Abstract
Objective
Recent diagnostic criteria for functional movement disorders have proposed a “laboratory supported” level of diagnostic certainty where the clinical diagnosis is supported by a positive test. For functional myoclonus the Bereitschaftspotential (BP) is generally accepted as a positive laboratory test. We hypothesised that a different EEG measure, event-related desynchronisation (ERD), might be more effective.
Methods
We analysed 20 patients with functional propriospinal myoclonus (fPSM) and 9 controls with organic myoclonus and performed back-averaging for BPs plus time-frequency decomposition to assess ERD and calculated sensitivity and specificity for both techniques.
Results
The BP was present in only 25% of patients with fPSM while the majority showed a significant ERD (mean 38 Hz; sensitivity 65%). ERD was significant at the group level (p < 0.001), but not the BP (p > 0.05). Both BP and ERD were absent in our control group.
Conclusion
ERD in high-beta may be a useful new test for positive diagnosis of functional myoclonus.
Keywords: EEG, Myoclonus
1. Introduction
Functional (psychogenic) movement disorders (FMD) are common and disabling. There has been a shift in diagnostic criteria in recent times. Reliance on the presence of psychological comorbidity or of traumatic life events has been reduced in favour of positive clinical signs that differentiate such patients from those with organic movement disorders. This is a shift which mirrors that which has occurred in the diagnosis of functional neurological disorder/conversion disorder in general (DSMV, [1]).
Recent diagnostic criteria for FMD have proposed a “laboratory supported” level of diagnostic certainty via the use of specific positive laboratory tests to support the clinical diagnosis [2]. Tests have been proposed and validated for functional tremor [3]. For functional myoclonus, the presence of a pre-movement or Bereitschaftspotential (BP) is proposed as diagnostically useful [4]. The BP is a slow rising (negative) potential seen in the EEG about a second or so before self-paced movement in healthy people, but which is also seen in patients with functional myoclonus prior to their myoclonic jerks. Whilst BPs appear to be useful to support the diagnosis of functional myoclonus they are limited by low sensitivity and require multiple events for reliable interpretation. In addition, it is uncertain how neural activity represented by BPs relates to movement preparation and execution.
Recently, a new explanatory model for functional neurological symptoms including FMD has been proposed, highlighting the role of self-directed attention and expectations [5]. It proposes that triggering (physical/emotional) factors lead to the development of new expectations reflecting abnormal motor/sensory states residing in intermediate cortical levels (e.g. supplementary/premotor areas for motor symptoms). Directed attention can, via increasing the gain on these attended channels, cause movements or percepts in keeping with the abnormal expectations. Hierarchically higher cortical areas, such as the prefrontal cortex, do not predict these abnormal movements/percepts which leads to symptoms being perceived as involuntary by patients.
There is now empirical and theoretical evidence that gain in attentional networks may be indexed and in part contributed to by oscillations, with reductions in beta/low-gamma power occurring prior to cued or self-paced movements [6]. In light of this, and the theoretical model for FMD outlined above, we hypothesised that event related desynchronisation (ERD) might therefore precede movements in functional myoclonus. If this hypothesis is correct, then ERD might be a new diagnostic marker for functional myoclonus, as well as supporting an attention based pathophysiological model of FMD.
2. Methods
We selected 20 patients from a previously published cohort of patients with functional propriospinal myoclonus (fPSM) [7]. Propriospinal myoclonus is a hyperkinetic movement disorder characterised by axial jerks [8]. Recently, it has become clear that propriospinal myoclonus has a functional (psychogenic) origin in the majority of cases [9]. Patients included in this study were diagnosed with fPSM based on a range of features including BPs, variable recruitment pattern/long duration of movements, placebo response to botulinum toxin injection and resolution of symptoms with physical/cognitive rehabilitation. Nine patients with organic, electrophysiologically confirmed, cortical myoclonus were included as a control group as fPSM is believed to be cortically driven in light of the effects of attention and the presence of BPs in many patients. All patients had previously undergone EEG with simultaneous EMG recordings. EEG was recorded from frontocentral areas (F3,C1,Cz,C2,F4,FPz) with bilateral earlobe references. To focus on the motor network and reduce effects of eye movement artefacts, only central channels (C1,C2,Cz) were analysed. Electrical impedance was <5 kΩ and the bandpass filter was set at 0.05–350 Hz. EMG was recorded with surface electrodes located on muscles involved in the movements with bandpass filtering set at 25–1250 Hz.
