Abstract
We collected 2768 Influenza-like illness emergency public health incidents from April 1, 2005 to November 30, 2013reported in the Emergency Public Reporting System. After screening by strict inclusion and exclusion criteria, there were 613 outbreaks analyzed with susceptible–exposed–infectious/asymptomatic–removed model in order to estimate the proportion of asymptomatic individuals (p) and the effective reproduction number (Rt). The relation between Rt and viral subtypes, regions, outbreak sites, populations, and seasons were analyzed. The mean values of p of different subtypes ranged from 0.09 to 0.15, but could be as high as up to 0.94. Different subtypes, provinces, regions, and sites of outbreak had statistically significantly different Rt. In particular, the southern region also manifested different Rt by affected population size and seasonality. Our results provide China and also the rest of the world a reference to understand characteristics of transmission and develop prevention and control strategies.
Introduction
Influenza is a respiratory infectious disease which can result in annual infection of 5–15% of the population, leading to 250,000 and 500,000 deaths[1]. Surveillance of the disease agents at the human and animal interface facilitates early detection of influenza transmission. Numerous outbreaks of influenza/ILI are reported every year throughout China. From April 1, 2005 to November 30, 2013, there were 2,768 influenza/Influenza-like illness (ILI) outbreaks recorded in the Emergency Public Reporting System (EPRS).There are two systems in China for influenza surveillance: one is National Public Health Information System and the other is the National Sentinel Surveillance System for ILI. The two systems are linked, such that the influenza outbreak report in the first system is directly synchronized to the second system. Before November 2012 it is mandatory to report any incident with more than 30 cases of ILI weekly in the same unit (equivalent to class IV public health emergency). Since November 2012, the number has been changed from 30 to 10. This study adopted the definition of 30 cases for analysis.
To control outbreaks in schools or work place, the transmissibility of influenza and the proportion of asymptomatic infections are the key factors that should be taken into consideration of control strategies. There are two main categories responding to large-scale contagious outbreaks at city-, province- or region-scales: drug and non-drug intervention, for examples, antiviral treatment, prevention medication, vaccination; and advocate of social contact avoidance, school closure, and travel restrictions respectively. According to "National Health and Family Planning Commission of the People's Republic of China. Guidelines for Dispose of Influenza-like Illness Outbreak (2012th edition), 2012. (in Chinese)", the principal measure to contain school outbreaks is isolation. However, in the presence of asymptomatic infections, isolation can be inefficient because cases are not easy to be identified. Isolation can also become difficult when transmission is intense.
An England cohort study showed that the proportion of influenza asymptomatic infection can be as high as 77 percent [2], while Yang [3] and Longini [4, 5] obtained the estimate of only 33% in North American or Thai population. Therefore, characterization of the transmissibility and the proportion of asymptomatic infection of influenza virus shall provide a reference for policy making and evaluation of public health measures.
Ordinary differential equation (ODE) modeling was first applied to infectious disease modeling in 1927 by Kermack and McKendrick, who established the susceptible–infectious–removed (SIR) model [6]. ODE models have undergone continuous improvement and have been frequently used in mathematical modeling in influenza studies. For example, Stone et al. [7] and Dushoff et al. [8] used SIR and susceptible–infectious–removed–susceptible (SIRS) models, respectively, to simulate the seasonality and periodicity of influenza epidemics. Arino et al. [9] established the SEIAR model of influenza pandemics and simulated the effects of targeted prevention and control measures.
