Abstract
Background
At our institution, until April 2013, patients who showed early donor specific anti-HLA antibodies (DSA) after lung transplantation were preemptively treated with therapeutic plasma exchange (tPE) and a single dose of Rituximab. In April 2013, we moved to a therapy based on IgM-enriched human immunoglobulins (IVIG), repeated every 4 weeks, and a single dose of Rituximab.
Methods
This observational study was designed to evaluate the short-term patient and graft survival in patients who underwent IVIG-based DSA treatment (group A, n = 57) versus contemporary patients transplanted between April 2013 and January 2015 without DSA (group C, n = 180), as well as to evaluate DSA clearance in IVIG-treated patients versus historic patients who had undergone tPE-based treatment (group B, n = 56). Patient records were retrospectively reviewed. Follow-up ended on April 1, 2015.
Results
At 6 months and 1 year of follow-up, group A had a survival similar to group C (P = 0.81) but better than group B (P = 0.008). Group A showed statistically nonsignificant trends toward improved freedom from pulsed-steroid therapy and biopsy-confirmed rejection over groups B and C. The DSA clearance was better in group A than group B at treatment end (92% vs 64%; P = 0.002) and last DSA control (90% vs 75%; P = 0.04).
Conclusions
Patients with new early DSA but without graft dysfunction that are treated with IVIG and Rituximab have similarly good early survival as contemporary lung transplant recipients without early DSA. The IVIG yielded increased DSA clearance compared with historic tPE-based treatment, yet spontaneous clearance of new DSA also remains common.
Combination treatment of IgM enriched human immunoglobulins (IVIG) and a single dose of rituximab significantly reduced the incidence of de novo DSA production after lung transplantation compared to historical therapeutic plasma exchange and a single dose of rituximab. Supplemental digital content is available in the text.
In lung transplantation (LTX), treatment of donor-specific anti-HLA antibodies (DSA) appears justified, because DSA are risk factors for mortality and acute and chronic graft rejection.1-9 The DSA treatment protocols have usually been borrowed from other solid organ transplantations.10-19 However, overall experience with DSA treatment in LTX is scarce, and the available retrospective case series include limited numbers of patients. No randomized trials have been conducted. Therefore, the efficacy of DSA treatment remains controversial.20
At our institution, until April 2013, patients who developed DSA early after transplantation were treated with therapeutic plasma exchange (tPE) and Rituximab.18 In April 2013, we moved to an IgM-enriched IVIG (Pentaglobin; Biotest Pharma GmbH, Germany)–based treatment combined with Rituximab. In comparison to tPE, IVIG treatment is less invasive, because it does not require placement of a dialysis catheter and use of an extracorporeal blood pump. Moreover, it can be performed more easily in an outpatient setting and repeated at follow-up.
We designed a retrospective observational study to evaluate the IVIG-based treatment of early DSA in 2 ways. First, we compared patient and graft survival between DSA patients who were treated with IVIG and contemporary patients who were transplanted between April 2013 and January 2015 and did not develop early DSA. Second, we compared outcomes and efficacy in clearing early DSA between the IVIG-based and historic, tPE-based protocols.
MATERIALS AND METHODS
Patients
A retrospective observational study was performed in a single university center to evaluate the efficacy and safety of an IVIG-based treatment protocol of early DSA.
Three patient groups were defined. Patients who underwent LTX between April 2013 and January 2015, developed early DSA and underwent IVIG-based treatment, formed group A. Patients who showed de novo DSA or a positive complement-dependent cytotoxicity (CDC) crossmatch were included in group A. Instead, patients, who were transplanted during the same period, developed early DSA but were not treated, were excluded.
The DSA clearance in group A patients was compared with the DSA clearance in historic patients transplanted between January 2009 and June 2013 who developed early DSA or showed a positive CDC crossmatch but were treated with tPE. These patients formed group B.
Outcomes of group A patients were compared with the outcomes of the remaining contemporary patients who were transplanted between April 2013 and January 2015 but did not develop early DSA. These patients formed group C and served as the control group.
All patients received a single dose of anticytomegalovirus (CMV)-enriched human immunoglobulins immediately upon arrival at the intensive care unit (ICU), without any induction therapy. Posttransplant immunosuppressive therapy was based on a triple therapy (calcineurin inhibitor, mycophenolate mofetil, and prednisolone). Before January 2013, most patients received cyclosporine as first-line calcineurin inhibitor. However, all patients with assumed higher immunological risk, such as retransplants, patients' posthuman stem-cell transplant as well as children, were discharged on tacrolimus. Since January 2013, tacrolimus was used first line in all patients after LTX at our institution, but patients with assumed low immunological risk were later switched to cyclosporine in the outpatient setting 3 months after LTX. At our institution, patients usually received mycophenolate mofetil as cell cycle inhibitor after transplantation.
Patient records and outpatient visits were retrospectively reviewed. Follow-up was 100% completed and ended on April 1, 2015.
The hospital ethical review board, being a retrospective study, waived the need for patient consent to the study.
Variables
The CMV risk profiles, the need for postoperative or secondary extracorporeal membrane oxygenation support, bronchiolitis obliterans syndrome (BOS) and primary graft dysfunction (PGD), and the variables pulsed-steroid therapy, biopsy-confirmed acute rejection and infection requiring hospitalization and treatment, have been defined elsewhere.6,18,21-24 At our institution, patients do not undergo transbronchial biopsy during initial hospitalization after LTX. Thereafter, protocol surveillance transbronchial biopsies are performed at 1, 3, and 6 months and 1 year after transplantation and upon indication.
In this study, incidence of antibody-mediated rejection was not reported. Because of unspecific pathologic diagnostic criteria for antibody-mediated rejection,25 lung pathologists at our institution never made the diagnosis.
Early DSA were defined as DSA, which were detected during initial hospitalization after LTX, before hospital discharge. The DSA clearance was defined as the absence of DSA in 2 consecutive Luminex-based solid phase multiplex bead array (SPA) (LIFECODES; Immucore Transplant Diagnostics, Inc., Stamfort, CT) controls, to avoid false-negative results, because it has been demonstrated that, in kidney transplantation, DSA may be bound to the graft while at the same time not being present in serum.26 The DSA recurrence was defined as a renewed positivity of previously cleared DSA at Luminex-based SPA control.