All movement recordings were visually inspected for artefacts and EEG data was epoched from −6 to +6 s relative to movement onset. For analysis of the BP, EEG movement-locked back-averages were calculated across events. The BP was clinically defined, in keeping with conventional criteria, as a negative electrical shift that increased over time (amplitude 2:5 μV). Time frequency decomposition was performed using the Multitaper method [10] with logRatio baseline correction rescaling (−5 s to −4 s). In order to be able to reliably compare the two methods, events were then statistically analysed from 4 s before until 4 s after the onset of movement using Statistical Parametric Mapping (SPM, version 12b). Time frequency data were converted to statistical images and smoothed. A one-sample t-test was performed on all individual images to determine regions in time-frequency space where power was above (+ve contrast) or below baseline (−ve contrast). Significant changes in power were defined as a desynchronisation of beta/low gamma (13–45 Hz) at a statistical threshold of p < 0.05 (corrected at the cluster level) in each subject. A second one-sample t-test (p < 0.05, Family Wise Error (FWE) corrected for multiple comparisons) was then performed to assess group level spectral power decreases/increases including all single subject negative and positive contrasts, respectively. The negative electrical shift prior to the movements representing the BP was also statistically analysed by converting the data to images and using a one sample t-test to assess if the maximum negative deflection significantly deviates from the mean electrical potential prior to the jerks. Significant changes in the BP were assessed using the same statistical criteria as in the time frequency analysis at the individual and group levels, with the same multiple comparison correction.
3. Results
Mean ages in the fPSM and control groups were 44 and 48 years respectively. Gender distribution (M:F) was 10:10 in fPSM and 6:3 in controls. The mean number of events in the fPSM group was 63.2+ − 7.1 and 23+ − 1.4 for controls. The control group included cases of both primary and secondary cortical myoclonus (Table 1).
Table 1. Clinical characteristics of cortical myoclonus patients.
| Subject | Gender | Age | Diagnosis |
|---|---|---|---|
| 1 | M | 73 | Isolated cortical myoclonus |
| 2 | M | 77 | Cortical myoclonus with parkinsonism |
| 3 | M | 75 | Cortical myoclonus in MSA |
| 4 | M | 50 | Cortical myoclonus with progressive ataxia + epilepsy |
| 5 | F | 27 | Isolated cortical myoclonus |
| 6 | M | 57 | Cortical myoclonus in Unverricht Lundborg disease |
| 7 | M | 25 | Isolated cortical myoclonus |
| 8 | F | 20 | Isolated cortical myoclonus |
| 9 | F | 36 | Cortical myoclonus with epilepsy |
Using the 5 μV conventional criteria, 10 fPSM patients (50%) had a BP. However, when BPs were analysed statistically, only 5 subjects (25%) showed supra-threshold decreases in electrical potential at a p < 0.05 (cluster corrected) significance level. In the frequency domain using the same statistical criteria, 13 of fPSM patients (65%) had significant desynchronisation of high beta (n = 4), low gamma (n = 6) or both (n = 3). Most subjects with a BP also had desynchronisation except for two (subject 3 and 9). The average number of events was 71 in the BP-positive group (49 in BP-negative) and 65 in the ERD-positive group (51 in the ERD-negative), there were no remarkable differences in age or gender between positive and negative groups.
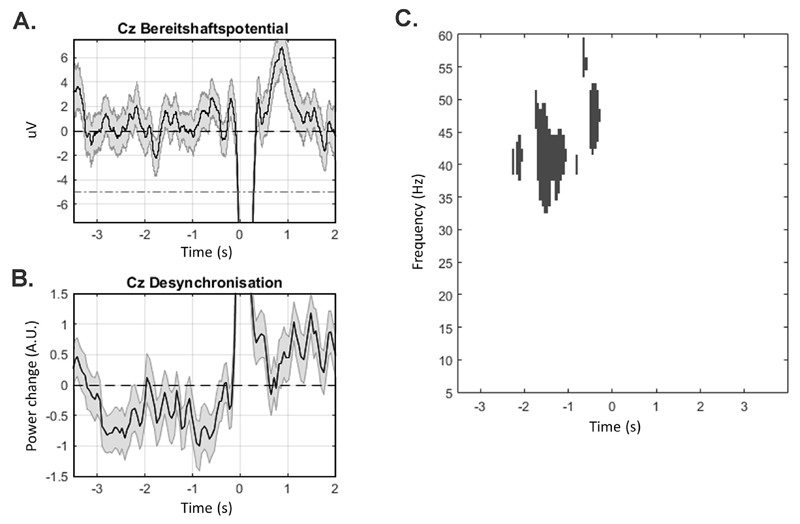
The BP and ERD results of subject 15 demonstrating an ERD in the absence of a BP are shown as an example (Fig. 1a and b).
Fig. 1. Bereitshaftspotential and Event Related Desynchronisation.
A. Single subject data (subject 15, 65 movements) demonstrating mean voltage + - SEM with absent Bereitshaftspotential prior to movement onset at channel Cz (red dashed line shows conventional deflection criteria for BP). B. Normalised high beta/gamma power (31–45 Hz) +- SEM demonstrating a significant reduction in power starting 3 s prior to movement in the same subject. C. Statistical image demonstrating significant group level (n = 20) reductions in spectral power relative to baseline with a significant cluster centred around 39 Hz (displayed clusters are significant at p < 0.05, FWE corrected).
