A distorted octahedral NS5 donor set is found in the binuclear title molecule which features an unprecedented [Cd(dithiocarbamate)2]2 core. Molecules are connected into a three-dimensional architecture by O—H⋯O,N hydrogen bonding.
Keywords: crystal structure, cadmium, dithiocarbamate, hydrogen bonding
Abstract
The asymmetric unit in the title binuclear compound, [Cd(C6H12NOS2)2(C12H10N4)]2·2H2O, comprises a CdII atom, two dithiocarbamate (dtc) anions, a monodentate 3-pyridinealdazine ligand and a lattice water molecule. The binuclear molecule is constructed by the application of inversion symmetry. One dtc ligand simultaneously chelates one cadmium atom and bridges the centrosymmetric mate, while the other dtc ligand is chelating only. This leads to a centrosymmetric [Cd(dtc)2]2 core to which are appended two 3-pyridinealdazine ligands. The resulting NS5 donor set is based on an octahedron. The three-dimensional molecular packing is sustained by hydroxyl-O—H(hydroxyl) and water-O—H⋯O(hydroxyl) hydrogen bonding, leading to supramolecular layers parallel to (101) which are connected by water-O—H⋯N(pyridyl) hydrogen bonding; additional C—H⋯O, S π(chelate ring) interactions are also evident. The retention of the central [Cd(dtc)2]2 core upon adduct formation is unprecedented in the structural chemistry of the zinc-triad dithiocarbamates.
Chemical context
The common feature of the structural chemistry of the binary cadmium dithiocarbamates, i.e. molecules of the general formula Cd(S2CNRR′)2 for R, R′ = alkyl, is the adoption of aggregated species in the solid state. The overwhelming majority of structures are binuclear, [Cd(S2CNRR′)2]2, arising from equal numbers of μ2-tridentate and bidentate (chelating) ligands (Tiekink, 2003 ▸; Tan, Halim et al., 2016 ▸). The exceptional structures are trinuclear {Cd[S2CN(p-tol)furan-2-ylmethyl]2}3 (Kumar et al., 2014 ▸), having two μ2-tridentate and four chelating ligands, and one-dimensional polymeric [Cd(S2CNMe2)2]n (Bing et al., 2010 ▸), {Cd[S2CN(iPr)CH2CH2OH]2}n (Tan et al., 2013 ▸; Tan, Halim et al., 2016 ▸) and {Cd[S2CN(Me)CH2CH(OMe)2]2}n (Ferreira et al., 2016 ▸), having all ligands μ2-tridentate. Interestingly, supramolecular isomers were found for the {Cd[S2CN(iPr)CH2CH2OH]2}n species (Tan et al., 2013 ▸; Tan, Halim et al., 2016 ▸), which were shown to adopt the common binuclear structural motif. Up to now, whenever Cd(S2CNRR′)2 is reacted with bases, e.g. pyridyl-donors, the original aggregate is disrupted in that no dtc links are retained between cadmium atoms. Thus, when archetypal, binuclear [Cd(S2CNEt2)2]2 (Domenicano et al., 1968 ▸; Dee & Tiekink, 2002 ▸) reacts with monodentate N-donors such as 2,6-dimethylpyridine, mononuclear, five-coordinate species result (Lennartson & Håkansson, 2009 ▸). Similarly, bidentate chelating ligands, such as 2,2′-bipyridyl, lead to mononuclear species but with formally six-coordinate cadmium atoms (Airoldi et al., 1990 ▸). Higher nuclearity structures are also formed with bridging, bidentate ligands such as in the one-dimensional coordination polymers formed with μ2-1,2-bis(4-pyridyl)ethylene (Chai et al., 2003 ▸) and μ2-1,2-bis(4-pyridyl)ethane (Avila et al., 2006 ▸). In the latter structures, six-coordinate, trans-N2S4 donor sets are found. In the present report, crystals of the 1:2 adduct between {Cd[S2CN(iPr)CH2CH2OH)]2}2 and 3-pyridinealdazine were isolated and shown by X-ray crystallography that despite having one potentially bidentate bi-pyridyl ligand per Cd[S2CN(iPr)CH2CH2OH)]2 unit, the central binuclear core (Tan et al., 2013 ▸; Tan, Halim et al., 2016 ▸) remained intact with the 3-pyridinealdazine molecules coordinating in a monodentate mode, thereby representing a new structural motif for this class of compound.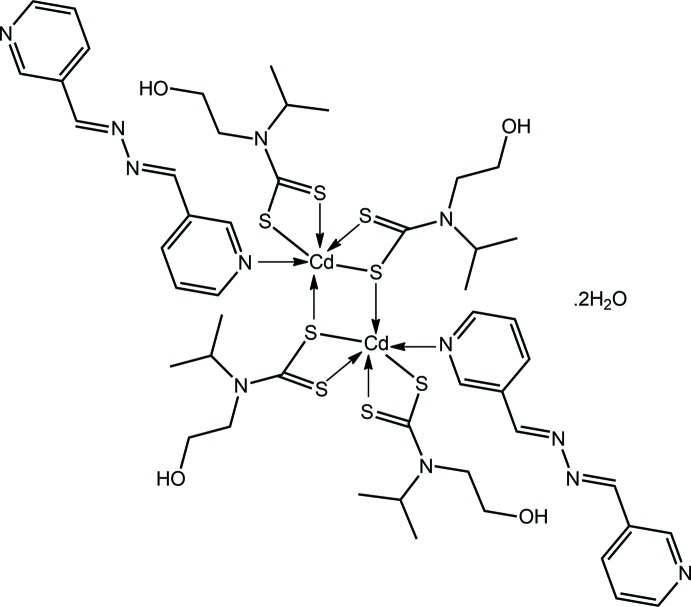
Structural commentary
The molecular structure of the binuclear title compound, isolated as a dihydrate, is shown in Fig. 1 ▸ and selected geometric parameters are collated in Table 1 ▸. The binuclear compound is disposed about a centre of inversion so the asymmetric comprises a Cd[S2CN(iPr)CH2CH2OH)]2 entity, a 3-pyridinealdazine ligand and one water molecule of solvation. One dithiocarbamate (dtc) ligand coordinates in a chelating mode forming very similar Cd—S bond lengths, i.e. the difference between the Cd—Sshort and Cd—Slong bond lengths is only 0.033 Å; this equivalence is reflected in the equivalence in the associated C1—S1, S2 bond lengths, Table 1 ▸. The second independent dtc chelates one cadmium atom and at the same time bridges the other cadmium atom. The Cd—S3bridging bond lengths are close to being equal, differing by only 0.010 Å, and are longer by ca 0.1 Å than the non-bridging Cd—S4 bond length, Table 1 ▸. The differences in the number and strength of the Cd—S bond lengths for the S3-dtc ligand is reflected in the C7—S3, S4 separations with the C7—S4 bond length of 1.714 (2) Å being the shortest across the series. The sixth position in the distorted octahedral coordination geometry is occupied by a nitrogen atom of the monodentate 3-pyridinealdazine ligand. Distortions in angles about the cadmium atom are largely related to the restricted bite distances of the dtc ligands, Table 1 ▸. While not having crystallographic symmetry, the 3-pyridinealdazine molecule adopts an anti disposition about both imine bonds, i.e. C18=N4 = 1.283 (3) Å and C19=N5 = 1.277 (3) Å; the central, azo bond is 1.415 (2) Å. The pyridyl-N atoms are also anti but there are twists in the 3-pyridinealdazine molecule, as seen in the value of the dihedral angle between the two pyridyl rings of 22.78 (12)°.