DSA Detection and Controls
The DSA detection protocol did not change during the study period. All groups A, B, and C patients were screened for anti-HLA antibodies at the time of listing to LTX, and, during hospitalization for LTX, immediately before LTX, on day 14 and before hospital discharge or upon indication using CDC (Bio-Rad Medical Diagnostics GmbH, Dreieich, Germany) and Luminex-based SPA assays. Both B- and T cell crossmatches were performed using pretransplant serum. A low threshold of 1000 mean fluorescence intensity (MFI) was used to detect early DSA. If the lower cutoff MFI was set at higher values, for example, at 2000 or 5000 MFI, many patients, which anyway were at risk of developing humoral rejection, would have been missed. Therefore, to pick up these cases early, once sensitization had taken place, but humoral rejection was not undergoing yet, the MFI threshold value was set low at 1000 MFI. The highest MFI value against donor antigens was considered if more than 1 HLA coating bead with the same HLA specificity was detected. However, MFI values are only a semiquantitative measure of antibodies levels.27 At our institution, DSA against HLA DP were not measured.
At follow-up, in group A patients, Luminex-based SPA DSA controls were performed, according to the treatment protocol, at the beginning of each treatment session, after treatment end, and every 6 months. In group B patients, Luminex-based SPA DSA controls had been performed usually once per year. Instead, in group C patients, Luminex-based SPA DSA controls were not regularly controlled, but only upon indication, for example, in case of graft dysfunction.
IVIG-Based DSA Treatment Protocol
In April 2013, an IVIG-based treatment protocol was established and agreed by all the clinicians at our institution (Figure 1). This protocol was used to treat lung-transplanted patients who developed early DSA or showed a positive CDC crossmatch, instead of the tPE-based protocol, which had been used so far. Therapeutic plasma exchange was still used in combination with IVIG in patients with a positive crossmatch or assumed acute rejection.
FIGURE 1.

The process map reports the actual DSA treatment protocol at our institution.
Immediately after evidence of early DSA, its confirmation through a control sample and evaluation of patient current clinical conditions, DSA treatment was initiated. The DSA treatment was usually performed preemptively, independent of graft dysfunction. In case of absence of lung dysfunction, treatment was performed only with IVIG and Rituximab. The first IVIG dose consisted of 2 g/kg of IgM-enriched human immunoglobulins. However, if graft dysfunction was suspected or in presence of a positive crossmatch, 3 or 5 sessions of tPE or, more recently, 2 sessions of immunoabsorption preceded the first IVIG dose, to accelerate DSA clearance. Then, a single dose of dose of Rituximab (375 mg/m2) was given. Originally, a second dose of Rituximab was considered in the protocol. However, because all peripheral blood fluorescence-activated cell-sorting assays for CD19+ and CD20+ lymphocytes were negative after the first dose, the second dose was never administered. If DSA control was still positive, treatment with an IVIG dose of 0.5 g/kg was performed every 4 weeks. Treatment was ended and considered successful only if 2 consecutive controls were negative for DSA.
The IVIG was given through a peripheral venous catheter. Premedication with an antihistaminic drug (usually 150 mg ranitidine and 10 mg clemastine fumarate) and 1 g paracetamol was given to avoid allergic reactions before Rituximab, and before IVIG, only if the patient had already shown allergic reactions against IVIG. No prophylactic antibiotic therapy was given during IVIG therapy.
The DSA treatment protocol based only on tPE and Rituximab, which was used between January 2009 and April 2013, has been recently described elsewhere.18
Statistics
Data collection and analysis were performed retrospectively using SPSS 22.0 (IBM, NY). Endpoints were DSA clearance at treatment end and last Luminex-based SPA control, mortality, and freedoms from pulsed-steroid therapy, biopsy-confirmed acute rejection, and infection requiring hospitalization. Because of the short follow-up period for groups A and C in comparison to B patients, only incidence of BOS for each group was reported, without quantifying statistically significant differences among groups.
Categorical and continuous variables were summarized as percentages and median ± interquartile range, respectively. The independent samples nonparametric Kruskal-Wallis 1-way ANOVA or Mann-Whitney tests and the χ2 test or the Fisher exact tests were used for group comparisons of continuous and categorical variables, respectively.
Survival estimates along with freedom from pulsed-steroid therapy, biopsy-confirmed acute rejection, BOS, and infection requiring hospitalization were calculated by the product-limit method of Kaplan-Meier. Differences among groups, except for BOS, were quantified using the log-rank test. Survival and freedom curves were truncated at 2 years follow-up for group B patients.
Some patient characteristics may have conferred a degree of heterogeneity to each patient group, which could have biased outcomes. Therefore, outcomes and DSA clearance were evaluated in groups A and B patients, after stratification according to presence of preformed versus de novo DSA; in group A patients, after stratification according to the presence of graft dysfunction or a positive crossmatch; in group B patients, after stratification according to cyclosporine use at discharge; and in group C patients, after stratification according to presence of preoperative donor nonspecific HLA antibodies.
Two-tailed P values of 0.05 or less were considered significant.
RESULTS
Patient Groups
Between April 1, 2013, and January 31, 2015, among 243 patients who underwent LTX at our institution, 63 (26%) patients developed early DSA or showed a positive crossmatch. Among these patients, 57 (90%) underwent IVIG-based treatment and formed group A.
Six (10%) patients were not treated, because the DSA report came when patients were already discharged to the rehabilitation clinic and were not recalled for treatment. These patients were transplanted from October 2013 to February 2014, at the beginning of our experience with the IVIG-based treatment. More recently, because our experience with IVIG treatment has grown, patients, where positive DSA evidence came after hospital discharge, were recalled and underwent treatment as well. Among these 6 patients, none showed DSA at the last Luminex-based SPA control, and all were alive at end follow-up. However, these patients were too few to form a control group and therefore excluded from the study.
The remaining 180 (74%) contemporary patients without early DSA formed group C.
