ABSTRACT
We observed the histopathological changes of retinal ganglion cells (RGCs), optic disc, and optic nerve in rabbit with advanced retinitis pigmentosa (RP). Wild-type (WT) and rhodopsin transgenic (Tg) of RP rabbits were used at age 24 months. Light and electron microscopy were used to observe the retina, optic disc, and optic nerve. RGCs were also confirmed by immunofluorescent staining with a TUJ-1 monoclonal antibody. In addition to the rod and cone degeneration, we observed the astrocyte infiltration of the optic disc due to the damage of small RGCs and nerve fibres and atrophy of small optic nerve fibres. They subsequently lead to the optic disc excavation and atrophy of the optic nerve. Consequently, our histopathological study clarified that not only the outer retina but also the inner retina, the optic disc, and the optic nerve were also affected in the late stages of RP rabbit.
KEYWORDS: Optic disc, optic nerve, rabbit, retinal ganglion cell, retinitis pigmentosa
Introduction
Retinitis pigmentosa (RP) is a group of inherited retinal diseases caused by mutations of genes related to the retina, and characterised by a progressive loss of retinal photoreceptor cells, mainly the rods, and represents the leading cause of blindness. These mutations result in degeneration of the rods followed by a gradual loss of cones and the death of the retinal ganglion cells (RGCs).1,2 In human RP, the initial histopathological changes occur in the proliferation of retinal pigment epithelium (RPE) cells with the alteration of the photoreceptor outer segment, followed by the disarray and roughness of the outer nuclear layer (ONL) that occurs while degeneration and loss of the inner segment begins. The loss apparently starts with displacement or migration of nuclei from the ONL into the photoreceptor layer, and the loss of nuclei of the ONL appears to be a primary retinal disorder associated with ageing.3 In the advanced stage, the ONL becomes thin and some cones disappear. Eventually, the retina becomes so thin that the cells of inner nuclear layer (INL) and Bruch’s membrane almost come into direct contact.
However, the aetiology and pathophysiology remain unknown. To overcome this difficulty, Kondo et al.4 recently created rhodopsin Pro347Leu transgenic (Tg) rabbits by using bacterial artificial chromosome transgenesis. In the Tg rabbits, detailed morphological observations have been previously presented by Jones et al.5 Retinal degeneration begins in the periphery, rods survive for up to 16 weeks, and cones survive for up to 40 weeks; however, rods degeneration is complete in regions by 40 weeks. Our previous morphological study showed pyknotic nuclei of the ONL and progressive loss of photoreceptor cells over time by 8 months of age, especially in the peripheral retina. In 12 months of age, we additionally found that there was a decrease in the number and density of rods and cones, although the cones were relatively well preserved.6
In contrast, no clear reports have been published as the morphological characteristics of inner retina, especially the RGCs, optic disc, and optic nerve in this animal model. Therefore, the aim of this study is to observe the histopathological change of RGCs, optic disc, and optic nerve in the rabbit with advanced RP.
Materials and methods
Animals
The experiments were performed on 2 eyes of Tg and 2 eyes of wild-type (WT) pigmented Dutch male rabbits. Both types of rabbits were 24 months old. From the changes in the pupil light response and the amplitude of the electroretinography, we determined the time point for the morphological observation.6 The Tg rabbit was generated in the Kitayama Labs Co., Ltd. (Ina, Nagano, Japan).
This experiment was conducted according to the Association of Research in
Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and with the approval of the Animal Experiment Ethics Committee of Kitasato University (approval number: 2015-017). All experiments were conducted under anaesthesia. We gave full consideration to ethical issues and paid careful attention to minimising pain.
Dissecting, light, and electron microscopy
The enucleated right eye was fixed overnight in 2.5% glutaraldehyde + 0.1 M phosphate-buffered saline (PBS). On the following day, the eye specimen was observed by dissecting microscope (SMZ-10; Nikon, Tokyo, Japan) with an Intralux 6000-1 Light Source (Volpi AG, Schlieren, Switzerland). Then, the sections of the eye specimen (nasal, temporal, superior [dorsal] to the optic disc, inferior [ventral] to the optic disc [visual streak], centre of the visual streak) were cut into smaller pieces (5 × 3 mm). The pieces were rinsed three times with 0.1 M PBS, immersed in 1% OsO4, dehydrated, and embedded in Quetol 812 resin. The resin was then polymerised and cured at 60°C for 48 h. Each block of retinal tissue was cut into 0.5-µm-thick sections, stained with toluidine blue, and examined under a light microscope (BX50; Olympus, Tokyo, Japan). Tissue was also cut into ultrathin sections at a thickness of 80 nm, double-stained with lead citrate and uranyl acetate, and examined using an electron microscope (JEM-1230; JEOL, Tokyo, Japan).
