In the title hydrated salt, which was obtained from the hydrothermal reaction between between potassium 1,1,3,3-tetracyano-2-ethoxypropenide and 4,4′-bipyridine in the presence of iron(II) sulfate heptahydrate, the ionic components are linked into a three-dimensional network by C—H⋯N hydrogen bonds.
Keywords: crystal structure, hydrothermal synthesis, polynitrile anions, molecular structure, hydrogen bonding
Abstract
The title hydrated salt, C30H26N6 2+·2C9H5N4O−·3H2O, was obtained as an unexpected product from the hydrothermal reaction between potassium 1,1,3,3-tetracyano-2-ethoxypropenide, 4,4′-bipyridine and iron(II) sulfate heptahydrate. The cation lies across a twofold rotation axis in the space group I2/a with the other components all in general positions. In the cation, the H atom linking the pyridine units is disordered over two adjacent sites having occupancies of 0.66 (4) and 0.36 (4), i.e. as N—H⋯N and N⋯H—N. The water molecules of crystallization are each disordered over two sets of atomic sites, having occupancies of 0.522 (6) and 0.478 (6) for one, and 0.34 (3) and 0.16 (3) for the other, and it was not possible to reliably locate the H atoms associated with these partial-occupancy sites. In the crystal, four independent C—H⋯N hydrogen bonds link the ionic components into a three-dimensional network.
Chemical context
In recent years, the use of polynitrile anions as coordinating ligands for the construction of polymeric architectures with interesting properties has been a burgeoning subject in materials and coordination chemistry (Thétiot et al., 2003 ▸; Benmansour et al., 2007 ▸; Atmani et al., 2008 ▸). These anions are versatile structural components, leading to many different architectures in zero, one, two or three dimensions, and incorporating most of the 3d transition metals (Benmansour et al., 2008 ▸, 2010 ▸, 2012 ▸; Yuste et al., 2009 ▸; Setifi, Domasevitch et al., 2013 ▸; Setifi, Setifi et al., 2013 ▸; Setifi, Lehchili et al., 2014 ▸). This versatility is based on two main properties of these ligands: (i) the ability to act as bridges, given the linear and rigid geometry of the cyano groups, and (ii) the possibility of functionalization with different potentially coordinating groups that leads to a high variety of coordination modes. To take advantage of this behaviour we have been using these organic anions in combination with other chelating or bridging neutral co-ligands to explore their structural and electronic characteristics of the resulting complexes, particularly with reference to molecular materials exhibiting interesting magnetic exchange coupling behaviour. During the course of attempts to prepare such complexes with 4,4′-bipyridine, we isolated the title compound (I) (Fig. 1 ▸ and Scheme 1), whose structure is reported here.
Figure 1.
The independent components of the structure of compound (I), showing the atom-labelling scheme, the complete central 4,4′-bipy unit and the hydrogen bond (shown as a dashed line) between the cation and anion within the selected asymmetric unit. Displacement ellipsoids are drawn at the 30% probability level and the atoms marked with ‘a’ are at the symmetry position (−x +  , y, −z + 1). The partially occupied water sites have refined occupancies as follows: O5A 0.522 (6), O5B 0.478 (6), O6A 0.34 (3) and O6B 0.16 (3).
, y, −z + 1). The partially occupied water sites have refined occupancies as follows: O5A 0.522 (6), O5B 0.478 (6), O6A 0.34 (3) and O6B 0.16 (3).
Structural commentary
The structure of compound (I) consists of a [tris(4,4′-bipyridine)]diium dication, [(4,4′-bipy)-H-(4,4′-bipy)-H-(4,4′-bipy)]2+, two 1,1,3,3-tetracyano-2-ethoxypropenide anions, [(NC)2CC(OEt)C(CN)2]−, and three water molecules. The cation lies across a twofold rotation axis, selected for the reference cation as that along (0.25, y, 0.5), while the other components all lie in general positions. Within the cation, the H atom linking the 4,4′-bipy units is disordered over two adjacent sites having occupancies of 0.66 (4) and 0.36 (4), and the two independent water molecules are also disordered, both over two atomic sites, with one having occupancies of 0.522 (6) and 0.478 (6) and the other having occupancies of 0.34 (3) and 0.16 (3).
