ABSTRACT
Microglia are recognized as major immune cells in the brain. They have been traditionally studied in various contexts of disease, where their activation has been assumed to induce mostly detrimental effects. Recent studies, however, have challenged the current view of microglia, clarifying their essential contribution to the development of neural circuits and brain function. In this review, we particularly discuss the role of microglia as the major orchestrators that regulate adult neurogenesis in the hippocampus. We also review the roles of microglia in seizure-induced adult neurogenesis in the epileptic dentate gyrus. Specifically, we introduce our recent study, in which we identified a novel mechanism by which viable newborn cells in the adult dentate gyrus are phagocytosed and eliminated by microglia after status epilepticus, maintaining homeostasis of the dentate circuitry. This review aims to reconsider the microglial function in adult neurogenesis, especially when they are activated during epileptogenesis, challenging the dogma that microglia are harmful neurotoxic cells.
KEYWORDS: adult neurogenesis, epilepsy, hippocampus, microglia, phagocytosis
Introduction
Microglia are recognized as resident immune cells in the brain that exhibit a phagocytic capacity and originate from erythromyeloid progenitors in the early embryonic yolk sac.1 Recent studies have revealed that microglia engulf and remove less active synapses2,3 in the healthy brain as well as dead cells and cell debris during inflammation, indicating their role in regulating homeostasis of the central nervous system (CNS). However, compared to neurons and astrocytes, the discovery and introduction of microglia into the neuroscience arena has been delayed half a century, which leaves microglia as one of the least-understood cell types in the brain. In particular, the specific molecular and cellular mechanisms through which microglia communicate and interact with other cell types are only beginning to be explored. Here, we summarize and discuss the data concerning the involvement of microglia in adult neurogenesis, a process of generating and functionally incorporating neurons into pre-existing neuronal circuits. Specifically, we focus on adult neurogenesis in the subgranular zone (SGZ) of the hippocampal dentate gyrus and discuss the role of microglia in both physiological and pathological conditions, which is related to our recently published paper entitled ‘Microglia engulf viable newborn cells in the epileptic dentate gyrus’ published in the journal Glia.4
In physiological conditions
Accumulating research has promoted our understanding of the origin of newborn neurons and their survival, maturation and integration into pre-existing neuronal circuits in the adult hippocampus (Fig. 1). Genetic fate-mapping studies have demonstrated that the radial glia-like neural stem cells in the SGZ, an intermittent zone between the granular cell layer and the hilus, are the source of newborn neurons in the dentate gyrus.5 These neural stem cells give rise to intermediate neural progenitor cells (NPCs), which in turn give rise to neuroblasts and immature neurons.6,7 Immature neurons migrate into the inner granule cell layer and differentiate into mature dentate granule cells.8 At each stage of neurogenesis, surveillant microglia are suggested to regulate the fate and development of adult-born neurons.
Figure 1.
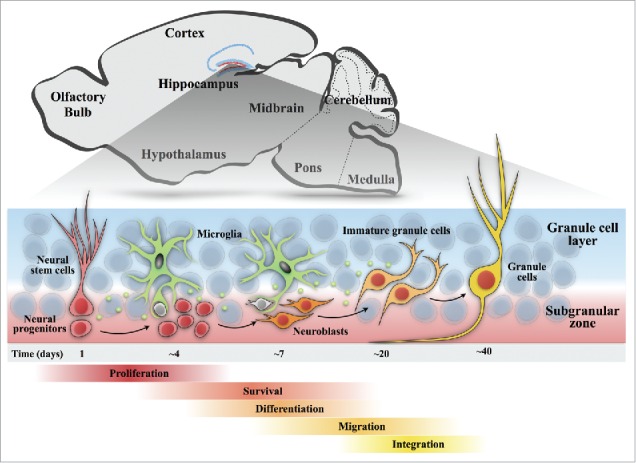
Schematic diagram of the process of adult neurogenesis in the hippocampus. A sagittal view of an adult rodent brain highlighting the dentate neurogenic niche. In the adult dentate gyrus, neurogenesis undergoes 5 continuous stages. Stage 1, proliferation: amplifying neural progenitors are generated from the neural stem cells with their cell bodies located within the subgranular zone and radial processes projecting through the granular cell layer. Stage 2, survival: a large proportion of progenitors undergoes apoptotic death (gray) in the early period of their life. Stage 3, differentiation: progenitors differentiate into immature neurons (orange). Stage 4, migration: immature neurons migrate a short distance into the granule cell layer. Stage 5, integration: new granule cells (yellow) receive inputs from the entorhinal cortex and send axons to synapse CA3 and hilar neurons. In each stage of neurogenesis, surveillant microglia regulate the fate and development of newborn neurons via the engulfment of apoptotic cells (Sierra et al., 2012) and secreting inflammatory and growth factors, such as IL-1β,19 TGF, PDGF, EGF and BDNF26,57.