Materials and Methods
Data collection criteria
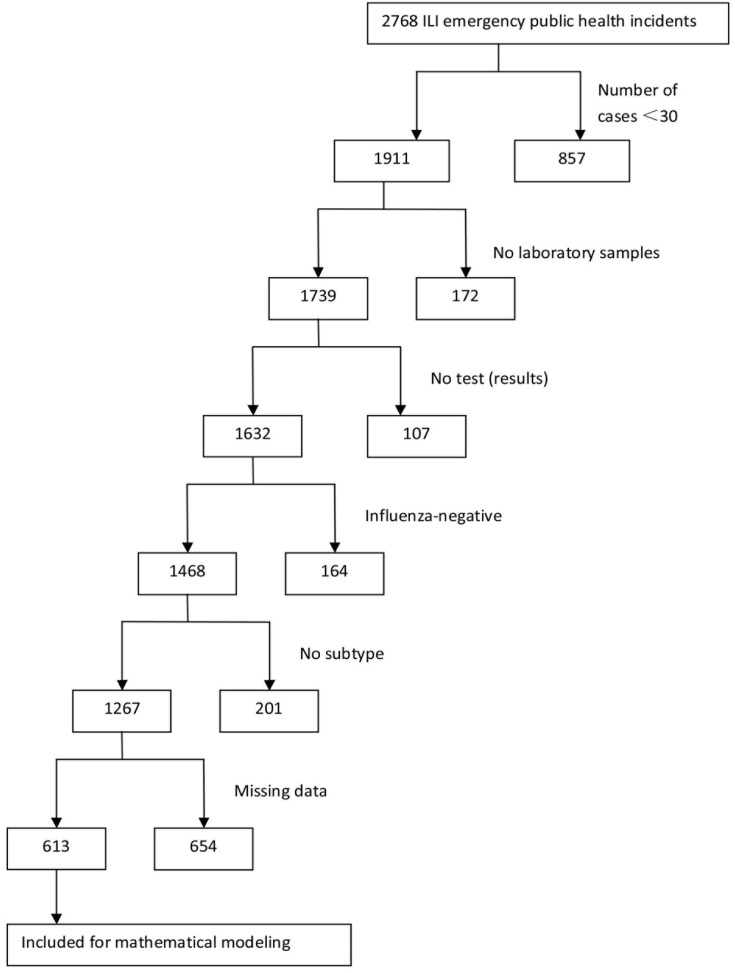
Information related to influenza outbreaks reported in China through the EPRS from April 1, 2005 to November 30, 2013 was collected. Influenza-like illness (ILI) refers to a fever (axillary temperature ≥38°C) accompanied by coughing or sore throat and a lack of a laboratory-confirmed diagnosis of the specific pathogen [10, 11]. The criteria for an influenza outbreak was defined as ≥30 ILIs occurring in the same school, preschool, or other collective organization within 1 week [10], with laboratory-confirmed influenza viruses through virus isolation or real-time reverse transcriptase polymerase chain reaction (RT-PCR) analysis. Subjects were included when the following inclusion criteria were met: 1) emergency public health incidents with ≥30 patients; 2) incidents with specific influenza subtypes verified by pathogen examinations including virus isolation and/or nucleic acid detection; and 3) a time interval between the first case and healthcare authority intervention of ≥5 days. The inclusion criteria are summarized as a flowchart in Fig 1.
Fig 1. Flowchart of influenza outbreak data inclusion criteria in this study.
Structure of the data and target data for modeling
We used each included outbreak as a unit and collected the names, reported areas, provinces, regions (southern or northern), time of occurrence, accumulated cases, location types of the incidents, number of people affected by a single incident, and laboratory test results of the incidents. We sorted the time distribution of the disease cases of each outbreak within the time interval between the date of onset in the first case and the date of disease control authority intervention as the epidemic outbreak data during a period of no intervention. The method of data capture in the no-intervention period in each outbreak is shown in Figure A in S2 File in the target data section.
Subtype of influenza
Based on the characteristics of the influenza viruses, we classified the influenza viruses as A (H3N2), A (H1N1) pdm, A (H1N1), B and mixed types. The mixed type refers to a mixture of ≥2 virus types in one case sample detected in a single outbreak; it can include ≥2 subtypes of type A or ≥1 subtype of type A in addition to type B influenza viruses.
The period between disease onset and recovery
We collected the disease course data from 8 outbreaks between January 1, 2011 and November 30, 2013, totaling 283 cases. The dataset contained the information of location, results of PCR test for influenza virus, the number of cases and the dates of disease onset and recovery. The infectious individual removal coefficient γ could then be obtained by taking the inverse of the period between disease onset and recovery. (S1 File).
Model establishment
We used an ODE model to estimate the effective reproduction number (Rt) of outbreak data recorded in EPRS and analyzed the differences in Rt by influenza viruses of various subtypes, areas, provinces, outbreak location types, initial susceptible population sizes, years, and seasons. Previous applications of ODE model to estimate Rt can be found in the work of Sertsou et al [12] and Tracht et al [13]. In this study, we applied a susceptible–exposed–infectious/asymptomatic–removed model to estimate Rt and the proportion of asymptomatic individuals in outbreaks happened in China in the past 8 years.