Group level (second-level) analysis demonstrated a significant ERD 1.5 s prior to movements in patients with fPSM centred at 39 Hz (t = 9.2, p < 0.001, FWE corrected) (Fig. 1c). At a less conservative threshold a significant peak of resynchronization in high beta centred around 27 Hz (t = 5.1, p < 0.001, uncorrected) was also seen 1.5–2 s after the movements. There was no significant group level effect in the BP data at either p < 0.05 FWE corrected or p < 0.001 uncorrected. Neither BPs nor ERDs were present in the control subjects at the individual or group levels.
4. Discussion
Event related desynchronisation (ERD) appears to have potential as an electrophysiological measure capable of providing a “laboratory supported” level of diagnostic certainty in patients with functional myoclonus. Using rigorous, matched, statistical methods, 25% of patients demonstrated a statistically significant BP, whereas 65% of patients showed a significant ERD prior to their movements. We propose that the ERD could therefore be a valuable diagnostic marker to positively identify patients with functional myoclonus. Due to its better sensitivity compared to BP, it may be particularly useful for assessing patients with infrequent movements. Furthermore, an optimal, statistically validated, threshold could be used instead of the full statistical methodology utilised here to simplify clinical implementation. The research priority is now for a prospective study to attempt validation of these findings and to provide further comparison of sensitivity and specificity with BP assessments. Although a control group with myoclonus of non-cortical origin would theoretically be suitable as well, we think that the absence of (cortically generated) ERD in patients with cortical myoclonus strengthens our conclusions. Oscillations in the beta/gamma frequency ranges are the product of synchronisation across populations of neurons, modulate prior to movements and may control gain in the motor network related to attention [11]. The finding of ERD prior to functional myoclonic jerks is predicted by the attention based model of functional neurological disorders recently proposed [5,6,12].
These spectral techniques may be beneficial for positive diagnosis (and pathophysiological investigation) of other paroxysmal functional neurological conditions with few events, for example psychogenic non-epileptic seizures. In addition to its diagnostic potential, if this biomarker is validated, exploration of techniques that could directly influence spectral power such as EEG biofeedback, non-invasive stimulation and specific medication, could provide interesting new avenues for treatment.
Acknowledgements
We thank Nathan Toms for his help with collecting the EEG and EMG data.
Funding sources
Institutional (Sobell Department of Motor Neuroscience and Movement Disorders, University College London, UCL).
Footnotes
Financial disclosure/conflict of interest
None reported.
References
- [1].Stone J, Hallett M, Carson A, Bergen D, Shakir R. Functional disorders in the Neurology section of ICD-11 A landmark opportunity. Neurology. 2014;83:2299–2301. doi: 10.1212/WNL.0000000000001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gupta A, Lang AE. Psychogenic movement disorders. Curr Opin Neurology. 2009;22:430–436. doi: 10.1097/WCO.0b013e32832dc169. [DOI] [PubMed] [Google Scholar]
- [3].Schwingenschuh P, Saifee TA, Katschnig-Winter P, Macerollo A, Koegl-Wallner M, Culea V, et al. Validation of “laboratory-supported” criteria for functional (psychogenic) tremor. Mov Disord. 2016 doi: 10.1002/mds.26525. [DOI] [PubMed] [Google Scholar]
- [4].Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001;16:595–599. doi: 10.1002/mds.1145. [DOI] [PubMed] [Google Scholar]
- [5].Edwards MJ, Adams RA, Brown H, Parees I, Friston KJ. A Bayesian account of “hysteria.”. Brain. 2012;135:3495–3512. doi: 10.1093/brain/aws129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kilner J, Bott L, Posada A. Modulations in the degree of synchronization during ongoing oscillatory activity in the human brain. Eur J Neurosci. 2005;21:2547–2554. doi: 10.1111/j.1460-9568.2005.04069.x. [DOI] [PubMed] [Google Scholar]
- [7].Erro R, Bhatia KP, Edwards MJ, Farmer SF, Cordivari C. Clinical diagnosis of propriospinal myoclonus is unreliable: an electrophysiologic study. Mov Disord. 2013;28:1868–1873. doi: 10.1002/mds.25627. [DOI] [PubMed] [Google Scholar]
- [8].Brown P, Thompson PD, Rothwell JC, Day BL, Marsden CD. Axial myoclonus of propriospinal origin. Brain. 1991;114(Pt 1A):197–214. [PubMed] [Google Scholar]
- [9].van der Salm SMA, Erro R, Cordivari C, Edwards MJ, Koelman JHTM, Ende TVD, et al. Propriospinal myoclonus: clinical reappraisal and review of literature. Neurology. 2014;83:1862–1870. doi: 10.1212/WNL.0000000000000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mitra PP, Pesaran B. Analysis of dynamic brain imaging data. Biophys J. 1999;76:691–708. doi: 10.1016/S0006-3495(99)77236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jenkinson N, Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011;34:611–618. doi: 10.1016/j.tins.2011.09.003. [DOI] [PubMed] [Google Scholar]
- [12].Gómez CM, Vaquero E, López-Mendoza D, González-Rosa J, Vázquez-Marrufo M. Reduction of EEG power during expectancy periods in humans. Acta Neurobiol Exp (Wars) 2004;64:143–151. doi: 10.55782/ane-2004-1500. [DOI] [PubMed] [Google Scholar]