Abstract
Hepatitis C virus (HCV) infection is one of the major causes of advanced liver disease and hepatocellular carcinoma (HCC) worldwide. While the knowledge about the molecular virology of HCV infection has markedly advanced, the molecular mechanisms of disease progression leading to fibrosis, cirrhosis and HCC are still unclear. Accumulating experimental and clinical studies indicate that HCV may drive hepatocarcinogenesis directly via its proteins or transcripts, and/or indirectly through induction of chronic liver inflammation. Despite the possibility to eradicate HCV infection through direct-acting antiviral treatment, the risk of HCC persists although specific biomarkers to estimate this risk are still missing. Thus, a better understanding of HCV-induced HCC and more physiological liver disease models are required to prevent cancer development.
Keywords: Hepatitis C virus, hepatocellular carcinoma, fibrosis, cancer hallmarks, direct-acting antiviral-based therapies
Introduction
Hepatitis C virus (HCV) is single-strand RNA virus from the Flaviviridae family targeting hepatocytes. Chronic HCV infection induces immune dysfunctions such as impaired T-cell functions and inefficient antibody responses, metabolic disorders such as hepatic steatosis, iron accumulation, and insulin resistance often associated with type 2 diabetes. More importantly, HCV is one of the major etiologies of chronic hepatitis and progressive liver fibrosis that lead to development of lethal complications, i.e., cirrhosis and hepatocellular carcinoma (HCC), the second leading cause of cancer mortality worldwide and the only and most rapidly increasing cancer death in the U.S. [1,2]. Chronic HCV infection is highly prevalent globally, including developed countries [3]. In the U.S., more than 1 million individuals, representing the “baby boomer” population, are estimated to develop HCV-related liver cirrhosis and/or HCC by 2020. Recently developed direct-acting antivirals (DAAs) for HCV effectively cure HCV infection, i.e., they enable to achieve sustained virologic response (SVR), but the high costs will limit their wide-spread use [4]. Of note, HCC risk remains high for decades even after SVR, and HCV-related HCC is predicted to increase until 2030 despite improved viral cure by DAAs [5,6].
HCV does not integrate its genetic material into the host genome, and therefore requires continuous replication to maintain chronic infection. Many host factors, playing essential roles in the HCV life cycle and immune evasion, have been identified as candidate targets for antiviral interventions (reviewed in [7]). However, disease pathogenesis that ultimately causes HCC is still unclear. Experimental studies to date have suggested models of viral carcinogenesis unique to HCV [8]. Increasing evidence shows that HCV transmits signals and modulates hepatocyte gene expression following engagement with cellular receptors [9,10]. Moreover, viral proteins have been involved in disrupting signal transduction pathways that affect cell survival, proliferation, and transformation [8]. This suggests that virus-host interactions and signaling during viral infection contribute to cellular transformation and development of HCC directly via HCV proteins or RNA, and/or indirectly through induction of chronic inflammation. Additionally, the genetic background of the host may play a role in HCC pathogenesis. Genetic analyses in HCV-infected patients have unraveled specific mutation or polymorphisms in MICA/HCP5, LEPR and IFNL3 loci that are associated with the development of HCC [11–16], indicating that genetic variation may contribute to individual susceptibility for HCV-driven HCC.
Of note, the persisting risk of HCC development even after viral cure suggests that HCV leaves molecular imprinting in the host genome that keeps driving carcinogenesis. Management of post-SVR HCC will be increasingly relevant as more patients achieve SVR by the DAA treatment in clinic. Here, we review several examples of mechanisms that may contribute to HCV-induced HCC and discuss the clinical challenges to prevent HCC development in at-risk patients in the era of DAA-based anti-HCV therapies.
Viral factors directly driving hepatocarcinogenesis
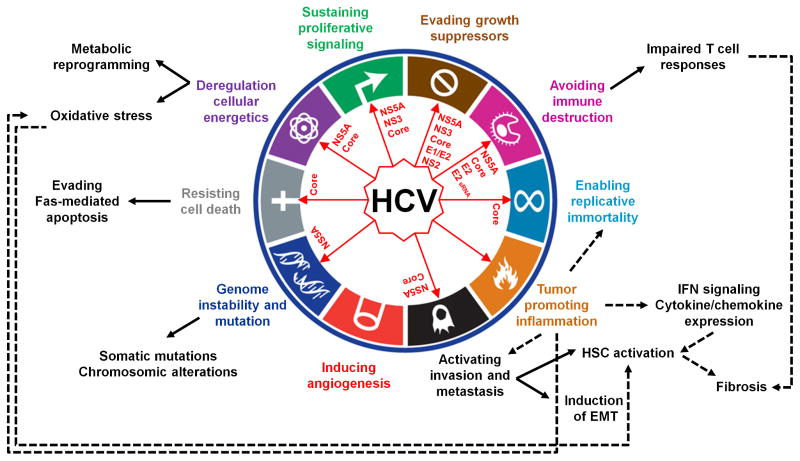
The strong and reproducible association of HCV genotype 3 with development of steatosis and HCC, genotype 1b with more frequent progression to HCC, and HCV core gene variants with post-SVR HCC suggests that specific viral factors influence or determine progression of liver disease [17–19]. The viral genome encodes for three structural (core, E1, E2) and seven non-structural proteins (p7, NS2, NS3, NS4A, NS4B, NS5A and NS5B). Several in vivo studies in transgenic mouse models reported direct induction of liver disease by the expression of viral proteins (reviewed in [20]). Although none of these models could faithfully recapitulate the full features of human disease, some of the phenotypes were consistent with epidemiological data from HCV-infected patients. Interestingly, these studies highlighted that HCV RNA and proteins can perturb hepatocellular homeostasis by driving several major cancer hallmarks (Figure 1).