Fifty-six historic DSA patients who had undergone tPE-based treatment formed group B. Among these patients, 54 (96%) were transplanted between January 2009 and April 2013. The remaining 2 (4%) patients were transplanted between April and June 2013, during a transition phase from the tPE to IVIG treatment protocols.
Pre-, Intra- and Postoperative Patient Characteristics
Preoperative patient characteristics were mostly similar among groups (Tables 1 and 2), without any significant difference in the prevalence of preoperative anti-HLA antibodies, which have been demonstrated to be a risk factor for mortality and graft dysfunction elsewhere,8 but not at our institution.6 Groups A, B, and C showed a different pattern of cumulative HLA mismatches.
TABLE 1.
Preoperative data
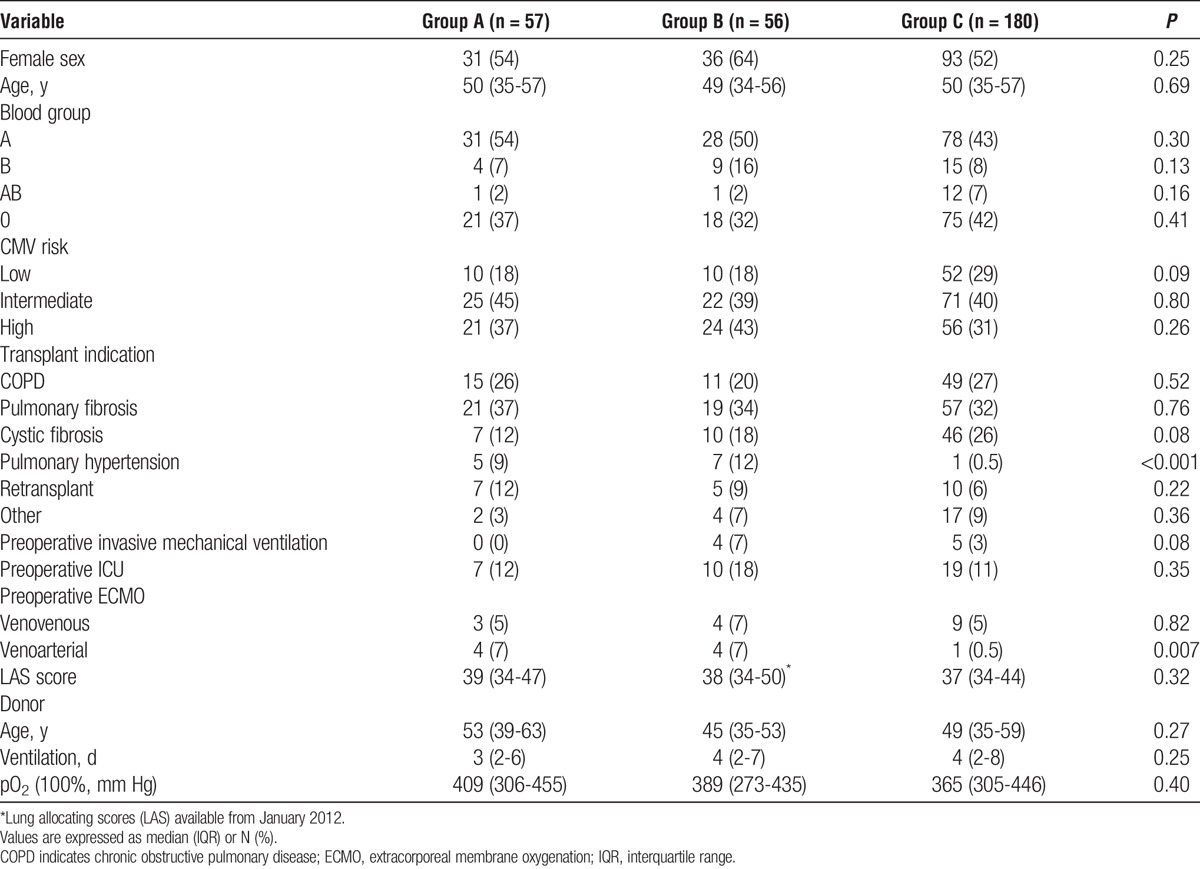
TABLE 2.
Immunology before and after transplantation

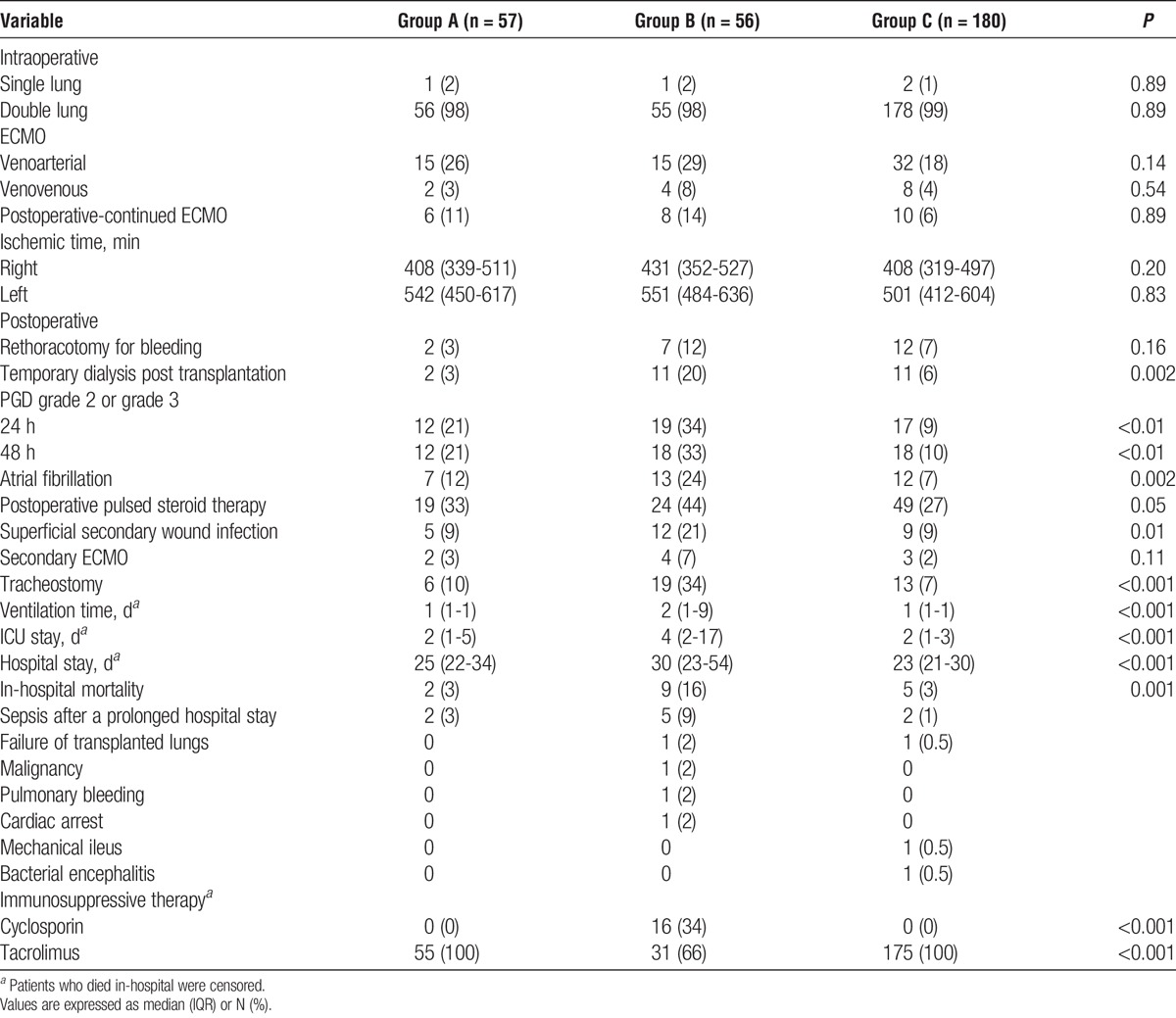
Postoperatively, group B showed a higher prevalence of complications and in-hospital mortality, as well as longer ICU and hospital stay times (Table 3). This difference was not due to the presence of graft dysfunction, because the prevalence of postoperative need for pulsed-steroid therapy for presumed rejection was similar among groups. Moreover, prevalence of PGD was higher in groups A and B than group C, but similar among groups A and B (P = 0.12 and 0.16 for PGD scores 2-3 at 24 and 48 hours, respectively).
TABLE 3.
Intraoperative and postoperative data
DSA and Therapy
The DSA characteristics and treatment are reported in Table 4. Fifty-one (90%) group A versus 48 (86%) group B patients developed de novo DSA (P = 0.36) after transplantation. More group A than group B patients developed DSA against recipient HLA DQ antigen (P = 0.004). No patient had undergone desensitization therapy with IVIG or tPE before transplantation.
TABLE 4.
Anti-HLA DSA: immunology and treatment.

The first dose of IVIG was preceded by tPE or immunoabsorption in 10 group A patients, due to positive crossmatch (n = 5) or assumed acute rejection (n = 5), according to the treatment protocol reported in Figure 1. Among patients with assumed acute rejection, 2 patients showed worsening of lung function tests and increase of infection parameters without evidence of active bacterial, fungal, or viral infection. The remaining 3 patients, during their initial ICU stay after transplantation, developed respiratory insufficiency with hypoxemia requiring reinstitution of mechanical ventilation, without evidence of infection. Two patients were successfully weaned from ventilation. The remaining patient died of sepsis 3 weeks after treatment.
