ABSTRACT
The morphogenetic movement associated with neural tube closure (NTC) requires both positive and negative regulations of cell proliferation. The dual requirement of cell division control during NTC underscores the importance of the developmental control of cell division. In the chordate ascidian, midline fusions of the neural ectoderm and surface ectoderm (SE) proceed in the posterior-to-anterior direction, followed by a single wave of asynchronous and patterned cell division in SE. Before NTC, SE exhibits synchronous mitoses; disruption of the synchrony causes a failure of NTC. Therefore, NTC is the crucial turning point at which SE switches from synchronous to patterned mitosis. Our recent work discovered that the first sign of patterned cell division in SE appears was an asynchronous S-phase length along the anterior-posterior axis before NTC: the asynchrony of S-phase is offset by the compensatory G2-phase length, thus maintaining the apparent synchrony of cell division. By the loss of compensatory G2 phase, the synchronized cell division harmoniously switches to a patterned cell division at the onset of NTC. Here we review the developmental regulation of rate and pattern of cell division during NTC with emphasis on the switching mechanism identified in our study.
KEYWORDS: ascidian, cdc25, cell cycle, chordate, Ciona, epidermis, G2 phase, morphogenesis, neural folds, neural tube closure
Introduction
The dorsal hollow neural tube is a shared characteristic of chordates and formed in large part by the formation, shaping and folding of neural plate followed by midline fusion of neural folds.1-4 Studies performed mainly in mice and chicks have indicated that the multistep morphogenetic events of neural tube closure (NTC) require the tight spatiotemporal regulation of cell division.1,2 The importance of the spatiotemporal regulation of cell division for NTC can be partly explained by the dual effects of cell division on the morphogenetic movement; cell division can facilitate or inhibit morphogenetic movement in a context-depending manner. In both cases, the spatiotemporal regulation of cell division is the key factor for the proper progression and completion of NTC as we briefly review in the following sections.
Cell proliferations that facilitate neural tube morphogenesis
The neural plate of vertebrates is a neuroepithelium that undergoes symmetrical proliferative cell divisions (progenitor expansion) during NTC; only after the completion of NTC, the neural plate undergoes asymmetric cell divisions and differentiation (neurogenesis).1 If the switch from symmetric to asymmetric divisions (i.e., the balance of cell division and differentiation) in the neural plate is accelerated or delayed, defects in NTC arise.5-7 In addition, proliferation of the tissues that have physical contact with the cranial and spinal regions of neural plate, namely mesenchyme and hindgut endoderm, respectively, are important for the proper NTC in each region. These notions are suggested by the defects of NTC in mouse mutants for Twist and Grainyhead-3 transcription factors, which are required for the proliferation of mesenchyme and hindgut endoderm, respectively.8,9 Moreover, requirement of surface ectoderm for NTC has been suggested by the NTC defects in the mouse mutant for transcription factor AP-2α, whose epidermal expression is thought to be crucial for NTC.10,11 Although the AP-2α-regulated cellular process required for NTC remains unclear,12 cell proliferation is one possible target.13,14 On a related note, a study in chicks has suggested that the orientation of cell division in surface ectoderm is important for the bending of neural plate.2,15 Overall, proper NTC requires the coordinated proliferation of multiple tissues. Understanding how the deregulation of proliferation in tissues other than the neural plate leads to the defects of NTC, probably through an imbalance in the mechanical forces acting between tissues,8,9 is an important future challenge.
Morphogenetic cell shape changes that require a temporal cell division arrest
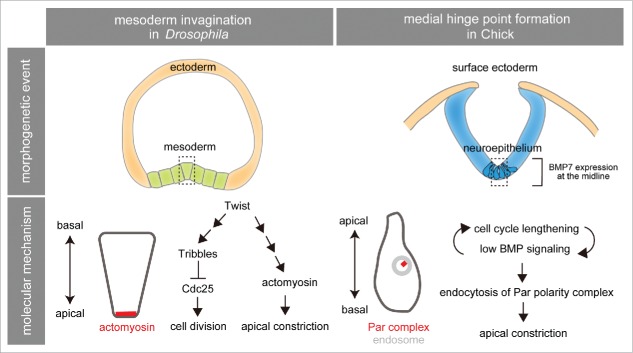
An important effect of cell division on the morphogenetic movement has been suggested from the studies of Drosophila mesoderm invagination (Fig. 1, left).16-18 In this process, mesodermal cells constrict their apical surface in an actomyosin-dependent manner. Since this important cell shape change (called ‘apical constriction’) depends on the actomyosin, which is differently organized with the actomyosin required for cytokinesis, apical constriction is incompatible with cell division. Therefore, the cell division of the mesoderm is transiently arrested during the invagination in order to avoid the competition between the apical constriction and cell division against the reorganization of actomyosin. The cell cycle phase responsible for this temporal cell division arrest is the G2 phase, since the genetic screening for genes that compromise the coordination of cell division and invagination of mesodermal cells identified the putative serine/threonine kinase Tribbles,16-18 whose major target is the activator of G2/M transition, Cdc25.