Proliferation
In normal physiological conditions, newborn cells are constantly generated in the adult SGZ, but this proliferating rate is altered by changes in the surrounding milieu, which are induced by environmental enrichment, voluntary exercise, and aging. Rodents with a free access to a running-wheel exhibit significantly enhanced cell proliferation, as well as improved performance in spatial memory and learning tasks.9 The effects of exercise are not restricted to the proliferation of NPCs. For example, running also increases the activation of both cortical and hippocampal microglia.10,11 Thus, it is possible that the exercise-induced activation of microglia contributes to enhanced NPCs.
Vukovic et al. found that microglia were able to activate latent NPCs through the C-X3-C chemokine receptor 1 (CX3CR1) pathway in the hippocampus of mice that underwent exercise (wheel-running).12 The C-X3-C motif chemokine 1 (CX3CL1)-CX3CR1 signaling is important in regulating the neurotoxic effects of microglia, such as releasing cytokines and abnormal engulfment. Although it is debatable which cell type expresses CX3CL1 and CX3CR1, CX3CL1 is principally expressed in neurons, while CX3CR1 is expressed in microglia.13-15 Previous reports have determined that interactions between CX3CL1 and CX3CR1 contribute to maintaining microglia in a resting phase, partially controlling their neurotoxicity.16,17 In addition to the exercise-related neurogenesis, the CX3CL1-CX3CR1 axis is suggested to be involved in the aging-related reduction of neurogenesis. Both the proliferation rate of NPCs and the expression levels of CX3CL1 decrease with aging, and Bachstetter et al. investigated the relationship between CX3CL1-CX3CR1 signaling and aging-induced effects on neurogenesis.18 They found that the disruption in CX3CL1-CX3CR1 signaling in young adult rodents decreased the proliferation of NPCs by activating interleukin-1β (IL-1β) signaling. The activation of the IL-1 receptor (IL-1R) in NPCs decreases the level of cyclin D1, a regulator of the G1 cell cycle expressed in NPCs, which suppresses NPC proliferation without affecting apoptosis.19
Survival
Ninety percent of newborn cells undergo apoptotic death in the first 1 to 4 d of their life, during the transition from amplifying NPCs to neuroblasts. Thus, the survival rate of newborn cells critically affects the number of neurons incorporated into the hippocampal circuitry. It has been shown that these apoptotic NPCs during the early stage of neurogenesis are effectively and rapidly cleared through phagocytosis by non-activated microglia.20 However, the consequences of microglial phagocytosis on adult hippocampal neurogenesis remain elusive. Treatment of mice with annexin V, which binds to the phosphatidylserine (PS) receptor and prevents the recognition of PS on the surface of apoptotic cells, presumably blocking phagocytosis, increases the number of apoptotic cells and reduces neurogenesis in the SGZ.21 This indicates that blocking microglial-mediated phagocytosis alone is not able to increase the survival rate of NPCs. Indeed, annexin V reduced neurogenesis by decreasing the survival of neuroblasts without affecting the proliferation of NPCs.22 These findings suggest that the clearance of apoptotic newborn cells by microglia promotes the survival rate of NPCs.