Figure 1. HCV RNA and proteins perturb hepatocellular homeostasis by driving major cancer hallmarks.
The diagram (adapted from [8]) shows the HCV-host interactions and signaling upon viral infection that contribute to cellular transformation and development of HCC. The red arrows indicate HCV RNA and proteins exerting a direct effect on a specific hallmark. Black arrows link specific hallmarks to examples of mechanisms of HCV-driven HCC, which were observed in both clinical and in vivo experimental models. Regarding the tumor promoting inflammation hallmark (in orange in the diagram), this is activated by the pathogen recognition receptors that sense HCV infection. Dotted lines indicate examples of inflammation-driven carcinogenesis. sRNA, small RNA.
First, metabolic reprogramming including disturbance of lipid metabolism and mitochondrial dysfunction were shown to play an important role in HCV-driven HCC (Figure 1). Indeed, chronic HCV infection enhances mitochondrial liver injury together with oxidative stress in human as well as experimental models [21]. Several studies highlight a role of the HCV core protein in steatosis and HCC nodule development as well as in insulin resistance, which is accompanied with intrahepatic lipid accumulation [20,22]. The alteration of lipid metabolism is induced by an HCV core-mediated impairment of lipid β-oxidation, which is associated with a reduced activity of the mitochondrial electron transport chain [20]. Recently, HCV core protein was also shown to contribute to mitochondrial damage by impairing mitophagy [23]. The resulting oxidative stress is regarded as a key trigger of HCC initiation and development (Figure 1). Imbalance of the oxidant/antioxidant state in the liver was shown to induce HCC in HCV core transgenic mice in the absence of inflammation [24]. Moreover, generation of reactive oxygen species (ROS) in the course of HCV infection was associated with genomic instability, a hallmark of cancer cells [20]. Indeed, accumulation of genetic mutations as well as chromosomal alterations crucially drive the development of HCC in patients [25]. By inducing a β-Catenin-dependent upregulation of c-Myc via NS5A, HCV was shown to perturb ROS production in association with enhanced DNA damage and aberrant cell-cycle arrest (Figure 1) [26]. In addition, increased telomerase (TERT) activity, a characteristic of transforming or transformation-prone cells, was observed in HCV core-transfected primary human hepatocytes that acquired an immortalized phenotype [27]. In line with this observation, somatic mutations in the TERT promoter that enhance TERT expression were shown to be among the earliest and most prevalent neoplastic event in HCC associated with all major etiologies including HCV [28].
Another major hallmark of cancer that is directly affected by HCV is evasion from cell death and senescence (Figure 1). Although HCV proteins were reported to have both pro-apoptotic and anti-apoptotic properties [8], HCV is likely involved in evasion from apoptosis in vivo. A number of studies indicate that Fas-mediated apoptosis is directly inhibited by different HCV proteins [20,29–31]. Given that the Fas system accounts for T cell-mediated cytotoxicity, suppression of cell death is not only a mechanism of sustained cell proliferation but also one strategy that enables HCV to escape immune surveillance by T cells and thus to establish persistent infection [32].
Finally, recent evidence indicates that viral proteins impact on epithelial mesenchymal transition (EMT) pathway, which promotes fibrogenesis, tumor development and metastases (Figure 1). HCV NS5A was shown to activate Twist2, a transcriptional regulator of EMT, and to cooperate with Ras oncogene to enhance tumor cell invasiveness in xenograft mouse models [33]. Furthermore, expression of HCV core in transgenic mice enhances intrahepatic TGF-β signaling, a key regulator of EMT driving the activation of human stellate cells (HSCs) [34]. Further studies showed that induction of EMT by HCV core is mediated by at least two mechanisms: i) the inhibition of E-cadherin expression by a complex comprising HCV core, Snail and the histone deacetylases HDAC1/HDAC2 [35]; ii) the HCV core-induced epigenetic silencing of SFRP1 via DNA methylation and histone modifications that in turn activates Wnt/β-catenin signaling [36]. Yet the clinical relevance of these recent findings remains to be determined.
HCV-induced inflammatory responses indirectly driving hepatocarcinogenesis
HCV infection can induce chronic hepatic inflammation with varying activity, which causes progressive liver fibrogenesis and leads to development of cirrhosis (Figure 1). Clinically, the majority of HCV-related HCC tumors develop in livers with cirrhosis established after decades of chronic inflammation, underscoring the key role of virus-induced inflammatory responses, besides the viral materials themselves, in HCC pathogenesis. Several inflammatory pathways have been implicated in HCC. First, the sensing of HCV infection by pathogen recognition receptors of the innate immune system activates the NF-κB signaling and downstream proinflammatory chemokines and cytokines including type III interferon (IFN), which is associated with HCC development (Figure 1) [37–39]. Ectopic lymphoid structure aggregated near the portal tract was reported as a niche of HCC initiation associated with striking NF-κB activation in a subset of HCV-infected human livers [40]. Approximately in 70 % of chronic hepatitis C (CHC) patients the immune response fails to eradicate the virus due to impaired T cell and antibody responses, and little antiviral efficacy of IFN-stimulated genes (ISGs) [41]. The adaptive immune response mediated by cytotoxic T cells has been suggested to contribute to liver injury by triggering repeated cycles of hepatocyte death and regeneration/proliferation. The inflammatory response also exacerbates oxidative stress in the liver (Figure 1). Cytokines, ROS and apoptotic signals contribute to HSC activation, which triggers aberrant deposition of extracellular matrix proteins and progressive fibrosis (Figure 1). As such, the functional liver parenchyma is progressively replaced by non-functional fibrotic tissue. Overall, this pattern of chronic inflammation, unresolved wound healing response and increased hepatocellular proliferation in CHC is thought to generate an environment highly permissive for hepatocarcinogenesis.