In the cation, the dihedral angle between the two symmetry-related rings of the central unit is 37.60 (4)°, the dihedral angle between the rings containing atoms N11 and N21 is 85.96 (5)° and that between the rings containing atoms N21 and N31 is 29.33 (3)° (cf. Fig. 1 ▸). In the anion, the corresponding pairs of bond distances and bond angles associated with the two C—C(CN)2 units containing the atoms C41 and C43 are very similar. In addition, the C—C distances in the C(CN)2 fragments are all short for their type [mean value (Allen et al., 1987 ▸) 1.431 Å, lower quartile value 1.425 Å], while the C—N distances are all long for their type (mean value 1.136 Å, upper quartile value 1.142 Å). These observations indicate that there is considerable delocalization of the negative charge within the anion, not just over the central propenide fragment, resonance forms (a) and (b) (see Scheme 2), but also onto the N atoms of the four cyano substituents, forms (c)–(f). Despite this, the core skeleton of the anion is not planar, as the two C(CN)2 units are rotated in conrotatory fashion out of the plane of the propenide unit; this central C3 fragment makes dihedral angles of 10.39 (13) and 16.71 (18)°, respectively, with the C(CN)2 units containing atoms C41 and C43.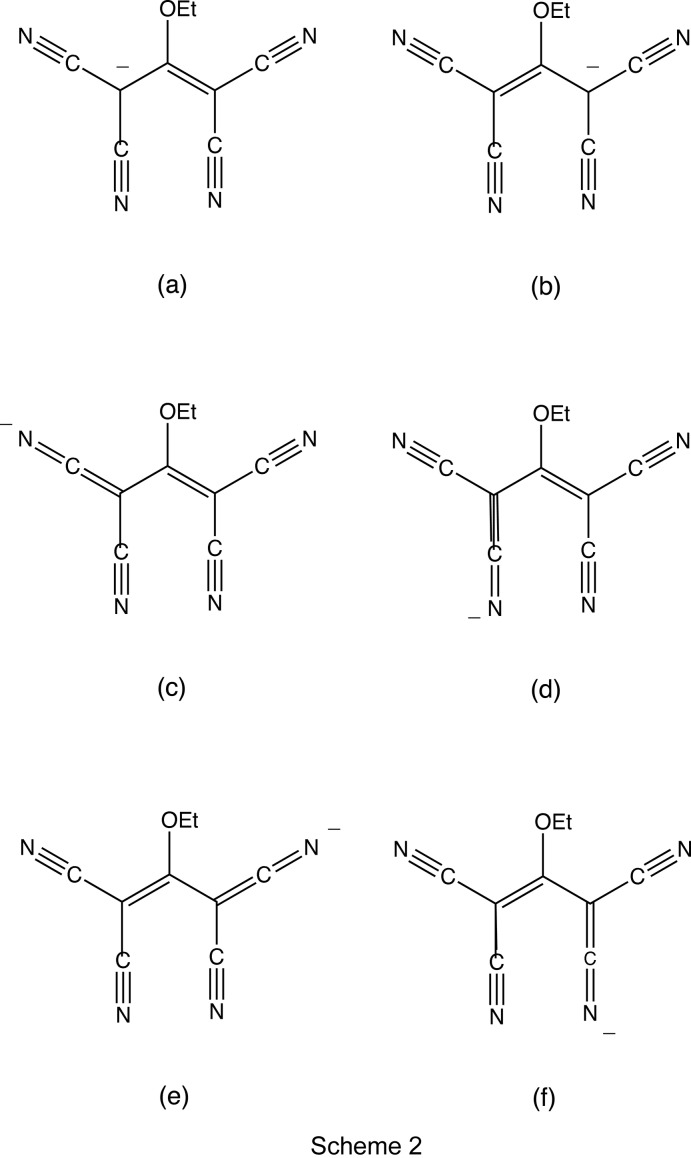
Supramolecular interactions
The two independent 4,4′-bipy units are linked by disordered N—H⋯N hydrogen bonds, both of which are almost linear (Table 1 ▸). In addition, there are four C—H⋯N hydrogen bonds in the structure: two of these have donor atoms, C13 and C15, which are part of the 4,4′-bipy unit containing N11 and acceptors in the anion, one has an acceptor in the 4,4′-bipy unit containing N21 and N31, and the fourth involves only the anion. Of these four interactions, the first two can be regarded as charge-assisted hydrogen bonds (Gilli et al., 1994 ▸) and it is interesting to note that the ethoxy O atom in the anion plays no role in the supramolecular assembly.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N11—H11⋯N21 | 0.98 (4) | 1.69 (4) | 2.6655 (18) | 175 (3) |
| N21—H21⋯N11 | 0.90 (7) | 1.78 (7) | 2.6655 (18) | 172 (5) |
| C12—H12⋯N31i | 0.95 | 2.57 | 3.4248 (19) | 150 |
| C13—H13⋯N411ii | 0.95 | 2.56 | 3.434 (2) | 154 |
| C15—H15⋯N411 | 0.95 | 2.38 | 3.249 (2) | 152 |
| C25—H25⋯O5B | 0.95 | 2.56 | 3.355 (4) | 141 |
| C35—H35⋯O6A | 0.95 | 2.53 | 3.474 (13) | 176 |
| C35—H35⋯O6B | 0.95 | 2.54 | 3.484 (16) | 170 |
| C421—H41A⋯N431iii | 0.99 | 2.61 | 3.589 (2) | 172 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
These six hydrogen bonds link the cations and anions into a three-dimensional network whose formation is readily analysed in terms of substructures (Ferguson et al., 1998a
▸,b
▸; Gregson et al., 2000 ▸) in zero, one and two dimensions. It is convenient to consider firstly the hydrogen bonds between cations and anions. The anions and the central 4,4′-bipy units containing atom N11 which are related by translation along the [010] direction are linked to form the one-dimensional substructure in the form of a ribbon of edge-fused  (14) loops (Fig. 2 ▸). Ribbons of this type, which are related by translation along [1
(14) loops (Fig. 2 ▸). Ribbons of this type, which are related by translation along [1 1], are linked by the 4,4′-bipy units containing atoms N21 and N31 to form the two-dimensional substructure, a sheet lying parallel to (10
1], are linked by the 4,4′-bipy units containing atoms N21 and N31 to form the two-dimensional substructure, a sheet lying parallel to (10 ) (Fig. 3 ▸). Adjacent sheets are linked by the zero-dimensional substructure which involves inversion-related pairs of anions forming a centrosymmetric motif characterized by an
) (Fig. 3 ▸). Adjacent sheets are linked by the zero-dimensional substructure which involves inversion-related pairs of anions forming a centrosymmetric motif characterized by an  (14) ring (Fig. 4 ▸).
(14) ring (Fig. 4 ▸).
Figure 2.
Part of the crystal structure of compound (I), showing the formation of a hydrogen-bonded ribbon of edge-fused  (14) rings along the [010] direction. For the sake of clarity, the 4,4′-bipy units containing atoms N21 and N31, the partial-occupancy water molecules, and the H atoms not involved in the motif shown have been omitted.
(14) rings along the [010] direction. For the sake of clarity, the 4,4′-bipy units containing atoms N21 and N31, the partial-occupancy water molecules, and the H atoms not involved in the motif shown have been omitted.
Figure 3.