A single dose of Rituximab was given at the end of the first cycle of IVIG and tPE in 52 (91%) group A and 51 (91%) group B patients, respectively. Five group A patients did not receive Rituximab due to recent infection (n = 4) or abdominal surgery (n = 1). Instead, 5 group B patients did not receive Rituximab due to evidence of carcinoma in the explanted lungs (n = 1) or concomitant steroid treatment for acute rejection (n = 4).
At follow-up, 8 (15%) group A patients did not require any further admission for IVIG treatment, because these patients did not show DSA at discharge any more (n = 7) or showed evidence of malignancy (n = 1).
Complications associated with IVIG therapy are reported in Table 4.
DSA Clearance
At treatment end, group A showed a better DSA clearance than group B (92% vs 64%; P = 0.002). Among the 4 group A patients who still showed DSA, 1 patient had evidence of malignancy soon after transplantation, and did not undergo further IVIG treatment. Another patient had undergone retransplantation. The remaining 2 patients showed persistent DSA against HLA-DQ antigen after 5 and 7 admissions for IVIG treatment.
At the last Luminex-based SPA control, performed after a median of 13 (6-26) months, DSA clearance was better in group A than group B (90% vs 75%; P = 0.04). Among group A patients, only one showed DSA recurrence 11 months after treatment end. The patient had developed chronic rejection due to unsatisfactory adherence to immunosuppressive therapy.
At treatment end and at last Luminex-based SPA control, DSA clearance showed no difference in groups A and B, after intragroup stratification according to the presence of preformed versus de novo DSA (Table S1, SDC, http://links.lww.com/TP/B222); in group A, after stratification according to presence of graft dysfunction or positive crossmatch (Table S2, SDC, http://links.lww.com/TP/B222); in group B, after stratification according to therapy with cyclosporine at discharge (Table S3, SDC, http://links.lww.com/TP/B222); and among group A versus excluded patients (Table S4, SDC, http://links.lww.com/TP/B222). Moreover, among groups A and B patients, DSA clearance at treatment end and at last control showed a similar trend before and after stratification according to the presence of preformed versus de novo DSA (Table S1, SDC, http://links.lww.com/TP/B222), with better clearance in group A patients.
Survival, Acute Rejection, and Infection
Median follow-up amounted to 12 (7-18) months for group A, 37 (25-43) months for group B, and 12 (7-18) months for group C, and is reported in Figures 2 and 3, Table 5, and Tables S1-S5 (SDC, http://links.lww.com/TP/B222).
FIGURE 2.