Despite the growing knowledge, many open questions remain unanswered. The molecular bases of the interplay between the innate and adaptive immune responses in the course of CHC and their relevance for HCC development are still largely unclear [41]. IFN pathway activation is one of the key components of the host responses to HCV, although cell types secreting IFN as well as types of secreted IFN stimulating specific subset of ISGs are still elusive. This is partly because of the lack of a robust immunocompetent in vivo HCV infection model that mirrors the cell circuits of HCC development as well as the crosstalk between parenchymal and non-parenchymal cell types driving disease progression under physiological condition as in human [20]. Transgenic mouse models coupled with epidemiological data in patients have provided important insights into mechanistic investigation. This approach was successful in unraveling a pathway of hepatocarcinogenesis driven by the pro-inflammatory cytokine lymphotoxin (LT) α and β [42]. By using transgenic mice for either LTs expression or NF-κB signaling components, Haybeack et al. discovered that LTs overexpression induces chronic hepatitis and HCC by altering NF-κB signaling in both hepatocytes and lymphocytes. These observations were corroborated by the enhanced LT expression in clinical liver specimens from virus-induced chronic hepatitis and HCC as compared to healthy liver [42]. More recently, HCV NS5B was shown to promote pro-inflammatory LTβ signaling in liver cells [43]. Likewise, two recent studies casted new light on novel mechanisms of HSC activation and liver fibrogenesis in CHC. The first involves the acetylation of HMGB1 by extracellular osteopontin (OPN), a stress sensor protein that is enhanced in liver disease and elevated in serum of patients who are at risk of HCC development [44]. Acetylated HMGB1 interacts with HDAC1/HDAC2 to promote collagen-I expression by HSCs and increase its histological deposition [45]. The second mechanism relies on the upregulation of the Gas6/Axl pathway in HSC leading to activation of these cells and liver fibrogenesis upon carbon tetrachloride-induced injury in mice [46]. Importantly, the clinical relevance of both mechanisms was evidenced by the correlation between the severity of liver injury and increased expression of OPN/HMGB1 or Gas6/Axl, respectively, in HCV-infected patients [45,46]. However, additional clinical cohort studies may be required to corroborate the involvement of these processes in HCV pathogenesis.
Treatment of HCV infection and prevention of HCC
Rapidly evolving DAA-based anti-HCV therapies now enable more than 90% of SVR rate with all-oral regimens even in the cases hard to cure before [47]. In patients previously treated with older, IFN-based regimens, SVR was significantly associated with reduced but not eliminated future risk of HCC development over a decade [48]. In the retrospective studies, several clinical characteristics such as more advanced liver fibrosis, older age, and male sex among others have been suggested as predisposing factors for post-SVR HCC (Table 1). However, estimation of HCC risk in patients newly achieving an SVR is still infeasible and the mechanisms of carcinogenesis are totally unknown. Given the annual incidence of post-SVR HCC, which is likely below the threshold that rationalizes regular HCC surveillance, HCC risk biomarkers or indices will play a critical role to perform cost-effective and practically feasible HCC surveillance by triaging the patients according to the predicted HCC risk [21]. Also, such biomarkers may provide clues to targets of HCC chemopreventive interventions. It is still unanswered question whether HCC risk after DAA-based or other types of anti-HCV therapies such as viral entry inhibition [49] is comparable to that of IFN-based therapies. Modulation of cellular signaling pathways such as IFN, EGF, mTOR, and retinoid X receptor-α pathways and drugs for metabolic disorder, some of which have been already clinically evaluated, may serve as alternative options of HCC chemoprevention for broader etiologies, including post-SVR HCC [50–56]. Experimental systems that allow mechanistic assessments of the carcinogenic drivers will be critical in identifying and developing rational molecular-targeted HCC chemoprevention therapies.
Table 1.
