Summary
The ability of animals to flexibly navigate through complex environments depends on the integration of sensory information with motor commands. The sensory modality most tightly linked to motor control is mechanosensation. Adaptive motor control depends critically on an animal’s ability to respond to mechanical forces generated both within and outside the body. The compact neural circuits of insects provide appealing systems to investigate how mechanical cues guide locomotion in rugged environments. Here, we review our current understanding of mechanosensation in insects and its role in adaptive motor control. We first examine the detection and encoding of mechanical forces by primary mechanoreceptor neurons. We then discuss how central circuits integrate and transform mechanosensory information to guide locomotion. Because most studies in this field have been performed in locusts, cockroaches, crickets, and stick insects, the examples we cite here are drawn mainly from these ‘big insects’. However, we also pay particular attention to the tiny fruit fly, Drosophila, where new tools are creating new opportunities, particularly for understanding central circuits. Our aim is to show how studies of big insects have yielded fundamental insights relevant to mechanosensation in all animals, and also to point out how the Drosophila toolkit can contribute to future progress in understanding mechanosensory processing.
Introduction
The unpredictable structure of the natural world poses a problem for motor control systems. Because the environment and the body itself are always changing, a given motor command signal will not always result in an identical movement. A related problem is that internal frames of reference also shift during movement — for example, when limbs move relative to each other. In the terminology of control theory, flexible movements cannot be reliably executed in open-loop, but instead require closed-loop feedback from both external and internal sensors [1].
An important source of feedback is mechanosensation. Mechanical forces on the body are an inevitable consequence of self-movement. Mechanotransduction is also faster than phototransduction, and it is equally effective throughout the day, whatever the ambient light level. Perhaps for these reasons, most animals are absolutely reliant on mechanosensation for normal movement—for example, human patients that lack mechanosensory feedback can generate coarse limb movements, but are unable to execute fine motor tasks [2].
Many robotic systems also use mechanical sensors to guide the control of mechanical actuators. Nonetheless, modern robots lack the motor flexibility of biological systems [3]. Although robots can excel at individual tasks, they frequently fail when placed in novel or unpredictable situations. One reason is that robots lack sensorimotor control circuits commensurate with those of animals. An important difference is that robots typically switch between explicit kinematic models to achieve different high-level behaviors, while biological systems appear to use modulation of low-level sensorimotor control loops [4]. As a result, it is difficult for a robot to find sound footholds on uneven terrain, or to extricate itself when it becomes stuck. The fact that engineers have not yet succeeded in fully solving these problems is a powerful reminder that we do not fully understand how mechanosensory feedback interacts with top-down commands to control movement in animals.
Insects are particularly interesting exemplars of flexible sensorimotor control. Although early descriptions often portrayed insects as simple automatons, “denying them any portion of intellect”, careful observation has since revealed that insect motor patterns are, in fact, remarkably adaptable [5]. Insects execute a dizzying variety of complex behaviors, including running, hunting, flying, courting, fighting, foraging, building, and grooming. Moreover, each of these behaviors is robust to variability in the mechanical forces acting on the body. For example, cockroaches can react to an unexpected mechanical stimulus in less than 20 ms [6, 7]. On a longer timescale, insects must be tolerant to the natural wear and tear of life. Foraging bumblebees collide with vegetation about 60 times a minute, sometimes resulting in a 5–10% loss of wing area in a single day [8]. Somehow, bumblebees are able to compensate for these dramatic changes in the structure and aerodynamics of their wings, not to mention the added weight from the pollen and nectar they have collected. Even the most promising insect-inspired robots, such as the running VelociRoACH [9] and the flying RoboBee [10], can mimic only a small fraction of the motor behaviors executed by real insects.
In this review, we will consider how specific design features of insect mechanosensation might contribute to the rapid and flexible control of movement. We will first examine the structure and function of mechanoreceptor organs, paying particular attention to how sensory neurons are optimized for speed, sensitivity, and robustness. Next, we will discuss how central circuits integrate and transform mechanosensory signals, in order to produce appropriate motor commands.
Our discussion will refer to many different insect species. The majority of the work in this field has been performed in what we will call big insects – mainly locusts, cockroaches, crickets, and stick insects. However, there is also a small but growing body of work in the fruit fly, Drosophila melanogaster. Until recently, work on mechanosensation in Drosophila focused mainly on the development of peripheral organs [11, 12] and the molecular genetics of mechanotransduction [13]. Recently, however, Drosophila has also become a major model for studying neural coding, central circuits, and the neural correlates of behavior. The Drosophila genetic toolkit now provides access to many specific cell types, and the major connectomics effort underway in Drosophila will soon tell us the synaptic connectivity of these cell types. To use these tools intelligently, we should consider the lessons already learned from studies of big insects. Because big insect studies have mainly been performed in adults rather than larvae, we have focused our discussion of Drosophila on the adult stage as well.
We hope this review will be useful for three types of readers. First, as Drosophila neuroscientists, we aim to provide a primer on big insect mechanosensation for the benefit of other Drosophilists. Second, in addressing readers familiar with big insects, we will indicate toward the end of the review how the Drosophila toolbox can help fill gaps in the existing literature. Third, for readers unfamiliar with insects of any kind, we hope to clarify how the study of insects is providing insights into the fundamental problems of mechanosensation and motor control that have relevance to all animals.
Insect mechanoreceptors
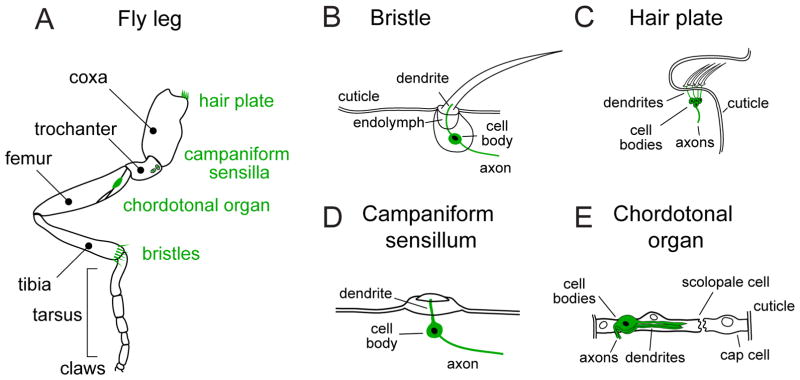
Within a given region of the body – e.g., the leg (Figure 1A) – one typically finds multiple mechanoreceptor types located in close proximity. Each mechanoreceptor type is sensitive to a particular range of mechanical stimuli, such that a naturalistic and complex mechanical stimulus will often co-activate multiple receptor types. Below we will summarize the distinctions between different mechanoreceptor types. Toward the end of this review, we will examine how signals from co-activated mechanoreceptor types are integrated in the central nervous system (CNS).
Figure 1. Anatomy of mechanoreceptor organs on the fly leg.
(A) Schematic of the Drosophila leg, illustrating the four classes of mechanoreceptor organs. Note that these examples do not represent the full complement of leg mechanoreceptors, but rather illustrative examples of each type of organ.
(B) Mechanosensory bristles are the primary exteroceptive organs, densely tiling the fly cuticle. Deflection of the bristle leads to firing in the bristle sensory neuron. In other insects, bristles are known as tactile hairs.
(C) Campaniform sensilla are small domes, which detect tension and compression in the surrounding cuticle. They are often found clumped in fields where strains on the cuticle are likely to be high, such as on proximal regions of the leg.
(D) Hair plates are tightly packed groups of small, stiff, parallel hairs, each of which is innervated by a single sensory neuron. They are often positioned next to folds within the cuticle, so that the hairs are deflected during joint movement. They function as proprioceptors, sensing movements of one joint segment relative to the adjoining segment.
(E) Chordotonal organs are stretch-sensitive mechanoreceptors that contain many individual sensory neurons with diverse mechanical sensitivities. They are found at leg joints, where they encode the angle and movement of the leg, as well as Johnston’s organ in the fly antenna, where they encode auditory signals.
As in vertebrates, the different types of insect mechanoreceptors are traditionally divided into two functional groups: exteroceptors and proprioceptors. Exteroceptors directly detect mechanical forces generated in the external world, while proprioceptors detect the position or movement of body parts. By convention, we will generally treat mechanically isolated (i.e., not tightly clustered) tactile hairs on the external body surface as exteroceptors, and all other mechanoreceptors as proprioceptors. However, the distinction between exteroceptors and proprioceptors is murky. Exteroceptors can be stimulated during self-generated movement, and, conversely, proprioceptors can be stimulated when external stimuli cause body parts to move. Indeed, as we will see below, some neurons that are typically characterized as proprioceptors are in fact primarily dedicated to sensing external stimuli, because they monitor body parts that generate relatively large movements in response to small external forces.
Tactile Hairs (Bristles)
The most visible and abundant of the mechanosensory structures in the adult insect are the trichoid sensilla, or tactile hairs. In the Drosophila literature, tactile hairs are referred to as bristles. A fruit fly has about 6000 bristles which fall into two discrete classes, macrochaetes and microchaetes, based on their size and developmental origins [14, 15]. The larger bristles (macrochaetes, from the Greek macros [large] + khaitē [long hair]) are consistent in number and position across individuals within a species, and even exhibit striking similarity across fly species that diverged 50 million years ago [16]. The smaller and more numerous microchaetes exhibit more variability in their distribution, but are typically arranged in regularly spaced rows.
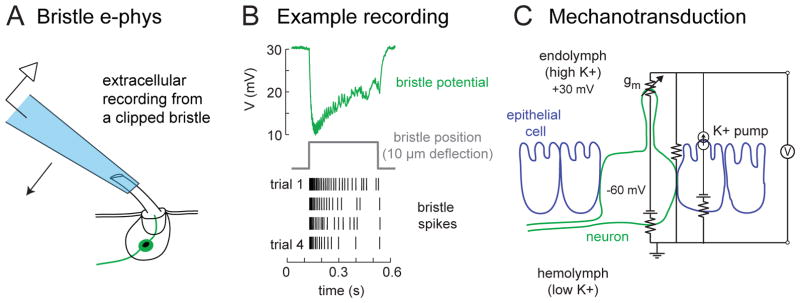
Each tactile hair is composed of a hollow hair shaft whose base is fixed to the dendritic tip of a single bipolar sensory neuron (Figure 1B). The hair acts as a lever arm that exerts forces on the tip of the sensory neuron dendrites, where mechanotransduction channels open to produce electrical currents [17]. Electrophysiological recordings from tactile hairs can be performed by clipping the hair shaft distal to the dendrite and simply placing an electrode in contact with the extracellular fluid that fills the shaft [Figure 2A; 18, 19]. The recorded extracellular signal is dominated by the receptor potential, which appears as a downward deflection, and action potentials appear as small superimposed transients (Figure 2B).
Figure 2. Mechanosensitive properties of tactile hair neurons.
(A) Extracellular electrophysiological recordings from tactile hairs can be made by inserting the shaft of a clipped hair in a recording pipette, which is mounted on a piezoelectric actuator so that the pipette can be used to move the hair.
(B) An example extracellular recording from a fly femur bristle [166] shows a downward field potential deflection (the receptor potential) and a burst of superimposed action potentials. Repeated movement of the bristle leads to adaptation in the bristle response, depicted as a spike raster.
(C) The equivalent circuit of the tactile hair epithelium recording [adapted from 20]. The hair neuron dendrite is bathed in endolymph (high K+), which is separated from the circulating hemolymph (low K+) by an electrically tight layer of epithelial cells. The K+ gradient is established by pumps in the apical membranes of epithelial cells, depicted here as a current source. The transepithelial potential (measured in the endolymph relative to the hemolymph) is about +30 mV. If the neuron rests at about −60 mV, then the total driving force pushing K+ into the neuron’s dendrite through mechanotransduction channels (gm) would be about 90 mV. It should be noted that the tactile hair neuron is separated from the hemolymph by a layer of glial cells (not depicted here). Membrane capacitance is also omitted for clarity.
The ionic basis of mechanoreceptor transduction is probably similar for all insect mechanoreceptor types, but recordings from tactile hairs illustrate the relevant facts particularly clearly. In an extracellular recording from a tactile hair, the receptor potential reflects the inward flow of current into the mechanosensory cell dendrite from the endolymph that surrounds the dendrite (Figure 2C). The driving force for this current is mainly the large K+ gradient that is maintained across the high-resistance layer of epithelial cells between the endolymph and the central hemolymph [20]. In Drosophila bristles, this ionic gradient gives rise to a standing transepithelial potential of approximately +30 mV [21]. Because only the dendrite is bathed in endolymph, the transepithelial potential and the transmembrane potential of the neuron act in series to drive K+ into the neuron when mechanotransduction channels open, thereby depolarizing the neuron [20]. This arrangement transfers much of the energetic burden of mechanotransduction to the pumps in the sheath cells that pump K+ into the endolymph. This arrangement also permits reliable, low latency transduction because the total driving force for mechanotransduction currents is very large; in Drosophila bristles, the latency of mechanotransduction is ~0.1 ms [22]. Interestingly, the same strategy is used by vertebrate epithelial mechanoreceptors, such as the hair cells of the cochlea [23].
Tactile hairs are directionally selective, and their preferred direction can be predicted from the bristle’s orientation in the cuticle. Fly bristles are typically positioned with their long axis oriented approximately 45° relative to the cuticle, and most bristle neurons are most sensitive to mechanical stimuli that push the bristle toward the cuticle [18, 22]. Direction selectivity of tactile hairs is thought to be related to the morphology of the hair and socket [24, 25], but other mechanisms may be involved, such as the identity and distribution of mechanotransduction channels.
