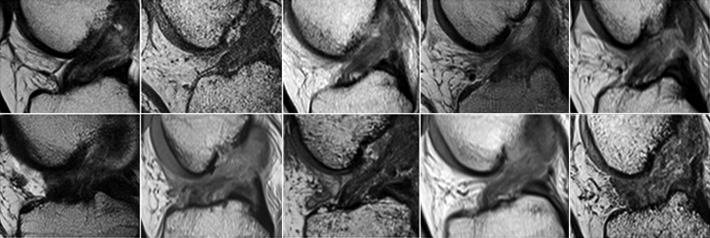
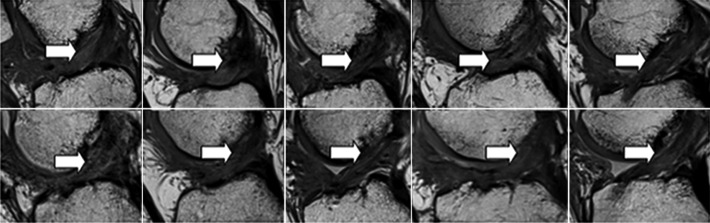
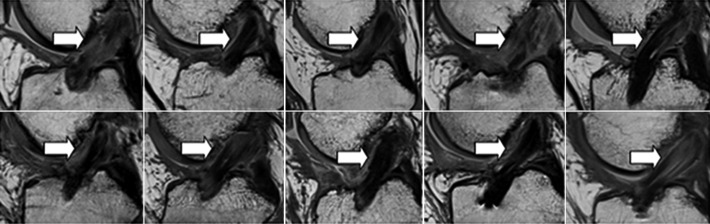
All 10 patients in the BEAR group exhibited continuous tissue in the region of the ACL at 3 months (Figure 5). The ACL grafts appeared to be intact in all 10 ACL reconstruction patients, with no gap between the proximal and distal limbs (Figure 6). There was no evidence of new meniscal or chondral injuries in any of the 3-month scans.
Figure 5.
Sagittal proton density (intermediate-weighted) images from all 10 subjects in the bridge-enhanced ACL repair (BEAR) group at 3 months after surgery show intact ACL fibers from the femoral to the tibial attachment sites (arrows). The intact fibers are low signal intensity (black), reflecting highly organized tissue with little free water. The peripheral higher signal intensity (lighter gray) indicates increased higher water content in the tissues surrounding the repaired ACL. ACL, anterior cruciate ligament.
Figure 6.
Sagittal proton density (intermediate-weighted) images from all 10 subjects in the hamstring autograft reconstruction group at 3 months after surgery show intact graft from the femoral to the tibial tunnels (arrows). The signal intensity within the graft is variable. The homogeneous low signal intensity (black) in some subjects (eg, top row second from left and bottom row third from left) is typical of the normal in situ hamstring tendon due to highly organized connective tissue with little free water. A more heterogeneous appearance is present in several subjects (eg, top row fourth from left) with central low signal tendon and peripheral high signal (lighter gray) indicating surrounding edema. Other subjects show intrasubstance higher signal within the graft itself (eg, bottom right) reflecting increased fluid within the graft.
Discussion
In this first-in-human study, we found the BEAR scaffold to be well tolerated by the synovial environment of the knee, with no deep infections or serious inflammatory reactions noted in the early postoperative period during which the scaffold is resorbed. No patients required return to the operating room for removal of the scaffold. Eight of 10 BEAR patients had grade A Lachman examinations, and 2 had a grade B. None of the BEAR patients had a grade C or D Lachman examination, and all had firm endpoints. On the 3-month MRIs, all BEAR patients had continuous tissue in the area of the ACL, and there was no difference in the amount of increased synovial fluid or synovium in the BEAR group compared with the ACL reconstruction group. The observed ACL tissue bridged the femoral and tibial insertion sites of the native ACL rather than coursing between the small tunnels drilled for suture placement. These findings validated our hypothesis that this uncrosslinked, low DNA–content extracellular matrix scaffold would have a tolerable immunogenicity profile. In addition, these data suggest that the BEAR technique would be reasonably safe to study in a larger number of patients in a subsequent randomized control trial.