Apoptosis of newborn cells may be directly induced by microglia-released cytokines, such as tumor necrosis factor-α (TNF-α). TNF-α triggers the apoptosis of hippocampal neurons via NF-κB translocation.23 The TNF-α receptor tumor necrosis factor receptor 1 (TNF-R1) is expressed in proliferating NPCs, and the survival rate of these cells in SGZ was increased in TNF-R1 knockout mice.24 Another study showed that TNF-α released by microglia induces the apoptosis of NPCs, a process mediated by Bcl-2-associated X (bax) protein, which functions as an apoptotic activator.25 In contrast, microglia-released growth factors not only enhance neuronal proliferation but also neuronal survival. The immunodepletion of transforming growth factor (TGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), or brain-derived neurotrophic factor (BDNF) from microglial conditioned medium resulted in a significant reduction in neuronal survival.26 Furthermore, in the context of the enriched environment, the expression level of insulin-like growth factor-1 (IGF-1) was elevated in microglia in the dentate gyrus,27 which suggested that the effects of an enriched environment on neurogenesis are partially mediated by microglia.
Maturation
In addition to proliferation and survival, microglia have the capacity to guide the differentiation of precursor cells isolated from the embryonic brain and adult mouse NPCs toward a neuronal phenotype. An in vitro study showed that NPC cultures grown in conditioned media from microglia contain a higher proportion of neurons. Furthermore, microglia-released soluble factors, which have not yet been molecularly identified, direct the migration of NPCs.28
When newborn granule cells are synaptically integrated into pre-existing neural circuits, they need to compete with mature granule cells to invade and replace pre-existing synapses29,30 probably in an activity-dependent manner.31,32 Because adult-born immature granule cells are more excitable than mature granule cells,33,34 they are more efficient in generating action potentials, even with weak glutamatergic inputs,35 and probably have a greater chance to win the synaptic sites. Recent studies have demonstrated that microglia preferentially engulf weak or less active synapses, contributing to the development of refined functional neural circuits with strong or more active synapses.2,3 Thus, the microglial-mediated engulfment of pre-existing synapses may be involved in the efficient formation of synapses by newborn granule cells.
Together, in the hippocampal neurogenic niche, microglia are versatile modulators of neurogenesis. Importantly, microglia can either enhance or suppress neurogenesis in response to the environmental milieu.
In epileptic conditions
In the healthy CNS, microglia are in a “surveillance state,” in which they exhibit a highly ramified morphology with thin processes that dynamically move in the brain parenchyma.36 In contrast, upon a pathological insult, such as infection or brain injury, microglia rapidly retract their processes, proliferate and start releasing neurotoxic factors, such as proinflammatory cytokines.37-39
It has been shown that epileptic seizures induce microglial activation in the hippocampus, partly mediated by the activation of Toll-like receptor 9, an innate immune sensor known to recognize microbial DNA.40 In animal models of temporal lobe epilepsy, status epilepticus (SE) acutely enhances adult neurogenesis, which results in an increased number of newborn granule cells.41,42 However, the functional properties of these extra granule cells after SE remain to be clarified. Accumulating evidence suggests that many newborn granule cells exhibit abnormal differentiation after SE and display hilar basal dendrites and aberrant axonal sprouting as well as ectopic settling in the dentate hilus.43-46 Furthermore, the elimination of newborn granule cells after SE decreased both the abnormal sprouting of the granule cell axons and the ectopic positioning of granule cells,46 attenuating spontaneous recurrent seizures.47 In contrast, Jakubs et al. suggested that SE-induced newborn granule cells are normally incorporated into the dentate circuits, possibly serving a compensatory role to restore inhibition.48 Thus, the selective elimination of SE-induced newborn granule cells is likely critical to homeostasis of the activity level of the dentate gyrus.