Clinical risk factors for post-SVR HCC development
| Type of anti- HCV therapy | No. SVR patients | Follow-up period (y) | Risk factors for HCC development | References |
|---|---|---|---|---|
| IFN-based | 1197 | 5.9 | Age ≥ 50 y, male, F3/4 fibrosis | [57] |
| IFN-based | 1056 | 4.7 | Age ≥ 60 y, AST ≥ 100 U/L, platelets < 150 ×109/L | [58] |
| IFN-based | 871 | 3.4 | F3/4 fibrosis, age ≥ 60 y, post-SVR AFP ≥ 20 ng/mL, platelets < 150 ×109/L | [59] |
| IFN-based | 1751 | 8.1 | Diabetes, male, alcohol, age (every 10 y) | [60] |
| IFN-based | 1425 | 3.3 | Post-SVR AFP ≥ 5 ng/mL, Age ≥ 65 y | [61] |
| IFN-based | 562 | 4.8 | F2/3/4 fibrosis, age ≥ 50 y, ethanol ≥ 30 g/d, pre-SVR AFP ≥ 8 ng/mL | [62] |
| IFN-based | 642 | 4.4 | GGT ≥ 75 U/L, age ≥ 65 y, F2/3 fibrosis | [63] |
| IFN-based | 522 | 7.2 | Diabetes, FIB-4 index | [64] |
| IFN-based | 801 | 5.0 | Age ≥ 60 y, post-SVR AFP ≥ 20 ng/mL platelets < 150 ×109/L, F3/4 fibrosis | [15] |
| IFN-based | 83 HCC among 2152 SVR | 6.7 | No surveillance (for risk of advanced HCC) | [65] |
| IFN-based | 10817 | 2.8 | cirrhosis, age ≥ 65 y, diabetes, HCV genotype 3 | [66] |
| IFN-based | 1351 | n.a. | Pre/post-SVR AFP ≥ 15 ng/mL, pre/post-SVR APRI ≥ 0.7 | [67] |
| IFN-based | 399 | 7.8 | Cirrhosis, diabetes | [68] |
| IFN-based | 24 SVR HCC cases vs. 96 matched controls | n.a. | Compensated cirrhosis, post-SVR albumin ≤ 36 g/L | [69] |
| IFN-based | 376 | 7.6 | Advanced fibrosis/cirrhosis, diabetes, LSM > 12 kPa | [70] |
| IFN-based | 1094 | 4.2 | Age ≥ 60 y, male, F3/4 fibrosis, post-SVR AFP ≥ 10 ng/mL | [71] |
| IFN-based | 598 | 5.1 | Pre/post-SVR APRI ≥ 1.0 | [72] |
SVR: sustained virologic response, HCC: hepatocellular carcinoma, HCV: hepatitis C virus, y: years, IFN: interferon, AFP: alpha-fetoprotein, GGT: gamma-glutamyl transpeptidase, FIB-4: fibrosis-4, LSM: liver stiffness measurement, APRI: aspartate aminotransferase-to-platelet ratio index, n.a.: not available.
Highlights.
The molecular mechanisms of HCV-driven HCC are still elusive.
HCV perturbs hepatocellular homeostasis by driving several major cancer hallmarks.
HCV-induced inflammatory responses indirectly drive hepatocarcinogenesis.
Biomarkers to predict HCC risk in patients after HCV cure are missing.
HCV may leave a cancer-prone molecular imprinting in the host genome.
Novel experimental systems are needed to assess HCC drivers mechanistically.
Acknowledgments
The authors acknowledge grant support of the European Union (ERC-2014-AdG-671231-HEPCIR (T.F.B, Y.H.), H2020-667273-HEPCAR (T.F.B.), EU-ANR ERA-NET Infect-ERA hepBccc (T.F.B.), ANRS (T.F.B., M.B.Z.), the French Cancer Agency (ARC IHU201301187 (T.F.B.)), the IdEx program of the University of Strasbourg (M.B.Z., T.F.B.), the Foundation University of Strasbourg (T.F.B.), NIH/NIDDK R01 DK099558 (Y.H.), and Irma T. Hirschl Trust (Y.H.). This work has been published under the framework of the LABEX ANR-10-LAB-28 and benefits from a funding from the state managed by the French National Research Agency as part of the Investments for the future program.
Footnotes
Declaration of interest
The authors do not have any conflict of interest and did not receive writing assistance.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest
** of outstanding interest
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM, et al. Annual Report to the nation on the status of cancer, 1975–2012; featuring the increasing incidence of liver cancer. Cancer. 2016 doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 4.Chung RT, Baumert TF. Curing chronic hepatitis C--the arc of a medical triumph. N Engl J Med. 2014;370:1576–1578. doi: 10.1056/NEJMp1400986. [DOI] [PubMed] [Google Scholar]
- 5.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 6.Harris RJ, Thomas B, Griffiths J, Costella A, Chapman R, Ramsay M, De Angelis D, Harris HE. Increased uptake and new therapies are needed to avert rising hepatitis C-related end stage liver disease in England: Modelling the predicted impact of treatment under different scenarios. J Hepatol. 2014;61:530–537. doi: 10.1016/j.jhep.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Zeisel MB, Lupberger J, Fofana I, Baumert TF. Host-targeting agents for prevention and treatment of chronic hepatitis C - perspectives and challenges. J Hepatol. 2013;58:375–384. doi: 10.1016/j.jhep.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Mesri EA, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe. 2014;15:266–282. doi: 10.1016/j.chom.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang X, Zeisel MB, Wilpert J, Gissler B, Thimme R, Kreutz C, Maiwald T, Timmer J, Kern WV, Donauer J, et al. Host cell responses induced by hepatitis C virus binding. Hepatology. 2006;43:1326–1336. doi: 10.1002/hep.21191. [DOI] [PubMed] [Google Scholar]