Figure 1.
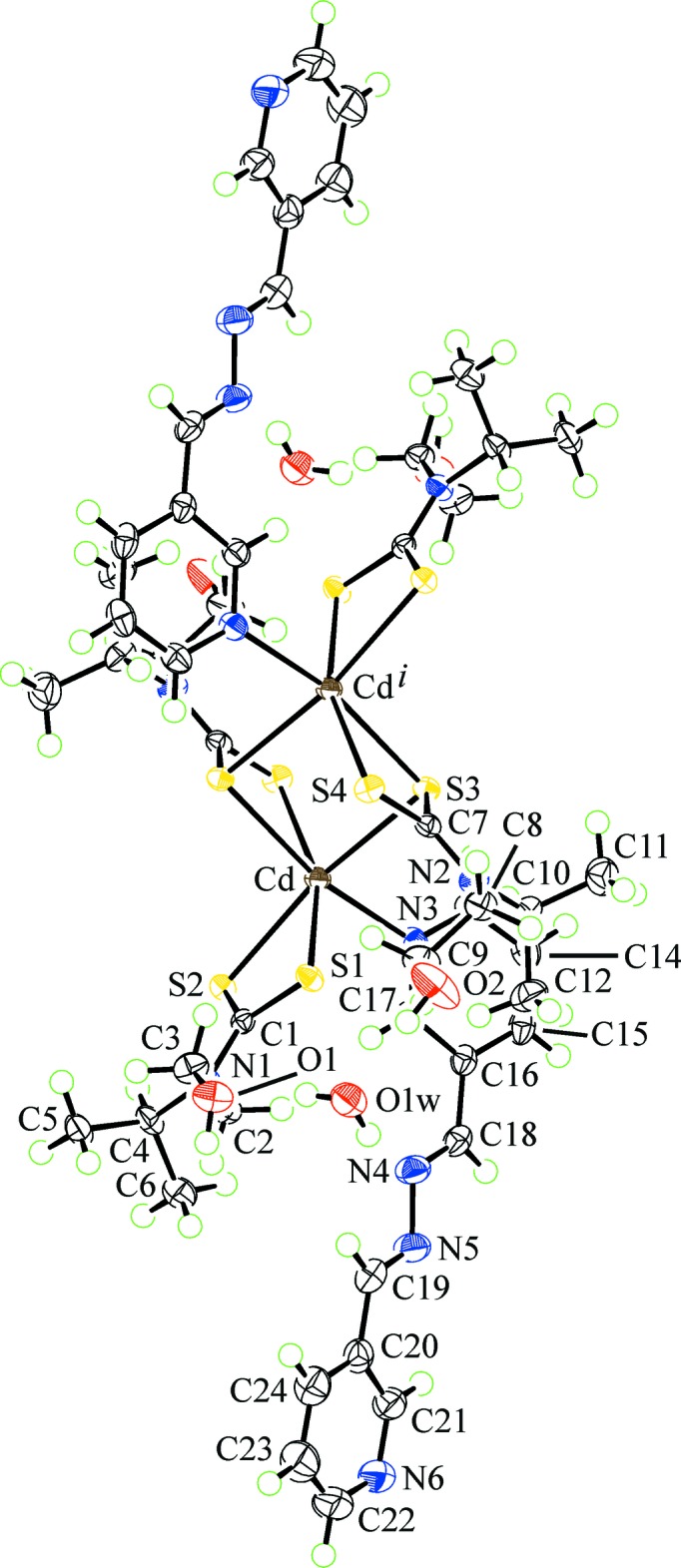
The molecular structure of the binuclear title compound, showing the atom-labelling scheme and displacement ellipsoids at the 50% probability level. [Symmetry code: (i) 1 − x, −y, 1 − z.]
Table 1. Selected geometric parameters (Å, °).
| Cd—S1 | 2.6444 (5) | Cd—N3 | 2.3811 (18) |
| Cd—S2 | 2.6768 (5) | S1—C1 | 1.7267 (19) |
| Cd—S3 | 2.7422 (5) | S2—C1 | 1.7231 (18) |
| Cd—S3i | 2.7317 (6) | S3—C7 | 1.7404 (19) |
| Cd—S4i | 2.6342 (5) | S4—C7 | 1.714 (2) |
| S1—Cd—S2 | 67.824 (14) | S2—Cd—S3 | 167.393 (15) |
| S4i—Cd—S3i | 67.343 (17) | N3—Cd—S3i | 166.35 (4) |
| S4i—Cd—S1 | 160.481 (17) |
Symmetry code: (i) 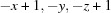 .
.
Supramolecular features
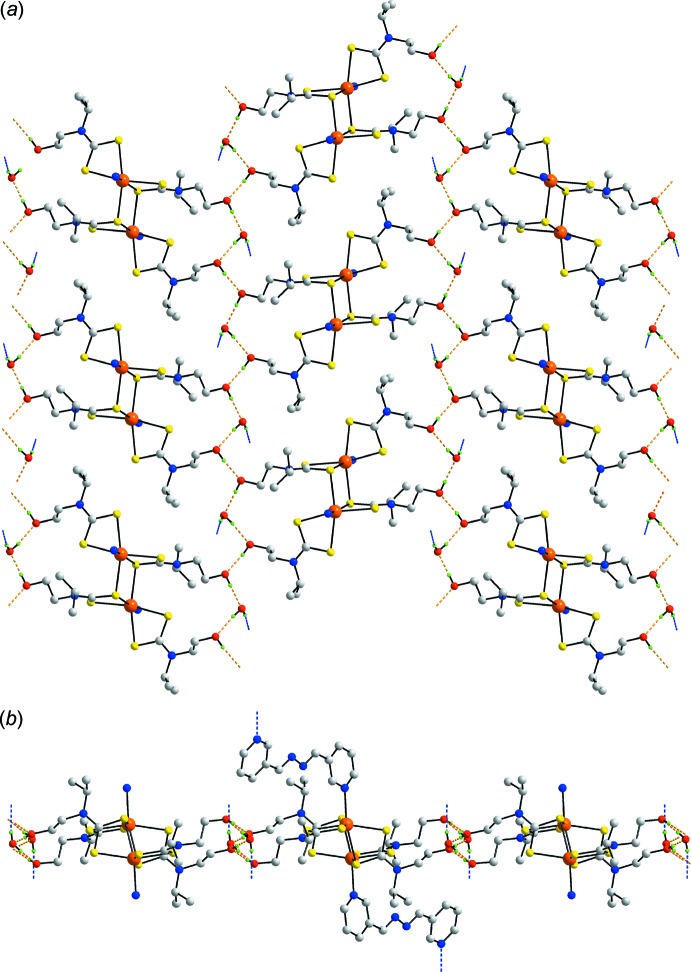
Significant O—H⋯O hydrogen bonding is found in the molecular packing of the binuclear title compound as would be expected from the chemical composition. Thus, molecules are assembled into layers approximately parallel to (101) by hydroxy-O—H⋯O(hydroxyl) and hydroxy-O—H⋯O(water) hydrogen bonds as detailed in Table 2 ▸. Thus, strings of {⋯Ohydroxy—H⋯Ohydroxy—H⋯Owater—H}n chains are formed as shown in Fig. 2 ▸ a. The water molecules also form water-O—H⋯N(pyridyl) hydrogen bonds on either side of the supramolecular layers sustained by O—H⋯O hydrogen bonds, Fig. 2 ▸ b. The pendent pyridyl-N atoms of Fig. 2 ▸ b are coordinating to cadmium atoms of successive layers so that a three-dimensional architecture results. Globally, and as seen from Fig. 3 ▸, the molecular packing comprises alternating layers of {Cd[S2CN(iPr)CH2CH2OH)]2}2 and 3-pyridinealdazine with the key links between them being hydrogen and coordinate bonding. Within this framework stabilized primarily by hydrogen-bonding interactions, there are some second tier interactions worthy of comment (Spek, 2009 ▸). Thus, referring to data in Table 2 ▸, the hydroxyl-O1 atom also accepts a contact from a pyridyl-C—H atom as the 3-pyridinealdazine ligand is orientated so that the non-coordinating end is directed over the hydroxy/water-rich region of the structure. Within the layers shown in Fig. 2 ▸ a, methine-C—H⋯S interactions are seen and between layers pyridyl-C—H⋯S contacts, interestingly, both involving the S2 atom. Finally, as has increasingly been noted in recent descriptions of the structural chemistry of metal dithiocarbamates, C—H⋯π(chelate) interactions are present (Tiekink & Zukerman-Schpector, 2011 ▸). Here, a pyridyl-C—H atom sits almost perpendicular to the chelate ring involving the S1-dithiocarbamate ligand, i.e. the C—H⋯ring centroid(chelate ring) angle is 178°, in the inter-layer region, Table 2 ▸.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1O⋯O2ii | 0.83 (2) | 1.83 (3) | 2.655 (3) | 172 (3) |
| O2—H2O⋯O1W | 0.85 (3) | 1.80 (3) | 2.640 (3) | 180 (6) |
| O1W—H1W⋯O1 | 0.84 (3) | 1.92 (3) | 2.750 (3) | 172 (3) |
| O1W—H2W⋯N6iii | 0.85 (2) | 2.00 (2) | 2.840 (3) | 172 (2) |
| C23—H23⋯O1ii | 0.95 | 2.50 | 3.295 (3) | 141 |
| C4—H4⋯S2iv | 1.00 | 2.79 | 3.599 (2) | 139 |
| C15—H15⋯S2v | 0.95 | 2.84 | 3.714 (2) | 153 |
| C15—H15⋯Cg(Cd,S1,S2,C1)vi | 0.95 | 2.79 | 3.737 (2) | 173 |