In our recent study published in Glia,4 we found that microglia selectively engulf extensively proliferating cells after SE to suppress the number of newborn cells to control levels to ensure homeostasis of the dentate circuitry. However, it should be noted that newborn cells were labeled only at 4 d after SE and traced until 11 d after SE. In addition, we found that microglial activation gradually increased after SE, peaking at 6 d after SE and lasting for 2 weeks. Thus, we do not exclude the possibility that seizure-induced newborn cells that were born other than 4 d post-SE survived microglial engulfment more efficiently. These survived newborn cells may account for increased cell numbers after SE in most of long-term tracing studies, in which newborn cells were labeled several times after SE.
It has previously been assumed that microglia engulf dead or dying cells but not living cells49 and that microglia engulf dead newborn neural progenitor cells and their cell debris in the SGZ;20 however, recent studies have indicated that microglia can also engulf living cells during development,50 inflammation,51 and under neuropathological conditions.52,53 This type of phagocytosis is referred to as “primary phagocytosis” because phagocytosis itself is the primary cause of cell death. In contrast, “secondary phagocytosis” refers to the phagocytosis of dying and dead cells (both apoptotic and necrotic) as well as cell debris.
In our study,4 we immunohistochemically analyzed the expression of the apoptosis marker cleaved (active) caspase-3 in the DG and found that microglia are rapidly activated and engulf caspase-3 negative post-SE-born viable cells via primary phagocytosis (Fig. 2). In addition, we showed that the suppression of microglial activation by minocycline resulted in an increase of ectopic newborn cells in the dentate hilus. Although activation of caspase-3 occurs at the beginning of apoptotic processes, leaving the possibility that the caspase-3 negative cells were also undergoing apoptotic processes, we confirmed that caspase-3 negative cells being engulfed by microglia exhibited normal nucleus morphologies. Consistent with our study, a recent study by Abiega et al. showed that microglia engulf viable neurons after SE in the hippocampus.54 Thus, the activation of microglia might be a promising strategy to eliminate ectopic newborn cells, which could provide aberrant excitatory networks in the dentate gyrus after SE, to prevent the resultant epileptogenic processes.
Figure 2.
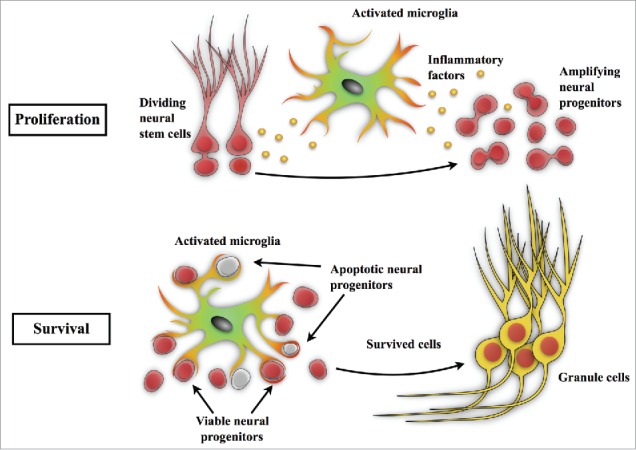
Microglia in seizure-induced neurogenesis. Epileptic seizures increase neurogenesis as well as microglial activation in the dentate gyrus. In the stage of proliferation, activated microglia produce inflammatory cytokines, such as TNFα,40 which suppress the aberrant proliferation of neural stem/progenitor cells. In the survival stage, our recent study4 revealed that both apoptotic and viable newborn cells in the dentate gyrus are phagocytosed and eliminated by microglia after status epilepticus. These studies demonstrate that microglia play a pivotal role in neurogenesis and in maintaining homeostasis of the dentate circuitry after epileptic seizures.