- 10.Zona L, Lupberger J, Sidahmed-Adrar N, Thumann C, Harris Helen J, Barnes A, Florentin J, Tawar Rajiv G, Xiao F, Turek M, et al. HRas signal transduction promotes hepatitis C virus cell entry by triggering assembly of the host tetraspanin receptor complex. Cell Host Microbe. 2013;13:302–313. doi: 10.1016/j.chom.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Goto K, Kato N. MICA SNPs and the NKG2D system in virus-induced HCC. J Gastroenterol. 2015;50:261–272. doi: 10.1007/s00535-014-1000-9. [DOI] [PubMed] [Google Scholar]
- 12.Lange CM, Bibert S, Dufour JF, Cellerai C, Cerny A, Heim MH, Kaiser L, Malinverni R, Mullhaupt B, Negro F, et al. Comparative genetic analyses point to HCP5 as susceptibility locus for HCV-associated hepatocellular carcinoma. J Hepatol. 2013;59:504–509. doi: 10.1016/j.jhep.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 13.Kumar V, Kato N, Urabe Y, Takahashi A, Muroyama R, Hosono N, Otsuka M, Tateishi R, Omata M, Nakagawa H, et al. Genome-wide association study identifies a susceptibility locus for HCV-induced hepatocellular carcinoma. Nat Genet. 2011;43:455–458. doi: 10.1038/ng.809. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda A, Shimizu T, Matsumoto Y, Fujii Y, Eso Y, Inuzuka T, Mizuguchi A, Shimizu K, Hatano E, Uemoto S, et al. Leptin receptor somatic mutations are frequent in HCV-infected cirrhotic liver and associated with hepatocellular carcinoma. Gastroenterology. 2014;146:222–232. e235. doi: 10.1053/j.gastro.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Chang KC, Tseng PL, Wu YY, Hung HC, Huang CM, Lu SN, Wang JH, Lee CM, Chen CH, Tsai MC, et al. A polymorphism in interferon L3 is an independent risk factor for development of hepatocellular carcinoma after treatment of hepatitis C virus infection. Clin Gastroenterol Hepatol. 2015;13:1017–1024. doi: 10.1016/j.cgh.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 16.Asahina Y, Tsuchiya K, Nishimura T, Muraoka M, Suzuki Y, Tamaki N, Yasui Y, Hosokawa T, Ueda K, Nakanishi H, et al. Genetic variation near interleukin 28B and the risk of hepatocellular carcinoma in patients with chronic hepatitis C. J Gastroenterol. 2014;49:1152–1162. doi: 10.1007/s00535-013-0858-2. [DOI] [PubMed] [Google Scholar]
- 17.Goossens N, Hoshida Y. Hepatitis C virus-induced hepatocellular carcinoma. Clin Mol Hepatol. 2015;21:105–114. doi: 10.3350/cmh.2015.21.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Akuta N, Suzuki F, Kobayashi M, Sezaki H, Kawamura Y, Hosaka T, Kobayashi M, Saitoh S, Suzuki Y, Arase Y, et al. Impact of mutations at amino acid 70 in HCV genotype 1b core region on hepatocarcinogenesis following eradication of HCV RNA. J Clin Microbiol. 2015;53:3039–3041. doi: 10.1128/JCM.01457-15. Clinical report indicating the oncogenic potential of the mutant HCV core in a large cohort of patients cleared for chronic HCV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Shamy A, Eng FJ, Doyle EH, Klepper AL, Sun X, Sangiovanni A, Iavarone M, Colombo M, Schwartz RE, Hoshida Y, et al. A cell culture system for distinguishing hepatitis C viruses with and without liver cancer-related mutations in the viral core gene. J Hepatol. 2015;63:1323–1333. doi: 10.1016/j.jhep.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerat H, Higgs M, Pawlotsky JM. Animal models in the study of hepatitis C virus-associated liver pathologies. Expert Rev Gastroenterol Hepatol. 2011;5:341–352. doi: 10.1586/egh.11.14. [DOI] [PubMed] [Google Scholar]
- 21.Hoshida Y, Fuchs BC, Bardeesy N, Baumert TF, Chung RT. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J Hepatol. 2014;61:S79–90. doi: 10.1016/j.jhep.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto H, Moriishi K, Moriya K, Murata S, Tanaka K, Suzuki T, Miyamura T, Koike K, Matsuura Y. Involvement of the PA28gamma-dependent pathway in insulin resistance induced by hepatitis C virus core protein. J Virol. 2007;81:1727–1735. doi: 10.1128/JVI.01683-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hara Y, Yanatori I, Ikeda M, Kiyokage E, Nishina S, Tomiyama Y, Toida K, Kishi F, Kato N, Imamura M, et al. Hepatitis C virus core protein suppresses mitophagy by interacting with parkin in the context of mitochondrial depolarization. Am J Pathol. 2014;184:3026–3039. doi: 10.1016/j.ajpath.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Moriya K, Nakagawa K, Santa T, Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Miyazawa T, Ishibashi K, Horie T, et al. Oxidative stress in the absence of inflammation in a mouse model for hepatitis C virus-associated hepatocarcinogenesis. Cancer Res. 2001;61:4365–4370. [PubMed] [Google Scholar]