Symmetry codes: (ii) 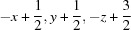 ; (iii)
; (iii) 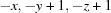 ; (iv)
; (iv) 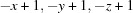 ; (v)
; (v) 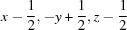 ; (vi)
; (vi) 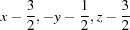 .
.
Figure 2.
Molecular packing: (a) view of the supramolecular layer sustained by hydroxy-O—H⋯O(hydroxy) and hydroxy-O—H⋯O(water) hydrogen bonds, shown as orange dashed lines. Only the pyridyl N atoms of the 3-pyridinealdazine ligands are shown. (b) A side-on view of the layer in (a) extended to show the two central 3-pyridinealdazine ligands (see text). The putative water-O—H⋯N(pyridyl) hydrogen bonds are shown as blue dashed lines. In both images, only acidic hydrogen atoms are included.
Figure 3.
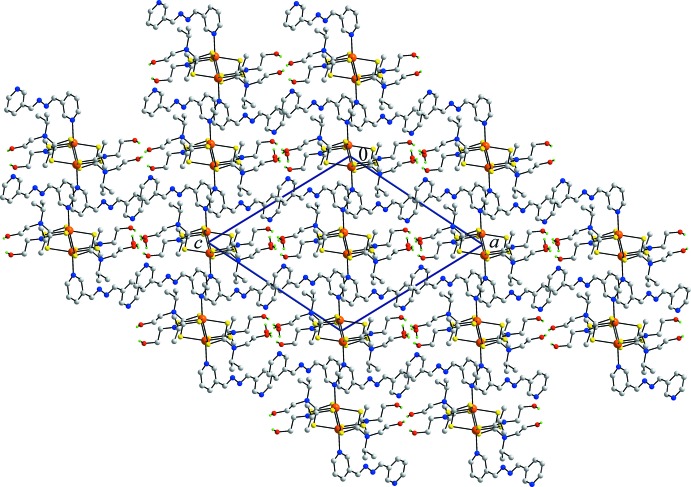
A view of the unit cell contents shown in projection down the b axis, highlighting the alternating layers of {Cd[S2CN(iPr)CH2CH2OH)]2}2 and 3-pyridinealdazine molecules. Water molecules are located in the hydroxy-rich regions, i.e. the key interfaces between layers.
Database survey
There are 14 examples of cadmium compounds with 3-pyridinealdazine in the crystallographic literature (Groom et al., 2016 ▸). Most of these feature μ2-bridging 3-pyridinealdazine such as in the two most relevant compounds to the present study, namely {Cd[S2P(O-iPr)2]2(μ2-3-pyridinealdazine)}n (Lai & Tiekink, 2006a ▸) and bulky analogue {Cd[S2P(O-cHex)2]2(μ2-3-pyridinealdazine)}n (Lai & Tiekink, 2006b ▸). In the structure of {Cd[O2P(O-tBu)2]2(3-pyridinealdazine)2(μ2-3-pyridinealdazine)·H2O}n (Rajakannu et al., 2015 ▸), both bridging and monodentate 3-pyridinealdazine ligands, in a 1:2 ratio, are observed. Underscoring the flexibility in mode of association of 3-pyridinealdazine in their crystal structures, in {[Cd(3-pyridinealdazine)2(μ2-3-pyridinealdazine)(OH2)2](3-pyridinealdazine)·2ClO4}n (Bhattacharya et al., 2013 ▸), bridging, monodentate and non-coordinating 3-pyridinealdazine ligands, in a 1:2:1 ratio, are noted.
The most curious feature of the structure of the title compound is the retention of the central binuclear core. This is unprecedented in the structural chemistry of cadmium dithiocarbamates (see Chemical context). A good number of zinc and mercury binary dithiocarbamates are also known to adopt related binuclear [M(S2CNRR’)2]2 aggregates owing to the presence of equal numbers of μ2-tridentate and chelating ligands (Tiekink, 2003 ▸; Jotani et al., 2016 ▸). Without exception, these are broken down upon adduct formation, regardless of the nature of the donor atom(s) (Groom et al., 2016 ▸). This makes more curious the recent report of the molecular structure of a cadmium xanthate adduct, [Cd(S2CO-iPr)2(hmta)]2, where hmta is hexamethylenetetramine, for which an analogous centrosymmetric core and NS5 donor set as in the title compound was observed (Tan, Azizuddin et al., 2016 ▸). This is quite unusual as there are no precedents for such [Cd(S2COR)2]2 cores in the structural chemistry of metal xanthates (Tiekink & Haiduc, 2005 ▸). Clearly, as more study continues in this field, more interesting outcomes will be noted and rationalizations emerge.