In addition to their phagocytic capacity, microglia contribute to homeostasis of neural circuits through the release of both neurotoxic and neuroprotective factors. Recently, Matsuda et al. reported that after SE, activated microglia secrete TNF-α to attenuate the proliferation of neural progenitor cells in the SGZ.40 Thus, microglia perform a stepwise regulatory mechanism to maintain the number of newly incorporated cells in the dentate circuitry. However, whether microglia regulate the other processes of neurogenesis, including the migration and integration of NPCs in the dentate gyrus, remains to be clarified.
Future studies are necessary to examine whether and how microglia choose which cells to kill and which to keep alive. Several ‘don't-eat-me’ and ‘eat-me’ signals, such as PS and complement molecules, have been suggested to modulate the phagocytic capacity of microglia.2,55,56 Whether these molecules are involved in triggering the primary phagocytosis of progenitor cells during the process of neurogenesis remains to be investigated.
Funding
This work was supported by JSPS KAKENHI Grant Numbers 26460094 and 26117504, and Brain Science Foundation.
References
- [1].Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al.. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010; 330:841-5; PMID:20966214; http://dx.doi.org/ 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres B a, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012. [cited 2014July10]; 74:691-705; PMID:22632727; http://dx.doi.org/ 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al.. Synaptic pruning by microglia is necessary for normal brain development. Science 2011; 333:1456-8; PMID:21778362; http://dx.doi.org/ 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]
- [4].Luo C, Koyama R, Ikegaya Y. Microglia engulf viable newborn cells in the epileptic dentate gyrus. Glia 2016; 64:1508-1517; PMID:27301702; http://dx.doi.org/ 10.1002/glia.23018 [DOI] [PubMed] [Google Scholar]
- [5].Dhaliwal J, Lagace DC. Visualization and genetic manipulation of adult neurogenesis using transgenic mice. Eur J Neurosci 2011; 33:1025-36; PMID:21395845; http://dx.doi.org/ 10.1111/j.1460-9568.2011.07600.x [DOI] [PubMed] [Google Scholar]
- [6].Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson Da, Suhr ST, Ray J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A 1995; 92:11879-83; PMID:8524867; http://dx.doi.org/ 10.1073/pnas.92.25.11879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ming G li, Song H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011; 70:687-702; PMID:21609825; http://dx.doi.org/ 10.1016/j.neuron.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature 2002; 415:1030-4; PMID:11875571; http://dx.doi.org/ 10.1038/4151030a [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 1999; 2:266-70; PMID:10195220; http://dx.doi.org/ 10.1038/6368 [DOI] [PubMed] [Google Scholar]
- [10].Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex 2003; 13:845-51; PMID:12853371; http://dx.doi.org/ 10.1093/cercor/13.8.845 [DOI] [PubMed] [Google Scholar]
- [11].Oláh S, Füle M, Komlósi G, Varga C, Báldi R, Barzó P, Tamás G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 2009; 461:1278-81; PMID:Can't; http://dx.doi.org/ 10.1038/nature08503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vukovic J, Colditz MJ, Blackmore DG, Ruitenberg MJ, Bartlett PF. Microglia Modulate Hippocampal Neural Precursor Activity in Response to Exercise and Aging. J Neurosci 2012; 32:6435-43; PMID:22573666; http://dx.doi.org/ 10.1523/JNEUROSCI.5925-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, et al.. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 2006; 9:917-24; PMID:16732273; http://dx.doi.org/ 10.1038/nn1715 [DOI] [PubMed] [Google Scholar]