- 25.Tornesello ML, Buonaguro L, Izzo F, Buonaguro FM. Molecular alterations in hepatocellular carcinoma associated with hepatitis B and hepatitis C infections. Oncotarget. 2016 doi: 10.18632/oncotarget.17837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgs MR, Lerat H, Pawlotsky JM. Hepatitis C virus-induced activation of beta-catenin promotes c-Myc expression and a cascade of pro-carcinogenetic events. Oncogene. 2013;32:4683–4693. doi: 10.1038/onc.2012.484. [DOI] [PubMed] [Google Scholar]
- 27.Ray RB, Meyer K, Ray R. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology. 2000;271:197–204. doi: 10.1006/viro.2000.0295. [DOI] [PubMed] [Google Scholar]
- 28*.Nault JC, Zucman-Rossi J. TERT promoter mutations in primary liver tumors. Clin Res Hepatol Gastroenterol. 2016;40:9–14. doi: 10.1016/j.clinre.2015.07.006. This review provides an overview on the role of TERT promoter mutations in HCC development. [DOI] [PubMed] [Google Scholar]
- 29.Machida K, Tsukiyama-Kohara K, Seike E, Tone S, Shibasaki F, Shimizu M, Takahashi H, Hayashi Y, Funata N, Taya C, et al. Inhibition of cytochrome c release in Fas-mediated signaling pathway in transgenic mice induced to express hepatitis C viral proteins. J Biol Chem. 2001;276:12140–12146. doi: 10.1074/jbc.M010137200. [DOI] [PubMed] [Google Scholar]
- 30.Machida K, Tsukamoto H, Liu JC, Han YP, Govindarajan S, Lai MM, Akira S, Ou JH. c-Jun mediates hepatitis C virus hepatocarcinogenesis through signal transducer and activator of transcription 3 and nitric oxide-dependent impairment of oxidative DNA repair. Hepatology. 2010;52:480–492. doi: 10.1002/hep.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zemel R, Gerechet S, Greif H, Bachmatove L, Birk Y, Golan-Goldhirsh A, Kunin M, Berdichevsky Y, Benhar I, Tur-Kaspa R. Cell transformation induced by hepatitis C virus NS3 serine protease. J Viral Hepat. 2001;8:96–102. doi: 10.1046/j.1365-2893.2001.00283.x. [DOI] [PubMed] [Google Scholar]
- 32.Nagao M, Nakajima Y, Hisanaga M, Kayagaki N, Kanehiro H, Aomatsu Y, Ko S, Yagita H, Yamada T, Okumura K, et al. The alteration of Fas receptor and ligand system in hepatocellular carcinomas: how do hepatoma cells escape from the host immune surveillance in vivo? Hepatology. 1999;30:413–421. doi: 10.1002/hep.510300237. [DOI] [PubMed] [Google Scholar]
- 33.Akkari L, Gregoire D, Floc’h N, Moreau M, Hernandez C, Simonin Y, Rosenberg AR, Lassus P, Hibner U. Hepatitis C viral protein NS5A induces EMT and participates in oncogenic transformation of primary hepatocyte precursors. J Hepatol. 2012;57:1021–1028. doi: 10.1016/j.jhep.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 34.Benzoubir N, Lejamtel C, Battaglia S, Testoni B, Benassi B, Gondeau C, Perrin-Cocon L, Desterke C, Thiers V, Samuel D, et al. HCV core-mediated activation of latent TGF-beta via thrombospondin drives the crosstalk between hepatocytes and stromal environment. J Hepatol. 2013;59:1160–1168. doi: 10.1016/j.jhep.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 35.Nie D, Shan X, Nie L, Duan Y, Chen Z, Yang Y, Li Z, Tian L, Gao Q, Shan Y, et al. Hepatitis C virus core protein interacts with Snail and histone deacetylases to promote the metastasis of hepatocellular carcinoma. Oncogene. 2015;35:3626–35. doi: 10.1038/onc.2015.428. [DOI] [PubMed] [Google Scholar]
- 36.Quan H, Zhou F, Nie D, Chen Q, Cai X, Shan X, Zhou Z, Chen K, Huang A, Li S, et al. Hepatitis C virus core protein epigenetically silences SFRP1 and enhances HCC aggressiveness by inducing epithelial-mesenchymal transition. Oncogene. 2014;33:2826–2835. doi: 10.1038/onc.2013.225. [DOI] [PubMed] [Google Scholar]
- 37.Luedde T, Schwabe RF. NF-kappaB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang D, Liu N, Zuo C, Lei S, Wu X, Zhou F, Liu C, Zhu H. Innate host response in primary human hepatocytes with hepatitis C virus infection. PLoS One. 2011;6:e27552. doi: 10.1371/journal.pone.0027552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas E, Gonzalez VD, Li Q, Modi AA, Chen W, Noureddin M, Rotman Y, Liang TJ. HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons. Gastroenterology. 2012;142:978–988. doi: 10.1053/j.gastro.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40**.Finkin S, Yuan D, Stein I, Taniguchi K, Weber A, Unger K, Browning JL, Goossens N, Nakagawa S, Gunasekaran G, et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat Immunol. 2015;16:1235–1244. doi: 10.1038/ni.3290. In vivo study demonstrating that ectopic lymphoid-like structures within the liver form an immunopathological microenvironment, which possibly serves as niche to promote HCC initiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heim MH, Thimme R. Innate and adaptive immune responses in HCV infections. J Hepatol. 2014;61:S14–25. doi: 10.1016/j.jhep.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 42.Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, Bremer J, Iezzi G, Graf R, Clavien PA, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16:295–308. doi: 10.1016/j.ccr.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simonin Y, Vegna S, Akkari L, Gregoire D, Antoine E, Piette J, Floc’h N, Lassus P, Yu GY, Rosenberg AR, et al. Lymphotoxin signaling is initiated by the viral polymerase in HCV-linked tumorigenesis. PLoS Pathog. 2013;9:e1003234. doi: 10.1371/journal.ppat.1003234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.da Costa AN, Plymoth A, Santos-Silva D, Ortiz-Cuaran S, Camey S, Guilloreau P, Sangrajrang S, Khuhaprema T, Mendy M, Lesi OA, et al. Osteopontin and latent-TGF beta binding-protein 2 as potential diagnostic markers for HBV-related hepatocellular carcinoma. Int J Cancer. 2015;136:172–181. doi: 10.1002/ijc.28953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arriazu E, Ge X, Leung TM, Magdaleno F, Lopategi A, Lu Y, Kitamura N, Urtasun R, Theise N, Antoine DJ, et al. Signalling via the osteopontin and high mobility group box-1 axis drives the fibrogenic response to liver injury. Gut. 2016 doi: 10.1136/gutjnl-2015-310752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barcena C, Stefanovic M, Tutusaus A, Joannas L, Menendez A, Garcia-Ruiz C, Sancho-Bru P, Mari M, Caballeria J, Rothlin CV, et al. Gas6/Axl pathway is activated in chronic liver disease and its targeting reduces fibrosis via hepatic stellate cell inactivation. J Hepatol. 2015;63:670–678. doi: 10.1016/j.jhep.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Majumdar A, Kitson MT, Roberts SK. Systematic review: current concepts and challenges for the direct-acting antiviral era in hepatitis C cirrhosis. Aliment Pharmacol Ther. 2016;43:1276–1292. doi: 10.1111/apt.13633. A comprehensive overview of the antiviral effects of DAA treatments in patients with HCV-induced cirrhosis as assessed during the past 3 years. [DOI] [PubMed] [Google Scholar]
- 48.Li DK, Chung RT. Impact of hepatitis C virus eradication on hepatocellular carcinogenesis. Cancer. 2015;121:2874–2882. doi: 10.1002/cncr.29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee CJ, Turek M, Gorke S, et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17:589–595. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lok AS, Everhart JE, Wright EC, Di Bisceglie AM, Kim HY, Sterling RK, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL, et al. Maintenance Peginterferon Therapy and Other Factors Associated With Hepatocellular Carcinoma in Patients With Advanced Hepatitis C. Gastroenterology. 2011;140:840–849. doi: 10.1053/j.gastro.2010.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruix J, Poynard T, Colombo M, Schiff E, Burak K, Heathcote EJ, Berg T, Poo JL, Mello CB, Guenther R, et al. Maintenance therapy with peginterferon alfa-2b does not prevent hepatocellular carcinoma in cirrhotic patients with chronic hepatitis C. Gastroenterology. 2011;140:1990–1999. doi: 10.1053/j.gastro.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 52.Hoshida Y, Fuchs BC, Tanabe KK. Prevention of hepatocellular carcinoma: potential targets, experimental models, and clinical challenges. Curr Cancer Drug Targets. 2012;12:1129–1159. [PMC free article] [PubMed] [Google Scholar]
- 53.Menon KV, Hakeem AR, Heaton ND. Meta-analysis: recurrence and survival following the use of sirolimus in liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2013;37:411–419. doi: 10.1111/apt.12185. [DOI] [PubMed] [Google Scholar]
- 54.Emberson JR, Kearney PM, Blackwell L, Newman C, Reith C, Bhala N, Holland L, Peto R, Keech A, Collins R, et al. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849. doi: 10.1371/journal.pone.0029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:881–891. doi: 10.1038/ajg.2013.5. quiz 892. [DOI] [PubMed] [Google Scholar]
- 56**.Fuchs BC, Hoshida Y, Fujii T, Wei L, Yamada S, Lauwers GY, McGinn CM, Deperalta DK, Chen X, Kuroda T, et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59:1577–1590. doi: 10.1002/hep.26898. This study provides in vivo evidence supporting that EGFR signaling could be an HCC chemoprevention target in cirrhotic liver. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Makiyama A, Itoh Y, Kasahara A, Imai Y, Kawata S, Yoshioka K, Tsubouchi H, Kiyosawa K, Kakumu S, Okita K, et al. Characteristics of patients with chronic hepatitis C who develop hepatocellular carcinoma after a sustained response to interferon therapy. Cancer. 2004;101:1616–1622. doi: 10.1002/cncr.20537. [DOI] [PubMed] [Google Scholar]
- 58.Ikeda M, Fujiyama S, Tanaka M, Sata M, Ide T, Yatsuhashi H, Watanabe H. Risk factors for development of hepatocellular carcinoma in patients with chronic hepatitis C after sustained response to interferon. J Gastroenterol. 2005;40:148–156. doi: 10.1007/s00535-004-1519-2. [DOI] [PubMed] [Google Scholar]