In many insects, tactile hair neurons are of two physiological types: rapidly and slowly adapting. In the locust, slowly adapting hairs are more common, and possess a lower mechanical threshold (10°), while rapidly adapting hairs have been found only on the tibia, and have a higher mechanical threshold (~40 °) [25]. Crickets [26], and cockroaches [27] also possess rapidly and slowly adapting tactile hairs with different mechanical thresholds. The same distinction pertains to cutaneous mechanoreceptors in mammals. In mammals, slowly adapting receptors, such as Merkel cells and Ruffini endings, respond best to sustained stimuli. By contrast, rapidly adapting receptors, such as Meissner’s corpuscles, respond only to the onset or offset of a stimulus [28]. In both insects and mammals, rapidly and slowly adapting mechanoreceptors are not spatially segregated, but are instead intermingled across the body surface.
In comparison to what is known about tactile hairs in locusts, crickets, and cockroaches, far less is known about the physiological types of bristles found in the fly. Recordings from macrochaete bristles on the head of blowflies [24] and the notum of Drosophila [18, 21, 29, 30] have identified only slowly adapting bristles with a very low mechanical threshold of ~1° (Figure 2). These bristles are probably not sensitive to wind or sound [24], but rather respond to transient mechanical deflections such as those created by contact with external objects or during grooming behavior. It is not known whether flies also possess high threshold, rapidly adapting bristles.
In addition to the purely tactile hairs, insects also possess sensory hairs that contain taste receptors. These taste hairs, also known as basiconic sensilla, are each innervated by multiple gustatory neurons (typically 4 in Drosophila) and one mechanosensory neuron. In the fly, taste hairs are found on the labial palps of the proboscis, the pharynx, the legs, wings, and genitals [31]. Whereas tactile hairs are thick, sharply pointed, and accompanied by a spine-like structure called a bract, the gustatory hairs are thin, have a blunt tip, and lack the bract cell [15]. In the locust, mechanosensory neurons within taste hairs are directionally selective and rapidly adapting, with a lower mechanical threshold than purely tactile hairs [32]. Their response properties in Drosophila are not known.
As the primary external mechanoreceptors in insects, tactile hairs contribute to a range of behaviors. For example, mechanical stimulation of small numbers of bristles on the Drosophila leg triggers appropriate postural changes, away from the site of stimulation [33]. In locusts, similar avoidance reflexes may contribute to object avoidance during walking [34].
Perhaps the most critical function of tactile hairs is to alert the animal to the presence of foreign objects, such as filth and parasites, on the body surface. In flies, stimulation of just one or two bristles is sufficient to trigger complex grooming sequences, in which the animal attempts to remove debris from its body [33, 35, 36]. These grooming behaviors involve precisely directed direct leg movements, which can be generated entirely by thoracic circuits, even in the absence of descending input from the brain [33, 37]. Careful analysis of grooming behavior in locusts has shown that leg grooming movements are precisely targeted to the site of hair stimulation [38], irrespective of the initial position or mechanical loading of the leg [39].
Although most tactile hairs are excited by contact with solid objects, some specialized hairs are so sensitive that they can detect air particle fluctuations. In the locust, a small number of hairs on the head can respond to strong air currents, and are thought to play a role in flight control [40, 41]. Indeed, some hairs are almost entirely dedicated to detecting air currents: in crickets and cockroaches, bristles on specialized abdominal appendages, known as cerci, can detect wind, courtship song, and the wingbeats of a predator [reviewed by 42]. Depending on context and behavioral state, cercal stimulation can trigger rapid escape responses and jumping, as well as grooming and aggression [43]. Thus, tactile hairs can either drive or modulate many different motor programs in insects, in keeping with their role as the primary transducers of external mechanical stimuli.
Hair plates
In addition to occurring as individual sensory hairs, tactile hairs also occur as tightly packed groups known as hair plates (Figure 1C). Proprioceptive hair plates are often positioned at folds in the cuticle, so that they will be deflected during joint movements [44]. However, they may also function as exteroceptors, as in the case of hair plates at the base of the cockroach antenna, which play an important role in active sensing and object localization [45]. In Drosophila, hair plates can be found at most leg joints [46, 47], but have not been identified on the antenna.
Like a tactile hair, each individual sensillum within a hair plate is innervated by a single sensory neuron. Hair plate sensilla occur as two physiological types: rapidly adapting neurons that respond phasically to hair movements, and slowly adapting neurons that respond tonically to maintained deflections [48, 49].
Studies in big insects have shown that hair plates at leg joints provide sensory feedback to motor neurons that control walking. In the cockroach, a hair plate at the most proximal leg joint provides direct excitatory input to extensor motor neurons of the trochanter, and inhibitory input to the motor neurons that control flexion [50]. Ablation of this hair plate causes the leg to overstep and collide with the more anterior leg, indicating that proprioceptive signals from the hair plate limit the forward movement of the leg during the swing phase of the walking cycle [51].
In the fly, two hair plates located on the ventral surface of the neck form the prosternal organ, which encodes head rotations along all three axes and contributes to compensatory head movements [52]. Shaving the prosternal organ hairs on one side causes flies to compensate by rolling the head toward the operated side [53], supporting the hypothesis that the function of the prosternal organ is to monitor head position and provide feedback for the control of head posture.
Campaniform sensilla
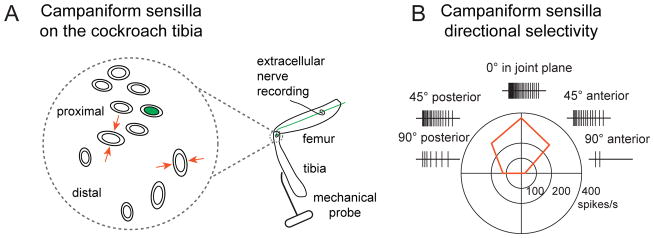
Campaniform sensilla are round or oval-shaped mechanosensory organs that respond to stress and strain within the cuticle [54]. Each sensillum consists of a socket spanned by a flexible cuticular dome that is innervated by the dendrite of a single bipolar sensory neuron (Figure 1D). The neuron is excited whenever the dome flattens [55], which occurs in response to compression and tension in the surrounding cuticle [56]. The elliptical shape of many campaniform sensilla endows them with directional selectivity— a fact first recognized by J.W.S. Pringle, based on his experiments with rubber-and-paper models of the dome [57]. The dome is specifically elongated by tension along its long axis, or else compression along its short axis [Figure 3A; 55, 58]. The orientation of the sensillum within the cuticle therefore dictates which stimuli will preferentially excite it [58].
Figure 3. Directional selectivity of campaniform sensilla.
(A) The diagram on the left shows the location and orientation of campaniform sensilla on the cockroach tibia [adapted from 58]. The two fields of sensilla are located on the tibia, close to the joint with the femur, with one cluster very close to the joint and one slightly more distal. The red arrows indicate that each campaniform sensillum’s preferred direction is compression along the dome’s short axis. At right is a schematic showing how extracellular recordings from campaniform sensillum afferents were made from the femoral nerve using a wire hook electrode, while forces were applied to the leg with a piezoelectric actuator.
(B) Schematic of an extracellular recording showing directional selectivity of an individual campaniform sensillum neuron. Here, a campaniform sensillum on the cockroach tibia responds maximally to dorsal movement in the plane of the femur/tibia joint [adapted from 58]. This recording corresponds to a campaniform sensillum in the more proximal cluster (labeled green in A), oriented with its long axis perpendicular to the leg axis. In contrast, campaniform sensilla in the more distal cluster respond maximally to ventral movements of the tibia [58].
Approximately 1200 campaniform sensilla are distributed over the legs, wings, halteres, and antennae of the fly [59], and exhibit considerable structural diversity [60, 61]. Campaniform sensory neurons in the fly can be classified into two physiological categories; rapidly and slowly adapting [62]. Groups of campaniform sensilla with similar directional sensitivities and response characteristics are often found clustered together in areas likely to encounter cuticular strains, such as joints [Figure 3A; 57].
Interestingly, the specific orientations of many campaniform sensilla position them to respond preferentially to self-generated force [58]. For example, campaniform sensilla on the stick insect trochanter are positioned to encode increases and decreases in mechanical load at the nearby leg joint [63]. Campaniform sensilla neurons are active when leg movements are resisted with a mechanical probe, but do not fire during unresisted leg movements, indicating that they encode mechanical load as resistance to muscle contraction [63, 64]. In this regard, the campaniform sensilla perform a function similar to that of vertebrate Golgi tendon organs [4]. However, unlike tendon organs, the coupling of campaniform sensilla to muscle tension is indirect, and depends on joint position [65].
The functional contributions of insect campaniform sensilla have been studied in the context of both walking and flight control. In particular, the stick insect has served as an important model system for investigating the role of sensory feedback from campaniform sensilla in postural control and walking [reviewed by 66]. For example, campaniform sensilla on the trochanter, the second leg segment, contribute to joint coordination during walking by setting the firing phase of motor neurons that control movement of the first leg segment, the coxa [67]. Under normal conditions, input from trochanter campaniform sensilla terminates activity in the protractor motor neuron of the coxa, and initiates activity in the corresponding retractor motor neuron. Accordingly, ablation of the trochanteral campaniform sensilla substantially decreases the magnitude of bursts in coxal motoneurons [67]. Campaniform sensilla can also contribute to inter-leg coordination by producing muscle bursts at appropriate phases of the step cycle, after a leg is placed on the substrate [68]. These studies have shown that sensory feedback from campaniform sensilla plays an important role in coordinating joint movement both within a leg and across legs, and that natural walking gaits are shaped by sensory feedback to motor circuits.
In flies, campaniform sensilla also play an important role in flight control, through feedback from both the halteres and wings. Halteres are small dumb-bell shaped organs, derived through evolutionary transformation of the hindwings, which beat back and forth in antiphase to the wings. When the body rotates during flight, arrays of campaniform sensilla at the base of the halteres detect inertial (Coriolis) forces that are linearly proportional to the angular velocity of the body [69]. Campaniform sensilla on the wings of locusts [70], moths [71], and flies [72] also encode wing bending forces.
Fast sensory feedback from haltere campaniform sensilla is thought to mediate compensatory flight control reflexes in flies [73]. For example, one haltere campaniform field (dF2) provides direct synaptic input to a motor neuron (mnb1) that controls a flight steering muscle [74]. The mnb1 motor neuron can also be entrained by direct input from wing campaniform sensilla [75]. Wing and haltere campaniform sensilla each fire a single action potential at unique phases within a wing stroke cycle, which in Drosophila lasts about 4–5 ms. Together, the relative phase difference between wing and haltere feedback may contribute to maintaining flight equilibrium by tuning the firing phase of steering muscles during flight [75]. Haltere campaniform sensilla exhibit diverse phase sensitivity [76], suggesting that sensory feedback is capable of fine-tuning flight control throughout the wingstroke.
Proprioceptive chordotonal organs
Chordotonal organs are internal mechanoreceptors found at nearly every joint, and between joints within individual limb and body segments. The fundamental unit of a chordotonal organ is the scolopidium, which is composed of 1–3 bipolar mechanosensory neurons and 2 accessory cell types, the scolopale cells and cap cells, which envelop and anchor the sensory neurons, respectively (Figure 1E). The chordotonal organ is attached to the cuticle wall or muscles by connective tissue, and or can be linked to a joint by an outgrowth of cuticle called an apodeme. Within a given chordotonal organ, there is diversity in the structure and attachment of individual scolopidia, which may be related to the diverse mechanical tuning of the chordotonal sensory neurons [77].
Most chordotonal organs are proprioceptors, but some (discussed separately below) function as exteroceptors. In flies, proprioceptive chordotonal organs are associated with the legs, wings, halteres, and mouthparts. Genetic experiments in the fly have demonstrated the important proprioceptive role of chordotonal neurons in many motor functions. Perturbation of the chordotonal organs in adult Drosophila produces deficits in locomotion and posture [78–82].
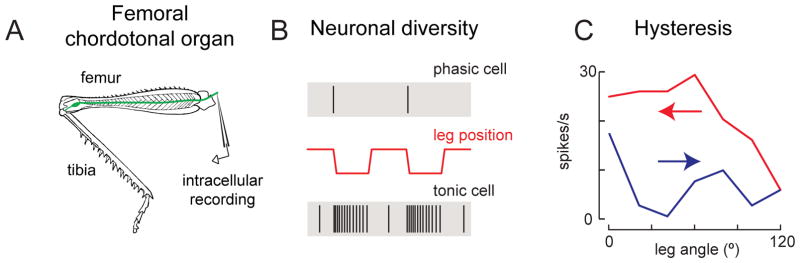
Although relatively little is known about the physiology of proprioceptive chordotonal neurons in adult Drosophila, there is an extensive literature about these neurons in big insects [reviewed by 83]. In particular, the femoral chordotonal organ (fCO) of the locust and stick insect has been investigated in great detail. The fCO is comprised of several hundred chordotonal neurons that are located in the femur and mechanically coupled to the tibia [77, 84, 85]. Neurons of the fCO are organized into two distinct clusters: the larger cluster (~400 neurons) is more distal and ventral within the femur, and the smaller (~80 neurons) is more proximal and dorsal [77, 86]. This organization is similar to that found in Drosophila, though the fly fCO contains fewer neurons [87].