Synthesis and crystallization
Cd[S2CN(iPr)CH2CH2OH)]2 (235 mg, 0.5 mmol) and 3-pyridinealdazine (110 mg, 0.5 mmol) were dissolved in 1-propanol (15 ml). The solution was carefully covered with hexanes. Yellow blocks were obtained via slow diffusion of hexanes into the 1-propanol solution over two weeks. m.p. 389–391 K. IR (cm−1): 1449 (m) ν(C=N), 1173 (s) ν(C—S). 1H NMR: δ 9.04 (d, Ar, J = 1.46 Hz), 8.81 (s, Ar), 8.72 (d, Ar, J = 1.46 Hz), 8.29 (d, Ar, J = 1.95 Hz), 7.56 (qd, HC=CH, J = 4.88 Hz), 5.22 [sept, CH(CH3)2, J = 6.83 Hz], 4.83 (t, OH, J = 5.37 Hz), 3.74 (d, CH2O, J = 6.83 Hz), 3.68 (d, NCH2, J = 6.83 Hz), 1.18 (d, CH3, J = 6.84 Hz). TGA: three steps, corresponding to loss of water (calculated weight loss 2.6%; experimental weight loss 2.3%; onset 352 K, mid-point 364 K, endset 378 K), loss of the 3-pyridinealdazine ligand (calculated weight loss 30.2%; experimental weight loss 30.5%; onset 418 K, mid-point 496 K, endset 511 K), and decomposition down to cadmium sulfide (calculated weight loss 79.3; experimental weight loss 75.1%; onset 542 K, mid-point 576 K, endset 620 K).
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 3 ▸. The carbon-bound H atoms were placed in calculated positions (C—H = 0.95–1.00 Å) and were included in the refinement in the riding model approximation, with U iso(H) set at 1.2–1.5U eq(C). The oxygen-bound H atoms were located in a difference Fourier map but were refined with a distance restraint of O—H = 0.84±0.01 Å, and with U iso(H) set at 1.5U eq(O).
Table 3. Experimental details.
| Crystal data | |
| Chemical formula | [Cd(C6H12NOS2)2(C12H10N4)]2·2H2O |
| M r | 1394.48 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 98 |
| a, b, c (Å) | 16.4700 (18), 12.2257 (12), 17.0862 (19) |
| β (°) | 114.932 (2) |
| V (Å3) | 3119.8 (6) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ (mm−1) | 1.00 |
| Crystal size (mm) | 0.40 × 0.30 × 0.08 |
| Data collection | |
| Diffractometer | AFC12K/SATURN724 |
| Absorption correction | Multi-scan (ABSCOR; Higashi, 1995 ▸) |
| T min, T max | 0.661, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 22846, 7133, 6807 |
| R int | 0.029 |
| (sin θ/λ)max (Å−1) | 0.650 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.029, 0.065, 1.08 |
| No. of reflections | 7133 |
| No. of parameters | 359 |
| No. of restraints | 4 |
| Δρmax, Δρmin (e Å−3) | 0.54, −0.39 |
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2056989016012214/wm5312sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016012214/wm5312Isup2.hkl
CCDC reference: 1496352
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Crystal data
| [Cd(C6H12NOS2)2(C12H10N4)2]2·2H2O | F(000) = 1432 |
| Mr = 1394.48 | Dx = 1.484 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 16.4700 (18) Å | Cell parameters from 19902 reflections |
| b = 12.2257 (12) Å | θ = 2.7–40.8° |
| c = 17.0862 (19) Å | µ = 1.00 mm−1 |
| β = 114.932 (2)° | T = 98 K |
| V = 3119.8 (6) Å3 | Plate, yellow |
| Z = 2 | 0.40 × 0.30 × 0.08 mm |
Data collection
| AFC12K/SATURN724 diffractometer | 7133 independent reflections |
| Radiation source: fine-focus sealed tube | 6807 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.029 |
| ω scans | θmax = 27.5°, θmin = 2.7° |
| Absorption correction: multi-scan (ABSCOR; Higashi, 1995) | h = −20→21 |
| Tmin = 0.661, Tmax = 1.000 | k = −15→15 |
| 22846 measured reflections | l = −22→19 |
Refinement
| Refinement on F2 | 4 restraints |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.029 | w = 1/[σ2(Fo2) + (0.0243P)2 + 2.9797P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.065 | (Δ/σ)max = 0.001 |
| S = 1.08 | Δρmax = 0.54 e Å−3 |
| 7133 reflections | Δρmin = −0.39 e Å−3 |
| 359 parameters |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cd | 0.43075 (2) | 0.13535 (2) | 0.44655 (2) | 0.01368 (5) | |
| S1 | 0.36321 (3) | 0.19066 (4) | 0.55658 (3) | 0.01568 (10) | |
| S2 | 0.43474 (3) | 0.35177 (4) | 0.47165 (3) | 0.01378 (9) | |
| S3 | 0.39975 (3) | −0.08571 (4) | 0.43612 (3) | 0.01393 (9) | |
| S4 | 0.45562 (3) | −0.11726 (4) | 0.62449 (3) | 0.01652 (10) | |
| O1 | 0.36420 (11) | 0.30641 (13) | 0.79528 (10) | 0.0241 (3) | |
| H1O | 0.3348 (17) | 0.3606 (15) | 0.797 (2) | 0.036* | |
| O2 | 0.24285 (16) | −0.03206 (14) | 0.70325 (14) | 0.0425 (5) | |
| H2O | 0.241 (3) | 0.0271 (17) | 0.728 (2) | 0.064* | |