Recent in vivo animal studies have suggested that 1 principal reason for the failure of the ACL to heal may be the premature loss of a scaffold bridging the 2 torn ACL ends.23 Currently, for very proximal tears where the tibial stump can be compressed to the femoral insertion site, it may be possible to get the ACL to heal with microfracture or sutures alone. Steadman has demonstrated some success with his “healing response” technique in skeletally immature and older active patients.30,31,35 There has also been recent interest in treating these proximal tears with suture repair techniques.6 However, the majority of ACL tears do not produce a tibial stump that is long enough for reapproximation to the femoral footprint, and thus, this technique can only be used in a small fraction of patients.5 The BEAR technique does not require exact tissue reapproximation as it fills the gap between the torn ligament ends with a biologically active scaffold. Autologous blood is added to an extracellular matrix scaffold and held in place in the fluid environment of the knee, where the cells in the blood stimulate ligament healing.10,11,28,37 Thus, the BEAR technique is designed for tears in which the 2 ends of the torn ligament cannot be reapproximated under compression. In this study, only 2 of the 242 screened patients were thought to have insufficient tissue on their preoperative MRI for the BEAR procedure, and no patients were found at surgery to have insufficient tissue (<50%) for the BEAR technique. Only patients with a maximum of 50% loss of the length of the ACL were included in the study; whether this technique would be effective in patients with a greater percentage loss or resorption of ACL tissue is not known.
It is unclear why some scaffolding materials, when placed in the joint environment, causes a significant immune or inflammatory response. Until now, the majority of collagen scaffolds used in musculoskeletal indications have been crosslinked, a process that binds the collagen molecules together, making the material mechanically strong. However, materials that have been crosslinked also may contain residual amounts of the crosslinking agent.6 The high stiffness of the crosslinked material would also discourage cell ingrowth and remodeling of the material and may stimulate an immune reaction characterized by foreign-body giant cells and chronic inflammation.32 The BEAR scaffold is not crosslinked. Preclinical studies have shown that cells permeate through the scaffold within 1 week of implantation,18 and that those cells have already started to remodel the BEAR material, which is completely resorbed by 8 weeks after surgery.25 In contrast, crosslinked scaffold materials are still present in the surgical site for months and sometimes years after implantation. Thus, they may continue to act as a potential inflammatory stimulant, which may require surgical removal.24 Second, some implanted collagen materials contain a relatively high DNA content from the host source—human, bovine, or porcine9—which may also contribute to the high rate of rejection seen when these materials are implanted into the human joint.34 In contrast, the BEAR implant is processed to reduce the bovine DNA content to less than 50 ng per milligram of scaffold. This high degree of xenogenic DNA clearing may also contribute to the lack of synovial or inflammatory response seen in the preclinical testing16,25 and now in the early clinical testing of this scaffold. Third, until now, collagen implants for enhancing ligament or tendon repair have been made using the entire collagen molecule.4 While the center portion of collagen is known to be very similar between species, the 3′ and 5′ ends of the collagen molecule are species specific. The BEAR implant has collagen that has been treated with a specific enzyme to remove the 3′ and 5′ ends of the collagen, leaving the center section of the collagen molecule that is least likely to stimulate an immune reaction. Last, the BEAR scaffold is designed to be hydrophilic to easily absorb the patient’s own blood and to encourage it to clot within the BEAR scaffold. We believe this is the first scaffold to be designed to be used with autologous blood to create a healing environment. While the BEAR scaffold is only a carrier for the autologous blood, it is able to hold the platelets, red blood cells, and white blood cells in the wound site of the ACL, allowing those cells to stimulate the ligament cells around them to invade the scaffold and regenerate the injured ligament tissue.14,19,25
Prior in vitro and preclinical animal studies have demonstrated consistently that whole blood is an excellent biologic stimulating agent for ACL repair. Stem cell enrichment of whole blood had no effect on in vivo ligament healing in a large animal model,28 and both in vitro and in vivo studies have shown that physiologic concentrations of platelets work as well or better than platelet-rich plasma in stimulating ACL repair.19,36 In addition, the inclusion of erythrocytes in the biologic stimulus has been found to increase collagen production within the ligament scar, and thus, erythrocyte removal during the process of making platelet-rich plasma may be detrimental to stimulating ligament healing.11 Thus, whole blood was used for this initial study, although it is certainly possible that other biologics may be found to be more beneficial in follow-on studies.