Immunofluorescent staining with neuronal class III beta-tubulin monoclonal antibody
Alexa Fluor®–labelled neuronal class III beta-tubulin (TUJ-1; Molecular Probes, Eugene, OR, USA) 1:500 monoclonal antibodies were used for immunofluorescent staining of the RGCs. After the eyeballs had been enucleated, a sample of the retina was carefully peeled off into 0.01 M phosphate buffer (PB). The sample was fixed in 4% paraformaldehyde solution for 60 min and rinsed with 0.01 M PB. For permeabilisation, the sample was then placed in 100% methanol at −20°C for 10 min. The specimens were blocked at room temperature for 60 min using 10% normal goat serum and 5% bovine serum albumin in PBS with 0.1% Tween 20 (PBS 2T). The retinal samples were incubated at 4°C overnight. Once the samples had been stained, they were then rinsed three times with PBS 2T and attached to a gelatin-coated glass slide. After drying, the retinal tissue was examined using a confocal laser scanning microscope with 488 nm laser lines (LSM 700 Meta; Carl Zeiss Meditec, Inc., Dublin, CA, USA).
Results
Dissecting microscopy
By using dissecting microscopy, the rabbit fundus was found to have an opaque myelinated portion that extends horizontally from the optic nerve head. The changes of the optic nerve head, that is, the signposts marking the border of the cup, are the flexion of vessels and vessel bayoneting. Compared with the WT rabbit (Figure 1A), optic disc cupping, optic atrophy, and decreasing of nerve fibre were seen in the Tg rabbit (Figure 1B).
Figure 1.
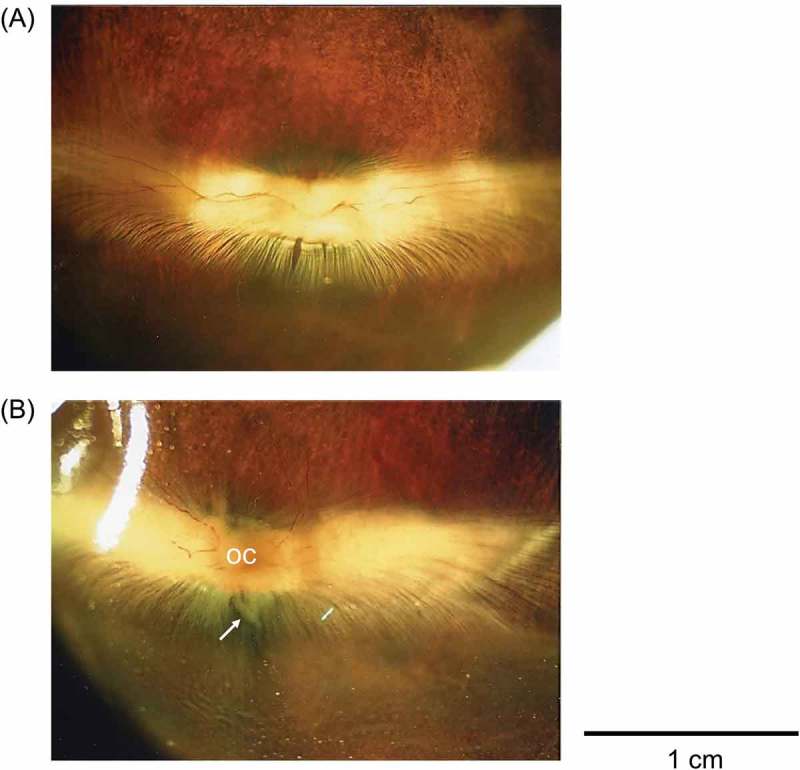
Dissecting microscopy. (A) Compared with the Wild-type (WT) rabbit, (B) optic disc cupping, optic atrophy, and decreasing of nerve fibers (white arrow) are seen in the transgenic (Tg) rabbit.