- [14].Harrison GP, Miele G, Hunter E, Lever AML. Functional analysis of the core human immunodeficiency virus type 1 packaging signal in a permissive cell line. J Virol 1998; 72:5886-96; PMID:9621050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lauro C, Di Angelantonio S, Cipriani R, Sobrero F, Antonilli L, Brusadin V, Ragozzino D, Limatola C. Activity of adenosine receptors type 1 Is required for CX3CL1-mediated neuroprotection and neuromodulation in hippocampal neurons. J Immunol 2008; 180:7590-6; PMID:18490761; http://dx.doi.org/ 10.4049/jimmunol.180.11.7590 [DOI] [PubMed] [Google Scholar]
- [16].Zujovic V, Benavides J, Vigé X, Carter C, Taupin V. Fractalkine modulates TNF-α secretion and neurotoxicity induced by microglial activation. Glia 2000; 29:305-15; PMID:10652441; http://dx.doi.org/ 10.1002/(SICI)1098-1136(20000215)29:4%3c305::AID-GLIA2%3e3.0.CO;2-V [DOI] [PubMed] [Google Scholar]
- [17].Zujovic V, Schussler N, Jourdain D, Duverger D, Taupin V. In vivo neutralization of endogenous brain fractalkine increases hippocampal TNF?? and 8-isoprostane production induced by intracerebroventricular injection of LPS. J Neuroimmunol 2001; 115:135-43; PMID:11282163; http://dx.doi.org/ 10.1016/S0165-5728(01)00259-4 [DOI] [PubMed] [Google Scholar]
- [18].Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, et al.. Fractalkine and CX3CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging 2011; 32:2030-44; PMID:20018408; http://dx.doi.org/ 10.1016/j.neurobiolaging.2009.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A 2008; 105:751-6; PMID:18178625; http://dx.doi.org/ 10.1073/pnas.0708092105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sierra A, Encinas JM, Deudero JJP, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 2010. [cited 2014November26]; 7:483-95; PMID:20887954; http://dx.doi.org/ 10.1016/j.stem.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lue LF, Rydel R, Brigham EF, Yang LB, Hampel H, Murphy GM, Brachova L, Yan S Du, Walker DG, Shen Y, et al.. Inflammatory repertoire of Alzheimer's disease and nondemented elderly microglia in vitro. Glia 2001; 35:72-9; PMID:11424194; http://dx.doi.org/ 10.1002/glia.1072 [DOI] [PubMed] [Google Scholar]
- [22].Lu Z, Elliott MR, Chen Y, Walsh JT, Klibanov AL, Ravichandran KS, Kipnis J. Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nat Cell Biol 2011; 13:1076-83; PMID:21804544; http://dx.doi.org/ 10.1038/ncb2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang L, Lindholm K, Konishi Y, Li R, Shen Y. Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J Neurosci 2002; 22:3025-32; PMID:11943805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJH, Bonde S, Kokaia Z, Jacobsen S-EW, Lindvall O. Tumor Necrosis Factor Receptor 1 Is a Negative Regulator of Progenitor Proliferation in Adult Hippocampal Neurogenesis. J Neurosci 2006; 26:9703-12; PMID:16988041; http://dx.doi.org/ 10.1523/JNEUROSCI.2723-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Guadagno J, Xu X, Karajgikar M, Brown a, Cregan SP. Microglia-derived TNFα induces apoptosis in neural precursor cells via transcriptional activation of the Bcl-2 family member Puma. Cell Death Dis 2013; 4:e538; PMID:23492769; http://dx.doi.org/ 10.1038/cddis.2013.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Morgan SC, Taylor DL, Pocock JM. Microglia release activators of neuronal proliferation mediated by activation of mitogen-activated protein kinase, phosphatidylinositol-3-kinase/ Akt and delta-Notch signalling cascades. J Neurochem 2004; 90:89-101; PMID:15198670; http://dx.doi.org/ 10.1111/j.1471-4159.2004.02461.x [DOI] [PubMed] [Google Scholar]