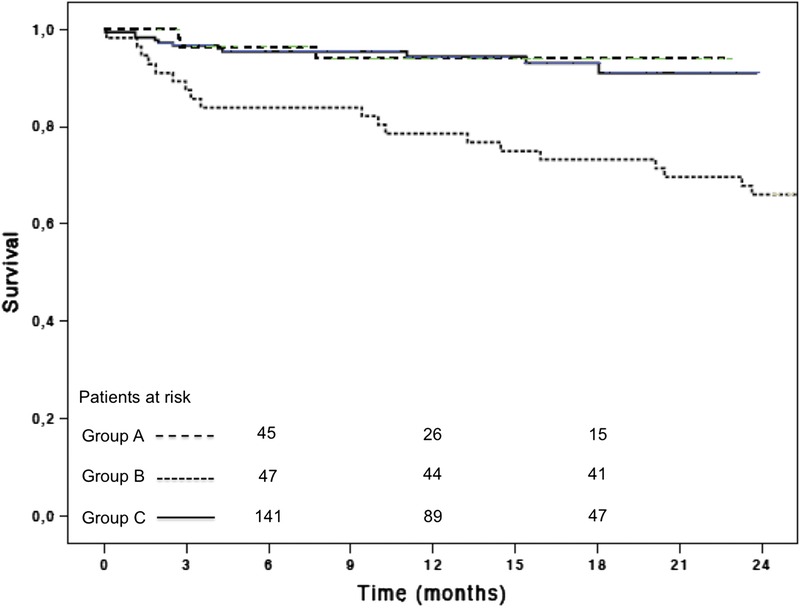
Overall survival. Group A patients showed better survival than group B patients and similar to group C patients. Patients at risk are reported above the x-axis. Survival curve for group B patients is truncated at 2 years of follow-up.
FIGURE 3.
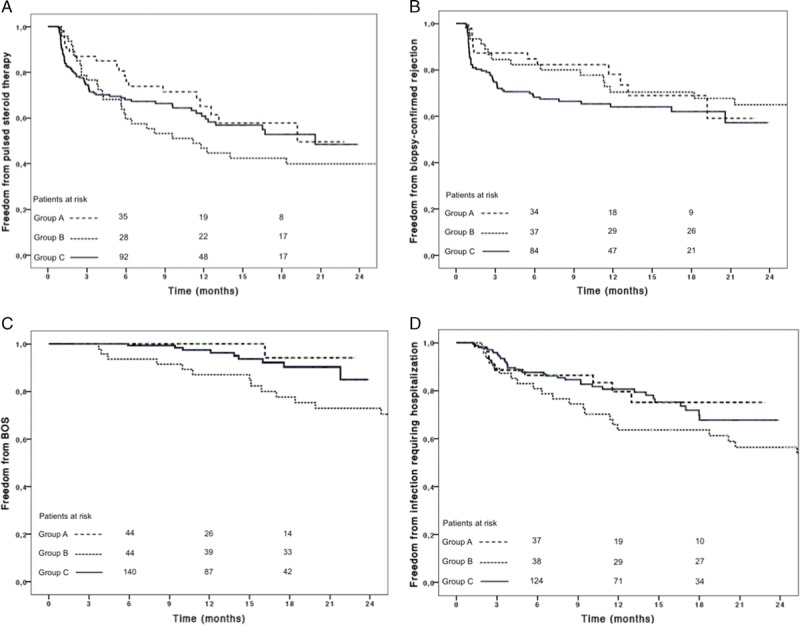
Freedom from pulse steroid therapy (A), biopsy-confirmed rejection (B), BOS (C), and from infection requiring hospitalization (D) are reported. Patient at risk are reported above the x-axis. The curves for group B patients have been truncated at 2 years of follow-up.
TABLE 5.
Outcomes among groups at follow-Up without any stratification
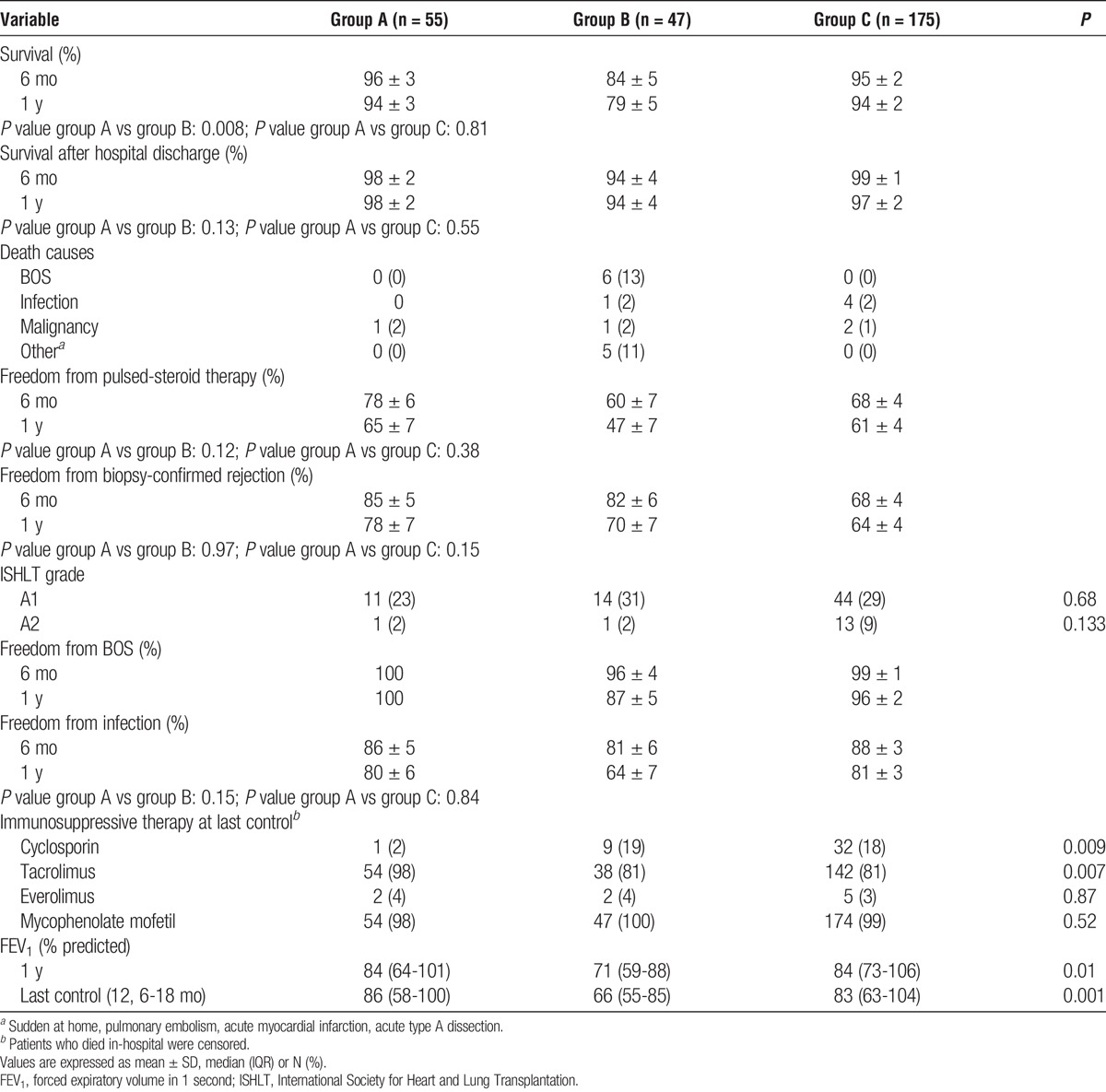
At 6 months and 1 year follow-up, group A showed better overall survival than group B and similar to group C (Table S1, SDC, http://links.lww.com/TP/B222). Sixteen patients died in-hospital (Table 3). Survival conditioned to hospital discharge was similar among groups (Table 5; Table S1, SDC, http://links.lww.com/TP/B222).
At 6 months and 1 year follow-up, there was no statistically significant difference among groups regarding freedom from pulsed-steroid therapy, biopsy-confirmed rejection and infection requiring hospitalization, although group A showed a trend toward better freedom from pulsed-steroid therapy and biopsy-confirmed rejection than the other 2 groups (Table 5; Table S1, SDC, http://links.lww.com/TP/B222).
Endpoints showed no difference in groups A and B after intragroup stratification according to presence of preformed versus de novo DSA (Table S1, SDC, http://links.lww.com/TP/B222); in group A, according to presence of graft dysfunction or positive crossmatch (Table S2, http://links.lww.com/TP/B222); in group B, after stratification according to therapy with cyclosporine at discharge (Table S3, SDC, http://links.lww.com/TP/B222); in group C, after stratification according to preoperative evidence of DSA (Table S5, SDC, http://links.lww.com/TP/B222); and among group A and the 6 excluded patients (Table S4, SDC, http://links.lww.com/TP/B222). Moreover, outcomes among groups A and B patients were similar before and after stratification according to presence of preformed versus de novo DSA (Tables S1, SDC, http://links.lww.com/TP/B222 and Table 5).
DISCUSSION
This study presents the short-term results of an IVIG-based treatment with Rituximab in patients with early humoral sensitization after LTX.