Part of the crystal structure of compound (I), showing the formation of a hydrogen-bonded sheet lying parallel to (10 ). For the sake of clarity, the partial-occupancy water molecules, and the H atoms not involved in the motif shown have been omitted.
). For the sake of clarity, the partial-occupancy water molecules, and the H atoms not involved in the motif shown have been omitted.
Figure 4.
Part of the crystal structure of compound (I), showing the formation by pairs of anions of a hydrogen-bonded  (14) ring. The atoms marked with an asterisk (*) are at the symmetry position (−x, −y + 1, −z + 1). For the sake of clarity, the unit-cell outline, the 4,4′-bipy units and the partial-occupancy water molecules have all been omitted.
(14) ring. The atoms marked with an asterisk (*) are at the symmetry position (−x, −y + 1, −z + 1). For the sake of clarity, the unit-cell outline, the 4,4′-bipy units and the partial-occupancy water molecules have all been omitted.
Three of the partially occupied water sites are linked by C—H⋯O hydrogen bonds (Table 1 ▸) within the selected asymmetric unit to one of the 4,4′-bipy components, while the fourth, O5A, lies 2.54 (3) Å from atom O6A at (−x + 1, y +  , −z +
, −z +  ), i.e. within the reference (10
), i.e. within the reference (10 ) sheet and comfortably within O—H⋯O hydrogen-bonding range.
) sheet and comfortably within O—H⋯O hydrogen-bonding range.
Database survey
The 1,1,3,3-tetracyano-2-ethoxypropenide unit, here conveniently denoted as X −, has been reported in a number of structures. These include salts of organic cations, including [(2,2′-bipy)H]+·X −, (II) (Setifi, Valkonen et al., 2015 ▸), [(4,4′-bipy)H2]2+·2X −, (III) (Setifi, Geiger et al., 2015 ▸), and [(4,4′-bipy)Et2]2+·2X −, (IV) (Setifi, Lehchili et al., 2014 ▸); salts of mononuclear metal complexes in which the 1,1,3,3-tetracyano-2-ethoxypropenide unit is not ccordinated to the metal centre, including [(2,2′-bi-1H-imidazole)2Cu]2+·2X −, (V) (Gaamoune et al., 2010 ▸), [(1,10-phen)3Fe]2−·2X −·0.5H2O, (VI) (Setifi, Setifi et al., 2013 ▸), [(1,10-phen)3Fe]2−·2X −·H2O, and (VII) (Setifi, Domasevitch et al., 2013 ▸); and compounds where the 1,1,3,3-tetracyano-2-ethoxypropenide unit acts as a ligand including a binuclear Cu complex in which it acts both as a bridging ligand between two CuII centres and as a monodentate terminal ligand, thus [(2,2′-bipy)XCu]2(μ-X)2, (VIII) (Addala et al., 2015 ▸), and a two-dimensional coordination polymer [X(1,10-phen)ClCu]n, (IX) (Setifi, Setifi et al., 2014 ▸).
Of these examples, compounds (II), (III) and (IV) are most closely related to compound (I) reported here. In the structure of compound (II), a combination of N—H⋯N and C—H⋯N hydrogen bonds links the ions into ribbons containing alternating  (18) and
(18) and  (26) rings; in (IV), where there are no N—H⋯N hydrogen bonds, the ions are linked into sheets by C—H⋯N hydrogen bonds, and in (III), an extensive series of N—H⋯N and C—H⋯N hydrogen bonds generates a three-dimensional network, so that the supramolecular aggregation is one-, two- and three-dimensional in compounds (II), (IV) and (III), respectively.
(26) rings; in (IV), where there are no N—H⋯N hydrogen bonds, the ions are linked into sheets by C—H⋯N hydrogen bonds, and in (III), an extensive series of N—H⋯N and C—H⋯N hydrogen bonds generates a three-dimensional network, so that the supramolecular aggregation is one-, two- and three-dimensional in compounds (II), (IV) and (III), respectively.
Synthesis and crystallization