- [27].Kohman RA, DeYoung EK, Bhattacharya TK, Peterson LN, Rhodes JS. Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav Immun 2012; 26:803-10; PMID:22056294; http://dx.doi.org/ 10.1016/j.bbi.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Aarum J, Sandberg K, Haeberlein SLB, Persson M a a. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A 2003; 100:15983-8; PMID:14668448; http://dx.doi.org/ 10.1073/pnas.2237050100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Toni N, Laplagne D a, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci 2008. [cited 2014November17]; 11:901-7; PMID:18622400; http://dx.doi.org/ 10.1038/nn.2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci 2007; 10:727-34; PMID:17486101; http://dx.doi.org/ 10.1038/nn1908 [DOI] [PubMed] [Google Scholar]
- [31].Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci 2007; 10:355-62; PMID:17277773; http://dx.doi.org/ 10.1038/nn1847 [DOI] [PubMed] [Google Scholar]
- [32].Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci 2007; 27:3252-9; PMID:17376985; http://dx.doi.org/ 10.1523/JNEUROSCI.4941-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dieni CV, Nietz AK, Panichi R, Wadiche JI, Overstreet-Wadiche L. Distinct Determinants of Sparse Activation during Granule Cell Maturation. J Neurosci 2013; 33:19131-42; PMID:24305810; http://dx.doi.org/ 10.1523/JNEUROSCI.2289-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mongiat LA, Espósito MS, Lombardi G, Schinder AF. Reliable activation of immature neurons in the adult hippocampus. PLoS One 2009; 4; PMID:19399173; http://dx.doi.org/ 10.1371/journal.pone.0005320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Marin-Burgin A, Mongiat LA, Pardi MB, Schinder AF. Unique Processing During a Period of High Excitation/Inhibition Balance in Adult-Born Neurons. Science 2012; 335:1238-42; http://dx.doi.org/ 10.1126/science.1214956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nimmerjahn A, Kirchhoff F, Helmchen F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005; 308:1314-9; PMID:15831717 [DOI] [PubMed] [Google Scholar]
- [37].Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 2009; 29:13435-44; PMID:19864556; http://dx.doi.org/ 10.1523/JNEUROSCI.3257-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012; 43:3063-70; PMID:22933588; http://dx.doi.org/ 10.1161/STROKEAHA.112.659656 [DOI] [PubMed] [Google Scholar]
- [39].Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, Liou AK-F, Leak RK, Gao Y, Chen J. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab 2013; 33:1864-74; PMID:23942366; http://dx.doi.org/ 10.1038/jcbfm.2013.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Matsuda T, Murao N, Katano Y, Juliandi B, Kohyama J, Akira S, Kawai T, Nakashima K. TLR9 signalling in microglia attenuates seizure-induced aberrant neurogenesis in the adult hippocampus. Nat Commun 2015; 6:6514; PMID:25751136; http://dx.doi.org/ 10.1038/ncomms7514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate Granule Cell Neurogenesis Is Increased by Seizures and Contributes to Aberrant Network Reorganization in the Adult Rat Hippocampus. 1997; 17:3727-38; PMID:9133393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jessberger S, Römer B, Babu H, Kempermann G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp Neurol 2005; 196:342-51; PMID:16168988; http://dx.doi.org/ 10.1016/j.expneurol.2005.08.010 [DOI] [PubMed] [Google Scholar]
- [43].Scharfman HE, Goodman JH, Sollas AL. Granule-Like Neurons at the Hilar / CA3 Border after Status Epilepticus and Their Synchrony with Area CA3 Pyramidal Cells: Functional Implications of Seizure-Induced Neurogenesis. 2000; 20:6144-58; PMID:10934264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol 2006; 59:81-91; PMID:16261566; http://dx.doi.org/ 10.1002/ana.20699 [DOI] [PubMed] [Google Scholar]