The IVIG therapy yielded optimal DSA clearance (92%), which persisted at last DSA control (90%) and was better than that in tPE-treated patients. Prevalence of IVIG-related side effects was low. Patients with DSA, which confer a higher risk for mortality and acute and chronic rejection,1-9 showed survival, freedom infection and forced expiratory volume in 1 second similar to transplanted patients without DSA, after IVIG therapy. Although IVIG patients showed better survival than tPE patients, results were confounded by the higher prevalence of postoperative complications in tPE than IVIG patients and different immunotherapy at discharge. In this last case, however, DSA clearance and outcomes were not significantly different in tPE patients after stratification according to cyclosporine use at discharge (Table S3, SDC, http://links.lww.com/TP/B222). Moreover, it remains controversial whether the immunosuppressive therapy has an impact on DSA clearance28-30 and survival.31
In solid-organ transplantation, immunoglobulins have been used successfully for clearing DSA or desensitizing patients with DSA before transplantation, either alone or in combination with other treatment modalities, such as tPE and immunoabsorption.10,11,15-17,19 Hachem et al17 reported the first case series on preemptive DSA treatment with IVIG and Rituximab in LTX.
In comparison to the study of Hachem et al, however, we included patients with preformed DSA or positive CDC crossmatch. This intragroup heterogeneity might have influenced the DSA clearance and outcomes in IVIG and tPE patients. In fact, in kidney transplantation, patients, who before transplantation were sensitized against donor HLA antigens, were at significant high risk of humoral rejection and graft loss after transplantations.32,33 Moreover, mechanisms and strength of antibody production in patients with preformed versus de novo DSA are different.2,8 In our study, outcomes and DSA clearance did not differ in patients with preformed DSA versus patients with de novo DSA in each group (Table S1, SDC, http://links.lww.com/TP/B222). In addition, outcomes and DSA clearance among IVIG and tPE patients showed similar results before and after stratification according to presence of preformed versus de novo DSA (Table S1, SDC, http://links.lww.com/TP/B222 and Table 5).
The improved DSA clearance in IVIG versus tPE patients may be due to the IVIG pleiotropic and long-lasting immunomodulatory effects: IVIG can downregulate B-cell activation and antibody production and provoke apoptosis of mature B cells; they can induce anti-inflammatory cytokines and contain blocking antiidiotypic antibodies to anti-HLA antibodies; and they can block complement-mediated injury through inhibition of C3 activation.11-16,34 Meanwhile, tPE, as well as immunoabsorption, only remove DSA from plasma. Therefore, we deem that the combination of the immunomodulatory effects of IVIG with the apoptotic effects of Rituximab on pre-B and mature B cells35,36 yielded the optimal DSA clearance obtained in our study. We still use tPE but only in combination with IVIG at our center.