The dorsal and ventral clusters of the fCO are thought to detect distinct mechanical stimuli and contribute to different behaviors [77, 86]. Neurons in the larger, dorsal cluster encode high-frequency (200–800 Hz) vibration of the tibia [88], and may play a role in sensing substrate vibration [89]. In contrast, neurons in the smaller, ventral cluster are sensitive to the position of the tibia; individual cells in this group are typically classified as either slowly adapting (tonically firing) neurons, or rapidly adapting (phasically firing) neurons (Figure 4A–B). Tonic neurons encode position of the joint between the femur and the tibia, while phasic neurons encode velocity and acceleration of joint movement [84, 90, 91]. Interestingly, individual neurons within the ventral cluster respond to rather specific features of the leg’s position and movement. Single neurons may strictly encode the leg’s position but only over a narrow range, or else may encode velocity invariant to position, or else position-and-velocity, or velocity-and-acceleration, etc. [84, 92–95].
Figure 4. Diverse selectivity in the neurons of the femoral chordotonal organ (fCO).
(A) Intracellular recordings can be made from individual chordotonal neurons of the ventral cluster in the hindleg of the locust femur. Neurons in the fCO are typically stimulated by movements of the receptor apodeme, and recordings are targeted to axons as they enter the VNC. These recordings have shown diversity in selectivity among individual neurons in the same organ.
(B) This example shows a phasic neuron, which exhibits transient responses to tibial movement, as well as a tonic neuron, which exhibits sustained firing [adapted from 91].
(C) Hysteresis of tibial position coding in a femoral chordotonal neuron. This neuron fires at higher rates when the preceding movement was flexion of the femur/tibia joint [adapted from 92]. Arrows indicate direction of preceding movements.
A notable property of chordotonal neuron encoding is hysteresis: the relationship between firing rate and joint position can depend on the preceding direction of movement [Figure 4C; 84, 90–92]. From a decoding perspective, hysteresis introduces ambiguity about joint position. However, it is also possible that hysteresis in sensory neurons may actually compensate for nonlinear dynamics in other parts of the circuit, such as muscles [96].
An important function of the fCO is to mediate basic resistance reflexes. For example, movement of the joint between the femur and the tibia is detected by chordotonal neurons within the ventral cluster, which directly excite flexor motor neurons and inhibit extensor motor neurons via polysynaptic pathways, leading to changes in muscle activity to oppose the movement [77, 86]. This reflex serves to stabilize the position of the joint and maintain the posture of the animal. Importantly, the reflex is reliable across a wide range of initial joint positions and movement directions and velocities [97, 98]. The sign and efficacy of the femoral resistance reflex is not fixed, but depends on behavioral context (see below). Given that individual chordotonal neurons are often narrowly tuned to particular combinations of position, velocity, and acceleration, it would seem that many chordotonal neurons must contribute to the control of these seemingly simple leg reflexes. However, the function of chordotonal neuron population coding remains relatively unexplored.
Studies in the locust have shown that the presynaptic terminals of fCO neurons receive rhythmic inhibitory synaptic input during walking [99]. This presynaptic inhibition can be driven by activity of chordotonal neurons [100] or other proprioceptors within the same leg [101], This inhibition does not arise from direct interactions between mechanosensory neurons, but more likely through GABAergic or glutamatergic interneurons whose identity is currently unknown [102]. Presynaptic inhibition may serve to maximize dynamic range by controlling the output gain of the chordotonal neuron population [103]. Alternatively, it may suppress expected mechanosensory input at particular phases within a motor rhythm, in order to increase sensitivity to unpredicted sensory signals [99]. Presynaptic modulation of neurotransmitter release is also a feature of vertebrate mechanosensory afferents, though its function remains poorly understood [104].
The activity of chordotonal neurons can be strongly influenced by the presence of neuromodulators. For example, neurons in the fCO of the stick insect [105] and the locusts [106] increase their firing in the presence of octopamine, the insect analog of noradrenaline. Interestingly, this gain increase is specific to tonically firing neurons that encode joint position [105]. Octopamine can act by directly increasing the excitability of chordotonal neurons in the leg, or by modulating presynaptic inhibition of chordotonal neuron axon terminals [106].
Exteroreceptive chordotonal organs
In addition to the proprioceptive chordotonal organs described above, many insects possess specialized chordotonal organs that are used to detect external mechanical signals. Although these organs are formally still proprioceptors – because they are located inside the body, and because they detect the position or movement of body parts – they function primarily as exteroceptors. This is because they monitor body parts that generate relatively large movements in response to small external forces.
In Drosophila, the most prominent example is Johnston’s organ, a chordotonal organ that resides inside the second-most distal segment of the antenna. It senses movements of the most distal antennal segment, which rotates easily about its long axis, and therefore generates relatively large movements in response to small fluctuations in air particle velocity. In Drosophila, Johnston’s organ comprises ~500 neurons [107]. This organ detects sound, including male courtship song, which is produced when the male fly extends and vibrates his wing while pursuing a female [reviewed by 108, 109]. Johnston’s organ also detects wind [110], and it even responds to the fly’s own wingbeats in flight [111]. Finally, there is some evidence that Johnston’s organ is involved in encoding gravity, because the distal antennal segment can move as much as 1 μm in response to changes in gravitational forces [112]. Johnston’s organ neurons seem to be divided into rapidly-adapting types and slowly-adapting types, some of which are also selective for the direction of antennal rotation (toward the head or away from the head) [110, 112, 113].
Many insects (including crickets, bees, locusts, and some flies) possess tympanal organs: specialized hearing structures that consist of a thin cuticular membrane stretched across an air-filled sac. Chordotonal organs attached to the tympanal membrane detect high frequency (2–100 kHz) mechanical vibrations, acting much like a vertebrate eardrum [reviewed by 114]. Drosophila do not have tympanal organs, and therefore rely on the Johnston’s organ for audition, limiting their hearing to lower frequencies (< 1 kHz).
Another example of a specialized chordotonal organ is the subgenual organ, a fan-shaped array of scolopidia in the tibia of most insects that is sensitive to vibrations of the substrate [115, 116]. Subgenual organs have not been reported in flies. However, Drosophila females appear to detect substrate-borne vibrations during courtship [117], possibly through the fCO or campaniform sensilla.
Multipolar receptor neurons
Multipolar receptors are non-ciliated neurons with multiple dendritic branches that terminate on a variety of internal organs and tissues. In Drosophila larvae, such multipolar neurons are commonly referred to as multidendritic neurons [118, 119], which are distinct from ciliated neurons with a single dendrite, such as those associated with tactile hairs, campaniform sensilla, and chordotonal organs. Multidendritic neurons in the larval body wall are active during peristaltic muscle contraction, indicating that they respond to selfgenerated forces during locomotion [81]. Some multidendritic neurons persist through metamorphosis, and are found in the abdomen of the adult fly [120].
Multipolar receptors are found throughout the insect body, typically embedded within an accessory structure such as a strand of tissue or muscle, or slung between protuberances of cuticle called sclerites. The proprioceptive role of multipolar receptors has been studied in great detail in larger insects. For example, in the locust, feedback from wing hinge multipolar receptors directly modulates the motor patterns that control flight [121, 122]. Multipolar stretch receptors in the moth wing signal the position of the wing during flight by encoding the amplitude, rather than the velocity, of stretch [123]. Ablation of wing hinge stretch receptors decreases the amplitude of visually-evoked flight responses, indicating that multipolar receptor feedback is integrated with descending motor commands to control wing movement [124].
Multipolar receptors are also found in the viscera. For example, in the blowfly, internal stretch receptor neurons monitor the distension of the fly gut, and provide a signal that terminates feeding behavior [125]. In adult Drosophila, multipolar receptors have been identified at leg joints, and in the most distal leg segment, the tarsus [126–128], though their function in the fly remains poorly understood.
Other mechanoreceptors
There are several mechanoreceptors found in insect legs whose physiology and function remain enigmatic – these include the strand receptors, muscle receptor organs, and tension receptors. None of these mystery mechanoreceptors has yet been described in Drosophila, but given the overall homology of mechanoreceptor structure and location in the legs of insects [129], genetic tools for labeling specific neuron types may soon enable their identification in the fly.
Strand receptors are unique among mechanoreceptor neurons, in that their cell bodies are located within the CNS, and they project dendrites into the periphery [130]. Like multipolar stretch receptors, they monitor stretch across joints or muscles, most notably at joints in the locust and cockroach legs [131].
Muscle receptor organs (MROs) are single multipolar receptor neurons associated with a modified muscle, which is itself innervated by a specialized motor neuron. MROs are thought to monitor changes in muscle position in a manner similar to vertebrate muscle spindles; for example, MROs that encode joint position have been identified in the locust [132]. However, in addition to detecting mechanical movements of the joint, the MRO motor neuron receives direct input from other proprioceptive organs, so that responses of muscle receptor organs result from both afferent and efferent signals from other mechanoreceptors [133].
Finally, some multipolar proprioceptors, referred to as tension receptors, can be found directly embedded in muscles. For example, in the locust, a single tension receptor is associated with the flexor tibiae muscle in the leg [134], while 200 tension receptors are located within a single ovipositor muscle that controls egg-laying behavior [135]. Tension receptors detect the forces generated by a muscle—they are most sensitive to active muscle contraction, rather than passive movement [134–136].
Central projections of peripheral mechanoreceptors
Peripheral mechanoreceptors on the insect body send direct axonal projections into the central nervous system. Detailed anatomical studies in a number of insect species have shown that these axons are systematically organized depending on mechanoreceptor location, physiological tuning, and receptor type.
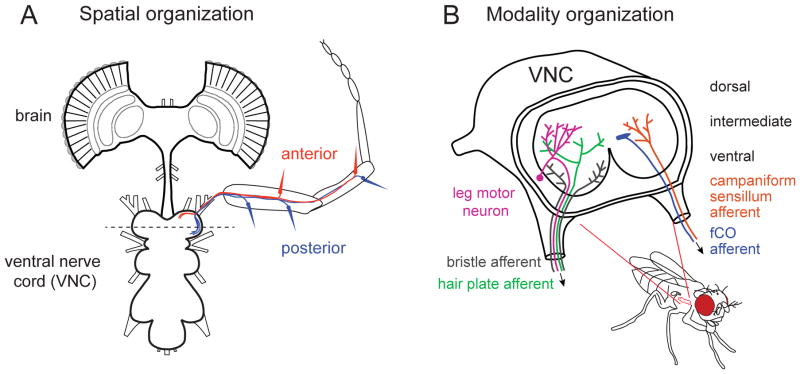
A well-studied example of axonal mapping is the somatotopic organization of afferents from leg bristle neurons. In Drosophila, bristles on each of the three pairs of legs project to a corresponding compartment, or neuromere, within the ventral nerve cord (VNC). Axons from bristle sensory neurons are then topographically organized within each neuromere – bristle neurons on the anterior surface of the front leg arborize along the anterior edge of the prothoracic neuromere, while posterior bristles arborize posteriorly [Figure 5A; 137]. This topographic map of the leg surface within each neuromere would allow central neurons to more easily sample inputs from groups of nearby bristles. This is relevant because, due to the structure of the natural world, nearby bristles are most likely to exhibit correlated activity.
Figure 5. Organization of fly mechanosensory afferents by topography and sensory modality.
(A) The arborizations of bristle axons in the VNC vary systematically with the location of the mechanoreceptor on the fly leg. Specifically, anterior bristles project to the anterior region of their respective neuromere, while posterior bristles project to the posterior region. This organization creates a map of the leg surface within the VNC [adapted from 137].
(B) Axons from different mechanoreceptor classes project to distinct regions of the fly CNS. Bristle neurons arborize in the ventral region of the VNC, while proprioceptive organs (such as hair plates, campaniform sensilla, and the fCO) project to intermediate layers. The most dorsal layers are occupied by motor neurons [schematic adapted from 46, 47].
Similar somatopic maps of tactile hair axons have been described in big insect species, including the locust [138], cockroach [139], and cricket [140]. A topographic map of mechanoreceptor afferents also exists in the dorsal horn of the mammalian spinal cord [141] and the trigeminal nuclei of the brainstem [142]. The existence of such maps across distantly related species suggests that topographic organization of primary sensory afferents plays an important functional role in central processing of somatosensory signals.
Roughly orthogonal to the somatotopic map, there is another axis of organization in the fly VNC. Namely, different sensory modalities arborize in different layers along the dorsal-ventral axis of the VNC (Figure 5B). Axons from bristle neurons arborize along the ventral edge of the neuropil, while campaniform sensilla and hair plate axons terminate in a more dorsal region termed the intermediate layer [47]. Leg chordotonal organs arborize along the medial border of this intermediate layer [126]. The most dorsal region of the VNC is dominated by motor neurons [143, 144]. Thus, proprioceptive afferents are positioned close to dorsal motor neuron dendrites, while tactile signals from hairs are segregated in more ventral layers. Again, the organization described here for Drosophila is roughly similar to that in other insects, such as the locust [reviewed by 145]. As a general rule, mechanoreceptors are not thought to make synapses outside the CNS. An exception is the fCO of Drosophila; although the majority of their synaptic output is probably in the VNC, some femoral chordotonal neurons also form a peripheral ‘glomerulus’ of synapses within the leg nerve [87].