The salt K(tcnoet) was prepared according to a published method (Middleton et al., 1958 ▸). The title compound was synthesized hydrothermally under autogenous pressure from a mixture of iron(II) sulfate heptahydrate (56 mg, 0.2 mmol), 4,4′-bipyridine (32 mg, 0.2 mmol) and K(tcnoet) (90 mg, 0.4 mmol) in water–methanol (4:1 v/v, 20 ml). This mixture was sealed in a Teflon-lined autoclave and held at 423 K for 2 d, and then cooled to ambient temperature at a rate of 10 K h−1 (yield 25%). Pale-yellow blocks of the title compound suitable for single-crystal X-ray diffraction were selected directly from the synthesized product.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. The H atoms bonded to C or N atoms were all located in difference maps. The H atoms bonded to C atoms were subsequently treated as riding atoms in geometrically idealised positions, with C—H = 0.95 (pyridyl), 0.98 (CH3) or 0.99 Å (CH2), and with U iso(H) = kU eq(C) where k = 1.5 for the methyl group, which was permitted to rotate but not to tilt, and 1.2 for all other H atoms bonded to C atoms. The unique H atom bonded to N was disordered over two atomic sites, labeled H11 and H21, adjacent to atoms N11 and N21, respectively, and having unequal occupancies; for these two sites, the atomic coordinates were refined with U iso(H) = 1.2U eq(N), leading to the N—H distances shown in Table 1 ▸ and to refined site occupancies of 0.66 (4) and 0.36 (4) for H11 and H21, respectively. No H-atom sites associated with water atoms O5 and O6 could be located. Each of these water O atoms is disordered over two atomic sites: O5 is disordered over two sites, labelled O5A and O5B, which are separated by 0.963 (4) Å, while O6 is disordered over two sites, labelled O6A and O6B, which are separated by 0.627 (9) Å. Free refinement of the site occupancies of O5A and O5B gave values of 0.579 (7) and 0.512 (7), respectively; these values are not physically possible and both are over-estimates because of the lack of H atoms in the modelling of the water sites. Accordingly, the occupancies of O5A and O5B were constrained to sum to unity, giving values of 0.522 (6) and 0.478 (6). Free refinement of the site occupancies for O6A and O6B gave values of 0.36 (3) and 0.16 (3), and these values were subsequently restrained to sum to 0.500 (2), giving final values of 0.34 (3) and 0.16 (3). The final analysis of variance showed a large value, 4.522, of K = [mean(F o 2)]/[mean(F c 2)] for the group of 541 very weak reflections having F c/F c(max) in the range 0.000 < F c/F c(max) < 0.014.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C30H26N6 2+·2C9H5N4O−·3H2O |
| M r | 894.95 |
| Crystal system, space group | Monoclinic, I2/a |
| Temperature (K) | 123 |
| a, b, c (Å) | 18.1861 (2), 7.1187 (1), 35.7070 (4) |
| β (°) | 100.448 (1) |
| V (Å3) | 4546.03 (10) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.09 |
| Crystal size (mm) | 0.45 × 0.38 × 0.31 |
| Data collection | |
| Diffractometer | Bruker–Nonius Kappa CCD with APEXII detector |
| Absorption correction | Multi-scan (SADABS; Sheldrick, 2003 ▸) |
| T min, T max | 0.907, 0.973 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 35680, 5197, 4559 |
| R int | 0.039 |
| (sin θ/λ)max (Å−1) | 0.650 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.049, 0.115, 1.09 |
| No. of reflections | 5197 |
| No. of parameters | 335 |
| No. of restraints | 1 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.32, −0.23 |
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2056989016012160/hb7603sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016012160/hb7603Isup2.hkl
CCDC reference: 1496221
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors acknowledge the Algerian MESRS (Ministère de l’Enseignement Supérieur et de la Recherche Scientifique), the DGRSDT (Direction Générale de la Recherche Scientifique et du Développement Technologique) and Université Ferhat Abbas Sétif 1 for financial support.
supplementary crystallographic information
Crystal data
| C30H26N62+·2C9H5N4O−·3H2O | F(000) = 1872 |
| Mr = 894.95 | Dx = 1.299 Mg m−3 |
| Monoclinic, I2/a | Mo Kα radiation, λ = 0.71073 Å |
| a = 18.1861 (2) Å | Cell parameters from 5197 reflections |
| b = 7.1187 (1) Å | θ = 2.3–27.5° |
| c = 35.7070 (4) Å | µ = 0.09 mm−1 |
| β = 100.448 (1)° | T = 123 K |
| V = 4546.03 (10) Å3 | Block, pale yellow |