- [45].Jessberger S, Zhao C, Toni N, Clemenson GD, Li Y, Gage FH. Seizure-Associated, Aberrant Neurogenesis in Adult Rats Characterized with Retrovirus-Mediated Cell Labeling. J Neurosci 2007; 27:9400-7; PMID:17728453; http://dx.doi.org/ 10.1523/JNEUROSCI.2002-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kron MM, Zhang H, Parent JM. The Developmental Stage of Dentate Granule Cells Dictates Their Contribution to Seizure-Induced Plasticity. J Neurosci 2010; 30:2051-9; PMID:20147533; http://dx.doi.org/ 10.1523/JNEUROSCI.5655-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jung KH, Chu K, Kim M, Jeong SW, Song YM, Lee ST, Kim JY, Lee SK, Roh JK. Continuous cytosine-b-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur J Neurosci 2004; 19:3219-26; PMID:15217378; http://dx.doi.org/ 10.1111/j.0953-816X.2004.03412.x [DOI] [PubMed] [Google Scholar]
- [48].Jakubs K, Nanobashvili A, Bonde S, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Environment Matters: Synaptic Properties of Neurons Born in the Epileptic Adult Brain Develop to Reduce Excitability. Neuron 2006; 52:1047-59; PMID:17178407; http://dx.doi.org/ 10.1016/j.neuron.2006.11.004 [DOI] [PubMed] [Google Scholar]
- [49].Neumann H, Kotter MR, Franklin RJM. Debris clearance by microglia: An essential link between degeneration and regeneration. Brain 2009; 132:288-95; PMID:18567623; http://dx.doi.org/ 10.1093/brain/awn109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cunningham CL, Martinez-Cerdeno V, Noctor SC. Microglia Regulate the Number of Neural Precursor Cells in the Developing Cerebral Cortex. J Neurosci 2013; 33:4216-33; PMID:23467340; http://dx.doi.org/ 10.1523/JNEUROSCI.3441-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Neher JJ, Neniskyte U, Zhao J-W, Bal-Price A, Tolkovsky AM, Brown GC. Inhibition of Microglial Phagocytosis Is Sufficient To Prevent Inflammatory Neuronal Death. J Immunol 2011; 186:4973-83; PMID:21402900; http://dx.doi.org/ 10.4049/jimmunol.1003600 [DOI] [PubMed] [Google Scholar]
- [52].Fuhrmann M, Bittner T, Jung CKE, Burgold S, Page RM, Mitteregger G, Haass C, LaFerla FM, Kretzschmar H, Herms J. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease. Nat Neurosci 2010; 13:411-3; PMID:20305648; http://dx.doi.org/ 10.1038/nn.2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Neher JJ, Emmrich J V. Fricker M, Mander PK, Thery C, Brown GC. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc Natl Acad Sci 2013; 110:E4098-107; PMID:24101459; http://dx.doi.org/ 10.1073/pnas.1308679110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Abiega O, Beccari S, Diaz-Aparicio I, Nadjar A, Layé S, Leyrolle Q, Gómez-Nicola D, Domercq M, Pérez-Samartín A, Sánchez-Zafra V, et al.. Neuronal Hyperactivity Disturbs ATP Microgradients, Impairs Microglial Motility, and Reduces Phagocytic Receptor Expression Triggering Apoptosis/Microglial Phagocytosis Uncoupling. PLOS Biol 2016; 14:e1002466; PMID:27228556; http://dx.doi.org/ 10.1371/journal.pbio.1002466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Claude J, Linnartz-Gerlach B, Kudin AP, Kunz WS, Neumann H. Microglial CD33-related Siglec-E inhibits neurotoxicity by preventing the phagocytosis-associated oxidative burst. J Neurosci 2013; 33:18270-6; PMID:24227736; http://dx.doi.org/ 10.1523/JNEUROSCI.2211-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci 2014; 15:209-16; PMID:24646669; http://dx.doi.org/ 10.1038/nrn3710 [DOI] [PubMed] [Google Scholar]
- [57].Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 2006; 9:268–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16415867 [DOI] [PubMed] [Google Scholar]