Sensory axons from a single receptor type may also form maps that correspond to their physiological tuning. This organization may facilitate the integration of correlated signals in downstream neurons. For example, the neurons that make up the cricket tympanal organ arborize in a tonotopic pattern related to their frequency sensitivity [146–148]. There is also evidence for a correlation between the physiological tuning of neurons in the locust fCO and their axonal morphology in the CNS [149]. Similarly, in the Drosophila brain, the axonal projections of chordotonal neurons from Johnston’s organ are organized according to their tuning [110, 150].
Compared to mechanoreceptors on the legs, less is known about the central projections of mechanoreceptor neurons on other regions of the insect body. One well-characterized set of central projections are those of the large filiform hairs of the locust head and thorax, whose axonal projections into the VNC have been used to study activity-dependent circuit formation [reviewed by 151]. Similarly, the central projections of Drosophila thorax bristles have been characterized in genetic studies of axon targeting [152] and synaptogenesis [153]. However, the central organization of head mechanoreceptors remains poorly characterized. In the few cases that have been examined, dye fills have shown that the arborizations of individual head mechanoreceptor neurons can be extensive. For example, tactile hairs on the cricket eye [154], filiform hairs on the locust head [155], and campaniform sensilla on the blowfly antennae [156] send axonal projections to both the brain and the VNC. In these examples, arborizations in the brain appear to be concentrated in ventral regions, including the subesophegeal ganglion and the antennal mechanosensory and motor center.
From mechanosensation to action: the problems faced by the central nervous system
In turning to the topic of central mechanosensory circuits, it is useful to consider the universal ‘problems’ that the mechanosensory systems of all animals must solve. Below, we list some of these basic problems, and we briefly describe recent work that illustrates how insect nervous systems can solve these problems. We then focus on two of these problems in greater detail in the following sections. Readers wanting more detail on any of these topics should consult Malcolm Burrows’ book [145] and other classic reviews [157, 158], as well as some more recent perspectives [4, 42, 66, 159–162].
Problem 1: Mechanoreceptor spikes often must be processed rapidly
Some mechanical signals demand an immediate behavioral response. The fastest motor responses to mechanical perturbations are mediated by the intrinsic biomechanical properties of the body, prior to the involvement of any neurons at all [e.g., 163, 164]. But there is also evidence that neural mechanisms are often adapted for speed. The extreme diversity and specialization of mechanoreceptor cells is one such adaptation: in essence, the peripheral nervous system performs much of the computational work of the mechanosensory processing system, and in doing so, it eliminates the need for additional layers of CNS processing. Another adaptation for speed can be seen at the very first stage of processing in the CNS, where single mechanoreceptor axons can diverge to synapse onto central neurons that perform distinct computations in parallel [165, 166]. By parallelizing central computations in this manner, the CNS can extract information as rapidly as possible. Speed is also achieved in part by making computations very local, thus minimizing the number of long axons and synaptic relays in any given circuit. For example, in the extreme case, many proprioceptive mechanoreceptors synapse directly on motor neurons [145, 167, 168]. Finally, in some cases, neural processing delays can be avoided altogether by taking advantage of passive mechanical conduction. For example, a mechanical stimulus applied to the locust tarsus propagates through the leg cuticle to be detected by campaniform sensilla on the proximal femur and trochanter [169]. This mechanical conduction occurs in less than 1 ms, compared to the 8 ms delay for conduction in the axons of distal campaniform sensilla neurons.
Problem 2: The implications of a mechanoreceptor spike train for motor control can depend on the position of the body
An intuitive example of this problem can be seen whenever an insect makes searching movement with its forelegs or antennae. To accurately locate the position of an object, the nervous system must take into account the leg’s position when an object is contacted. A recent study shed light on this problem in stick insects. When a leg makes transient contact with an object, a stick insect responds by searching in a tight local pattern around the site of contact [170]. Accurate local searching behavior does not require visual input, but does require an intact hair plate at the coxa-trochanter joint, suggesting that this proprioceptive organ provides key information about the joint’s position at the moment of contact. Intriguingly, searching behavior can be evoked by depolarizing current injection into a single identified ‘command interneuron’ in the appropriate neuromere [171]. However, if the foot is in contact with the substrate, then depolarization of this command interneuron does not produce searching. It will be interesting to determine where foot touch receptor signals act to gate searching behavior, and to learn how proprioceptors determine the coordinate frame of searching patterns. The coordinate frame problem also applies to the relationships between different appendages. For example, when a stick insect is exploring a gap with its antenna, a contact between the antenna and the far edge of the gap is sufficient to initiate a directed movement of the forelegs to the far side the gap, implying that the antenna’s position at the moment of contact is being relayed to the leg control system [172].
Problem 3: Mechanosensory cues must be integrated with cues from other sensory modalities
In many cases, motor actions are guided by signals from multiple sensory modalities, including mechanosensation, vision, and olfaction. The integration of cues from different sensors is a hard problem because different sensory modalities operate on different timescales, and are also formatted within different coordinate frames (e.g., retina-centric versus leg-centric coordinates). The solution thus requires both temporal integration and a spatial coordinate transformation. Recent studies in insects illustrate specific instances where mechanosensory cues are integrated with cues from other modalities. For example, cockroaches respond to an obstacle with a range of different climbing strategies depending on both antennal mechanoreceptor signals and visual cues [173]. Similarly, the wings of flying flies are controlled by both visual and mechanosensory cues operating on different timescales [174–176]. Multi-sensory integration may occur at multiple sites within the central nervous system. In flies, for example, visual commands from the brain descend to the VNC, but campaniform sensilla neurons on the wings and halteres also project directly to the brain [177].
Problem 4: Mechanoreceptors must modify the activity of synergistic muscle groups
Most movements involve the synergistic action of multiple muscles, with different motor patterns engaging different synergies (Figure 6A). This situation creates a constraint on mechanosensory feedback. Specifically, it means that mechanosensory feedback will be most effective when it specifically targets the muscles that participate synergistically in the same locomotor pattern. Below we will discuss the topic in more detail in a section dedicated to muscle synergies.
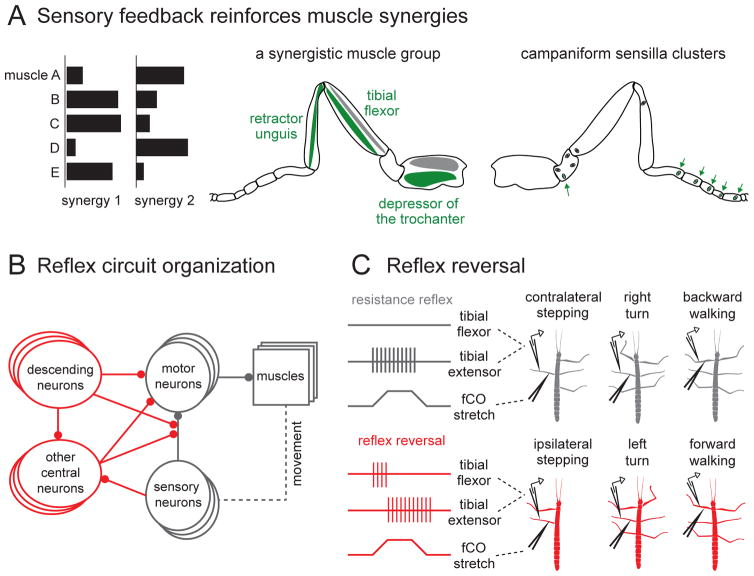
Figure 6. Mechanosensory processing in reflex circuits of insects.
(A) Left: schematic illustrating the concept that a muscle synergy involves multiple co-activated muscles, and different behaviors recruit different muscle synergies. Middle: a specific muscle synergy in the insect leg. Muscles indicated in green are co-activated when leg grips the substrate. (Antagonist muscles are shown in gray.) Right: campaniform sensilla on the leg provide positive force feedback to motor neurons that participate in this muscle synergy. Specific clusters of campaniform sensilla on the tarsus and trochanter (green arrows) are activated when the leg grips the substrate, and their activation leads to increased grip. Adapted from [180].
(B) Schematic connectivity diagram of mechanosensory reflex circuits in the insect VNC. The basic circuit supporting the resistance reflex is outlined in gray, and inputs that modulate the sign and efficacy of the reflex are outlined in red.
(C) Reflex reversal in the stick insect is specific to behavioral context [adapted from 186]. Extracellular recordings were made from the tibial flexor and extensor muscles and motor neurons while mechanically stimulating the femoral chordotonal organ (fCO) by pulling on the receptor apodeme. During contralateral stepping, turning to the right, or backward walking, fCO stretch excited the tibial extensor (top row), but during stepping of the ipsilateral front leg, leftward turning, or straight forward walking, fCO stretch produced a distinct pattern of tibial muscle activity (bottom row).
Problem 5: There is a fundamental tradeoff between stability and maneuverability
For example, when an animal is standing still, postural reflexes counteract disturbances in order to ensure a robust stance. However, when the organism needs to execute a motor pattern such as walking, postural reflexes may actively oppose the movements required for swinging the leg. The solution to this problem is to suppress (or even reverse) postural reflexes during voluntary movement. In essence, reflexes are managed by the CNS to promote stability in some cases and maneuverability in others. Below we will examine this topic in more detail in a section dedicated to ‘reflex reversal’.
Solving the muscle coordination problem: feedback loops targeting muscle synergies
Movements are executed by the coordinated action of multiple muscles. For example, when a flexor is activated, its corresponding extensor is often relaxed. These ‘muscle synergies’ may extend across multiple muscle groups that control different segments of a limb [reviewed by 178, 179]. For example, to pull an object toward our body, we synergistically activate flexors in both our arm and hand. Because mechanosensation is important for fine-tuning motor control, mechanosensory feedback signals might engage these same muscle synergies. Alternatively, mechanosensory signals might recruit other, distinct muscle synergies, or even modulate the activity of individual muscles.
A recent study approached this question by studying how mechanosensory feedback engages muscle synergies in both the cockroach and the stick insect. Zill et al. [180] focused on a muscle synergy involving three muscles of the same leg – namely, the depressor of the trochanter, the tibial flexor within the femur, and the retractor unguis within the tibia (Figure 6A). In order to exert a strong inward grip on the substrate underneath the foot, these three muscles must contract together.
This study found that when specific groups of leg campaniform sensilla were stimulated, the motor neurons innervating all three of the relevant muscles were co-activated (Figure 6A). For example, stimulation of campaniform sensilla on the foot had this effect, as did stimulation of campaniform sensilla on the proximal trochanter. Thus, distinct peripheral mechanoreceptors can generate the same pattern of muscle co-contraction – i.e., the same muscle synergy.
Importantly, the campaniform sensilla that had these effects are normally stimulated when the animal grips the substrate. The sensilla on the foot detect resisted forces exerted by the retractor muscle [181, 182]. Similarly, the sensilla on the proximal trochanter detect resisted forces exerted by the trochanteral depressor. Thus, this circuit consists of multiple positive feedback loops, which operate in parallel to increase grip.
Compared to vertebrates, insects have far fewer motor neurons per muscle. For example, Drosophila leg muscles are typically innervated by less than five motor neurons, and sometimes as few as two [143, 144]. Invertebrates also make extensive use of inhibition and neuromodulation to fine tune muscle activity [reviewed by 183]. In the future, it will be interesting to explore whether the organizing principle of muscle synergies extends to these inhibitory and neuromodulatory motor neurons.
Solving the stability-mobility tradeoff problem: reflex reversal
We define a reflex as a behavior mediated by signaling from sensory neurons to motor neurons via a direct or nearly direct pathway (i.e., with few intervening synapses). Naively, we might imagine that reflex behaviors are immutable, and that the neurons that mediate them have fixed relationships. In reality, however, most reflexes are flexible, and can be dramatically modified depending on behavioral context.
For example, stretch reflexes, or resistance reflexes, serve to stabilize posture in both invertebrates and vertebrates. These resistance reflexes are mediated by feedback circuits. The most basic implementation requires just three components: a sensory neuron, a motor neuron, and a muscle (Figure 6B). When the muscle is stretched, the stretch is detected by the sensory neuron, which provides excitation to the motor neuron, leading to contraction of the muscle, thereby opposing stretch. The function of this reflex is to help maintain posture during changes in mechanical load.
The circuits that underlie resistance reflexes can be remarkably flexible, exhibiting both short-term modulation as well as long-term plasticity [reviewed by 184]. One of the best examples of reflex flexibility is that of the stick insect tibia, originally described by Bassler [185]. In a stationary stick insect, stretching of the fCO excites tibia extensor motor neurons and inhibits tibia flexor motor neurons (Figure 6C). During voluntary leg movements, however, the sign of this reflex flips. Now, stretch of the fCO receptor apodeme leads to increased tibia flexion. This phenomenon is known as reflex reversal [reviewed by 157]. Reflex reversal is an important element of maneuverability: without it, the resistance reflex would oppose voluntary movement. Because the reversed reflex now reinforces rather than opposes movement, it is sometimes called an ‘assistance reflex’.
A recent study revealed the contextual specificity of the stick insect reflex reversal. Hellekes and colleagues [186] recorded activity from tibia motor neurons of the stick insect’s middle leg. They then mechanically stretched the fCO of the middle leg while the animal was walking. They found that reflex reversal did not occur during all active movements, but depended on the particular task being executed. For example, they observed reflex reversal during stepping of the ipsilateral front leg, but not stepping of the contralateral legs (Figure 6C). For the front leg, reflex reversal only occurred during forward walking; when the insect walked backward, this particular reflex did not reverse. This is likely related to the fact that femur-tibia joint is flexed during stance in forward walking, but is extended during stance in backward walking. In other words, during forward and backward walking, the leg muscles work together differently to create propulsion, and so the nature of reflex modification would naturally also be different.