| Z = 4 | 0.45 × 0.38 × 0.31 mm |
Data collection
| Bruker–Nonius Kappa CCD with APEXII detector diffractometer | 5197 independent reflections |
| Radiation source: fine focus sealed tube | 4559 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.039 |
| φ and ω scans | θmax = 27.5°, θmin = 2.3° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2003) | h = −23→23 |
| Tmin = 0.907, Tmax = 0.973 | k = −8→9 |
| 35680 measured reflections | l = −42→46 |
Refinement
| Refinement on F2 | 1 restraint |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.049 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.115 | w = 1/[σ2(Fo2) + (0.0362P)2 + 5.2868P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.09 | (Δ/σ)max = 0.001 |
| 5197 reflections | Δρmax = 0.32 e Å−3 |
| 335 parameters | Δρmin = −0.23 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| N11 | 0.34943 (7) | 0.62579 (18) | 0.59359 (3) | 0.0268 (3) | |
| H11 | 0.3778 (17) | 0.621 (4) | 0.6196 (11) | 0.032* | 0.66 (4) |
| C12 | 0.34888 (8) | 0.7810 (2) | 0.57234 (4) | 0.0260 (3) | |
| H12 | 0.3753 | 0.8893 | 0.5830 | 0.031* | |
| C13 | 0.31054 (7) | 0.7862 (2) | 0.53523 (4) | 0.0234 (3) | |
| H13 | 0.3109 | 0.8967 | 0.5204 | 0.028* | |
| C14 | 0.27134 (7) | 0.62756 (19) | 0.51984 (3) | 0.0192 (3) | |
| C15 | 0.27272 (8) | 0.4676 (2) | 0.54248 (4) | 0.0244 (3) | |
| H15 | 0.2466 | 0.3574 | 0.5327 | 0.029* | |
| C16 | 0.31261 (8) | 0.4715 (2) | 0.57924 (4) | 0.0276 (3) | |
| H16 | 0.3140 | 0.3625 | 0.5947 | 0.033* | |
| N21 | 0.42091 (7) | 0.62202 (17) | 0.66569 (4) | 0.0269 (3) | |
| H21 | 0.400 (3) | 0.616 (8) | 0.641 (2) | 0.032* | 0.34 (4) |
| C22 | 0.49341 (8) | 0.5785 (2) | 0.67630 (4) | 0.0276 (3) | |
| H22 | 0.5217 | 0.5488 | 0.6572 | 0.033* | |
| C23 | 0.52856 (8) | 0.5754 (2) | 0.71399 (4) | 0.0256 (3) | |
| H23 | 0.5802 | 0.5455 | 0.7206 | 0.031* | |
| C24 | 0.48726 (8) | 0.61675 (19) | 0.74231 (4) | 0.0219 (3) | |
| C25 | 0.41210 (8) | 0.6605 (2) | 0.73098 (4) | 0.0258 (3) | |
| H25 | 0.3821 | 0.6887 | 0.7494 | 0.031* | |
| C26 | 0.38118 (8) | 0.6626 (2) | 0.69263 (4) | 0.0277 (3) | |
| H26 | 0.3298 | 0.6940 | 0.6852 | 0.033* | |
| N31 | 0.58874 (8) | 0.61811 (19) | 0.86102 (3) | 0.0331 (3) | |
| C32 | 0.51622 (9) | 0.5772 (2) | 0.84948 (4) | 0.0327 (3) | |
| H32 | 0.4872 | 0.5488 | 0.8683 | 0.039* | |
| C33 | 0.48101 (9) | 0.5739 (2) | 0.81152 (4) | 0.0281 (3) | |
| H33 | 0.4294 | 0.5438 | 0.8049 | 0.034* | |
| C34 | 0.52250 (8) | 0.61556 (19) | 0.78334 (4) | 0.0227 (3) | |
| C35 | 0.59782 (8) | 0.6576 (2) | 0.79502 (4) | 0.0270 (3) | |
| H35 | 0.6283 | 0.6867 | 0.7768 | 0.032* | |
| C36 | 0.62787 (9) | 0.6565 (2) | 0.83360 (4) | 0.0313 (3) | |
| H36 | 0.6795 | 0.6848 | 0.8410 | 0.038* | |
| C41 | 0.15157 (8) | −0.1715 (2) | 0.58797 (4) | 0.0272 (3) | |
| C42 | 0.11579 (7) | −0.3411 (2) | 0.57565 (4) | 0.0234 (3) | |
| C43 | 0.11642 (8) | −0.5041 (2) | 0.59765 (4) | 0.0249 (3) | |
| C411 | 0.16626 (8) | −0.0335 (2) | 0.56164 (4) | 0.0297 (3) | |
| N411 | 0.17940 (8) | 0.0773 (2) | 0.54035 (4) | 0.0382 (3) | |
| C412 | 0.17576 (9) | −0.1283 (2) | 0.62709 (5) | 0.0347 (4) | |
| N412 | 0.19482 (10) | −0.0875 (3) | 0.65855 (5) | 0.0528 (4) | |
| C431 | 0.06545 (8) | −0.6532 (2) | 0.58565 (4) | 0.0262 (3) | |
| N431 | 0.02403 (7) | −0.77540 (19) | 0.57765 (4) | 0.0333 (3) | |
| C432 | 0.16949 (9) | −0.5343 (2) | 0.63136 (4) | 0.0321 (3) | |
| N432 | 0.21320 (9) | −0.5619 (3) | 0.65832 (4) | 0.0496 (4) | |
| O421 | 0.08005 (6) | −0.35955 (15) | 0.53932 (3) | 0.0283 (2) | |
| C421 | 0.02174 (9) | −0.2228 (2) | 0.52443 (4) | 0.0330 (3) | |
| H41A | 0.0143 | −0.2200 | 0.4963 | 0.040* | |
| H41B | 0.0372 | −0.0959 | 0.5341 | 0.040* | |
| C422 | −0.04964 (9) | −0.2764 (3) | 0.53674 (5) | 0.0427 (4) | |
| H42A | −0.0886 | −0.1850 | 0.5268 | 0.064* | |
| H42B | −0.0650 | −0.4016 | 0.5269 | 0.064* | |
| H42C | −0.0421 | −0.2778 | 0.5646 | 0.064* | |
| O5A | 0.2512 (2) | 0.9317 (7) | 0.73743 (12) | 0.0915 (17) | 0.522 (6) |