Light microscopy
In the WT rabbit, the structure of retina appeared almost normal (Figure 2A). The Tg rabbit showed that rods and cones disappeared in peripheral retina, and the remaining cones exhibited ballooning degeneration in the visual streak (Figure 2B). In the optic disk, myelinated axons of the WT rabbit were densely crowded (Figure 2C), while those of the Tg rabbit were sparsely distributed, and growth of astrocytes, i.e., astrocyte infiltration was observed (Figure 2D). Comparing with the cross-section of the optic nerve of the WT rabbit (Figure 2E), that of the Tg rabbit showed enlargement of interstitial spaces due to the decrease of axons (Figure 2F).
Figure 2.
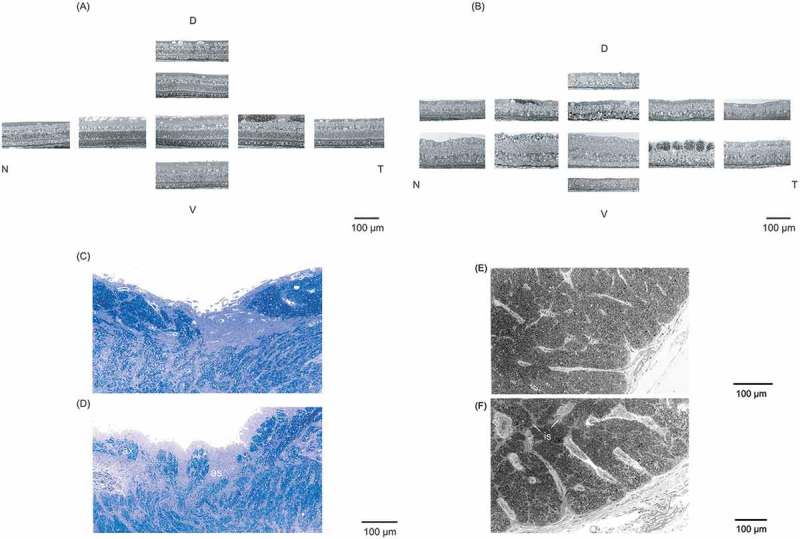
Light microscopy. (A) The WT rabbit’s retina has a normal structure. (B) The Tg rabbit shows that rods and cones almost disappear. In the optic disc, (C) myelinated axons of the WT rabbit are densely crowded. (D) Those of the Tg rabbit are sparsely distributed, and the astrocyte infiltration are observed. In the optic nerve, (E) compared with the WT rabbit, (F) the interstitial spaces are enlarged. D = dorsal; V = ventral; N = nasal; T = temporal; ax = axon; as = astrocyte infiltration; is = interstitial spaces.
Electron microscopy
Rods and cones were clearly delineated, and they were regularly distributed in the electron microscopy image of the WT rabbit (Figure 3A). Irregular distribution of pigment granules in the RPE, degeneration of cone nuclei, clump of mitochondria, and debris of outer segments were observed in the Tg rabbit (Figure 3B). In the periphery of the retina, there is a vacuolar degeneration of RGCs, and hypertrophy and proliferation of Müller cells (Figure 3C). Degeneration of myelinated axons in the optic disc (Figure 3D) and damage of the myelin sheath of small axons in the optic nerve, (Figure 3E) respectively, were observed.
Figure 3.
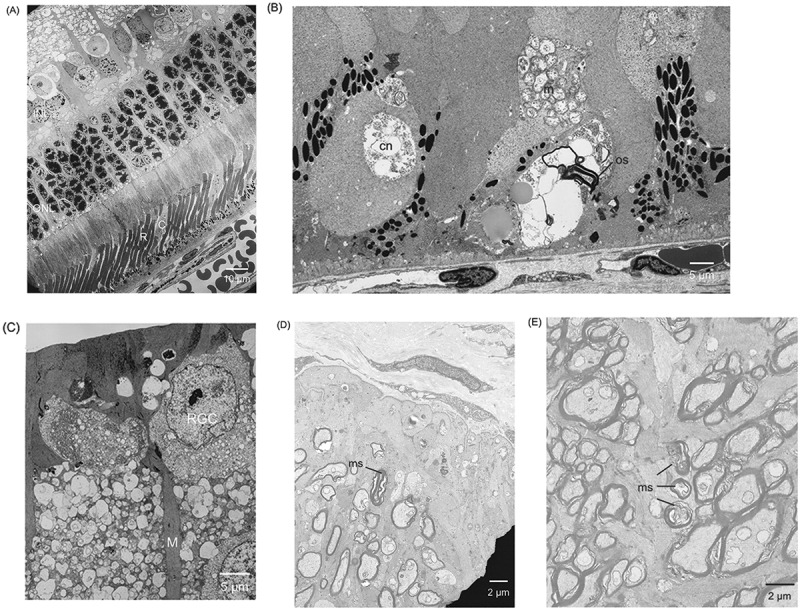
Electron microscopy. A) Rods and cones are clearly delineated in the WT rabbit. (B) Retinal pigment epithelium pigment granules irregularly distributed. Degeneration of cone nuclei, clump of mitochondria, and debris of outer segments are observed in the Tg rabbit. (C) Vacuolar degeneration of retinal ganglion cells (RGCs) and hypertrophic proliferation of Müller cells are observed. (D) Degeneration of myelinated axons in the optic disc. (E) Damage of the ring-like structure of the myelin sheath of small axons in the optic nerve. INL = inner nuclear layer; ONL = outer nuclear layer; R = rod; C = cone; cn = cone nuclei; m = mitochondria; os = outer segment; RGC = retinal ganglion cell; M = Müller cell; ms = myelin sheath.