| O1W | 0.23612 (12) | 0.15309 (13) | 0.77985 (12) | 0.0276 (4) | |
| H1W | 0.2791 (15) | 0.195 (2) | 0.787 (2) | 0.041* | |
| H2W | 0.1898 (13) | 0.185 (2) | 0.7437 (16) | 0.041* | |
| N1 | 0.28639 (11) | −0.08842 (14) | 0.51175 (11) | 0.0165 (3) | |
| N2 | 0.37924 (11) | 0.40314 (13) | 0.59396 (11) | 0.0150 (3) | |
| N3 | 0.29195 (12) | 0.15099 (13) | 0.32251 (11) | 0.0177 (3) | |
| N4 | 0.11563 (13) | 0.38202 (15) | 0.34081 (13) | 0.0242 (4) | |
| N5 | 0.05594 (13) | 0.46819 (16) | 0.33476 (13) | 0.0260 (4) | |
| N6 | −0.08839 (14) | 0.74203 (17) | 0.35311 (15) | 0.0324 (5) | |
| C1 | 0.39084 (12) | 0.32403 (15) | 0.54493 (12) | 0.0126 (3) | |
| C2 | 0.33799 (13) | 0.37919 (16) | 0.65353 (13) | 0.0165 (4) | |
| H2A | 0.2888 | 0.3259 | 0.6262 | 0.020* | |
| H2B | 0.3119 | 0.4472 | 0.6646 | 0.020* | |
| C3 | 0.40587 (15) | 0.33254 (18) | 0.73950 (14) | 0.0218 (4) | |
| H3A | 0.4335 | 0.2658 | 0.7284 | 0.026* | |
| H3B | 0.4540 | 0.3868 | 0.7680 | 0.026* | |
| C4 | 0.40109 (14) | 0.51947 (15) | 0.58531 (14) | 0.0178 (4) | |
| H4 | 0.4361 | 0.5203 | 0.5497 | 0.021* | |
| C5 | 0.46023 (15) | 0.56990 (17) | 0.67257 (15) | 0.0227 (4) | |
| H5A | 0.5143 | 0.5255 | 0.7010 | 0.034* | |
| H5B | 0.4273 | 0.5723 | 0.7087 | 0.034* | |
| H5C | 0.4771 | 0.6443 | 0.6640 | 0.034* | |
| C6 | 0.31638 (15) | 0.58567 (17) | 0.53696 (15) | 0.0238 (4) | |
| H6A | 0.2798 | 0.5495 | 0.4821 | 0.036* | |
| H6B | 0.3325 | 0.6592 | 0.5256 | 0.036* | |
| H6C | 0.2823 | 0.5909 | 0.5720 | 0.036* | |
| C7 | 0.37201 (13) | −0.09619 (15) | 0.52349 (13) | 0.0144 (4) | |
| C8 | 0.26157 (15) | −0.10857 (17) | 0.58411 (14) | 0.0198 (4) | |
| H8A | 0.2988 | −0.1687 | 0.6203 | 0.024* | |
| H8B | 0.1982 | −0.1319 | 0.5609 | 0.024* | |
| C9 | 0.27426 (16) | −0.00701 (17) | 0.63969 (15) | 0.0237 (4) | |
| H9A | 0.3383 | 0.0135 | 0.6676 | 0.028* | |
| H9B | 0.2400 | 0.0551 | 0.6037 | 0.028* | |
| C10 | 0.21194 (14) | −0.07222 (17) | 0.42431 (14) | 0.0207 (4) | |
| H10 | 0.2392 | −0.0431 | 0.3861 | 0.025* | |
| C11 | 0.16893 (16) | −0.1815 (2) | 0.38682 (16) | 0.0305 (5) | |
| H11A | 0.2142 | −0.2307 | 0.3832 | 0.046* | |
| H11B | 0.1211 | −0.1701 | 0.3290 | 0.046* | |
| H11C | 0.1438 | −0.2141 | 0.4241 | 0.046* | |
| C12 | 0.14460 (15) | 0.0123 (2) | 0.42532 (17) | 0.0299 (5) | |
| H12A | 0.1756 | 0.0812 | 0.4491 | 0.045* | |
| H12B | 0.1154 | −0.0143 | 0.4612 | 0.045* | |
| H12C | 0.0994 | 0.0245 | 0.3663 | 0.045* | |
| C13 | 0.26289 (15) | 0.08493 (17) | 0.25306 (14) | 0.0201 (4) | |
| H13 | 0.3011 | 0.0281 | 0.2507 | 0.024* | |
| C14 | 0.17944 (15) | 0.09657 (18) | 0.18503 (14) | 0.0227 (4) | |
| H14 | 0.1606 | 0.0482 | 0.1371 | 0.027* | |
| C15 | 0.12347 (14) | 0.18035 (17) | 0.18798 (14) | 0.0209 (4) | |
| H15 | 0.0665 | 0.1912 | 0.1414 | 0.025* | |
| C16 | 0.15226 (13) | 0.24779 (16) | 0.26003 (13) | 0.0170 (4) | |
| C17 | 0.23735 (14) | 0.22991 (16) | 0.32592 (13) | 0.0180 (4) | |
| H17 | 0.2573 | 0.2758 | 0.3753 | 0.022* | |
| C18 | 0.09590 (14) | 0.33688 (17) | 0.26722 (14) | 0.0199 (4) | |
| H18 | 0.0452 | 0.3609 | 0.2178 | 0.024* | |
| C19 | 0.07322 (14) | 0.50864 (18) | 0.40903 (15) | 0.0234 (4) | |
| H19 | 0.1193 | 0.4773 | 0.4588 | 0.028* | |
| C20 | 0.02300 (15) | 0.60274 (18) | 0.41864 (15) | 0.0231 (4) | |
| C21 | −0.04395 (15) | 0.65334 (19) | 0.34710 (16) | 0.0256 (5) | |
| H21 | −0.0585 | 0.6232 | 0.2915 | 0.031* | |
| C22 | −0.06761 (18) | 0.7822 (2) | 0.4324 (2) | 0.0400 (6) | |
| H22 | −0.0991 | 0.8450 | 0.4374 | 0.048* | |
| C23 | −0.00341 (19) | 0.7380 (2) | 0.50682 (18) | 0.0378 (6) | |
| H23 | 0.0087 | 0.7693 | 0.5615 | 0.045* | |
| C24 | 0.04320 (17) | 0.6467 (2) | 0.50016 (17) | 0.0301 (5) | |
| H24 | 0.0882 | 0.6145 | 0.5503 | 0.036* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cd | 0.01391 (8) | 0.01315 (7) | 0.01351 (8) | 0.00222 (5) | 0.00533 (6) | −0.00025 (5) |
| S1 | 0.0203 (2) | 0.0118 (2) | 0.0179 (2) | −0.00160 (17) | 0.0110 (2) | −0.00003 (17) |
| S2 | 0.0168 (2) | 0.0131 (2) | 0.0133 (2) | −0.00056 (17) | 0.00812 (18) | −0.00001 (16) |
| S3 | 0.0158 (2) | 0.0136 (2) | 0.0135 (2) | 0.00129 (17) | 0.00717 (18) | 0.00009 (16) |
| S4 | 0.0174 (2) | 0.0196 (2) | 0.0135 (2) | 0.00195 (18) | 0.00735 (19) | 0.00139 (18) |
| O1 | 0.0322 (9) | 0.0246 (8) | 0.0226 (8) | 0.0050 (7) | 0.0187 (7) | 0.0051 (6) |