Figure 1.
The temporal cell division arrest is important for 2 types of apical constriction, the mesoderm invagination in Drosophila and the medial hinge point formation in chicks. In the upper column, the regions of apical constriction are indicated by the dotted boxes. In the bottom column, the molecular events regulating the apical constriction are shown schematically.
The incompatibility of morphogenetic cell shape change and cell division has not been extensively studied during NTC compared to the mesodermal invagination in Drosophila. Nonetheless, stepwise process of NTC in vertebrate embryos, which involves various cell shape changes that are dependent on actomyosin, may also be incompatible with cell division as we explain below. During NTC, the neural plate is narrowed along the medio-lateral axis and concomitantly elongated along the anterior-posterior (A-P) axis by the convergent extension of neural plate.1-4 This process can be cooperatively promoted by apical junction rearrangement under the control of planar cell polarity (PCP) signaling.19,20 At the molecular level, the PCP-related cadherin Celsr1 causes an apical junction rearrangement through the DAAM1/PDZ-RhoGEF/Rho-kinase pathway to activate the actomyosin-dependent contraction of cell junction.20 Whether or not cell division control is important for the apical junction rearrangement remains unknown. Interestingly, however, the convergent extension of the paraxial mesoderm in Xenopus gastrula, which also depends on the PCP signaling and actomyosin, requires a reduction of cell division.21,22 These studies point to the possibility that the PCP-dependent convergent extension during NTC may also be incompatible with cell division.
At the second step of NTC, neural plate is folded at the medial hinge point (MHP).1-4 This process depends on both PCP signaling20 and the actin-binding protein Shroom, which activates the contractility of actomyosin bundles at adherens junction,23,24 leading to shrinkage of the apical area in the MHP cells. Nevertheless, the formation of MHP could not be blocked even if the actin filament is disrupted by cytochalasin D treatment during NTC in both mice and chicks, suggesting that the bending is promoted by another mechanism that is independent of actin filament organization.25,26 The neural plates of mice and chicks are pseudostratified neuroepithelia in which the bipolar neural progenitors perform cell division at the apical side.27 A lengthening of the cell cycle is observed in MHP. Based on this observation, it was proposed that the cell cycle lengthening is responsible for facilitating the basal positioning of the nucleus and concomitant apical constriction of MHP cells.27 Indeed, recent studies in chicks revealed interesting links between cell cycle lengthening, apical constriction and BMP signaling in MHP (Fig. 1, right).28-30 According to these studies, the cell cycle lengthening is intimately coupled with low BMP signaling at MHP. The low BMP-signaling state also facilitates endocytosis of PAR3-PAR6-aPKC complex, thereby causing the apical constriction of MHP cells.28-30 The upstream mechanism regulating the variation of cell cycle length along the dorsoventral axis including the cell cycle lengthening at MHP is not well understood; however, one gene responsible for the defects in NTC in mice encodes Phactr4 protein phosphatase 1. The mutants of this gene exhibit increased proliferation in the ventral side of neural tube.31 The NTC defects in this mouse can be rescued by the mutation of E2F, which promotes cell cycle progression. This study suggests the importance of dorsoventrally regulated proliferation for proper NTC. Although the bending of neural plate at MHP is promoted by multiple mechanisms and more complex than the mesoderm invagination in Drosophila, temporal cell division arrest by cell cycle lengthening is important for both types of apical constriction (Fig. 1).