Finally, Hellekes et al. [186] observed reflex reversal when the animal was attempting to turn to the same side as the manipulated leg, but not when the animal was turning in the opposite direction (Figure 6C). Again, the sign of the reflex is matched to the requirements of the behavior: to execute a turn, the middle leg on the inside must pull by flexing the femur-tibia joint, whereas the middle leg on the outside must push. Overall, these experiments show that the resistance reflex is not simply reversed whenever the animal is active, but can be specifically modulated during particular locomotor patterns. Indeed, it has been suggested that reflex modification is not simply a corollary of switching locomotor patterns, but is the essence of the switch. For example, in a walking insect, a command to turn may consist simply of the modification of leg mechanosensory reflexes such that turning is the natural result [187, 188].
What are the underlying sites and mechanisms of reflex reversal? As described above, there is some evidence that the output of mechanoreceptor neurons is directly modulated during behavior, — e.g., through presynaptic inhibition of the chordotonal neuron axon terminals by GABAergic feedback neurons [102]. However, it is also likely that the effect of sensory input upon motor neurons is influenced by VNC interneurons. One population of non-spiking interneurons in the stick insect has been shown to receive input from chordotonal neurons and provide input to tibia motor neurons [189, 190]. It is thought that the balance of excitation and inhibition within this population regulates the sign of chordotonal feedback within leg motor neurons [191].
Descending signals from the brain may also be involved in modulating reflex reversals during locomotion. For example, in the cockroach, removing the descending projections from the brain alters certain mechanosensory reflexes [187]. A recent study extended this finding to show that electrical stimulation of neurons in the cockroach central complex can alter the tibial resistance reflex [192]. An appealing hypothesis is that diverse motor patterns can be generated by top-down modulation of basic sensorimotor loops like the resistance reflex. This is a very different strategy than that implemented in robots, in which high-level behaviors are typically achieved with explicit kinematic models [4].
The ‘hard problems’ of sensorimotor control and the value of insect models
One way to identify compelling scientific problems is to ask which features of biological systems are most difficult to replicate in artificial systems. Thirty years ago, a reasonable answer might have been the problem of visual object recognition, which motivated many neurophysiological and computational studies of the primate visual cortex [194]. However, recent advances in artificial neural networks have made it possible for a computer to automatically classify natural images with accuracies that match, and sometimes surpass, human performance [195]. A plausible argument can be made that the efforts of both neuroscientists and computer scientists contributed to these advances in object recognition technology.
Today, flexible sensorimotor control represents a set of problems of a similar scale. Studies of insects are particularly well-suited to understanding the solutions to these problems, because insect nervous systems are compact, relatively stereotyped, and amenable to in vivo recordings— even intracellular recordings from targeted cell types in alert, behaving animals. As detailed above, there are some explicit and fundamental ‘hard problems’ of sensorimotor control that are faced by insects as well as other organisms. Already, neurophysiological and behavioral studies have taught us a great deal about how insects solve these problems. These biological insights are now providing inspiration for a new generation of artificial systems [4].
The role of Drosophila in the study of mechanosensory processing
There are many outstanding questions in insect mechanosensation. One of the most critical concerns the role of CNS circuits. Certainly, there is already a literature on CNS circuitry, and we have noted highlights from this literature. However, we are far from understanding how central circuits select and control complex motor behaviors in the face of external mechanical perturbations. One difficulty is the complexity of central circuits. In an insect, each thoracic neuromere – the central circuitry controlling a single leg – contains >100 motor neurons and several times as many local interneurons [4]. To understand central circuits of this complexity, we should aim to (1) identify neurons that are active during a particular behavior, (2) map their connections, (3) inject signals at particular locations in the circuit, and finally (4) break connections (and especially loops) in the circuit. Given this mission, Drosophila offers some powerful emerging tools.
Chief among these is the availability of many cell-type specific ‘driver’ lines that allow transgenes of interest to be expressed in specific neurons. Thousands of these lines are publicly available [196]. For example, a recent study identified driver lines targeting each of the major mechanoreceptor types in the Drosophila leg [166]. These sorts of driver lines are already being used to determine how gait is affected by silencing specific mechanoreceptor neurons [78, 79, 82]. Driver lines are also being identified that allow genetic access to specific central neurons of the VNC [166, 197, 198]. Meanwhile, another effort is currently underway to generate genetic driver lines that individually target many of the ~350 pairs of descending neurons that project from the brain to the VNC [199–201]. Descending signals from the brain are known to modulate the sign and amplitude of mechanosensory reflexes [187], for example, but the format of these signals is not well understood in any insect species. A collection of driver lines targeting descending neurons will be an important tool for answering this question.
Driver lines have two broad applications. One application is to perturb specific circuit elements – using optogenetic tools, or other genetic perturbations [reviewed by 202]. For example, driver lines targeting descending neurons will make it possible to inject signals directly into descending pathways, thereby showing whether these signals are sufficient to reverse sensorimotor loops like the leg resistance reflex, and whether this modulation is specific to particular reflexes and behaviors. The other application is equally important: driver lines allow neuroscientists to target electrodes and fluorescent activity reporters to almost any cell type of interest. Historically, insect neurophysiology was limited to recording from cell types that could be targeted using sharp glass microelectrodes – generally neurons with large-diameter dendrites or axons that could be stably penetrated with a sharp electrode. Now, in Drosophila, it is possible to label almost any cell type with a fluorescent protein, and thus make targeted patch-clamp recordings from virtually any central neuron of interest [reviewed by 203]. Another powerful tool in Drosophila is the ability to image neural activity in vivo using genetically-encoded calcium or voltage indicators.
Studies in Drosophila will also benefit from a major ongoing effort to map the synaptic connections in the adult fruit fly. This effort uses serial-section electron microscopy combined with cell-type specific genetic tools to map synaptic connections between morphologically identifiable cell types [204, 205]. Cell types identified morphologically can then be matched with driver lines using new bioinformatic tools [206]. This effort to map synaptic connections using large-scale electron microscopy has focused on the brain thus far, but it is currently being extended to the VNC as well.
The challenge, then, is to apply these tools to determine how central circuits integrate mechanosensory information to select different actions, such as grooming, walking, and take-off. To identify neurons that initiate changes in behavioral state, one could use pan-neuronal imaging to record activity while tracking behavior. Once relevant neurons have been identified, targeted in vivo electrophysiological recordings could then be used to understand how mechanosensory signals are integrated with other sensory cues and behavioral state changes to select appropriate behavioral responses. To test specific models of action selection, one could then bias behavioral transitions through targeted optogenetic stimulation in freely behaving flies. In order to make wise use of these tools, it is important to consider the lessons learned from work in big insects.
Indeed, although Drosophila presents many experimental advantages, there are also many questions that are still more amenable to investigation in other insect species. For example, some experiments are simply more feasible in larger insects. Ultimately, the identification of general principles for sensorimotor control will require investigation of multiple species and circuits. In the future, genome-editing tools like CRISPR/Cas9 may permit the use of genetic tools for targeted circuit manipulation in larger insects like the locust and cockroach. All of these considerations emphasize the broader point that insects are likely to be a source of future insights into mechanosensory processing, as well as a source of inspiration for roboticists and engineers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John C. Tuthill, Email: tuthill@uw.edu.
Rachel I. Wilson, Email: rachel_wilson@hms.harvard.edu.
References
- 1.Wiener N. Cybernetics: Or Control and Communication in the Animal and the Machine. Cambridge: MIT Press; 1948. [Google Scholar]
- 2.Sanes JN, Mauritz KH, Evarts EV, Dalakas MC, Chu A. Motor deficits in patients with large-fiber sensory neuropathy. Proc Natl Acad Sci USA. 1984;81:979–982. doi: 10.1073/pnas.81.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ijspeert AJ. Biorobotics: Using robots to emulate and investigate agile locomotion. Science. 2014;346:196–203. doi: 10.1126/science.1254486. [DOI] [PubMed] [Google Scholar]
- 4.Buschmann T, Ewald A, von Twickel A, Buschges A. Controlling legs for locomotion-insights from robotics and neurobiology. Bioinspir Biomim. 2015;10 doi: 10.1088/1748-3190/10/4/041001. [DOI] [PubMed] [Google Scholar]
- 5.Kirby W, Spence W. An introduction to entomology, or Elements of the natural history of insects. Vol. 4. London: Longman, Hurst, Rees, Orme, and Brown; 1822. [Google Scholar]
- 6.Schaefer PL, Kondagunta GV, Ritzmann RE. Motion analysis of escape movements evoked by tactile stimulation in the cockroach Periplaneta americana. J Exp Biol. 1994;190:287–294. doi: 10.1242/jeb.190.1.287. [DOI] [PubMed] [Google Scholar]
- 7.Camhi JM, Nolen TG. Properties of the escape system of cockroaches during walking. J Comp Physiol A. 1981;142:339–346. [Google Scholar]
- 8.Foster DJ, Cartar RV. What causes wing wear in foraging bumble bees? J Exp Biol. 2011;214:1896–1901. doi: 10.1242/jeb.051730. [DOI] [PubMed] [Google Scholar]
- 9.Haldane DW, Fearing RS. Running beyond the bio-inspired regime. IEEE International Conference on Robotics and Automation (ICRA) 2015:4539–4546. [Google Scholar]
- 10.Chirarattananon P, Ma KY, Wood RJ. Adaptive control of a millimeter-scale flapping-wing robot. Bioinspir Biomim. 2014;9:025004. doi: 10.1088/1748-3182/9/2/025004. [DOI] [PubMed] [Google Scholar]
- 11.Hartenstein V. Development of Insect Sensilla. In: Gilbert LI, editor. Comprehensive Molecular Insect Science. Vol. 1. Amsterdam: Elsevier; 2005. pp. 379–419. [Google Scholar]
- 12.Singhania A, Grueber WB. Development of the embryonic and larval peripheral nervous system of Drosophila. WIREs Dev Biol. 2014;3:193–210. doi: 10.1002/wdev.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kernan MJ. Mechanotransduction and auditory transduction in Drosophila. Pflugers Arch. 2007;454:703–720. doi: 10.1007/s00424-007-0263-x. [DOI] [PubMed] [Google Scholar]
- 14.Sturtevant AH. The North American species of Drosophila. Washington, D.C: The Carnegie institution of Washington; 1921. [Google Scholar]
- 15.Hannah-Alava A. Morphology and chaetotaxy of the legs of Drosophila melanogaster. J Morph. 1958;103:281–310. [Google Scholar]
- 16.Sturtevant AH. Studies on bristle pattern of Drosophila. Dev Biol. 1970;21:48-&. doi: 10.1016/0012-1606(70)90060-6. [DOI] [PubMed] [Google Scholar]
- 17.Thurm U. An insect mechanoreceptor. I Fine structure and adequate stimulus. Cold Spring Harb Symp Quant Biol. 1965;30:75–82. doi: 10.1101/sqb.1965.030.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Corfas G, Dudai Y. Adaptation and fatigue of a mechanosensory neuron in wild-type Drosophila and in memory mutants. J Neurosci. 1990;10:491–499. doi: 10.1523/JNEUROSCI.10-02-00491.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolbarsht ML. Electrical characteristics of insect mechanoreceptors. J Gen Physiol. 1960;44:105–122. doi: 10.1085/jgp.44.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurm U, Kuppers J. Epithelial physiology of insect sensilla. In: Locke M, Smith D, editors. Insect Biology in the Future. New York: Academic Press, Inc; 1980. pp. 735–763. [Google Scholar]