TUJ-1 Immunofluorescent staining
Subtype of the RGCs showed both large cells and small cells in the WT rabbit (Figure 4A). In the Tg rabbit, the remaining large cells, which have an extensively branching dendritic structure with long dendrites, were more clearly observed, whereas the number of small cells has decreased (Figure 4B).
Figure 4.
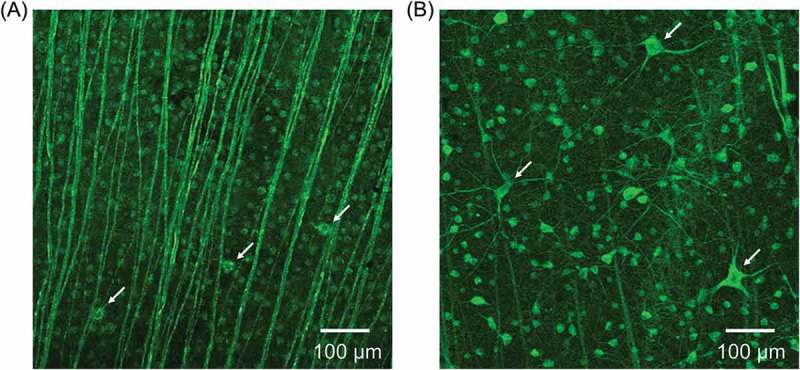
TUJ-1 immunofluorescent staining of RGCs. (A) Large cells (white arrows) and small cells in the WT rabbit. (B) Some remaining RGCs have large cell bodies with long dendrites (white arrows), whereas the number of small cells has decreased in the Tg rabbit.
Discussion
In our study, distribution of pigment granules seemed irregularly scattered, in the epithelial cells making up the RPE layer that thinned out. Rods and cones were lost in certain portions other than those of the visual streak. In the regions where retinal photoreceptor cells were clearly lost, RGCs also degenerated.
The morphology of outer retina in the Tg rabbit has been previously reported in various papers.4–6 The RP model rabbit shows, similar to human RP, progressive and mosaicked rod-specific degeneration, with some regions maintaining relative rod survival, others showing cone sparing, and still other regions losing all retinal photoreceptors. However, the morphological observations of the RGCs, optic disc, and optic nerve remain unclear.
Clinically, it is known that RGCs remain until after the late stage of RP in the inner retinal layers. Regarding the RGCs, a consensus has yet to be obtained in human RP. RGCs are not detectable due to complete degeneration and loss7; RGC degeneration is found in some patients8 and some regions9; and RGC degeneration at the early or late stage10,11 is detected. Contrastingly, nerve fibre layers were thickened, those for which the area sometimes extended to more than a half of the total retina, in which the nerve fibres became atrophic and sparsely distributed, and the glia tissues were proliferated.