| O5B | 0.28182 (16) | 0.9204 (5) | 0.76234 (11) | 0.0533 (13) | 0.478 (6) |
| O6A | 0.7139 (8) | 0.743 (4) | 0.7296 (2) | 0.055 (4) | 0.34 (3) |
| O6B | 0.7007 (9) | 0.824 (4) | 0.7286 (3) | 0.031 (4) | 0.16 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N11 | 0.0253 (6) | 0.0364 (7) | 0.0166 (5) | 0.0030 (5) | −0.0018 (5) | 0.0009 (5) |
| C12 | 0.0241 (7) | 0.0309 (8) | 0.0210 (6) | −0.0013 (6) | −0.0013 (5) | −0.0028 (6) |
| C13 | 0.0248 (6) | 0.0246 (7) | 0.0199 (6) | −0.0014 (5) | 0.0015 (5) | 0.0007 (5) |
| C14 | 0.0165 (6) | 0.0233 (7) | 0.0169 (6) | 0.0020 (5) | 0.0011 (5) | 0.0004 (5) |
| C15 | 0.0244 (6) | 0.0254 (7) | 0.0224 (7) | −0.0016 (6) | 0.0013 (5) | 0.0016 (5) |
| C16 | 0.0295 (7) | 0.0306 (8) | 0.0217 (7) | 0.0026 (6) | 0.0020 (5) | 0.0063 (6) |
| N21 | 0.0325 (6) | 0.0262 (6) | 0.0188 (6) | −0.0020 (5) | −0.0036 (5) | 0.0016 (5) |
| C22 | 0.0322 (7) | 0.0283 (7) | 0.0216 (7) | −0.0004 (6) | 0.0034 (6) | 0.0011 (6) |
| C23 | 0.0259 (7) | 0.0255 (7) | 0.0235 (7) | 0.0015 (6) | −0.0002 (5) | 0.0007 (5) |
| C24 | 0.0269 (7) | 0.0174 (6) | 0.0197 (6) | −0.0014 (5) | 0.0001 (5) | 0.0006 (5) |
| C25 | 0.0270 (7) | 0.0265 (7) | 0.0228 (7) | −0.0006 (6) | 0.0016 (5) | −0.0024 (5) |
| C26 | 0.0260 (7) | 0.0280 (8) | 0.0259 (7) | −0.0003 (6) | −0.0035 (5) | −0.0002 (6) |
| N31 | 0.0437 (8) | 0.0285 (7) | 0.0227 (6) | 0.0020 (6) | −0.0053 (5) | 0.0004 (5) |
| C32 | 0.0466 (9) | 0.0287 (8) | 0.0216 (7) | −0.0022 (7) | 0.0029 (6) | 0.0032 (6) |
| C33 | 0.0332 (7) | 0.0253 (7) | 0.0239 (7) | −0.0026 (6) | 0.0003 (6) | 0.0014 (6) |
| C34 | 0.0295 (7) | 0.0174 (6) | 0.0193 (6) | 0.0035 (5) | −0.0010 (5) | −0.0005 (5) |
| C35 | 0.0292 (7) | 0.0249 (7) | 0.0250 (7) | 0.0031 (6) | 0.0001 (6) | −0.0028 (5) |
| C36 | 0.0324 (8) | 0.0294 (8) | 0.0280 (7) | 0.0037 (6) | −0.0054 (6) | −0.0041 (6) |
| C41 | 0.0283 (7) | 0.0256 (7) | 0.0286 (7) | −0.0013 (6) | 0.0074 (6) | −0.0023 (6) |
| C42 | 0.0226 (6) | 0.0266 (7) | 0.0217 (6) | 0.0015 (5) | 0.0060 (5) | −0.0009 (5) |
| C43 | 0.0246 (7) | 0.0266 (7) | 0.0233 (7) | −0.0006 (6) | 0.0038 (5) | 0.0012 (5) |
| C411 | 0.0284 (7) | 0.0245 (7) | 0.0379 (8) | −0.0001 (6) | 0.0108 (6) | −0.0039 (6) |
| N411 | 0.0419 (8) | 0.0267 (7) | 0.0495 (8) | −0.0033 (6) | 0.0181 (7) | 0.0013 (6) |
| C412 | 0.0343 (8) | 0.0332 (9) | 0.0375 (9) | −0.0080 (7) | 0.0084 (7) | −0.0069 (7) |
| N412 | 0.0583 (10) | 0.0593 (11) | 0.0399 (9) | −0.0190 (8) | 0.0065 (7) | −0.0157 (8) |
| C431 | 0.0278 (7) | 0.0265 (7) | 0.0250 (7) | 0.0036 (6) | 0.0068 (5) | 0.0037 (6) |
| N431 | 0.0345 (7) | 0.0284 (7) | 0.0366 (7) | −0.0030 (6) | 0.0053 (6) | 0.0006 (6) |
| C432 | 0.0305 (8) | 0.0326 (8) | 0.0325 (8) | −0.0031 (6) | 0.0036 (6) | 0.0064 (6) |
| N432 | 0.0429 (8) | 0.0560 (10) | 0.0432 (9) | −0.0030 (7) | −0.0096 (7) | 0.0147 (7) |
| O421 | 0.0348 (5) | 0.0277 (5) | 0.0211 (5) | 0.0027 (4) | 0.0019 (4) | −0.0006 (4) |
| C421 | 0.0368 (8) | 0.0334 (8) | 0.0263 (7) | 0.0047 (7) | −0.0011 (6) | 0.0061 (6) |
| C422 | 0.0353 (9) | 0.0423 (10) | 0.0489 (10) | 0.0042 (8) | 0.0031 (7) | −0.0003 (8) |
| O5A | 0.059 (2) | 0.166 (4) | 0.054 (3) | 0.015 (2) | 0.021 (2) | 0.037 (2) |
| O5B | 0.0324 (15) | 0.082 (2) | 0.043 (2) | 0.0148 (14) | 0.0002 (14) | −0.0208 (16) |
| O6A | 0.058 (4) | 0.072 (11) | 0.038 (2) | −0.021 (6) | 0.015 (2) | −0.009 (4) |
| O6B | 0.047 (5) | 0.023 (9) | 0.027 (4) | 0.002 (5) | 0.016 (3) | 0.000 (4) |
Geometric parameters (Å, º)
| N11—C16 | 1.339 (2) | C33—C34 | 1.395 (2) |
| N11—C12 | 1.3393 (19) | C33—H33 | 0.9500 |
| N11—H11 | 0.98 (4) | C34—C35 | 1.390 (2) |
| C12—C13 | 1.3813 (19) | C35—C36 | 1.387 (2) |
| C12—H12 | 0.9500 | C35—H35 | 0.9500 |
| C13—C14 | 1.3938 (19) | C36—H36 | 0.9500 |
| C13—H13 | 0.9500 | C41—C42 | 1.404 (2) |
| C14—C15 | 1.3943 (19) | C41—C411 | 1.418 (2) |
| C14—C14i | 1.487 (2) | C41—C412 | 1.420 (2) |
| C15—C16 | 1.3802 (19) | C42—O421 | 1.3480 (16) |
| C15—H15 | 0.9500 | C411—N411 | 1.151 (2) |