| O2 | 0.0775 (15) | 0.0232 (8) | 0.0574 (13) | −0.0139 (9) | 0.0583 (13) | −0.0092 (8) |
| O1W | 0.0316 (9) | 0.0233 (8) | 0.0317 (10) | −0.0008 (7) | 0.0172 (8) | −0.0012 (7) |
| N1 | 0.0162 (8) | 0.0177 (8) | 0.0177 (9) | 0.0016 (6) | 0.0091 (7) | 0.0033 (6) |
| N2 | 0.0183 (8) | 0.0129 (7) | 0.0165 (8) | −0.0012 (6) | 0.0100 (7) | −0.0009 (6) |
| N3 | 0.0198 (8) | 0.0160 (8) | 0.0145 (8) | 0.0015 (7) | 0.0044 (7) | −0.0003 (6) |
| N4 | 0.0212 (9) | 0.0250 (9) | 0.0234 (10) | 0.0069 (7) | 0.0065 (8) | 0.0021 (7) |
| N5 | 0.0240 (9) | 0.0266 (9) | 0.0262 (10) | 0.0069 (8) | 0.0094 (8) | 0.0004 (8) |
| N6 | 0.0272 (10) | 0.0331 (11) | 0.0339 (12) | 0.0045 (9) | 0.0099 (9) | −0.0078 (9) |
| C1 | 0.0116 (8) | 0.0149 (8) | 0.0105 (9) | 0.0001 (7) | 0.0038 (7) | −0.0003 (7) |
| C2 | 0.0178 (9) | 0.0181 (9) | 0.0172 (10) | 0.0006 (7) | 0.0110 (8) | −0.0005 (7) |
| C3 | 0.0227 (10) | 0.0273 (10) | 0.0182 (10) | 0.0028 (9) | 0.0114 (9) | 0.0017 (8) |
| C4 | 0.0238 (10) | 0.0122 (8) | 0.0209 (10) | −0.0034 (8) | 0.0127 (9) | −0.0024 (7) |
| C5 | 0.0266 (11) | 0.0169 (9) | 0.0247 (11) | −0.0049 (8) | 0.0111 (9) | −0.0067 (8) |
| C6 | 0.0291 (11) | 0.0187 (10) | 0.0236 (11) | 0.0012 (9) | 0.0112 (10) | 0.0011 (8) |
| C7 | 0.0169 (9) | 0.0103 (8) | 0.0167 (9) | 0.0006 (7) | 0.0077 (8) | 0.0008 (7) |
| C8 | 0.0225 (10) | 0.0198 (9) | 0.0232 (11) | −0.0011 (8) | 0.0155 (9) | 0.0016 (8) |
| C9 | 0.0310 (11) | 0.0208 (10) | 0.0301 (12) | 0.0005 (9) | 0.0232 (10) | 0.0020 (9) |
| C10 | 0.0156 (9) | 0.0241 (10) | 0.0210 (11) | 0.0016 (8) | 0.0064 (8) | 0.0035 (8) |
| C11 | 0.0277 (12) | 0.0285 (12) | 0.0278 (13) | −0.0016 (10) | 0.0045 (10) | −0.0011 (10) |
| C12 | 0.0213 (11) | 0.0304 (12) | 0.0351 (14) | 0.0054 (9) | 0.0092 (10) | 0.0033 (10) |
| C13 | 0.0258 (11) | 0.0168 (9) | 0.0167 (10) | 0.0008 (8) | 0.0080 (9) | −0.0008 (8) |
| C14 | 0.0294 (11) | 0.0223 (10) | 0.0141 (10) | −0.0051 (9) | 0.0069 (9) | −0.0018 (8) |
| C15 | 0.0184 (10) | 0.0232 (10) | 0.0162 (10) | −0.0042 (8) | 0.0026 (8) | 0.0029 (8) |
| C16 | 0.0164 (9) | 0.0163 (9) | 0.0175 (10) | −0.0026 (7) | 0.0064 (8) | 0.0029 (7) |
| C17 | 0.0194 (10) | 0.0156 (9) | 0.0168 (10) | 0.0003 (8) | 0.0056 (8) | −0.0003 (7) |
| C18 | 0.0151 (9) | 0.0218 (10) | 0.0206 (10) | 0.0002 (8) | 0.0052 (8) | 0.0051 (8) |
| C19 | 0.0181 (10) | 0.0280 (11) | 0.0227 (11) | −0.0007 (8) | 0.0073 (9) | 0.0030 (9) |
| C20 | 0.0209 (10) | 0.0259 (10) | 0.0247 (11) | −0.0042 (9) | 0.0118 (9) | −0.0028 (9) |
| C21 | 0.0231 (11) | 0.0296 (11) | 0.0234 (12) | 0.0015 (9) | 0.0091 (9) | −0.0041 (9) |
| C22 | 0.0339 (14) | 0.0411 (14) | 0.0447 (16) | 0.0055 (12) | 0.0162 (13) | −0.0165 (13) |
| C23 | 0.0384 (14) | 0.0480 (15) | 0.0295 (14) | −0.0030 (12) | 0.0167 (12) | −0.0160 (12) |
| C24 | 0.0289 (12) | 0.0369 (13) | 0.0246 (12) | −0.0019 (10) | 0.0113 (10) | −0.0019 (10) |
Geometric parameters (Å, º)
| Cd—S1 | 2.6444 (5) | C5—H5B | 0.9800 |
| Cd—S2 | 2.6768 (5) | C5—H5C | 0.9800 |
| Cd—S3 | 2.7422 (5) | C6—H6A | 0.9800 |
| Cd—S3i | 2.7317 (6) | C6—H6B | 0.9800 |
| Cd—S4i | 2.6342 (5) | C6—H6C | 0.9800 |
| Cd—N3 | 2.3811 (18) | C8—C9 | 1.523 (3) |
| S1—C1 | 1.7267 (19) | C8—H8A | 0.9900 |
| S2—C1 | 1.7231 (18) | C8—H8B | 0.9900 |
| S3—C7 | 1.7404 (19) | C9—H9A | 0.9900 |
| S3—Cdi | 2.7317 (6) | C9—H9B | 0.9900 |
| S4—C7 | 1.714 (2) | C10—C11 | 1.521 (3) |
| S4—Cdi | 2.6343 (5) | C10—C12 | 1.521 (3) |
| O1—C3 | 1.426 (2) | C10—H10 | 1.0000 |
| O1—H1O | 0.830 (10) | C11—H11A | 0.9800 |
| O2—C9 | 1.419 (2) | C11—H11B | 0.9800 |
| O2—H2O | 0.846 (10) | C11—H11C | 0.9800 |
| O1W—H1W | 0.842 (10) | C12—H12A | 0.9800 |
| O1W—H2W | 0.847 (10) | C12—H12B | 0.9800 |
| N1—C7 | 1.341 (2) | C12—H12C | 0.9800 |
| N1—C8 | 1.477 (2) | C13—C14 | 1.383 (3) |
| N1—C10 | 1.493 (3) | C13—H13 | 0.9500 |
| N2—C1 | 1.345 (2) | C14—C15 | 1.393 (3) |
| N2—C2 | 1.472 (2) | C14—H14 | 0.9500 |
| N2—C4 | 1.490 (2) | C15—C16 | 1.388 (3) |
| N3—C17 | 1.337 (3) | C15—H15 | 0.9500 |
| N3—C13 | 1.346 (3) | C16—C17 | 1.396 (3) |
| N4—C18 | 1.283 (3) | C16—C18 | 1.469 (3) |
| N4—N5 | 1.415 (2) | C17—H17 | 0.9500 |
| N5—C19 | 1.277 (3) | C18—H18 | 0.9500 |
| N6—C21 | 1.336 (3) | C19—C20 | 1.466 (3) |
| N6—C22 | 1.342 (3) | C19—H19 | 0.9500 |
| C2—C3 | 1.532 (3) | C20—C24 | 1.396 (3) |
| C2—H2A | 0.9900 | C20—C21 | 1.399 (3) |
| C2—H2B | 0.9900 | C21—H21 | 0.9500 |