Relatively little is known about the final step of NTC, fusion of the neural folds, because genetic analyses have been complicated by the secondary defects in the earlier steps.1 The neural fold is made up of the borders of neural and surface ectoderms. The left and right neural folds form a new contact at the dorsal midline, thereby making a seamless neural tube and the overlying epidermal layer.1-4 It has been postulated that the cell membrane protrusions at the leading edge of the surface ectoderm are important for the fusion of neural folds.1,3 A recent study used conditional knockout of the Rho family small GTPases to demonstrate the essential role of the regulation of actomyosin in the surface ectoderm for forming the cell protrusions required for the fusion of neural folds.32 Although the regulation of cell division during the fusion of neural folds has not been well studied in vertebrates, our studies in the chordate ascidian revealed the crucial role of spatiotemporally regulated cell division for the proper cell shape changes during neural fold fusion as we describe in the following sections.33,34
Ascidian as a simple model of cell cycle regulation during NTC
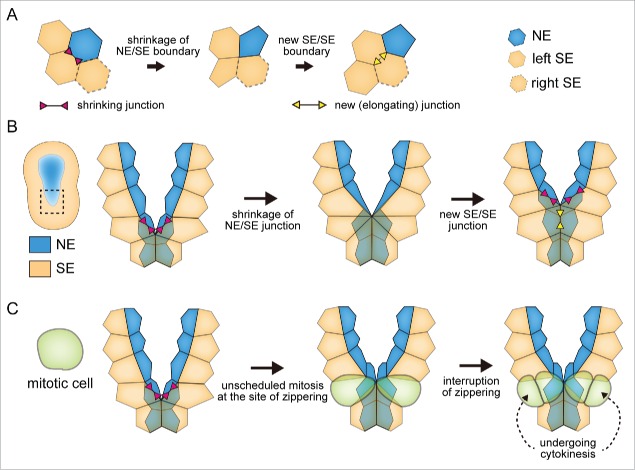
A prominent feature of NTC is its directional progression along the A-P axis,1-4 which is most apparent during the fusion of neural folds. The central nervous system of urochordate ascidian consists of fewer than 400 neural cells, and the cell lineages of neural cells and neighboring surface ectodermal cells have been well described.4,35,36 These characteristics are advantageous for the analysis of neural cell behaviors, and thus the cellular mechanism of neural fold fusion was recently revealed in the ascidian Ciona intestinalis.37 Actomyosin-driven rearrangement of the apical cell junction is a well-established mechanism of cell shape change that can promote morphogenesis (Fig. 2A).38 In Ciona, fusion of the neural ectoderm (NE) and surface ectoderm (SE) occurs simultaneously at the dorsal midline. Live analysis of the apical cell junction during this process revealed that the junction between NE and SE shrinks by the actomyosin contractility that is activated by RhoA/Rho-kinase signaling. As a result, cells of NE and SE are rearranged so that the NEs and SEs on the left and right neural folds form new contacts and cell junctions (Fig. 2B).37 These apical junction rearrangement events between NE and SE occur repeatedly, thereby zipping-up the neural plate into a tube. Although this mechanism is primarily important for the directional progression of the zippering in Ciona, filopodia is also observed, which may support the progression of the process of zippering.33
Figure 2.
(A) Basic process of apical junction rearrangement. The cells are labeled with NE, left SE or right SE to show that apical cell rearrangement similar to the one shown in A can be seen during the zippering shown in B. The shrinkage of NE/SE junction (inward double-arrowheads colored magenta) enables the subsequent formation of a new elongating junction between the left and right SEs (outward double-arrowheads colored yellow). (B) The rearrangement of apical junction during zippering in the Ciona embryo is shown in the same manner as in (A). (C) The cell shape change accompanying unscheduled cell division interrupts the shrinkage of apical junction.
Before the initiation of zippering, the epidermal cells in the surface ectoderm (SE) of ascidian embryos perform rapid and synchronous cell divisions, which may function as proliferative cell divisions required for expansion of the epidermis. Our study in 2011 revealed that the epidermal cell cycle is prolonged at the G2 phase during the zippering, and this prolongation is attributable to down-regulation of the G2/M regulator cdc25.33 The other key finding of this study was that the timings of the zippering and cell division along the A-P axis are tightly coordinated. Before the zippering, the epidermal cells perform synchronous cell division. After the zippering, however, the epidermal cells show a cell division wave in the posterior-to-anterior direction, which chases the progression of the zippering. More concretely, posterior epidermal cells tend to divide earlier than anterior cells. In this paper, we refer to the cell division wave characterized by asynchronous cell division timing in a single round of cell cycle as ‘patterned cell division’. The two changes occurring at the onset of zippering (1) lengthening of the G2 phase and (2) the switch from synchronous to patterned cell division were suggested to be important for postponing the occurrence of patterned cell division until the completion of zippering.33 Indeed, shortening of the G2 phase specifically in the epidermis by cdc25 overexpression resulted in a disruption of the zippering, because mitoses interrupted the morphogenetic movement (Fig. 2C),33 demonstrating the incompatibility of the zippering with the epidermal cell division. In contrast, the cell cycle phase and its regulator that were responsible for the switch from synchronous to patterned cell division at the onset of NTC remained unclear.33 Therefore, our study in 2016 elucidated the respective contribution of the S and G2 phases to the synchronous-to-patterned switch of the cell division pattern (Fig. 3).34
Figure 3.