Other types of immunoglobulins given for specific indications may also clear DSA, such as CMV immunoglobulins and antithymocyte globulins.12 The IVIG preparation mostly applied in kidney transplantation for treatment of DSA is not enriched for any specific components.10-12 At our institution, we use IgM enriched IVIG due to their largest inhibition of lymphocytoxicity12 and their immunomodulatory and antibacterial properties, because they have been used mainly in patients with sepsis so far.37 Thus, it is possible that IVIG did not only clear DSA in our patients but also protected them from infection. The IVIG patients, although potentially more immunosuppressed through repeated IVIG therapy and Rituximab, showed freedom from infection requiring hospitalization similar to patients without DSA.
We have recently shown in patients mainly transplanted before implementation of the IVIG protocol that development of early DSA was a risk factor for mortality.6 In the present study, although follow-up was short, survival of IVIG patients was similar to survival of patients without DSA. Moreover, interestingly, IVIG patients showed improved freedom from pulsed-steroid therapy and biopsy-confirmed rejection than patients without DSA, although not statistically significant, pointing at a potential immunoprotective effect of IVIG. Longer follow-up and more studies are required to evaluate this effect on graft and patient survival.
Finally, despite the good survival and DSA clearance results obtained with the present treatment protocol, IVIG may still provoke adverse effects. Moreover, prolonged IVIG treatment implies costs and impairs quality of life of patients who have to repeat treatment every 4 weeks. Regarding the former problem, careful control of patients during IVIG administration is paramount. Regarding the latter, a combination of tPE or immunoabsorption with IVIG in all patients with early DSA may accelerate their clearance. Therapy costs and side effects may raise some concerns about the necessity of DSA treatment,20 because some DSA patients cleared DSA without any treatment. However, there is enough available scientific evidence showing that DSA are risk factors for survival and acute and chronic rejection, not only in LTX, but also in other solid organ transplantation.1-9,14 A randomized trial will be helpful in clearing the real impact of DSA and their treatment on patient and graft survival in LTX.
STUDY LIMITATIONS
The retrospective nature of the analysis introduced inherent limitations. To obviate the lack of randomization and account for in-group heterogeneity, outcomes were evaluated after stratification according to presence of positive crossmatch or graft dysfunction in group A, preformed versus de novo DSA in and among groups A and B, preoperative donor nonspecific HLA antibodies in group C and baseline immunotherapy in group B.
Only patients who developed early DSA during initial hospitalization after LTX were allocated to IVIG treatment. The remaining patients (group C), who were discharged from the hospital without having developed early DSA, could have developed DSA thereafter. However, the prevalence of DSA at follow-up could not be estimated in these patients, because they did not routinely undergo DSA controls at our institution.
CONCLUSIONS
The present treatment protocol with IgM-enriched IVIG and Rituximab showed better long-lasting DSA clearance than our historic tPE-based treatment. However, spontaneous DSA clearance remains frequent. Although the better survival in IVIG patients in comparison with tPE patients was influenced by confounders related to the retrospective nature of this study, patients with early new DSA but without graft dysfunction treated with IVIG have midterm survival and lung function equivalent to patients not developing early DSA at all.
Supplementary Material
Footnotes
W.S., J.G., T.W., A.H., and G.W. are members of the German Centre for Lung Research (DZL/BREATH).
The authors declare no conflicts of interests.
F.I. and W.S. participated in the research design, performance of the research, writing of the article, and data analysis. D.K. participated in performance of the research and data analysis. I.T. participated in the performance of the research and critical revision of the article. C.K., M.A., T.S., J.S., C.E., and M.V. participated in the performance of the research. J.K. participated in the research design, performance of the research, and critical revision of the article. A.T. participated in critical revision of the article. M.G. participated in the performance of the research. M.H. participated in the research design and performance of the research. R.B. participated in the critical revision of the article. N.S. participated in the performance of the research. J.G. participated in the critical revision of the article. T.W. participated in the critical revision of the article. A.H. participated in research design and critical revision of the article. G.W. participated in research design, data analysis, writing of the article, and critical revision of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Hadjiliadis D, Chaparro C, Reinsmoen NL, et al. Pre-transplant panel reactive antibody in lung transplant recipients is associated with significantly worse post-transplant survival in a multicenter study. J Heart Lung Transplant. 2005;24:S249–S254. [DOI] [PubMed] [Google Scholar]
- 2.McManigle W, Pavlisko EN, Martinu T. Acute Cellular and Antibody-Mediated Allograft Rejection. Semin Respir Crit Care Med. 2013;34:320–335. [DOI] [PubMed] [Google Scholar]
- 3.Snyder LD, Wang Z, Chen DF, et al. Implications for human leukocyte antigen antibodies after lung transplantation: a 10 year experience in 441 patients. Chest. 2013;144:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lobo LJ, Aris RM, Schmitz J, et al. Donor-specific antibodies are associated with antibody-mediated rejection, acute cellular rejection, bronchiolitis obliterans syndrome, and cystic fibrosis after lung transplantation. J Heart Lung Transplant. 2013;32:70–77. [DOI] [PubMed] [Google Scholar]
- 5.Witt CA, Gaut JP, Yusen RD, et al. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant. 2013;32:1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ius F, Sommer W, Tudorache I, et al. Early donor-specific antibodies in lung transplantation: risk factors and impact on survival. J Heart Lung Transplant. 2014;33:1255–1263. [DOI] [PubMed] [Google Scholar]