| C3—H3A | 0.9900 | C22—C23 | 1.377 (4) |
| C3—H3B | 0.9900 | C22—H22 | 0.9500 |
| C4—C6 | 1.521 (3) | C23—C24 | 1.386 (4) |
| C4—C5 | 1.526 (3) | C23—H23 | 0.9500 |
| C4—H4 | 1.0000 | C24—H24 | 0.9500 |
| C5—H5A | 0.9800 | ||
| N3—Cd—S4i | 101.46 (4) | N1—C7—S4 | 120.60 (15) |
| N3—Cd—S1 | 94.50 (4) | N1—C7—S3 | 120.40 (15) |
| N3—Cd—S2 | 90.73 (4) | S4—C7—S3 | 119.00 (11) |
| S4i—Cd—S2 | 100.522 (15) | N1—C8—C9 | 111.86 (16) |
| S1—Cd—S2 | 67.824 (14) | N1—C8—H8A | 109.2 |
| S4i—Cd—S3i | 67.343 (17) | C9—C8—H8A | 109.2 |
| S4i—Cd—S1 | 160.481 (17) | N1—C8—H8B | 109.2 |
| S2—Cd—S3 | 167.393 (15) | C9—C8—H8B | 109.2 |
| N3—Cd—S3i | 166.35 (4) | H8A—C8—H8B | 107.9 |
| S1—Cd—S3i | 98.110 (17) | O2—C9—C8 | 107.62 (17) |
| S2—Cd—S3i | 98.831 (15) | O2—C9—H9A | 110.2 |
| N3—Cd—S3 | 86.49 (4) | C8—C9—H9A | 110.2 |
| S4i—Cd—S3 | 92.082 (15) | O2—C9—H9B | 110.2 |
| S1—Cd—S3 | 100.113 (14) | C8—C9—H9B | 110.2 |
| S3i—Cd—S3 | 86.184 (15) | H9A—C9—H9B | 108.5 |
| C1—S1—Cd | 87.09 (6) | N1—C10—C11 | 110.18 (17) |
| C1—S2—Cd | 86.13 (6) | N1—C10—C12 | 111.95 (18) |
| C7—S3—Cdi | 84.87 (7) | C11—C10—C12 | 112.89 (19) |
| C7—S3—Cd | 97.23 (6) | N1—C10—H10 | 107.2 |
| Cdi—S3—Cd | 93.816 (15) | C11—C10—H10 | 107.2 |
| C7—S4—Cdi | 88.51 (7) | C12—C10—H10 | 107.2 |
| C3—O1—H1O | 108 (2) | C10—C11—H11A | 109.5 |
| C9—O2—H2O | 107 (3) | C10—C11—H11B | 109.5 |
| H1W—O1W—H2W | 106 (3) | H11A—C11—H11B | 109.5 |
| C7—N1—C8 | 120.49 (17) | C10—C11—H11C | 109.5 |
| C7—N1—C10 | 121.81 (16) | H11A—C11—H11C | 109.5 |
| C8—N1—C10 | 117.33 (16) | H11B—C11—H11C | 109.5 |
| C1—N2—C2 | 121.05 (16) | C10—C12—H12A | 109.5 |
| C1—N2—C4 | 121.41 (16) | C10—C12—H12B | 109.5 |
| C2—N2—C4 | 117.38 (15) | H12A—C12—H12B | 109.5 |
| C17—N3—C13 | 118.42 (18) | C10—C12—H12C | 109.5 |
| C17—N3—Cd | 115.48 (14) | H12A—C12—H12C | 109.5 |
| C13—N3—Cd | 126.01 (14) | H12B—C12—H12C | 109.5 |
| C18—N4—N5 | 111.56 (18) | N3—C13—C14 | 122.51 (19) |
| C19—N5—N4 | 111.02 (19) | N3—C13—H13 | 118.7 |
| C21—N6—C22 | 117.4 (2) | C14—C13—H13 | 118.7 |
| N2—C1—S2 | 121.63 (14) | C13—C14—C15 | 118.9 (2) |
| N2—C1—S1 | 119.59 (14) | C13—C14—H14 | 120.5 |
| S2—C1—S1 | 118.77 (11) | C15—C14—H14 | 120.5 |
| N2—C2—C3 | 111.96 (16) | C16—C15—C14 | 119.0 (2) |
| N2—C2—H2A | 109.2 | C16—C15—H15 | 120.5 |
| C3—C2—H2A | 109.2 | C14—C15—H15 | 120.5 |
| N2—C2—H2B | 109.2 | C15—C16—C17 | 118.27 (19) |
| C3—C2—H2B | 109.2 | C15—C16—C18 | 121.51 (19) |
| H2A—C2—H2B | 107.9 | C17—C16—C18 | 120.22 (19) |
| O1—C3—C2 | 111.22 (17) | N3—C17—C16 | 122.88 (19) |
| O1—C3—H3A | 109.4 | N3—C17—H17 | 118.6 |
| C2—C3—H3A | 109.4 | C16—C17—H17 | 118.6 |
| O1—C3—H3B | 109.4 | N4—C18—C16 | 119.52 (19) |
| C2—C3—H3B | 109.4 | N4—C18—H18 | 120.2 |
| H3A—C3—H3B | 108.0 | C16—C18—H18 | 120.2 |
| N2—C4—C6 | 110.94 (17) | N5—C19—C20 | 121.0 (2) |
| N2—C4—C5 | 111.74 (17) | N5—C19—H19 | 119.5 |
| C6—C4—C5 | 112.17 (17) | C20—C19—H19 | 119.5 |
| N2—C4—H4 | 107.2 | C24—C20—C21 | 118.1 (2) |
| C6—C4—H4 | 107.2 | C24—C20—C19 | 120.3 (2) |
| C5—C4—H4 | 107.2 | C21—C20—C19 | 121.6 (2) |
| C4—C5—H5A | 109.5 | N6—C21—C20 | 123.2 (2) |
| C4—C5—H5B | 109.5 | N6—C21—H21 | 118.4 |
| H5A—C5—H5B | 109.5 | C20—C21—H21 | 118.4 |
| C4—C5—H5C | 109.5 | N6—C22—C23 | 123.9 (2) |
| H5A—C5—H5C | 109.5 | N6—C22—H22 | 118.0 |
| H5B—C5—H5C | 109.5 | C23—C22—H22 | 118.0 |
| C4—C6—H6A | 109.5 | C22—C23—C24 | 118.5 (2) |
| C4—C6—H6B | 109.5 | C22—C23—H23 | 120.8 |
| H6A—C6—H6B | 109.5 | C24—C23—H23 | 120.8 |
| C4—C6—H6C | 109.5 | C23—C24—C20 | 119.0 (2) |
| H6A—C6—H6C | 109.5 | C23—C24—H24 | 120.5 |
| H6B—C6—H6C | 109.5 | C20—C24—H24 | 120.5 |
| C18—N4—N5—C19 | 176.5 (2) | C7—N1—C10—C11 | 96.7 (2) |
| C2—N2—C1—S2 | 177.27 (14) | C8—N1—C10—C11 | −76.4 (2) |
| C4—N2—C1—S2 | 2.0 (3) | C7—N1—C10—C12 | −136.83 (19) |
| C2—N2—C1—S1 | −3.9 (3) | C8—N1—C10—C12 | 50.1 (2) |
| C4—N2—C1—S1 | −179.22 (14) | C17—N3—C13—C14 | 0.8 (3) |
| Cd—S2—C1—N2 | 174.63 (16) | Cd—N3—C13—C14 | 177.07 (15) |
| Cd—S2—C1—S1 | −4.16 (10) | N3—C13—C14—C15 | 0.6 (3) |
| Cd—S1—C1—N2 | −174.61 (16) | C13—C14—C15—C16 | −1.6 (3) |
| Cd—S1—C1—S2 | 4.21 (11) | C14—C15—C16—C17 | 1.3 (3) |
| C1—N2—C2—C3 | 82.7 (2) | C14—C15—C16—C18 | −179.43 (18) |
| C4—N2—C2—C3 | −101.9 (2) | C13—N3—C17—C16 | −1.1 (3) |
| N2—C2—C3—O1 | −178.05 (16) | Cd—N3—C17—C16 | −177.75 (15) |
| C1—N2—C4—C6 | 104.3 (2) | C15—C16—C17—N3 | 0.0 (3) |