- 21.Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 22.Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- 23.Corey DP, Hudspeth AJ. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979;281:675–677. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- 24.Theis J. Mechanoreceptive bristles on the head of the blowfly: mechanics and electrophysiology of the macrochaetae. J Comp Physiol A. 1979;132:55–68. [Google Scholar]
- 25.Newland PL. Physiological properties of afferents from tactile hairs on the hindlegs of the locust. J Exp Biol. 1991;155:487–503. doi: 10.1242/jeb.155.1.487. [DOI] [PubMed] [Google Scholar]
- 26.Kanou M, Shimozawa T. A threshold analysis of cricket cercal interneurons by an alternating air-current stimulus. J Comp Physiol A. 1984;154:357–365. [Google Scholar]
- 27.Westin J. Responses to wind recorded from the cercal nerve of the cockroach Periplaneta americana 1 Response properties of single sensory neurons. J Comp Physiol A. 1979;133:97–102. [Google Scholar]
- 28.Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013;79:618–639. doi: 10.1016/j.neuron.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engel JE, Wu CF. Altered mechanoreceptor response in Drosophila bang-sensitive mutants. J Comp Physiol A. 1994;175:267–278. doi: 10.1007/BF00192986. [DOI] [PubMed] [Google Scholar]
- 30.Chadha A, Cook B. Dissection of gain control mechanisms in Drosophila mechanotransduction. J Neurosci. 2012;32:13052–13061. doi: 10.1523/JNEUROSCI.2171-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tiss Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- 32.Newland PL, Burrows M. Processing of mechanosensory information from gustatory receptors on a hind leg of the locust. J Comp Physiol A. 1994;174:399–410. doi: 10.1007/BF00191706. [DOI] [PubMed] [Google Scholar]
- 33.Vandervorst P, Ghysen A. Genetic control of sensory connections in Drosophila. Nature. 1980;286:65–67. doi: 10.1038/286065a0. [DOI] [PubMed] [Google Scholar]
- 34.Siegler MV, Burrows M. Receptive fields of motor neurons underlying local tactile reflexes in the locust. J Neurosci. 1986;6:507–513. doi: 10.1523/JNEUROSCI.06-02-00507.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corfas G, Dudai Y. Habituation and dishabituation of a cleaning reflex in normal and mutant Drosophila. J Neurosci. 1989;9:56–62. doi: 10.1523/JNEUROSCI.09-01-00056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seeds AM, Ravbar P, Chung P, Hampel S, Midgley FM, Jr, Mensh BD, Simpson JH. A suppression hierarchy among competing motor programs drives sequential grooming in Drosophila. eLife. 2014;3:e02951. doi: 10.7554/eLife.02951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berkowitz A, Laurent G. Central generation of grooming motor patterns and interlimb coordination in locusts. J Neurosci. 1996;16:8079–8091. doi: 10.1523/JNEUROSCI.16-24-08079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page KL, Matheson T. Wing hair sensilla underlying aimed hindleg scratching of the locust. J Exp Biol. 2004;207:2691–2703. doi: 10.1242/jeb.01096. [DOI] [PubMed] [Google Scholar]
- 39.Durr V, Matheson T. Graded limb targeting in an insect is caused by the shift of a single movement pattern. J Neurophysiol. 2003;90:1754–1765. doi: 10.1152/jn.00416.2003. [DOI] [PubMed] [Google Scholar]
- 40.Camhi JM. Locust wind receptors. I Transducer mechanics and sensory response. J Exp Biol. 1969;50:335-&. doi: 10.1242/jeb.50.2.335. [DOI] [PubMed] [Google Scholar]
- 41.Camhi JM. Locust wind receptors. III Contribution to flight initiation and lift control. J Exp Biol. 1969;50:363-&. doi: 10.1242/jeb.50.2.363. [DOI] [PubMed] [Google Scholar]
- 42.Jacobs GA, Miller JP, Aldworth Z. Computational mechanisms of mechanosensory processing in the cricket. J Exp Biol. 2008;211:1819–1828. doi: 10.1242/jeb.016402. [DOI] [PubMed] [Google Scholar]
- 43.Baba Y, Shimozawa T. Diversity of motor responses Initiated by a wind stimulus in the freely moving cricket, Gryllus bimaculatus. Zool Sci. 1997;14:587–594. [Google Scholar]
- 44.Pringle JWS. Proprioception in insects III. The function of the hair sensilla at the joints. J Exp Biol. 1938;15:467–473. [Google Scholar]
- 45.Okada J, Toh Y. The role of antennal hair plates in object-guided tactile orientation of the cockroach (Periplaneta americana) J Comp Physiol A. 2000;186:849–857. doi: 10.1007/s003590000137. [DOI] [PubMed] [Google Scholar]
- 46.Murphey RK, Possidente D, Pollack G, Merritt DJ. Modality-specific axonal projections in the CNS of the flies Phormia and Drosophila. J Comp Neurol. 1989;290:185–200. doi: 10.1002/cne.902900203. [DOI] [PubMed] [Google Scholar]
- 47.Merritt DJ, Murphey RK. Projections of leg proprioceptors within the CNS of the fly Phormia in relation to the generalized insect ganglion. J Comp Neurol. 1992;322:16–34. doi: 10.1002/cne.903220103. [DOI] [PubMed] [Google Scholar]
- 48.French AS, Wong RKS. The responses of trochanteral hair plate sensilla in the cockroach to periodic and random displacements. Biol Cyber. 1976;22:33–38. [Google Scholar]
- 49.Newland PL, Watkins B, Emptage NJ, Nagayama T. The structure, response properties and development of a hair plate on the mesothoracic leg of the locust. J Exp Biol. 1995;198:2397–2404. doi: 10.1242/jeb.198.11.2397. [DOI] [PubMed] [Google Scholar]
- 50.Pearson KG, Wong RKS, Fourtner CR. Connexions between hair-plate afferents and motoneurones in the cockroach leg. J Exp Biol. 1976;64:251–266. doi: 10.1242/jeb.64.1.251. [DOI] [PubMed] [Google Scholar]
- 51.Wong RKS, Pearson KG. Properties of the trochanteral hair plate and its function in the control of walking in the cockroach. J Exp Biol. 1976;64:233–249. doi: 10.1242/jeb.64.1.233. [DOI] [PubMed] [Google Scholar]
- 52.Paulk A, Gilbert C. Proprioceptive encoding of head position in the black soldier fly, Hermetia illucens (L.) (Stratiomyidae) J Exp Biol. 2006;209:3913–3924. doi: 10.1242/jeb.02438. [DOI] [PubMed] [Google Scholar]
- 53.Preuss T, Hengstenberg R. Structure and kinematics of the prosternal organs and their influence on head position in the blowfly Calliphora erythrocephala Meig. J Comp Physiol A. 1992;171:483–493. [Google Scholar]
- 54.Pringle JWS. Proprioception in insects I. A new type of mechanical receptor from the palps of the cockroach. J Exp Biol. 1938;15:101–113. [Google Scholar]
- 55.Spinola SM, Chapman KM. Proprioceptive indentation of the campaniform sensilla of cockroach legs. J Comp Physiol A. 1975;96:257–272. [Google Scholar]
- 56.Chapman KM, Duckrow RB, Moran DT. Form and role of deformation in excitation of an insect mechanoreceptor. Nature. 1973;244:453–454. doi: 10.1038/244453a0. [DOI] [PubMed] [Google Scholar]
- 57.Pringle JWS. Proprioception in insects II. The action of the campaniform sensilla on the legs. J Exp Biol. 1938;15:114–131. [Google Scholar]
- 58.Zill SN, Moran DT. The Exoskeleton and Insect Proprioception. I Responses of Tibial Campaniform Sensilla to External and Muscle-Generated Forces in the American Cockroach, Periplaneta americana. J Exp Biol. 1981;91:1–24. [Google Scholar]
- 59.Gnatzy W, Grunert U, Bender M. Campaniform sensilla of Calliphora vicina (Insecta, Diptera) II Topography. Zoomorph. 1987;106:312–319. [Google Scholar]
- 60.Grunert U, Gnatzy W. Campaniform sensilla of Calliphora vicina (Insecta, Diptera)II Typology. Zoomorph. 1987;106:320–328. [Google Scholar]
- 61.Cole ES, Palka J. The pattern of campaniform sensilla on the wing and haltere of Drosophila melanogaster and several of its homeotic mutants. J Embryol Exp Morphol. 1982;71:41–61. [PubMed] [Google Scholar]
- 62.Dickinson MH, Palka J. Physiological properties, time of development, and central projection are correlated in the wing mechanoreceptors of Drosophila. J Neurosci. 1987;7:4201–4208. doi: 10.1523/JNEUROSCI.07-12-04201.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zill SN, Schmitz J, Chaudhry S, Buschges A. Force encoding in stick insect legs delineates a reference frame for motor control. J Neurophysiol. 2012;108:1453–1472. doi: 10.1152/jn.00274.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zill SN, Chaudhry S, Buschges A, Schmitz J. Directional specificity and encoding of muscle forces and loads by stick insect tibial campaniform sensilla, including receptors with round cuticular caps. Arthropod Struct Dev. 2013;42:455–467. doi: 10.1016/j.asd.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 65.Ridgel AL, Frazier SF, Zill SN. Dynamic responses of tibial campaniform sensilla studied by substrate displacement in freely moving cockroaches. J Comp Physiol A. 2001;187:405–420. doi: 10.1007/s003590100213. [DOI] [PubMed] [Google Scholar]
- 66.Zill S, Schmitz J, Buschges A. Load sensing and control of posture and locomotion. Arthropod Struct Dev. 2004;33:273–286. doi: 10.1016/j.asd.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Akay T, Haehn S, Schmitz J, Buschges A. Signals from load sensors underlie interjoint coordination during stepping movements of the stick insect leg. J Neurophysiol. 2004;92:42–51. doi: 10.1152/jn.01271.2003. [DOI] [PubMed] [Google Scholar]
- 68.Borgmann A, Hooper SL, Buschges A. Sensory feedback induced by front-leg stepping entrains the activity of central pattern generators in caudal segments of the stick insect walking system. J Neurosci. 2009;29:2972–2983. doi: 10.1523/JNEUROSCI.3155-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pringle JWS. The Gyroscopic Mechanism of the Halteres of Diptera. Philos Trans R Soc Lond B-Biol Sci. 1948;233:347–384. [Google Scholar]
- 70.Elson RC. Interneuronal processing of inputs from the campaniform sensilla of the locust hindwing. J Comp Physiol A. 1987;161:761–776. [Google Scholar]
- 71.Dickerson BH, Aldworth ZN, Daniel TL. Control of moth flight posture is mediated by wing mechanosensory feedback. J Exp Biol. 2014;217:2301–2308. doi: 10.1242/jeb.103770. [DOI] [PubMed] [Google Scholar]
- 72.Dickinson MH. Comparison of encoding properties of campaniform sensilla on the fly wing. J Exp Biol. 1990;151:245–261. [Google Scholar]
- 73.Dickinson MH. Haltere-mediated equilibrium reflexes of the fruit fly, Drosophila melanogaster. Philos Trans R Soc Lond B-Biol Sci. 1999;354:903–916. doi: 10.1098/rstb.1999.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fayyazuddin A, Dickinson MH. Haltere afferents provide direct, electrotonic input to a steering motor neuron in the blowfly, Calliphora. J Neurosci. 1996;16:5225–5232. doi: 10.1523/JNEUROSCI.16-16-05225.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fayyazuddin A, Dickinson MH. Convergent mechanosensory input structures the firing phase of a steering motor neuron in the blowfly, Calliphora. J Neurophysiol. 1999;82:1916–1926. doi: 10.1152/jn.1999.82.4.1916. [DOI] [PubMed] [Google Scholar]
- 76.Fox JL, Fairhall AL, Daniel TL. Encoding properties of haltere neurons enable motion feature detection in a biological gyroscope. Proc Natl Acad Sci USA. 2010;107:3840–3845. doi: 10.1073/pnas.0912548107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Field LH, Pfluger HJ. The femoral chordotonal organ: A bifunctional orthopteran (Locusta migratoria) sense organ? Comp Biochem Physiol. 1989;93:729–743. [Google Scholar]
- 78.Mendes CS, Bartos I, Akay T, Marka S, Mann RS. Quantification of gait parameters in freely walking wild type and sensory deprived Drosophila melanogaster. eLife. 2013;2:e00231. doi: 10.7554/eLife.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mendes CS, Rajendren SV, Bartos I, Marka S, Mann RS. Kinematic responses to changes in walking orientation and gravitational load in Drosophila melanogaster. PloS one. 2014;9:e109204. doi: 10.1371/journal.pone.0109204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Akitake B, Ren Q, Boiko N, Ni J, Sokabe T, Stockand JD, Eaton BA, Montell C. Coordination and fine motor control depend on Drosophila TRPγ. Nature communications. 2015;6 doi: 10.1038/ncomms8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng LE, Song W, Looger LL, Jan LY, Jan YN. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron. 2010;67:373–380. doi: 10.1016/j.neuron.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Isakov A, Buchanan SM, Sullivan B, Ramachandran A, Chapman JK, Lu ES, Mahadevan L, de Bivort B. Recovery of locomotion after injury in Drosophila depends on proprioception. J Exp Biol. 2016;219:1760–1771. doi: 10.1242/jeb.133652. [DOI] [PubMed] [Google Scholar]
- 83.Field LH, Matheson T. Chordotonal Organs of Insects. In: Evans PD, editor. Advances in Insect Physiology. Vol. 27. San Diego: Academic Press Inc; 1998. pp. 1–228. [Google Scholar]