- 7.Morrell M, Pilewski JM, Gries CJ, et al. De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. J Heart Lung Transplant. 2014;33:1288–1294. [DOI] [PubMed] [Google Scholar]
- 8.Smith JD, Ibrahim MW, Newell H, et al. Pre-transplant donor HLA-antibodies: characteristics causing detrimental effects on survival after lung transplantation. J Heart Lung Transplant. 2014;33:1074–1082. [DOI] [PubMed] [Google Scholar]
- 9.Safavi S, Robinson DR, Soresi S, et al. De novo donor HLA-specific antibodies predict development of bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2014;33:1273–1281. [DOI] [PubMed] [Google Scholar]
- 10.Zachary AA, Montgomery RA, Ratner LE, et al. Specific and durable elimination of antibody to donor HLA antigens in renal-transplant patients. Transplantation. 2003;76:1519–1525. [DOI] [PubMed] [Google Scholar]
- 11.Jordan SC, Vo AA, Toyoda M, et al. Post-transplant therapy with high-dose intravenous gammaglobulin: applications to treatment of antibody-mediated rejection. Pediatr Transplant. 2005;9:155–161. [DOI] [PubMed] [Google Scholar]
- 12.Singh N, Pirsch J, Samaniego M. Antibody-mediated rejection: treatment alternatives and outcomes. Transplant Rev (Orlando). 2009;23:34–46. [DOI] [PubMed] [Google Scholar]
- 13.Fehr T, Gaspert A. Antibody-mediated kidney allograft rejection: therapeutic options and their experimental rationale. Transpl Int. 2012;25:623–632. [DOI] [PubMed] [Google Scholar]
- 14.Mehra N, Siddiqui J, Baranwal A, et al. Clinical relevance of antibody development in renal transplantation. Ann NY Acad Sci. 2013;1283:30–42. [DOI] [PubMed] [Google Scholar]
- 15.Vo AA, Petrozzino J, Yeung K, et al. Efficacy, outcomes, and cost-effectiveness of desensitization using IVIG and rituximab. Transplantation. 2013;95:852–858. [DOI] [PubMed] [Google Scholar]
- 16.Vo AA, Choi J, Cisneros K, et al. Benefits of rituximab combined with intravenous immunoglobulin for desensitization in kidney transplant recipients. Transplantation. 2014;98:312–319. [DOI] [PubMed] [Google Scholar]
- 17.Hachem RR, Yusen RD, Meyers BF, et al. Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation. J Heart Lung Transplant. 2010;29:973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ius F, Sommer W, Tudorache I, et al. Preemptive treatment with therapeutic plasma exchange and rituximab for early donor-specific antibodies after lung transplantation. J Heart Lung Transplant. 2015;34:50–58. [DOI] [PubMed] [Google Scholar]
- 19.Tinckam KJ, Keshavjee S, Chaparro C, et al. Survival in sensitized lung transplant recipients with perioperative desensitization. Am J Transplant. 2015;15:417–426. [DOI] [PubMed] [Google Scholar]
- 20.Westall GP, Snell GI. Antibody-mediated rejection in lung transplantation: fable, spin or fact? Transplantation. 2014;98:927–930. [DOI] [PubMed] [Google Scholar]
- 21.Ius F, Kuehn C, Tudorache I, et al. Lung transplantation on cardiopulmonary support: venoarterial extracorporeal membrane oxygenation outperformed cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2012;144:1510–1516. [DOI] [PubMed] [Google Scholar]
- 22.Estenne M, Maurer J, Boehler A, et al. Bronchiolitis Obliterans Syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:27–310. [DOI] [PubMed] [Google Scholar]
- 23.Lee JC, Christie JD, Keshavjee S. Primary graft dysfunction: definition, risk factors, short- and long-term outcomes. Semin Respir Crit Care Med. 2010;31:161–171. [DOI] [PubMed] [Google Scholar]
- 24.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. [DOI] [PubMed] [Google Scholar]
- 25.Berry G, Burke M, Andersen C, et al. Pathology of pulmonary antibody-mediated rejection: 2012 update from the Pathology Council of the ISHLT. J Heart Lung Transplant. 2013;32:14–21. [DOI] [PubMed] [Google Scholar]
- 26.Archdeacon P, Chan M, Neuland C, et al. Summary of FDA antibody-mediated rejection workshop. Am J Transplant. 2011;11:896–906. [DOI] [PubMed] [Google Scholar]
- 27.Tambur AR, Herrera ND, Haarberg KM, et al. Assessing antibody strength: comparison of MFI, C1q, and titer information. Am J Transplant. 2015;15:2421–2430. [DOI] [PubMed] [Google Scholar]
- 28.Perbos E, Juinier E, Guidicelli G, et al. Evolution of donor-specific antibodies (DSA) and incidence of de novo DSA in solid organ transplant recipients after switch to Everolimus alone or associated with low dose of calcineurin inhibitors. Clin Transplant. 2014;28:1054–1060. [DOI] [PubMed] [Google Scholar]
- 29.Lee PC, Chang SS, Shieh SC, et al. Cyclosporine or tacrolimus: which is the better partner for myfortic or cellcept? Transplant Proc. 2012;44:137–139. [DOI] [PubMed] [Google Scholar]
- 30.Gareau AJ, Nashan B, Hirsch GM, et al. Cyclosporine immunosuppression does not prevent the production of donor-specific antibody capable of mediating allograft vasculopathy. J Heart Lung Transplant. 2012;31:874–880. [DOI] [PubMed] [Google Scholar]
- 31.Treede H, Glanville AR, Klepetko W, et al. Tacrolimus and cyclosporine have differential effects on the risk of development of bronchiolitis obliterans syndrome: results of a prospective, randomized international trial in lung transplantation. J Heart Lung Transplant. 2012;31:797–804. [DOI] [PubMed] [Google Scholar]
- 32.Lúcia M, Luque S, Crespo E, et al. Preformed circulating HLA-specific memory B cells predict high risk of humoral rejection in kidney transplantation. Kidney Int. 2015;88:874–887. [DOI] [PubMed] [Google Scholar]
- 33.Lefacheur C, Loupy A, Hill GS, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21:1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordan SC, Toyoda M, Vo AA. Regulation of immunity and inflammation by intravenous immunoglobulin: relevance to solid organ transplantation. Expert Rev Clin Immunol. 2011;7:341–348. [DOI] [PubMed] [Google Scholar]
- 35.Becker YT, Samaniego-Picota M, Sollinger HW. The emerging role of rituximab in organ transplantation. Transpl Int. 2006;19:621–628. [DOI] [PubMed] [Google Scholar]
- 36.Clatworthy MR. Targeting B Cells and Antibody in Transplantation. Am J Transplant. 2011;11:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shankar-Hari M, Spencer J, Sewell WA, et al. Bench-to-bedside review: immunoglobulin therapy for sepsis—biological plausibility from a critical care perspective. Crit Care. 2012;16:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