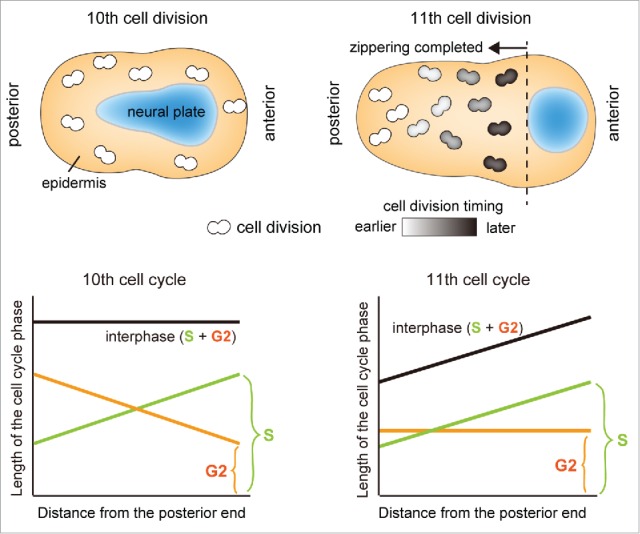
Difference in cell cycle length at the 10th cell cycle (before the zippering) and at the 11th cell cycle (after the zippering) in Ciona. In the upper row, the spatiotemporal variation of cell division is shown schematically. The epidermis and the neural plate uncovered by the epidermis are colored orange and blue, respectively. The epidermal cells undergoing cell division are colored with the gray scale according to the timing of mitosis. In the left side of the dotted line, zippering is completed and the epidermis overlaid the neural tissue. Note that the cell division wave does not cross the dotted line because 11th mitosis occurs after the zippering. In the bottom row, the dependencies of the S- and G2-phase length on the relative position of cells along the A-P axis are shown schematically. In the 10th cell cycle, the graded S phase length and compensatory G2 phase length summed up to the constant interphase length. In contrast, in the 11th cell cycle, the graded S phase length and constant G2 phase length summed up to the graded interphase length.
Distinct roles of the S and G2 phases in regulation of the cell division pattern
To distinguish the S and G2 phases in live embryos, we employed a live cell cycle marker composed of a proliferating cell nuclear antigen and fluorescent protein (PCNA-GFP fusion),39 which has been applied to cell-cycle analysis of the neuroepithelium in zebrafish.40 This method allowed us to quantify the lengths of S and G2 phases, and plot them as functions of the distance from the posterior end of the embryos (Fig. 3, bottom row). We found that the first sign of patterned epidermal cell division exists in the synchronous cell cycle that is one cell cycle earlier than the patterned cell division as an asynchronous S-phase length along the A-P axis that is offset by a compensatory G2-phase length (Fig. 3, left column).34 This compensation of S-phase asynchrony by G2 phase maintains the apparent synchrony of the epidermal cell division before the zippering. At the onset of zippering, loss of the compensatory G2 phase occurs, thereby converting the synchronized cell division into a patterned cell division corresponding to the S-phase length (Fig. 3, right column). The compensatory G2-phase length showed a correspondence with the asymmetric expression of the G2/M regulator cdc25 along the A-P axis, which is the key regulator of the timing of epidermal cell division in Ciona. Moreover, analyses of the cis-regulatory element of cdc25 revealed that the asymmetric expression of cdc25 requires the transcriptional activation by AP-2 and GATA, both of which show asymmetric gene expressions along the A-P axis by themselves.34 In vertebrates, transcription of AP-2 can be modulated by retinoic acid,41,42 which is the key morphogen regulating formation of the A-P axis. The asymmetric expression of GATA along the A-P axis has also been reported in vertebrates.43,44 These studies provide interesting parallels between the expression of these genes in ascidians and vertebrates. In ascidians, the disruption of AP-2 and GATA causes the loss of asymmetric cdc25 expression and the compensatory G2 phase, which in turn causes the premature patterned cell division before NTC. Premature loss of the compensatory G2 phase also resulted in the unscheduled cell division in the cell undergoing the shrinkage of apical junction at the NE/SE junction thereby interrupting the apical junction rearrangement and zippering (Fig. 2C).34 This result suggests that the switch from synchronous to patterned epidermal cell division must be tightly regulated for the normal progression of the zippering. In summary, this study demonstrated the distinct contributions of S and G2 phase for the switch in epidermal cell division pattern during NTC: S-phase asynchrony provides a trigger of the patterned cell division, and the developmentally regulated compensatory G2 phase determines the timing of the switch for patterned cell division. Interestingly, a similar compensatory relationship between the S and G2 phase was also observed in the neural plate, the analysis of which is a key future challenge.34