- 84.Matheson T. Responses and locations of neurones in the locust metathoracic femoral chordotonal organ. J Comp Physiol A. 1990;166:915–927. [Google Scholar]
- 85.Matheson T, Field LH. Innervation of the metathoracic femoral chordotonal organ of Locusta migratoria. Cell Tissue Res. 1990;259:551–560. [Google Scholar]
- 86.Kittmann R, Schmitz J. Functional specialization of the scoloparia of the femoral chordotonal organ in stick insects. J Exp Biol. 1992;173:91–108. [Google Scholar]
- 87.Shanbhag SR, Singh K, Naresh Singh R. Ultrastructure of the femoral chordotonal organs and their novel synaptic organization in the legs of Drosophila melanogaster Meigen (Diptera : Drosophilidae) Int J Insect Morphol Embyol. 1992;21:311–322. [Google Scholar]
- 88.Stein W, Sauer AE. Physiology of vibration-sensitive afferents in the femoral chordotonal organ of the stick insect. J Comp Physiol A. 1999;184:253–263. [Google Scholar]
- 89.Sauer EA, Stein W. Sensorimotor pathways processing vibratory signals from the femoral chordotonal organ of the stick insect. J Comp Physiol A. 1999;185:21–31. [Google Scholar]
- 90.Buschges A. The physiology of sensory cells in the ventral scoloparium of the stick insect femoral chordotonal organ. J Exp Biol. 1994;189:285–292. doi: 10.1242/jeb.189.1.285. [DOI] [PubMed] [Google Scholar]
- 91.Zill SN. Plasticity and proprioception in insects. I Responses and cellular properties of individual receptors of the locust metathoracic femoral chordotonal organ. J Exp Biol. 1985;116:435–461. doi: 10.1242/jeb.116.1.435. [DOI] [PubMed] [Google Scholar]
- 92.Matheson T. Range fractionation in the locust metathoracic femoral chordotonal organ. J Comp Physiol A. 1992;170:509–520. [Google Scholar]
- 93.Hofmann T, Koch UT. Acceleration receptors in the femoral chordotonal organ of the stick insect, Cuniculina impigra. J Exp Biol. 1985;114:225–237. [Google Scholar]
- 94.Hofmann T, Koch UT, Bassler U. Physiology of the femoral chordotonal organ in the stick insect, Cuniculina impigra. J Exp Biol. 1985;114:207–223. [Google Scholar]
- 95.Kondoh Y, Okuma J, Newland PL. Dynamics of neurons controlling movements of a locust hind leg: Wiener kernel analysis of the responses of proprioceptive afferents. J Neurophysiol. 1995;73:1829–1842. doi: 10.1152/jn.1995.73.5.1829. [DOI] [PubMed] [Google Scholar]
- 96.Zill SN, Jepson-Innes K. Evolutionary adaptation of a reflex system: sensory hysteresis counters muscle ‘catch’ tension. J Comp Physiol A. 1988;164:43–48. doi: 10.1007/BF00612716. [DOI] [PubMed] [Google Scholar]
- 97.Field LH, Coles MML. The position-dependent nature of postural resistance reflexes in the locust. J Exp Biol. 1994;188:65–88. doi: 10.1242/jeb.188.1.65. [DOI] [PubMed] [Google Scholar]
- 98.Field LH, Burrows M. Reflex Effects of the femoral chordotonal organ upon leg motor neurones of the locust. J Exp Biol. 1982;101:265–285. [Google Scholar]
- 99.Wolf H, Burrows M. Proprioceptive sensory neurons of a locust leg receive rhythmic presynaptic inhibition during walking. J Neurosci. 1995;15:5623–5636. doi: 10.1523/JNEUROSCI.15-08-05623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Burrows M, Laurent G. Synaptic potentials in the central terminals of locust proprioceptive afferents generated by other afferents from the same sense organ. J Neurosci. 1993;13:808–819. doi: 10.1523/JNEUROSCI.13-02-00808.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stein W, Schmitz J. Multimodal convergence of presynaptic afferent inhibition in insect proprioceptors. J Neurophysiol. 1999;82:512–514. doi: 10.1152/jn.1999.82.1.512. [DOI] [PubMed] [Google Scholar]
- 102.Sauer AE, Buschges A, Stein W. Role of presynaptic inputs to proprioceptive afferents in tuning sensorimotor pathways of an insect joint control network. J Neurobiol. 1997;32:359–376. doi: 10.1002/(sici)1097-4695(199704)32:4<359::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 103.Burrows M, Matheson T. A presynaptic gain control mechanism among sensory neurons of a locust leg proprioceptor. J Neurosci. 1994;14:272–282. doi: 10.1523/JNEUROSCI.14-01-00272.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goulding M, Bourane S, Garcia-Campmany L, Dalet A, Koch S. Inhibition downunder: an update from the spinal cord. Curr Opin Neurobiol. 2014;26:161–166. doi: 10.1016/j.conb.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramirez JM, Buschges A, Kittmann R. Octopaminergic modulation of the femoral chordotonal organ in the stick insect. J Comp Physiol A. 1993;173:209–219. [Google Scholar]
- 106.Matheson T. Octopamine modulates the responses and presynaptic inhibition of proprioceptive sensory neurones in the locust Schistocerca gregaria. J Exp Biol. 1997;200:1317–1325. doi: 10.1242/jeb.200.9.1317. [DOI] [PubMed] [Google Scholar]
- 107.Kamikouchi A, Shimada T, Ito K. Comprehensive classification of the auditory sensory projections in the brain of the fruit fly Drosophila melanogaster. J Comp Neurol. 2006;499:317–356. doi: 10.1002/cne.21075. [DOI] [PubMed] [Google Scholar]
- 108.Matsuo E, Kamikouchi A. Neuronal encoding of sound, gravity, and wind in the fruit fly. J Comp Physiol A. 2013;199:253–262. doi: 10.1007/s00359-013-0806-x. [DOI] [PubMed] [Google Scholar]
- 109.Murthy M. Unraveling the auditory system of Drosophila. Curr Opin Neurobiol. 2010;20:281–287. doi: 10.1016/j.conb.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 110.Yorozu S, Wong A, Fischer BJ, Dankert H, Kernan MJ, Kamikouchi A, Ito K, Anderson DJ. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature. 2009;458:201–205. doi: 10.1038/nature07843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mamiya A, Dickinson MH. Antennal mechanosensory neurons mediate wing motor reflexes in flying Drosophila. J Neurosci. 2015;35:7977–7991. doi: 10.1523/JNEUROSCI.0034-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Gopfert MC, Ito K. The neural basis of Drosophila gravity-sensing and hearing. Nature. 2009;458:165–171. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- 113.Lehnert BP, Baker AE, Gaudry Q, Chiang AS, Wilson RI. Distinct roles of TRP channels in auditory transduction and amplification in Drosophila. Neuron. 2013;77:115–128. doi: 10.1016/j.neuron.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hoy RR, Robert D. Tympanal hearing in insects. Annual review of entomology. 1996;41:433–450. doi: 10.1146/annurev.en.41.010196.002245. [DOI] [PubMed] [Google Scholar]
- 115.Shaw S. Detection of airborne sound by a cockroach ‘vibration detector’: a possible missing link in insect auditory evolution. J Exp Biol. 1994;193:13–47. doi: 10.1242/jeb.193.1.13. [DOI] [PubMed] [Google Scholar]
- 116.Moran DT, Rowley JC. The fine structure of the cockroach subgenual organ. Tissue Cell. 1975;7:91–105. doi: 10.1016/s0040-8166(75)80009-7. [DOI] [PubMed] [Google Scholar]
- 117.Fabre CC, Hedwig B, Conduit G, Lawrence PA, Goodwin SF, Casal J. Substrate-borne vibratory communication during courtship in Drosophila melanogaster. Curr Biol. 2012;22:2180–2185. doi: 10.1016/j.cub.2012.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bodmer R, Jan YN. Morphological differentiation of the embryonic peripheral neurons in Drosophila. Roux’s Arch Dev Biol. 1987;196:69–77. doi: 10.1007/BF00402027. [DOI] [PubMed] [Google Scholar]
- 119.Grueber WB, Ye B, Moore AW, Jan LY, Jan YN. Dendrites of distinct classes of Drosophila sensory neurons show different capacities for homotypic repulsion. Curr Biol. 2003;13:618–626. doi: 10.1016/s0960-9822(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 120.Shimono K, Fujimoto A, Tsuyama T, Yamamoto-Kochi M, Sato M, Hattori Y, Sugimura K, Usui T, Kimura K, Uemura T. Multidendritic sensory neurons in the adult Drosophila abdomen: origins, dendritic morphology, and segment- and age-dependent programmed cell death. Neural Dev. 2009;4:37. doi: 10.1186/1749-8104-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pearson KG, Ramirez JM. Influence of input from the forewing stretch receptors on motoneurones in flying locusts. J Exp Biol. 1990;151:317–340. [Google Scholar]
- 122.Burrows M. Monosynaptic connexions between wing stretch receptors and flight motoneurones of the locust. J Exp Biol. 1975;62:189–219. doi: 10.1242/jeb.62.1.189. [DOI] [PubMed] [Google Scholar]
- 123.Frye MA. Encoding properties of the wing hinge stretch receptor in the hawkmoth Manduca sexta. J Exp Biol. 2001;204:3693–3702. doi: 10.1242/jeb.204.21.3693. [DOI] [PubMed] [Google Scholar]
- 124.Frye MA. Effects of stretch receptor ablation on the optomotor control of lift in the hawkmoth Manduca sexta. J Exp Biol. 2001;204:3683–3691. doi: 10.1242/jeb.204.21.3683. [DOI] [PubMed] [Google Scholar]
- 125.Gelperin A. Stretch receptors in the foregut of the blowfly. Science. 1967;157:208–210. doi: 10.1126/science.157.3785.208. [DOI] [PubMed] [Google Scholar]
- 126.Smith SA, Shepherd D. Central afferent projections of proprioceptive sensory neurons in Drosophila revealed with the enhancer-trap technique. J Comp Neurol. 1996;364:311–323. doi: 10.1002/(SICI)1096-9861(19960108)364:2<311::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 127.Blagburn J. Engrailed expression in subsets of adult Drosophila sensory neurons: an enhancer-trap study. Invert Neurosci. 2008;8:133–146. doi: 10.1007/s10158-008-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Desai BS, Chadha A, Cook B. The stum gene is essential for mechanical sensing in proprioceptive neurons. Science. 2014;343:1256–1259. doi: 10.1126/science.1247761. [DOI] [PubMed] [Google Scholar]
- 129.Braunig P. Strand receptors with central cell bodies in the proximal leg joints of orthopterous insects. Cell Tiss Res. 1982;222:647–654. doi: 10.1007/BF00213862. [DOI] [PubMed] [Google Scholar]
- 130.Braunig P, Hustert R. Proprioceptors with central cell bodies in insects. Nature. 1980;283:768–770. [Google Scholar]
- 131.Braunig P. Strand receptors associated with the femoral chordotonal organs of locust legs. J Exp Biol. 1985;116:331–341. [Google Scholar]
- 132.Braunig P. The peripheral and central nervous organization of the locust coxo-trochanteral joint. J Neurobiol. 1982;13:413–433. doi: 10.1002/neu.480130504. [DOI] [PubMed] [Google Scholar]
- 133.Braunig P, Hustert R. Actions and interactions of proprioceptors of the locust hind leg coxo-trochanteral joint. J Comp Physiol A. 1985;157:73–82. [Google Scholar]
- 134.Theophilidis G, Burns MD. A muscle tension receptor in the locust leg. J Comp Physiol A. 1979;131:247–254. [Google Scholar]
- 135.Wanischeck M, Rose U. Unusual tension reception in an insect. J Neurobiol. 2005;65:115–124. doi: 10.1002/neu.20180. [DOI] [PubMed] [Google Scholar]
- 136.Matheson T, Field L. An elaborate tension receptor system highlights sensory complexity in the hind leg of the locust. J Exp Biol. 1995;198:1673–1689. doi: 10.1242/jeb.198.8.1673. [DOI] [PubMed] [Google Scholar]
- 137.Murphey RK, Possidente DR, Vandervorst P, Ghysen A. Compartments and the topography of leg afferent projections in Drosophila. J Neurosci. 1989;9:3209–3217. doi: 10.1523/JNEUROSCI.09-09-03209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Newland PL. Morphology and somatotopic organisation of the central projections of afferents from tactile hairs on the hind leg of the locust. J Comp Neurol. 1991;312:493–508. doi: 10.1002/cne.903120402. [DOI] [PubMed] [Google Scholar]
- 139.Collin SP. The central morphology of mechanoreceptor afferents in the metathoracic leg of the cockroach, Periplaneta americana (Insecta) J Neurobiol. 1985;16:269–282. doi: 10.1002/neu.480160403. [DOI] [PubMed] [Google Scholar]
- 140.Johnson SE, Murphey RK. The afferent projection of mesothoracic bristle hairs in the cricket, Acheta domesticus. J Comp Physiol A. 1985;156:369–379. [Google Scholar]
- 141.Brown AG, Rose PK, Snow PJ. The morphology of hair follicle afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1977;272:779–797. doi: 10.1113/jphysiol.1977.sp012073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Erzurumlu RS, Murakami Y, Rijli FM. Mapping the face in the somatosensory brainstem. Nat Rev Neurosci. 2010;11:252–263. doi: 10.1038/nrn2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brierley DJ, Rathore K, VijayRaghavan K, Williams DW. Developmental origins and architecture of Drosophila leg motoneurons. J Comp Neurol. 2012;520:1629–1649. doi: 10.1002/cne.23003. [DOI] [PubMed] [Google Scholar]
- 144.Baek M, Mann RS. Lineage and birth date specify motor neuron targeting and dendritic architecture in adult Drosophila. J Neurosci. 2009;29:6904–6916. doi: 10.1523/JNEUROSCI.1585-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Burrows M. Neurobiology of an Insect Brain. Oxford: Oxford University Press; 1996. [Google Scholar]
- 146.Oldfield BP. Central projections of primary auditory fibres in Tettigoniidae (Orthoptera: Ensifera) J Comp Physiol A. 1983;151:389–395. [Google Scholar]
- 147.Stumpner A. Tonotopic organization of the hearing organ in a bushcricket. Naturwissenschaften. 1996;83:81–84. [Google Scholar]