This study has several limitations. First, only a small number of patients were enrolled in the study to evaluate safety. Thus, adverse events expected to have relatively low occurrence rates (eg, deep joint infection occurs in less than 1% of patients undergoing ACL reconstruction) would not likely be detected in this cohort. However, for complications noted in prior studies of extracellular matrix patches,13 where a serious inflammatory response was seen in 20% of patients, there was an 89% chance of observing such a response in our cohort of 10 BEAR patients. Nonetheless, this study was designed with the guidance of the FDA to show that a long-term efficacy trial would be reasonably safe to perform. In addition, differences in outcomes between the ACL reconstruction and BEAR groups, which were relatively small in comparison to the standard deviations of the measurements, would also not be detectable in this sample size. The BEAR procedure is performed through an arthrotomy, while ACL reconstruction was performed arthroscopically. While this did not affect the primary or secondary outcome measures reported here, it may be a variable that contributes to efficacy differences in longer-term and larger studies that may have the power to detect such differences. Thus, conclusions about efficacy or performance cannot be drawn in this study. Finally, the length of follow-up was only 3 months. While this is long enough to detect complications related to the resorbable scaffold (ie, an inflammatory reaction to the material), it is not long enough to begin to see if performance of the healing ligament can compare with that of a quadruple hamstring tendon autograft. Future work, including longer follow-up of this initial cohort, will be geared toward demonstrating the efficacy of the BEAR procedure.
However, with these data, we have demonstrated that the use of the BEAR scaffold in these 10 patients did not result in a large percentage of patients having an infection or a severe inflammatory reaction, arthrofibrosis, or a reaction that required scaffold removal. In addition, the Lachman testing results suggest the stability of the knee may be reasonable at this short time point. Finally, the continuous ACL fibers seen on the 3-month postoperative MRIs in all subjects suggest healing ACL tissue. Combined, these findings suggest that the BEAR technique may be a method deserving of further study in a larger number of patients.
ACKNOWLEDGMENT
The authors acknowledge the significant contributions of the scaffold development team, including Gabriel Perrone, Gordon Roberts, Doris Peterkin, and Jakob Sieker; the clinical trial team, including Bethany Trainor, Andrea Hale, and Shanika Coney; and the imaging team, including Robert Mulkern, Alison Biercevicz, and Ata Kiapour. In addition, they acknowledge the contributions of their medical safety monitoring team of Joseph DeAngelis and Peter Nigrovic, their data monitor Margaret Malsch, as well as the clinical care team for the trial patients, including Nicole Bottino, Stephanie Burgess, Casey Gavin, Andrea Cianci, Mariah Mullen, Elspeth Hart, Elizabeth Kramer, Patrick Vavken, Jennifer Beck, Melissa Christino, Dai Sugimoto, Emily Hanson, Stacey Murphy, Ariana Moccia, Andrea Stracciolini, Michael Beasley, William Meehan, Michael O’Brien, Cynthia Stein, Kathryn Ackerman, Ellen Geminiani, Bridget Quinn, Pierre D’Hemecourt, Lisa Vopat, Gianmichel Corrado, Stacey Gigante, Dylan Taylor, and Elizabeth Killkelley. The authors are also grateful for the study design guidance provided by the Division of Orthopedic Devices at the Center for Devices and Radiological Health at the US Food and Drug Administration, particularly the efforts of Jemin Dedania and Neil Barkin.