Cell cycle compensation as a coordinator of the timing of cell division with growth and morphogenesis
The phenomenon of cell cycle compensation that maintains the rate of cell division in spite of the changes in the length of each cell cycle phase has been recognized from the early days of the genetic analysis of the basic cell cycle control. For example, a temperature-sensitive fission-yeast mutant of the cell cycle-regulator gene wee1 shows the shortening of the G2 phase, but this is offset by the lengthening of the G1 phase at the permissive temperature,45 and therefore the yeast does not show any apparent change in the total cell cycle length at the permissive temperature. Likewise, shortening of the G1 phase by the overexpression of cyclins in certain mammalian cells causes lengthening of the G2 phase, thereby maintaining the total cell cycle length.46,47 More recently, a study on the Drosophila wing imaginal disc has shown that such cell cycle compensation can also be observed in a growing tissue of the multicellular organism.48 The study in Drosophila revealed that the cell cycle compensation depends on the negative feedback regulations of E2F activity. E2F promotes the activation of G1/S- and M-Cdks, both of which in turn inhibit the activity of E2F. These negative feedback regulations can compensate for the shortening of the G1 phase by the lengthening of G2 phase and vice versa, providing the first mechanistic insight into the phenomenon of cell cycle compensation. In the absence of E2F, cell cycle compensation fails, and the frequency of apoptosis is increased when the cells are forced to shorten or lengthen their G1 or G2 phases.48 Therefore, the authors of this study postulated that the cell cycle compensation is a homeostatic mechanism that insulates the regulation of the cell division rate from the effects elicited by growth factors that can shorten or lengthen a particular phase of the cell cycle. The compensation between the lengths of the S and G2 phases in the epidermis of ascidians is reminiscent of the concept of cell cycle compensations seen in other organisms, but the fundamental difference is that the compensatory G2 phase in the ascidian is developmentally regulated, since we showed that the transcription of cdc25 is regulated by tissue-specific transcription factors rather than a ubiquitous factor such as E2F.34,49 Our understanding of the mechanism of cell cycle compensation during the NTC of ascidians is still incomplete. Indeed, there are at least 2 key remaining questions in this system. First, what is the mechanism regulating the S-phase asynchrony of epidermal cells, which should also be regulated by the developmental mechanism relevant to the patterning along the A-P axis? And second, how generalizable is the developmentally regulated cell cycle compensation during NTC to other tissues or species? The latter question can be addressed by elucidating the cell cycle compensation in the neural plate of Ciona. It would also be important in the future to examine whether the 2 types of cell cycle compensation identified in the invertebrate model organisms namely Drosophila and Ciona, are also relevant to the coordination of growth and morphogenesis during the NTC of vertebrates.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Takaki Miyata and Dr. Tomoyasu Shinoda (Nagoya University) for their helpful comments on an earlier version of the manuscript.