- 148.Romer H. Tonotopic organization of the auditory neuropile in the bushcricket Tettigonia viridissima. Nature. 1983;306:60–62. [Google Scholar]
- 149.Matheson T. Morphology of the central projections of physiologically characterized neurons from the locust metathoracic femoral chordotonal organ. J Comp Physiol A. 1992;170:101–120. [Google Scholar]
- 150.Matsuo E, Yamada D, Ishikawa Y, Asai T, Ishimoto H, Kamikouchi A. Identification of novel vibration- and deflection-sensitive neuronal subgroups in Johnston’s organ of the fruit fly. Front Physiol. 2014;5:179. doi: 10.3389/fphys.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pfluger H-J, Wolf H. Developmental and activity-dependent plasticity of filiform hair receptors in the locust. Frontiers in Physiology. 2013;4 doi: 10.3389/fphys.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pfluger HJ, Hurdelbrink S, Czjzek A, Burrows M. Activity-dependent structural dynamics of insect sensory fibers. J Neurosci. 1994;14:6946–6955. doi: 10.1523/JNEUROSCI.14-11-06946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Urwyler O, Izadifar A, Dascenco D, Petrovic M, He H, Ayaz D, Kremer A, Lippens S, Baatsen P, Guerin CJ, et al. Investigating CNS synaptogenesis at single-synapse resolution by combining reverse genetics with correlative light and electron microscopy. Development. 2015;142:394–405. doi: 10.1242/dev.115071. [DOI] [PubMed] [Google Scholar]
- 154.Honegger HW. Interommatidial hair receptor axons extending into the ventral nerve cord in the cricket Gryllus campestris. Cell Tiss Res. 1977;182:281–285. doi: 10.1007/BF00220597. [DOI] [PubMed] [Google Scholar]
- 155.Tyrer NM, Bacon JP, Davies CA. Sensory projections from the wind-sensitive head hairs of the locust Schistocerca gregaria Distribution in the central nervous system. Cell Tiss Res. 1979;203:79–92. doi: 10.1007/BF00234330. [DOI] [PubMed] [Google Scholar]
- 156.Nassel DR, Hogmo O, Hallberg E. Antennal receptors in the blowfly Calliphora erythrocephala I The gigantic central projection of the pedicellar campaniform sensillum. J Morph. 1984;180:159–169. doi: 10.1002/jmor.1051800206. [DOI] [PubMed] [Google Scholar]
- 157.Bassler U, Buschges A. Pattern generation for stick insect walking movements - multisensory control of a locomotor program. Brain Research Reviews. 1998;27:65–88. doi: 10.1016/s0165-0173(98)00006-x. [DOI] [PubMed] [Google Scholar]
- 158.Dickinson MH, Farley CT, Full RJ, Koehl MA, Kram R, Lehman S. How animals move: an integrative view. Science. 2000;288:100–106. doi: 10.1126/science.288.5463.100. [DOI] [PubMed] [Google Scholar]
- 159.Buschges A, Gruhn M. Mechanosensory Feedback in Walking: From Joint Control to Locomotor Patterns. In: Casas J, Simpson SJ, editors. Advances in Insect Physiology. Vol. 34. Academic Press; 2007. pp. 193–230. [Google Scholar]
- 160.Ayali A, Couzin-Fuchs E, David I, Gal O, Holmes P, Knebel D. Sensory feedback in cockroach locomotion: current knowledge and open questions. J Comp Physiol A. 2015:1–10. doi: 10.1007/s00359-014-0968-1. [DOI] [PubMed] [Google Scholar]
- 161.Borgmann A, Buschges A. Insect motor control: methodological advances, descending control and inter-leg coordination on the move. Curr Opin Neurobiol. 2015;33:8–15. doi: 10.1016/j.conb.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 162.Ritzmann RE, Buschges A. Adaptive motor behavior in insects. Curr Opin Neurobiol. 2007;17:629–636. doi: 10.1016/j.conb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 163.Ache JM, Matheson T. Passive joint forces are tuned to limb use in insects and drive movements without motor activity. Curr Biol. 2013;23:1418–1426. doi: 10.1016/j.cub.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jindrich DL, Full RJ. Dynamic stabilization of rapid hexapedal locomotion. J Exp Biol. 2002;205:2803–2823. doi: 10.1242/jeb.205.18.2803. [DOI] [PubMed] [Google Scholar]
- 165.Burrows M. Parallel processing of proprioceptive signals by spiking local interneurons and motor neurons in the locust. J Neurosci. 1987;7:1064–1080. doi: 10.1523/JNEUROSCI.07-04-01064.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tuthill JC, Wilson RI. Parallel transformation of tactile signals in central circuits of Drosophila. Cell. 2016;164:1046–1059. doi: 10.1016/j.cell.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dickinson MH. The initiation and control of rapid flight maneuvers in fruit flies. Integr Comp Biol. 2005;45:274–281. doi: 10.1093/icb/45.2.274. [DOI] [PubMed] [Google Scholar]
- 168.Buschges A. Sensory control and organization of neural networks mediating coordination of multisegmental organs for locomotion. J Neurophysiol. 2005;93:1127–1135. doi: 10.1152/jn.00615.2004. [DOI] [PubMed] [Google Scholar]
- 169.Holtje M, Hustert R. Rapid mechano-sensory pathways code leg impact and elicit very rapid reflexes in insects. J Exp Biol. 2003;206:2715–2724. doi: 10.1242/jeb.00492. [DOI] [PubMed] [Google Scholar]
- 170.Berg E, Buschges A, Schmidt J. Single perturbations cause sustained changes in searching behavior in stick insects. J Exp Biol. 2013;216:1064–1074. doi: 10.1242/jeb.076406. [DOI] [PubMed] [Google Scholar]
- 171.Berg EM, Hooper SL, Schmidt J, Buschges A. A leg-local neural mechanism mediates the decision to search in stick insects. Curr Biol. 2015;25:2012–2017. doi: 10.1016/j.cub.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 172.Blasing B, Cruse H. Stick insect locomotion in a complex environment: climbing over large gaps. J Exp Biol. 2004;207:1273–1286. doi: 10.1242/jeb.00888. [DOI] [PubMed] [Google Scholar]
- 173.Harley CM, English BA, Ritzmann RE. Characterization of obstacle negotiation behaviors in the cockroach, Blaberus discoidalis. J Exp Biol. 2009;212:1463–1476. doi: 10.1242/jeb.028381. [DOI] [PubMed] [Google Scholar]
- 174.Bender JA, Dickinson MH. Visual stimulation of saccades in magnetically tethered Drosophila. J Exp Biol. 2006;209:3170–3182. doi: 10.1242/jeb.02369. [DOI] [PubMed] [Google Scholar]
- 175.Sherman A, Dickinson MH. A comparison of visual and haltere-mediated equilibrium reflexes in the fruit fly Drosophila melanogaster. J Exp Biol. 2003;206:295–302. doi: 10.1242/jeb.00075. [DOI] [PubMed] [Google Scholar]
- 176.Sherman A, Dickinson MH. Summation of visual and mechanosensory feedback in Drosophila flight control. J Exp Biol. 2004;207:133–142. doi: 10.1242/jeb.00731. [DOI] [PubMed] [Google Scholar]
- 177.Chan WP, Dickinson MH. Position-specific central projections of mechanosensory neurons on the haltere of the blow fly, Calliphora vicina. J Comp Neurol. 1996;369:405–418. doi: 10.1002/(SICI)1096-9861(19960603)369:3<405::AID-CNE6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 178.Ting LH. Dimensional reduction in sensorimotor systems: a framework for understanding muscle coordination of posture. Prog Brain Res. 2007;165:299–321. doi: 10.1016/S0079-6123(06)65019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tresch MC, Jarc A. The case for and against muscle synergies. Curr Opin Neurobiol. 2009;19:601–607. doi: 10.1016/j.conb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zill SN, Chaudhry S, Buschges A, Schmitz J. Force feedback reinforces muscle synergies in insect legs. Arthropod Struct Dev. 2015 doi: 10.1016/j.asd.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 181.Zill SN, Chaudhry S, Exter A, Buschges A, Schmitz J. Positive force feedback in development of substrate grip in the stick insect tarsus. Arthropod Struct Dev. 2014;43:441–455. doi: 10.1016/j.asd.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 182.Zill SN, Keller BR, Chaudhry S, Duke ER, Neff D, Quinn R, Flannigan C. Detecting substrate engagement: responses of tarsal campaniform sensilla in cockroaches. J Comp Physiol A. 2010;196:407–420. doi: 10.1007/s00359-010-0526-4. [DOI] [PubMed] [Google Scholar]
- 183.Wolf H. Inhibitory motoneurons in arthropod motor control: organisation, function, evolution. J Comp Physiol A. 2014;200:693–710. doi: 10.1007/s00359-014-0922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Clarac F, Cattaert D, Le Ray D. Central control components of a ‘simple’ stretch reflex. Trends Neurosci. 2000;23:199–208. doi: 10.1016/s0166-2236(99)01535-0. [DOI] [PubMed] [Google Scholar]
- 185.Bassler U. Reversal of a reflex to a single motoneuron in the stick insect Çarausius morosus. Biol Cyber. 1976;24:47–49. [Google Scholar]
- 186.Hellekes K, Blincow E, Hoffmann J, Buschges A. Control of reflex reversal in stick insect walking: effects of intersegmental signals, changes in direction, and optomotor-induced turning. J Neurophysiol. 2012;107:239–249. doi: 10.1152/jn.00718.2011. [DOI] [PubMed] [Google Scholar]
- 187.Mu L, Ritzmann RE. Interaction between descending input and thoracic reflexes for joint coordination in cockroach: I. descending influence on thoracic sensory reflexes. J Comp Physiol A. 2008;194:283–298. doi: 10.1007/s00359-007-0307-x. [DOI] [PubMed] [Google Scholar]
- 188.Szczecinski NS, Brown AE, Bender JA, Quinn RD, Ritzmann RE. A neuromechanical simulation of insect walking and transition to turning of the cockroach Blaberus discoidalis. Biol Cyber. 2014;108:1–21. doi: 10.1007/s00422-013-0573-3. [DOI] [PubMed] [Google Scholar]
- 189.Buschges A. Nonspiking pathways in a joint-control loop of the stick insect Carausius morosus. J Exp Biol. 1990;151:133–160. [Google Scholar]
- 190.Buschges A. Processing of sensory input from the femoral chordotonal organ by spiking interneurones of stick insects. J Exp Biol. 1989;144:81–111. [Google Scholar]
- 191.Driesang RB, Buschges A. Physiological changes in central neuronal pathways contributing to the generation of a reflex reversal. J Comp Physiol A. 1996;179:45–57. [Google Scholar]
- 192.Martin JP, Guo P, Mu L, Harley CM, Ritzmann RE. Central-complex control of movement in the freely walking cockroach. Curr Biol. 2015;25:2795–2803. doi: 10.1016/j.cub.2015.09.044. [DOI] [PubMed] [Google Scholar]
- 193.Daltorio KA, Mirletz BT, Sterenstein A, Cheng JC, Watson A, Kesavan M, Bender JA, Martin J, Ritzmann RE, Quinn RD. How cockroaches exploit tactile boundaries to find new shelters. Bioinspir Biomim. 2015;10:065002. doi: 10.1088/1748-3190/10/6/065002. [DOI] [PubMed] [Google Scholar]
- 194.Edelman S, Poggio T. Models of object recognition. Curr Opin Neurobiol. 1991;1:270–273. doi: 10.1016/0959-4388(91)90089-p. [DOI] [PubMed] [Google Scholar]
- 195.Yamins DL, DiCarlo JJ. Using goal-driven deep learning models to understand sensory cortex. Nat Neurosci. 2016;19:356–365. doi: 10.1038/nn.4244. [DOI] [PubMed] [Google Scholar]
- 196.Jenett A, Rubin GM, Ngo TT, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bidaye SS, Machacek C, Wu Y, Dickson BJ. Neuronal control of Drosophila walking direction. Science. 2014;344:97–101. doi: 10.1126/science.1249964. [DOI] [PubMed] [Google Scholar]
- 198.Mann K, Gordon MD, Scott K. A pair of interneurons influences the choice between feeding and locomotion in Drosophila. Neuron. 2013;79:754–765. doi: 10.1016/j.neuron.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Korff W, Namiki S, Rowell W, Dickinson M, Card GM. Functional dissection and organization of descending interneurons during flight control in Drosophila. International Society for Neuroethology, 12th Congress; Uruguay. 2016. [Google Scholar]
- 200.Ache JM, Namiki S, Lee A, Branson K, Card GM. Descending control of visually-evoked landing in Drosophila. International Society for Neuroethology, 12th Congress; Uruguay. 2016. [Google Scholar]
- 201.Cande J, Berman GJ, Korff W, Card G, Shaevitz J, Stern D. Optogenetic dissection of descending behavioral control in Drosophila. Cosyne Abstracts; Salt Lake City, USA. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kazama H. Systems neuroscience in Drosophila: Conceptual and technical advantages. Neuroscience. 2015;296:3–14. doi: 10.1016/j.neuroscience.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 203.Seelig JD, Jayaraman V. Studying sensorimotor processing with physiology in behaving Drosophila. Int Rev Neurobiol. 2011;99:169–189. doi: 10.1016/B978-0-12-387003-2.00007-0. [DOI] [PubMed] [Google Scholar]
- 204.Plaza SM, Scheffer LK, Chklovskii DB. Toward large-scale connectome reconstructions. Curr Opin Neurobiol. 2014;25:201–210. doi: 10.1016/j.conb.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 205.Takemura SY, Xu CS, Lu Z, Rivlin PK, Parag T, Olbris DJ, Plaza S, Zhao T, Katz WT, Umayam L, et al. Synaptic circuits and their variations within different columns in the visual system of Drosophila. Proc Natl Acad Sci USA. 2015;112:13711–13716. doi: 10.1073/pnas.1509820112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Costa M, Manton JD, Ostrovsky AD, Prohaska S, Jefferis GSXE. NBLAST: Rapid, sensitive comparison of neuronal structure and construction of neuron family databases. 2016 doi: 10.1016/j.neuron.2016.06.012. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]