- 59.Chang KC, Hung CH, Lu SN, Wang JH, Lee CM, Chen CH, Yen MF, Lin SC, Yen YH, Tsai MC, et al. A novel predictive score for hepatocellular carcinoma development in patients with chronic hepatitis C after sustained response to pegylated interferon and ribavirin combination therapy. J Antimicrob Chemother. 2012;67:2766–2772. doi: 10.1093/jac/dks269. [DOI] [PubMed] [Google Scholar]
- 60.Arase Y, Kobayashi M, Suzuki F, Suzuki Y, Kawamura Y, Akuta N, Sezaki H, Saito S, Hosaka T, Ikeda K, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology. 2013;57:964–973. doi: 10.1002/hep.26087. [DOI] [PubMed] [Google Scholar]
- 61.Oze T, Hiramatsu N, Yakushijin T, Miyazaki M, Yamada A, Oshita M, Hagiwara H, Mita E, Ito T, Fukui H, et al. Post-treatment levels of alpha-fetoprotein predict incidence of hepatocellular carcinoma after interferon therapy. Clin Gastroenterol Hepatol. 2014;12:1186–1195. doi: 10.1016/j.cgh.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 62.Yamashita N, Ohho A, Yamasaki A, Kurokawa M, Kotoh K, Kajiwara E. Hepatocarcinogenesis in chronic hepatitis C patients achieving a sustained virological response to interferon: significance of lifelong periodic cancer screening for improving outcomes. J Gastroenterol. 2014;49:1504–1513. doi: 10.1007/s00535-013-0921-z. [DOI] [PubMed] [Google Scholar]
- 63.Huang CF, Yeh ML, Tsai PC, Hsieh MH, Yang HL, Hsieh MY, Yang JF, Lin ZY, Chen SC, Wang LY, et al. Baseline gamma-glutamyl transferase levels strongly correlate with hepatocellular carcinoma development in non-cirrhotic patients with successful hepatitis C virus eradication. J Hepatol. 2014;61:67–74. doi: 10.1016/j.jhep.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 64.Toyoda H, Kumada T, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Kitabatake S, Ito T. Risk factors of hepatocellular carcinoma development in non-cirrhotic patients with sustained virologic response for chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2015;30:1183–1189. doi: 10.1111/jgh.12915. [DOI] [PubMed] [Google Scholar]
- 65.Toyoda H, Tada T, Tsuji K, Hiraoka A, Tachi Y, Itobayashi E, Takaguchi K, Senoh T, Takizawa D, Ishikawa T, et al. Characteristics and prognosis of hepatocellular carcinoma detected in patients with chronic hepatitis C after the eradication of hepatitis C virus: A multicenter study from Japan. Hepatol Res. 2016;46:734–742. doi: 10.1111/hepr.12613. [DOI] [PubMed] [Google Scholar]
- 66**.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology. 2016;64:130–137. doi: 10.1002/hep.28535. An interesting retrospective cohort study revealing that risk of post-SVR HCC, though markedly decreased by HCV cure, remains relatively high in aged patients with cirrhosis, diabetes or HCV (gen3) infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu CK, Chang KC, Hung CH, Tseng PL, Lu SN, Chen CH, Wang JH, Lee CM, Tsai MC, Lin MT, et al. Dynamic alpha-fetoprotein, platelets and AST-to-platelet ratio index predict hepatocellular carcinoma in chronic hepatitis C patients with sustained virological response after antiviral therapy. J Antimicrob Chemother. 2016;71:1943–1947. doi: 10.1093/jac/dkw097. [DOI] [PubMed] [Google Scholar]
- 68.Hedenstierna M, Nangarhari A, Weiland O, Aleman S. Diabetes and Cirrhosis Are Risk Factors for Hepatocellular Carcinoma After Successful Treatment of Chronic Hepatitis C. Clin Infect Dis. 2016:ciw362. doi: 10.1093/cid/ciw362. [pii] doi:310.1093/cid/ciw1362. [DOI] [PubMed] [Google Scholar]
- 69.Zeng QL, Li B, Zhang XX, Chen Y, Fu YL, Lv J, Liu YM, Yu ZJ. Clinical Model for Predicting Hepatocellular Carcinomas in Patients with Post-Sustained Virologic Responses of Chronic Hepatitis C: A Case Control Study. Gut Liver. 2016 doi: 10.5009/gnl15321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang JH, Yen YH, Yao CC, Hung CH, Chen CH, Hu TH, Lee CM, Lu SN. Liver stiffness-based score in hepatoma risk assessment for chronic hepatitis C patients after successful antiviral therapy. Liver Int. 2016 doi: 10.1111/liv.13179. [DOI] [PubMed] [Google Scholar]
- 71.Nagaoki Y, Aikata H, Nakano N, Shinohara F, Nakamura Y, Hatooka M, Morio K, Kan H, Fujino H, Kobayashi T, et al. Development of hepatocellular carcinoma in patients with hepatitis C virus infection who achieved sustained virological response following interferon therapy: A large-scale, long-term cohort study. J Gastroenterol Hepatol. 2016;31:1009–1015. doi: 10.1111/jgh.13236. [DOI] [PubMed] [Google Scholar]
- 72.Lee HW, Chon YE, Kim SU, Kim BK, Park JY, Kim do Y, Ahn SH, Jung KS, Park YN, Han KH. Predicting Liver-Related Events Using Transient Elastography in Chronic Hepatitis C Patients with Sustained Virological Response. Gut Liver. 2016;10:429–436. doi: 10.5009/gnl15021. [DOI] [PMC free article] [PubMed] [Google Scholar]