References
- [1].Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet 2003; 4:784-93; PMID:13679871; http://dx.doi.org/ 10.1038/nrg1181 [DOI] [PubMed] [Google Scholar]
- [2].Colas JF, Schoenwolf GC. Towards a cellular and molecular understanding of neurulation. Dev Dyn 2001; 221:117-45; PMID:11376482; http://dx.doi.org/ 10.1002/dvdy.1144 [DOI] [PubMed] [Google Scholar]
- [3].Wallingford JB. Neural tube closure and neural tube defects: studies in animal models reveal known knowns and known unknowns. Am J Med Genet C Semin Med Genet 2005; 135C:59-68; PMID:15806594; http://dx.doi.org/ 10.1002/ajmg.c.30054 [DOI] [PubMed] [Google Scholar]
- [4].Nicol D, Meinertzhagen IA. Development of the central nervous system of the larva of the ascidian, Ciona intestinalis L. II. Neural plate morphogenesis and cell lineages during neurulation. Dev Biol 1988; 130:737-66; PMID:3197930; http://dx.doi.org/ 10.1016/0012-1606(88)90364-8 [DOI] [PubMed] [Google Scholar]
- [5].Ishibashi M, Ang SL, Shiota K, Nakanishi S, Kageyama R, Guillemot F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev 1995; 9:3136-48; PMID:8543157; http://dx.doi.org/ 10.1101/gad.9.24.3136 [DOI] [PubMed] [Google Scholar]
- [6].Zhong W, Jiang MM, Schonemann MD, Meneses JJ, Pedersen RA, Jan LY, Jan YN. Mouse numb is an essential gene involved in cortical neurogenesis. Proc Natl Acad Sci U S A 2000; 97:6844-9; PMID:10841580; http://dx.doi.org/ 10.1073/pnas.97.12.6844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lardelli M, Williams R, Mitsiadis T, Lendahl U. Expression of the Notch 3 intracellular domain in mouse central nervous system progenitor cells is lethal and leads to disturbed neural tube development. Mech Dev 1996; 59:177-90; PMID:8951795; http://dx.doi.org/ 10.1016/0925-4773(96)00589-8 [DOI] [PubMed] [Google Scholar]
- [8].Chen ZF, Behringer RR. twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev 1995; 9:686-99; PMID:7729687; http://dx.doi.org/ 10.1101/gad.9.6.686 [DOI] [PubMed] [Google Scholar]
- [9].Gustavsson P, Greene ND, Lad D, Pauws E, de Castro SC, Stanier P, Copp AJ. Increased expression of Grainyhead-like-3 rescues spina bifida in a folate-resistant mouse model. Hum Mol Genet. 2007; 16:2640-6; PMID:17720888; http://dx.doi.org/ 10.1093/hmg/ddm221 [DOI] [PubMed] [Google Scholar]
- [10].Schorle H, Meier P, Buchert M, Jaenisch R, Mitchell PJ. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature 1996; 381:235-8; PMID:8622765; http://dx.doi.org/ 10.1038/381235a0 [DOI] [PubMed] [Google Scholar]
- [11].Zhang J, Hagopian-Donaldson S, Serbedzija G, Elsemore J, Plehn-Dujowich D, McMahon AP, Flavell RA, Williams T. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature 1996; 381:238-41; PMID:8622766; http://dx.doi.org/ 10.1038/381238a0 [DOI] [PubMed] [Google Scholar]
- [12].Pyrgaki C, Liu A, Niswander L. Grainyhead-like 2 regulates neural tube closure and adhesion molecule expression during neural fold fusion. Dev Biol 2011; 353:38-49. PMID:21377456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Eckert D, Buhl S, Weber S, Jäger R, Schorle H. The AP-2 family of transcription factors. Genome Biol 2005; 6:246; PMID:16420676; http://dx.doi.org/ 10.1186/gb-2005-6-13-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang X, Bolotin D, Chu DH, Polak L, Williams T, Fuchs E. AP-2alpha: a regulator of EGF receptor signaling and proliferation in skin epidermis. J Cell Biol 2006; 172:409-41; PMID:16449191; http://dx.doi.org/ 10.1083/jcb.200510002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sausedo RA, Smith JL, Schoenwolf GC. Role of nonrandomly oriented cell division in shaping and bending of the neural plate. J Comp Neurol 1997; 381:473-88; PMID:9136804; http://dx.doi.org/ 10.1002/(SICI)1096-9861(19970519)381:4%3c473::AID-CNE7%3e3.0.CO;2- [DOI] [PubMed] [Google Scholar]
- [16].Grosshans J, Wieschaus E. A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell 2000; 101:523-31. PMID:10850494; http://dx.doi.org/ 10.1016/S0092-8674(00)80862-4 [DOI] [PubMed] [Google Scholar]