Our findings in the Tg rabbit showed that the small type of RGCs and nerve fibres were particularly damaged, those with large cell bodies and long dendrites remained, and a hypertrophic proliferation of Müller cells was observed in some regions. The reason for this was considered to be that the bipolar cells and RGCs were lost associated with the loss of rods and cones by degeneration in the periphery of the retina, and those degenerated cells underwent phagocytosis by Müller cells subject to hypertrophic growth. These hypotheses strongly supported previous findings.12
Generally, it is well known that glial tissues around the optic disc and optic nerve consist of astrocytes and oligodendrocytes, among others. Among those, astrocytes are the glial tissues that provide nutrients to the optic nerves. Due to the degeneration of axons and loss in their number, along with the atrophy of nerve fibres, glial proliferation and enlargement of tissues occurred, which resulted in the optic disc excavation and optic nerve atrophy. Moreover, oligodendrocytes are known to be relevant to myelination in the glial tissues. In our study, the optic nerve became conspicuously atrophic, around which atrophic sites of nerve fibres and enlargement and proliferation of astrocytes were observed. These findings strongly helped explain a previous report.13 Of note, we also observed the disruption of the myelin sheath of small axons that occurred with the degeneration of axons and atrophy of the optic nerve. Therefore, optic disc cupping might closely be associated with the atrophy of the optic nerve.
Although the present study has the limitation of its small number of examples, the results are noteworthy. Thus, this limitation does not affect our conclusions, because the histopathological differences in the retina, the optic disc, and optic nerve between the WT rabbit and the Tg rabbit were obvious. Consequently, our present study clarified that not only the rods and cones but also the RGCs, the optic disc, and the optic nerve were affected in the late stages of RP in rabbit.
Funding Statement
This work was supported by JSPS KAKENHI, grant number JP16K21346.
Acknowledgement
The authors thank Mr. Robert E. Brandt for editing the manuscript.
Funding
This work was supported by JSPS KAKENHI, grant number JP16K21346.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- [1].Berson EL. Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci 1993;34:1659–1676. [PubMed] [Google Scholar]
- [2].Hartong DT, Berson EL, Dryja TP.. Retinitis pigmentosa. Lancet 2006;368:1795–1809. [DOI] [PubMed] [Google Scholar]
- [3].Gartner S, Henkind P.. Aging and degeneration of the human macula. 1. Outer nuclear layer and photoreceptors. Br J Ophthalmol 1981;65:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kondo M, Sakai T, Komeima K, Kurimoto Y, Ueno S, Nishizawa Y, Usukura J, Fujikado T, Tano Y, Terasaki H.. Generation of a transgenic rabbit model of retinal degeneration. Invest Ophthalmol Vis Sci 2009;50:1371–1377. [DOI] [PubMed] [Google Scholar]
- [5].Jones BW, Kondo M, Terasaki H, Watt CB, Rapp K, Anderson J, Lin Y, Shaw MV, Yang JH, Marc RE.. Retinal remodeling in the Tg P347L rabbit, a large-eye model of retinal degeneration. J Comp Neurol 2011;519:2713–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Asakawa K, Ishikawa H, Uga S, Mashimo K, Shimizu K, Kondo M, Terasaki H.. Functional and morphological study of retinal photoreceptor cell degeneration in transgenic rabbits with a Pro347Leu rhodopsin mutation. Jpn J Ophthalmol 2015;59:353–363. [DOI] [PubMed] [Google Scholar]
- [7].Sheth SS, Rush RB, Natarajan S.. Inner and outer retinal volumetric and morphologic analysis of the macula with spectral domain optical coherence tomography in retinitis pigmentosa. Middle East Afr J Ophthalmol 2012;19:227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hood DC, Lin CE, Lazow MA, Locke KG, Zhang X, Birch DG.. Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2009;50:2328–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stone JL, Barlow WE, Humayun MS, de Juan E Jr, Milam AH. Morphometric analysis of macular photoreceptors and ganglion cells in retinas with retinitis pigmentosa. Arch Ophthalmol 1992;110:1634–1639. [DOI] [PubMed] [Google Scholar]
- [10].Vámos R, Tátrai E, Németh J, Holder GE, DeBuc DC, Somfai GM.. The structure and function of the macula in patients with advanced retinitis pigmentosa. Invest Ophthalmol Vis Sci 2011;52:8425–8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Santos A, Humayun MS, de Juan E Jr, RJ Greenburg, MJ Marsh, IB Klock, AH Milam. Preservation of the inner retina in retinitis pigmentosa. A morphometric analysis. Arch Ophthalmol 1997;115:511–515. [DOI] [PubMed] [Google Scholar]
- [12].Szamier RB, Berson EL.. Histopathologic study of an unusual form of retinitis pigmentosa. Invest Ophthalmol Vis Sci 1982;22:559–570. [PubMed] [Google Scholar]
- [13].Lahav M, Craft J, Albert DM, Ishii Y.. Advanced pigmentary retinal degeneration: an ultrastructural study. Retina 1982;2:65–75. [DOI] [PubMed] [Google Scholar]