| C2—N2—C4—C6 | −71.1 (2) | C18—C16—C17—N3 | −179.23 (18) |
| C1—N2—C4—C5 | −129.72 (19) | N5—N4—C18—C16 | 178.04 (17) |
| C2—N2—C4—C5 | 54.8 (2) | C15—C16—C18—N4 | 165.2 (2) |
| C8—N1—C7—S4 | −6.0 (2) | C17—C16—C18—N4 | −15.5 (3) |
| C10—N1—C7—S4 | −178.81 (14) | N4—N5—C19—C20 | 176.96 (18) |
| C8—N1—C7—S3 | 173.68 (14) | N5—C19—C20—C24 | −179.4 (2) |
| C10—N1—C7—S3 | 0.9 (3) | N5—C19—C20—C21 | −1.6 (3) |
| Cdi—S4—C7—N1 | 174.52 (15) | C22—N6—C21—C20 | −1.0 (4) |
| Cdi—S4—C7—S3 | −5.15 (10) | C24—C20—C21—N6 | 0.8 (3) |
| Cdi—S3—C7—N1 | −174.68 (15) | C19—C20—C21—N6 | −177.0 (2) |
| Cd—S3—C7—N1 | 92.11 (15) | C21—N6—C22—C23 | 0.4 (4) |
| Cdi—S3—C7—S4 | 4.99 (10) | N6—C22—C23—C24 | 0.3 (5) |
| Cd—S3—C7—S4 | −88.22 (11) | C22—C23—C24—C20 | −0.4 (4) |
| C7—N1—C8—C9 | 84.0 (2) | C21—C20—C24—C23 | −0.1 (3) |
| C10—N1—C8—C9 | −102.9 (2) | C19—C20—C24—C23 | 177.8 (2) |
| N1—C8—C9—O2 | 175.92 (19) |
Symmetry code: (i) −x+1, −y, −z+1.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1O···O2ii | 0.83 (2) | 1.83 (3) | 2.655 (3) | 172 (3) |
| O2—H2O···O1W | 0.85 (3) | 1.80 (3) | 2.640 (3) | 180 (6) |
| O1W—H1W···O1 | 0.84 (3) | 1.92 (3) | 2.750 (3) | 172 (3) |
| O1W—H2W···N6iii | 0.85 (2) | 2.00 (2) | 2.840 (3) | 172 (2) |
| C23—H23···O1ii | 0.95 | 2.50 | 3.295 (3) | 141 |
| C4—H4···S2iv | 1.00 | 2.79 | 3.599 (2) | 139 |
| C15—H15···S2v | 0.95 | 2.84 | 3.714 (2) | 153 |
| C15—H15···Cg(Cd,S1,S2,C1)vi | 0.95 | 2.79 | 3.737 (2) | 173 |
Symmetry codes: (ii) −x+1/2, y+1/2, −z+3/2; (iii) −x, −y+1, −z+1; (iv) −x+1, −y+1, −z+1; (v) x−1/2, −y+1/2, z−1/2; (vi) x−3/2, −y−1/2, z−3/2.
References
- Airoldi, C., De Oliveira, S. F., Ruggiero, S. G. & Lechat, J. R. (1990). Inorg. Chim. Acta, 174, 103–108.
- Avila, V., Benson, R. E., Broker, G. A., Daniels, L. M. & Tiekink, E. R. T. (2006). Acta Cryst. E62, m1425–m1427.
- Bhattacharya, B., Dey, R., Maity, D. K. & Ghoshal, D. (2013). CrystEngComm, 15, 9457–9464.
- Bing, Y., Li, X., Zha, M. & Lu, Y. (2010). Acta Cryst. E66, m1500. [DOI] [PMC free article] [PubMed]
- Brandenburg, K. (2006). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Chai, J., Lai, C. S., Yan, J. & Tiekink, E. R. T. (2003). Appl. Organomet. Chem. 17, 249–250.
- Dee, C. M. & Tiekink, E. R. T. (2002). Z. Kristallogr. New Cryst. Struct 217, 85–86.
- Domenicano, A., Torelli, L., Vaciago, A. & Zambonelli, L. (1968). J. Chem. Soc. A, pp. 1351–1361.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Ferreira, I. P., de Lima, G. M., Paniago, E. B., Pinheiro, C. B., Wardell, J. L. & Wardell, S. M. S. V. (2016). Inorg. Chim. Acta, 441, 137–145.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Higashi, T. (1995). ABSCOR. Rigaku Corporation, Tokyo, Japan.
- Jotani, M. M., Tan, Y. S. & Tiekink, E. R. T. (2016). Z. Kristallogr 231, 403–413.
- Kumar, V., Singh, V., Gupta, A. N., Manar, K. K., Drew, M. G. B. & Singh, N. (2014). CrystEngComm, 16, 6765–6774.
- Lai, C. S. & Tiekink, E. R. T. (2006a). Z. Kristallogr 221, 288–293.
- Lai, C. S. & Tiekink, E. R. T. (2006b). J. Mol. Struct. 796, 114–118.
- Lennartson, A. & Håkansson, M. (2009). Angew. Chem. Int. Ed. 48, 5869–5871. [DOI] [PubMed]
- Molecular Structure Corporation & Rigaku (2005). CrystalClear. MSC, The Woodlands, Texas, USA, and Rigaku Corporation, Tokyo, Japan.
- Rajakannu, P., Howlader, R., Kalita, A. Ch., Butcher, R. J. & Murugavel, R. (2015). Inorg. Chem. Front. 2, 55–66.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Tan, Y. S., Azizuddin, A. D., Câmpian, M. V., Haiduc, I. & Tiekink, E. R. T. (2016). Z. Kristallogr 231, 155–165.
- Tan, Y. S., Halim, S. N. A. & Tiekink, E. R. T. (2016). Z. Kristallogr 231, 113–126.
- Tan, Y. S., Sudlow, A. L., Molloy, K. C., Morishima, Y., Fujisawa, K., Jackson, W. J., Henderson, W., Halim, S. N. B. A., Ng, S. W. & Tiekink, E. R. T. (2013). Cryst. Growth Des. 13, 3046–3056.
- Tiekink, E. R. T. (2003). CrystEngComm, 5, 101–113.
- Tiekink, E. R. T. & Haiduc, I. (2005). Prog. Inorg. Chem 54, 127–319.
- Tiekink, E. R. T. & Zukerman-Schpector, J. (2011). Chem. Commun. 47, 6623–6625. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2056989016012214/wm5312sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016012214/wm5312Isup2.hkl
CCDC reference: 1496352
Additional supporting information: crystallographic information; 3D view; checkCIF report