- [17].Mata J, Curado S, Ephrussi A, Rorth P. Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell 2000; 101:511-22; PMID:10850493; http://dx.doi.org/ 10.1016/S0092-8674(00)80861-2 [DOI] [PubMed] [Google Scholar]
- [18].Seher TC, Leptin M. Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation. Curr Biol 2000; 10:623-9; PMID:10837248; http://dx.doi.org/ 10.1016/S0960-9822(00)00502-9 [DOI] [PubMed] [Google Scholar]
- [19].Williams M, Yen W, Lu X, Sutherland A. Distinct apical and basolateral mechanisms drive planar cell polarity-dependent convergent extension of the mouse neural plate. Dev Cell 2014; 29:34-46; PMID:24703875; http://dx.doi.org/ 10.1016/j.devcel.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell 2012; 149:1084-1097; PMID:22632972; http://dx.doi.org/ 10.1016/j.cell.2012.04.021 [DOI] [PubMed] [Google Scholar]
- [21].Murakami MS, Moody SA, Daar IO, Morrison DK. Morphogenesis during Xenopus gastrulation requires Wee1-mediated inhibition of cell proliferation. Development 2004; 131:571-80; PMID:14711880; http://dx.doi.org/ 10.1242/dev.00971 [DOI] [PubMed] [Google Scholar]
- [22].Leise WF 3rd, Mueller PR. Inhibition of the cell cycle is required for convergent extension of the paraxial mesoderm during Xenopus neurulation. Development 2004; 131:1703-15; PMID:15084456; http://dx.doi.org/ 10.1242/dev.01054 [DOI] [PubMed] [Google Scholar]
- [23].Hildebrand JD, Soriano P. Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell 1999; 99:485-97; PMID:10589677; http://dx.doi.org/ 10.1016/S0092-8674(00)81537-8 [DOI] [PubMed] [Google Scholar]
- [24].Haigo SL, Hildebrand JD, Harland RM, Wallingford JB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol 2003; 13:2125-37; PMID:14680628; http://dx.doi.org/ 10.1016/j.cub.2003.11.054 [DOI] [PubMed] [Google Scholar]
- [25].Ybot-Gonzalez P, Copp AJ. Bending of the neural plate during mouse spinal neurulation is independent of actin microfilaments. Dev Dyn 1999; 215:273-83; PMID:10398537; http://dx.doi.org/ 10.1002/(SICI)1097-0177(199907)215:3%3c273::AID-AJA9%3e3.0.CO;2-H [DOI] [PubMed] [Google Scholar]
- [26].Schoenwolf GC, Folsom D, Moe A. A reexamination of the role of microfilaments in neurulation in the chick embryo. Anat Rec 1988; 220:87-102; PMID:3348489; http://dx.doi.org/ 10.1002/ar.1092200111 [DOI] [PubMed] [Google Scholar]
- [27].Smith JL, Schoenwolf GC. Role of cell-cycle in regulating neuroepithelial cell shape during bending of the chick neural plate. Cell Tissue Res 1988; 252:491-500; PMID:3396052; http://dx.doi.org/ 10.1007/BF00216636 [DOI] [PubMed] [Google Scholar]
- [28].Eom DS, Amarnath S, Fogel JL, Agarwala S. Bone morphogenetic proteins regulate neural tube closure by interacting with the apicobasal polarity pathway. Development 2011; 138:3179-88; PMID:21750029; http://dx.doi.org/ 10.1242/dev.058602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Eom DS, Amarnath S, Agarwala S. Apicobasal polarity and neural tube closure. Dev Growth Differ 2013; 55:164-72; PMID:23277919; http://dx.doi.org/ 10.1111/dgd.12030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Amarnath S, Agarwala S. Cell-cycle-dependent TGFβ-BMP antagonism regulates neural tube closure by modulating tight junctions. J Cell Sci 2016; PMID:27034139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim TH, Goodman J, Anderson KV, Niswander L. Phactr4 regulates neural tube and optic fissure closure by controlling PP1-, Rb-, and E2F1-regulated cell-cycle progression. Dev Cell 2007; 13:87-102; PMID:17609112; http://dx.doi.org/ 10.1016/j.devcel.2007.04.018 [DOI] [PubMed] [Google Scholar]
- [32].Rolo A, Savery D, Escuin S, de Castro SC, Armer HE, Munro PM, Molè MA, Greene N, Copp AJ. Regulation of cell protrusions by small GTPases during fusion of the neural folds. Elife 2016; 5:e13273; PMID:27114066; http://dx.doi.org/ 10.7554/eLife.13273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ogura Y, Sakaue-Sawano A, Nakagawa M, Satoh N, Miyawaki A, Sasakura Y. Coordination of mitosis and morphogenesis: role of a prolonged G2 phase during chordate neurulation. Development 2011; 138:577-87; PMID:21205801; http://dx.doi.org/ 10.1242/dev.053132 [DOI] [PubMed] [Google Scholar]