| C16—H16 | 0.9500 | C412—N412 | 1.151 (2) |
| N21—C26 | 1.335 (2) | C42—C43 | 1.400 (2) |
| N21—C22 | 1.3405 (19) | C43—C431 | 1.423 (2) |
| N21—H21 | 0.90 (8) | C43—C432 | 1.416 (2) |
| C22—C23 | 1.3813 (19) | C431—N431 | 1.152 (2) |
| C22—H22 | 0.9500 | C432—N432 | 1.149 (2) |
| C23—C24 | 1.396 (2) | O421—C421 | 1.4662 (18) |
| C23—H23 | 0.9500 | C421—C422 | 1.493 (2) |
| C24—C25 | 1.3887 (19) | C421—H41A | 0.9900 |
| C24—C34 | 1.4885 (18) | C421—H41B | 0.9900 |
| C25—C26 | 1.3826 (19) | C422—H42A | 0.9800 |
| C25—H25 | 0.9500 | C422—H42B | 0.9800 |
| C26—H26 | 0.9500 | C422—H42C | 0.9800 |
| N31—C36 | 1.339 (2) | O5A—O5B | 0.963 (4) |
| N31—C32 | 1.340 (2) | O6A—O6B | 0.627 (9) |
| C32—C33 | 1.390 (2) | O6A—O6Aii | 1.78 (2) |
| C32—H32 | 0.9500 | ||
| C16—N11—C12 | 120.54 (12) | C32—C33—C34 | 119.08 (14) |
| C16—N11—H11 | 118.5 (16) | C32—C33—H33 | 120.5 |
| C12—N11—H11 | 121.0 (17) | C34—C33—H33 | 120.5 |
| N11—C12—C13 | 121.05 (14) | C35—C34—C33 | 117.49 (13) |
| N11—C12—H12 | 119.5 | C35—C34—C24 | 121.27 (13) |
| C13—C12—H12 | 119.5 | C33—C34—C24 | 121.24 (13) |
| C12—C13—C14 | 119.33 (13) | C36—C35—C34 | 119.10 (14) |
| C12—C13—H13 | 120.3 | C36—C35—H35 | 120.5 |
| C14—C13—H13 | 120.3 | C34—C35—H35 | 120.5 |
| C13—C14—C15 | 118.63 (11) | N31—C36—C35 | 124.16 (14) |
| C13—C14—C14i | 121.13 (8) | N31—C36—H36 | 117.9 |
| C15—C14—C14i | 120.25 (9) | C35—C36—H36 | 117.9 |
| C16—C15—C14 | 119.06 (13) | C42—C41—C411 | 121.34 (13) |
| C16—C15—H15 | 120.5 | C42—C41—C412 | 122.53 (14) |
| C14—C15—H15 | 120.5 | C411—C41—C412 | 116.12 (14) |
| N11—C16—C15 | 121.39 (13) | O421—C42—C43 | 114.36 (12) |
| N11—C16—H16 | 119.3 | O421—C42—C41 | 120.05 (13) |
| C15—C16—H16 | 119.3 | C41—C42—C43 | 125.51 (13) |
| C26—N21—C22 | 118.63 (12) | C42—C43—C431 | 120.70 (13) |
| C26—N21—H21 | 123 (3) | C42—C43—C432 | 122.51 (13) |
| C22—N21—H21 | 119 (3) | C431—C43—C432 | 116.70 (13) |
| N21—C22—C23 | 122.47 (14) | N411—C411—C41 | 178.83 (16) |
| N21—C22—H22 | 118.8 | N412—C412—C41 | 177.9 (2) |
| C23—C22—H22 | 118.8 | N431—C431—C43 | 176.87 (15) |
| C22—C23—C24 | 119.19 (13) | N432—C432—C43 | 178.5 (2) |
| C22—C23—H23 | 120.4 | C42—O421—C421 | 118.36 (11) |
| C24—C23—H23 | 120.4 | O421—C421—C422 | 109.51 (13) |
| C25—C24—C23 | 117.80 (12) | O421—C421—H41A | 109.8 |
| C25—C24—C34 | 120.90 (12) | C422—C421—H41A | 109.8 |
| C23—C24—C34 | 121.30 (12) | O421—C421—H41B | 109.8 |
| C26—C25—C24 | 119.55 (13) | C422—C421—H41B | 109.8 |
| C26—C25—H25 | 120.2 | H41A—C421—H41B | 108.2 |
| C24—C25—H25 | 120.2 | C421—C422—H42A | 109.5 |
| N21—C26—C25 | 122.35 (13) | C421—C422—H42B | 109.5 |
| N21—C26—H26 | 118.8 | H42A—C422—H42B | 109.5 |
| C25—C26—H26 | 118.8 | C421—C422—H42C | 109.5 |
| C36—N31—C32 | 116.31 (13) | H42A—C422—H42C | 109.5 |
| N31—C32—C33 | 123.86 (15) | H42B—C422—H42C | 109.5 |
| N31—C32—H32 | 118.1 | O6B—O6A—O6Aii | 102 (2) |
| C33—C32—H32 | 118.1 | ||
| C16—N11—C12—C13 | 0.0 (2) | C25—C24—C34—C35 | −150.27 (14) |
| N11—C12—C13—C14 | 0.5 (2) | C23—C24—C34—C35 | 29.3 (2) |
| C12—C13—C14—C15 | −0.6 (2) | C25—C24—C34—C33 | 29.1 (2) |
| C12—C13—C14—C14i | 179.35 (14) | C23—C24—C34—C33 | −151.33 (14) |
| C13—C14—C15—C16 | 0.1 (2) | C33—C34—C35—C36 | −0.1 (2) |
| C14i—C14—C15—C16 | −179.83 (14) | C24—C34—C35—C36 | 179.35 (13) |
| C12—N11—C16—C15 | −0.5 (2) | C32—N31—C36—C35 | 0.6 (2) |
| C14—C15—C16—N11 | 0.4 (2) | C34—C35—C36—N31 | −0.4 (2) |
| C26—N21—C22—C23 | −0.4 (2) | C411—C41—C42—O421 | −14.7 (2) |
| N21—C22—C23—C24 | 0.8 (2) | C412—C41—C42—O421 | 165.94 (14) |
| C22—C23—C24—C25 | −0.3 (2) | C411—C41—C42—C43 | 161.79 (14) |
| C22—C23—C24—C34 | −179.91 (13) | C412—C41—C42—C43 | −17.6 (2) |
| C23—C24—C25—C26 | −0.3 (2) | O421—C42—C43—C432 | 158.96 (13) |
| C34—C24—C25—C26 | 179.24 (13) | C41—C42—C43—C432 | −17.7 (2) |
| C22—N21—C26—C25 | −0.3 (2) | O421—C42—C43—C431 | −17.47 (19) |
| C24—C25—C26—N21 | 0.7 (2) | C41—C42—C43—C431 | 165.87 (14) |
| C36—N31—C32—C33 | −0.3 (2) | C43—C42—O421—C421 | 127.63 (14) |