Footnotes
The content of this article solely the responsibility of the authors and does not necessarily represent the official views of the National Football League Players Association, Harvard University, the National Institutes of Health, or Boston Children’s Hospital.
One or more of the authors has declared the following potential conflict of interest or source of funding: Funding for this study was provided by the Translational Research Program at Boston Children’s Hospital, the Children’s Hospital Orthopaedic Surgery Foundation, the Children’s Hospital Sports Medicine Foundation as well as the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases through grant numbers R01-AR065462 and R01-AR056834. This work also was conducted with the additional support of the National Football League Players Association (NFLPA). M.M.M. is an inventor on patents held by Boston Children’s Hospital regarding the use of collagen materials to stimulate ligament repair.
References
- 1. Anderson AF, Irrgang JJ, Kocher MS, Mann BJ, Harrast JJ. The International Knee Documentation Committee Subjective Knee Evaluation Form: normative data. Am J Sports Med. 2006;34:128–135. [DOI] [PubMed] [Google Scholar]
- 2. Andersson C, Odensten M, Gillquist J. Knee function after surgical or nonsurgical treatment of acute rupture of the anterior cruciate ligament: a randomized study with a long-term follow-up period. Clin Orthop Relat Res. 1991;264:255–263. [PubMed] [Google Scholar]
- 3. Bray RC, Leonard CA, Salo PT. Correlation of healing capacity with vascular response in the anterior cruciate and medial collateral ligaments of the rabbit. J Orthop Res. 2003;21:1118–1123. [DOI] [PubMed] [Google Scholar]
- 4. Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am. 2006;88:2665–2672. [DOI] [PubMed] [Google Scholar]
- 5. DiFelice GS, Villegas C, Taylor S. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31:2162–2171. [DOI] [PubMed] [Google Scholar]
- 6. Duan X, Sheardown H. Crosslinking of collagen with dendrimers. J Biomed Mater Res A. 2005;75:510–518. [DOI] [PubMed] [Google Scholar]
- 7. Feagin JA, Jr, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1975;4:95–100. [DOI] [PubMed] [Google Scholar]
- 8. Fleming BC, Spindler KP, Palmer MP, Magarian EM, Murray MM. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37:1554–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilbert TW, Freund JM, Badylak SF. Quantification of DNA in biologic scaffold materials. J Surg Res. 2009;152:135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harrison S, Vavken P, Kevy S, Jacobson M, Zurakowski D, Murray MM. Platelet activation by collagen provides sustained release of anabolic cytokines. Am J Sports Med. 2011;39:729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harrison SL, Vavken P, Murray MM. Erythrocytes inhibit ligament fibroblast proliferation in a collagen scaffold. J Orthop Res. 2011;29:1361–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. [DOI] [PubMed] [Google Scholar]
- 13. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1238–1244. [DOI] [PubMed] [Google Scholar]
- 14. Jacobson M, Fufa D, Abreu EL, Kevy S, Murray MM. Platelets, but not erythrocytes, significantly affect cytokine release and scaffold contraction in a provisional scaffold model. Wound Repair Regen. 2008;16:370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Joshi SM, Mastrangelo AN, Magarian EM, Fleming BC, Murray MM. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med. 2009;37:2401–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Magarian EM, Vavken P, Connolly SA, Mastrangelo AN, Murray MM. Safety of intra-articular use of atelocollagen for enhanced tissue repair. Open Orthop J. 2012;6:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malcarney HL, Bonar F, Murrell GA. Early inflammatory reaction after rotator cuff repair with a porcine small intestine submucosal implant: a report of 4 cases. Am J Sports Med. 2005;33:907–911. [DOI] [PubMed] [Google Scholar]