- [34].Ogura Y, Sasakura Y. Developmental control of cell-cycle compensation provides a switch for patterned mitosis at the onset of chordate neurulation. Dev Cell 2016; 37:148-61; PMID:27093084; http://dx.doi.org/ 10.1016/j.devcel.2016.03.013 [DOI] [PubMed] [Google Scholar]
- [35].Sasakura Y, Mita K, Ogura Y, Horie T. Ascidians as excellent chordate models for studying the development of the nervous system during embryogenesis and metamorphosis. Dev Growth Differ 2012; 54:420-37; PMID:22524611; http://dx.doi.org/ 10.1111/j.1440-169X.2012.01343.x [DOI] [PubMed] [Google Scholar]
- [36].Pasini A, Amiel A, Rothbächer U, Roure A, Lemaire P, Darras S. Formation of the ascidian epidermal sensory neurons: insights into the origin of the chordate peripheral nervous system. PLoS Biol 2006; 4:e225; PMID:16787106; http://dx.doi.org/ 10.1371/journal.pbio.0040225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hashimoto H, Robin FB, Sherrard KM, Munro EM. Sequential contraction and exchange of apical junctions drives zippering and neural tube closure in a simple chordate. Dev Cell 2015; 32:41-55; PMID:25625209; http://dx.doi.org/ 10.1016/j.devcel.2014.12.017 [DOI] [PubMed] [Google Scholar]
- [38].Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 2004; 429:667-71; PMID:15190355; http://dx.doi.org/ 10.1038/nature02590 [DOI] [PubMed] [Google Scholar]
- [39].Leonhardt H, Rahn HP, Weinzierl P, Sporbert A, Cremer T, Zink D, Cardoso MC. Dynamics of DNA replication factories in living cells. J Cell Biol 2000; 149:271-80; PMID:10769021; http://dx.doi.org/ 10.1083/jcb.149.2.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Leung L, Klopper AV, Grill SW, Harris WA, Norden C. Apical migration of nuclei during G2 is a prerequisite for all nuclear motion in zebrafish neuroepithelia. Development 2011; 138:5003-13; PMID:22028032; http://dx.doi.org/ 10.1242/dev.071522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Williams T, Admon A, Lüscher B, Tjian R. Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Dev 1988; 2:1557-69; PMID:3063603; http://dx.doi.org/ 10.1101/gad.2.12a.1557 [DOI] [PubMed] [Google Scholar]
- [42].Shen H, Wilke T, Ashique AM, Narvey M, Zerucha T, Savino E, Williams T, Richman JM. Chicken transcription factor AP-2: cloning, expression and its role in outgrowth of facial prominences and limb buds. Dev Biol 1997; 188:248-66; PMID:9268573; http://dx.doi.org/ 10.1006/dbio.1997.8617 [DOI] [PubMed] [Google Scholar]
- [43].Read EM, Rodaway AR, Neave B, Brandon N, Holder N, Patient RK, Walmsley ME. Evidence for non-axial A/P patterning in the nonneural ectoderm of Xenopus and zebrafish pregastrula embryos. Int J Dev Biol 1998; 42:763-74; PMID:9727832 [PubMed] [Google Scholar]
- [44].Sheng G, Stern CD. Gata2 and Gata3: novel markers for early embryonic polarity and for non-neural ectoderm in the chick embryo. Mech Dev 1999; 87:213-6; PMID:10495290; http://dx.doi.org/ 10.1016/S0925-4773(99)00150-1 [DOI] [PubMed] [Google Scholar]
- [45].Nurse P, Thuriaux P. Regulatory genes controlling mitosis in the fission yeast Schizosaccharomyces pombe. Genetics 1980; 96:627-37; PMID:7262540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ohtsubo M, Roberts JM. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science 1993; 259:1908-12; PMID:8384376; http://dx.doi.org/ 10.1126/science.8384376 [DOI] [PubMed] [Google Scholar]
- [47].Resnitzky D, Gossen M, Bujard H, Reed SI. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol 1994; 14:1669-79; PMID:8114703; http://dx.doi.org/ 10.1128/MCB.14.3.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Reis T, Edgar BA. Negative regulation of dE2F1 by cyclin-dependent kinases controls cell cycle timing. Cell 2004; 117:253-64; PMID:15084262; http://dx.doi.org/ 10.1016/S0092-8674(04)00247-8 [DOI] [PubMed] [Google Scholar]
- [49].Ogura Y, Sasakura Y. Cell-cycle compensation coupled with developmental patterning. Cell Cycle 2016; 1-2; PMID:27359220 [DOI] [PMC free article] [PubMed] [Google Scholar]