| N31—C32—C33—C34 | −0.1 (2) | C41—C42—O421—C421 | −55.52 (18) |
| C32—C33—C34—C35 | 0.3 (2) | C42—O421—C421—C422 | −81.41 (17) |
| C32—C33—C34—C24 | −179.10 (13) |
Symmetry codes: (i) −x+1/2, y, −z+1; (ii) −x+3/2, −y+3/2, −z+3/2.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N11—H11···N21 | 0.98 (4) | 1.69 (4) | 2.6655 (18) | 175 (3) |
| N21—H21···N11 | 0.90 (7) | 1.78 (7) | 2.6655 (18) | 172 (5) |
| C12—H12···N31iii | 0.95 | 2.57 | 3.4248 (19) | 150 |
| C13—H13···N411iv | 0.95 | 2.56 | 3.434 (2) | 154 |
| C15—H15···N411 | 0.95 | 2.38 | 3.249 (2) | 152 |
| C25—H25···O5B | 0.95 | 2.56 | 3.355 (4) | 141 |
| C35—H35···O6A | 0.95 | 2.53 | 3.474 (13) | 176 |
| C35—H35···O6B | 0.95 | 2.54 | 3.484 (16) | 170 |
| C421—H41A···N431v | 0.99 | 2.61 | 3.589 (2) | 172 |
Symmetry codes: (iii) −x+1, y+1/2, −z+3/2; (iv) −x+1/2, y+1, −z+1; (v) −x, −y−1, −z+1.
References
- Addala, A., Setifi, F., Kottrup, K., Glidewell, C., Setifi, Z., Smith, G. & Reedijk, J. (2015). Polyhedron, 87, 307–310.
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–S19.
- Atmani, C., Setifi, F., Benmansour, S., Triki, S., Marchivie, M., Salaün, J.-Y. & Gómez-García, C. J. (2008). Inorg. Chem. Commun. 11, 921–924.
- Benmansour, S., Atmani, C., Setifi, F., Triki, S., Marchivie, M. & Gómez-García, C. J. (2010). Coord. Chem. Rev. 254, 1468–1478.
- Benmansour, S., Setifi, F., Gómez-García, C. J., Triki, S. & Coronado, E. (2008). Inorg. Chim. Acta, 361, 3856–3862.
- Benmansour, S., Setifi, F., Triki, S. & Gómez-García, C. J. (2012). Inorg. Chem. 51, 2359–2365. [DOI] [PubMed]
- Benmansour, S., Setifi, F., Triki, S., Salaün, J.-Y., Vandevelde, F., Sala-Pala, J., Gómez-García, C. J. & Roisnel, T. (2007). Eur. J. Inorg. Chem. pp. 186–194.
- Bruker (2008). COLLECT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Ferguson, G., Glidewell, C., Gregson, R. M. & Meehan, P. R. (1998a). Acta Cryst. B54, 129–138.
- Ferguson, G., Glidewell, C., Gregson, R. M. & Meehan, P. R. (1998b). Acta Cryst. B54, 139–150.
- Gaamoune, B., Setifi, Z., Beghidja, A., El-Ghozzi, M., Setifi, F. & Avignant, D. (2010). Acta Cryst. E66, m1044–m1045. [DOI] [PMC free article] [PubMed]
- Gilli, P., Bertolasi, V., Ferretti, V. & Gilli, G. (1994). J. Am. Chem. Soc. 116, 909–915.
- Gregson, R. M., Glidewell, C., Ferguson, G. & Lough, A. J. (2000). Acta Cryst. B56, 39–57. [DOI] [PubMed]
- Middleton, W. J., Little, E. L., Coffman, D. D. & Engelhardt, V. A. (1958). J. Am. Chem. Soc. 80, 2795–2806.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Setifi, Z., Domasevitch, K. V., Setifi, F., Mach, P., Ng, S. W., Petříček, V. & Dušek, M. (2013). Acta Cryst. C69, 1351–1356. [DOI] [PubMed]
- Setifi, F., Geiger, D. K., Abdul Razak, I. & Setifi, Z. (2015). Acta Cryst. C71, 658–663. [DOI] [PubMed]
- Setifi, Z., Lehchili, F., Setifi, F., Beghidja, A., Ng, S. W. & Glidewell, C. (2014). Acta Cryst. C70, 338–341. [DOI] [PubMed]
- Setifi, Z., Setifi, F., El Ammari, L., El-Ghozzi, M., Sopková-de Oliveira Santos, J., Merazig, H. & Glidewell, C. (2014). Acta Cryst. C70, 19–22. [DOI] [PubMed]
- Setifi, Z., Setifi, F., Ng, S. W., Oudahmane, A., El-Ghozzi, M. & Avignant, D. (2013). Acta Cryst. E69, m12–m13. [DOI] [PMC free article] [PubMed]
- Setifi, Z., Valkonen, A., Fernandes, M. A., Nummelin, S., Boughzala, H., Setifi, F. & Glidewell, C. (2015). Acta Cryst. E71, 509–515. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2003). SADABS. University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Thétiot, F., Triki, S. & Sala-Pala, J. (2003). Polyhedron, 22, 1837–1843.
- Yuste, C., Bentama, A., Marino, N., Armentano, D., Setifi, F., Triki, S., Lloret, F. & Julve, M. (2009). Polyhedron, 28, 1287–1294.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2056989016012160/hb7603sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016012160/hb7603Isup2.hkl
CCDC reference: 1496221
Additional supporting information: crystallographic information; 3D view; checkCIF report