- 18. Mastrangelo AN, Haus BM, Vavken P, Palmer MP, Machan JT, Murray MM. Immature animals have higher cellular density in the healing anterior cruciate ligament than adolescent or adult animals. J Orthop Res. 2010;28:1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mastrangelo AN, Vavken P, Fleming BC, Harrison SL, Murray MM. Reduced platelet concentration does not harm PRP effectiveness for ACL repair in a porcine in vivo model. J Orthop Res. 2011;29:1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murray MM, Fleming BC. Biology of anterior cruciate ligament injury and repair: Kappa Delta Ann Doner Vaughn Award paper 2013. J Orthop Res. 2013;31:1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41:1762–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murray MM, Magarian E, Zurakowski D, Fleming BC. Bone-to-bone fixation enhances functional healing of the porcine anterior cruciate ligament. Arthroscopy. 2010;26(suppl):S49–S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murray MM, Martin SD, Martin TL, Spector M. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82:1387–1397. [DOI] [PubMed] [Google Scholar]
- 24. Namdari S, Melnic C, Huffman GR. Foreign body reaction to acellular dermal matrix allograft in biologic glenoid resurfacing. Clin Orthop Relat Res. 2013;471:2455–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Proffen BL, Perrone GS, Fleming BC, et al. Electron beam sterilization does not have a detrimental effect on the ability of extracellular matrix scaffolds to support in vivo ligament healing. J Orthop Res. 2015;33:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Proffen BL, Perrone GS, Fleming BC, et al. Effect of low-temperature ethylene oxide and electron beam sterilization on the in vitro and in vivo function of reconstituted extracellular matrix-derived scaffolds. J Biomater Appl. 2015;30:435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Proffen BL, Perrone GS, Roberts G, Murray MM. Bridge-enhanced ACL repair: A review of the science and the pathway through FDA investigational device approval. Ann Biomed Eng. 2015;43:805–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Proffen BL, Vavken P, Haslauer CM, et al. Addition of autologous mesenchymal stem cells to whole blood for bioenhanced ACL repair has no benefit in the porcine model. Am J Sports Med. 2015;43:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Soler JA, Gidwani S, Curtis MJ. Early complications from the use of porcine dermal collagen implants (Permacol) as bridging constructs in the repair of massive rotator cuff tears. A report of 4 cases. Acta Orthop Belg. 2007;73:432–436. [PubMed] [Google Scholar]
- 30. Steadman JR, Cameron-Donaldson ML, Briggs KK, Rodkey WG. A minimally invasive technique (“healing response”) to treat proximal ACL injuries in skeletally immature athletes. J Knee Surg. 2006;19:8–13. [DOI] [PubMed] [Google Scholar]
- 31. Steadman JR, Matheny LM, Briggs KK, Rodkey WG, Carreira DS. Outcomes following healing response in older, active patients: a primary anterior cruciate ligament repair technique. J Knee Surg. 2012;25:255–260. [DOI] [PubMed] [Google Scholar]
- 32. Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg Am. 2006;88:2673–2686. [DOI] [PubMed] [Google Scholar]
- 33. Vavken P, Fleming BC, Mastrangelo AN, Machan JT, Murray MM. Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy. 2012;28:672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89:786–791. [DOI] [PubMed] [Google Scholar]
- 35. Wasmaier J, Kubik-Huch R, Pfirrmann C, Grehn H, Bieg C, Eid K. Proximal anterior cruciate ligament tears: the healing response technique versus conservative treatment. J Knee Surg. 2013;26:263–271. [DOI] [PubMed] [Google Scholar]
- 36. Yoshida R, Cheng M, Murray MM. Increasing platelet concentration in platelet-rich plasma inhibits anterior cruciate ligament cell function in three-dimensional culture. J Orthop Res. 2014;32:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoshida R, Murray MM. Peripheral blood mononuclear cells enhance the anabolic effects of platelet-rich plasma on anterior cruciate ligament fibroblasts. J Orthop Res. 2013;31:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoshida R, Vavken P, Murray MM. Decellularization of bovine anterior cruciate ligament tissues minimizes immunogenic reactions to alpha-gal epitopes by human peripheral blood mononuclear cells. Knee. 2012;19:672–675. [DOI] [PMC free article] [PubMed] [Google Scholar]