Based on the natural history of influenza [4–5, 9], we established the susceptible–exposed–infectious/asymptomatic–removed (SEIAR) model [9]. The model can be expressed using the following differential equations:
| (1) |
Where dS/dt, dE/dt, dI/dt, dA/dt, and dR/dt represent the rate of change at moment t in the various populations S, E, I, A, and R, respectively. β, κ, ω, ω', p, γ, and γ' represent the infection rate coefficient, transmission capacity coefficient of A in relation to I, incubation period coefficient, latent period coefficient, asymptomatic infection ratio, infectious individual removal coefficient, and asymptomatic individual removal coefficient.
The transmissibility of influenza in outbreaks
Effective reproduction number (Rt) is defined as the expected number of secondary infections that result from introducing a single infected individual into an otherwise susceptible population [14–15]. Rt is generally applied to determine the influenza transmission capacity. Rt refers to the number of new disease cases expected to be directly infected by one source of infection during the infection period in the susceptible population [14–15]. If Rt<1, the number of infected individuals would decrease toward zero. The disease would not be prevalent and would therefore be gradually eliminated. In contrast, if Rt>1, the disease would be prevalent. According to the definition of Rt and the methods reported by Chen et al. [16] and Arino et al. [9], the Rt expression in “model (1)” is as follows:
| (2) |
Other parameter estimation
Studies have shown that the influenza incubation period, mean latent period, and infection period of asymptomatic individuals are 1–7 days (with a mean of 1.9 days), 1.2 days and 4.2 days, respectively. The infection capacity of asymptomatic individuals is half that of infectious individuals [3–5]. Therefore, the values of the following four parameters were used: ω = 0.5263, ω' = 0.8333, γ' = 0.2439, and κ = 0.5. Coefficients β and p were obtained from model fitting.
Simulation methods and data analysis
We used SEIAR model to fit the target data (Figure A in S2 File) of all the 613 outbreaks to estimate β and p per incident. Thent of each outbreak was calculated accordingly. Model simulation was conducted using the software Berkeley Madonna 8.3.18 (University of California, Berkeley, Berkeley, USA). Differential equation fitting was performed using the fourth-order Runge-Kutta method with the tolerance set at 0.001. While the curve fit is in progress, Berkeley Madonna displays the root-mean-square deviation between the data and the best fit that has been run.
We analyzed the differences in p by subtypes and the differences in Rt by subtypes, provinces, regions, time, location types of infection occurrence, and initial susceptible population sizes. The last item refers to the number of persons affected in an influenza outbreak. For example, in a school, if the cases are limited to one class, then the class size is initial susceptible population size. If the cases happen in more than one class of the same study year, then the total size of all the classes of the same study year will be assumed. Finally, the cases are from different classes, different years, the total number of students in the school will be used. The software SPSS 13.0 was employed to run the data analysis by using t test, ANOVA analysis, χ2 test and the regression (curve estimation).
Sensitivity analysis
Of the coefficients in the model, the value κ (κ = 0.5) was obtained from existing literature [2–4]. But according to recent clinical evidence, the value of κ could be low down to 0.1, which suggests that asymptomatic infection might be less important in the spread of epidemics than previously thought [17]. To understand the impact of κ, we did a sensitivity analysis based on 10 randomly selected outbreaks in China. Based on the definition of κ, we used 0.1–0.9 as κ for the sensitivity analysis, and observed the influence of these changes to the Rt.
In addition, we analyzed the relationship between Rt and the susceptible population at the beginning of the outbreak (S0) by using the 10 selected outbreaks. We set 5, 10, 20, 50, 100 and 1000 to do the curve fitting of a same outbreak. A new indicator pR (where pR = [Rt(S0)-Rt(1000S0)]/Rt(S0)) was calculated. We then compared S0 with pR by using the following the ten functions (Linear, Quadratic, Compound, Growth, Logarithmic, Cubic, S, Exponential, Inverse and Power function) in the software SPSS 13.0.
To consider the robustness of the SEIAR model, and also because four parameters of the SEIAR model, i.e., ω, ω', γ' and k, were estimated by references, some uncertainty was present that may affect the results of the constructed models. Sensitivity was evaluated by varying these four parameters, which were divided into 1,000 values ranging from 0.1429 to 1 (indicating incubation periods ranging from 1–7 days), from 0.1429 to 1 (indicating latent periods ranging from 1–7 days), from 0.0833 to 1 (indicating infectious periods of asymptomatic individuals ranging from 1–12 days), and from 0 to 1 (indicating transmissibility of asymptomatic individuals ranging from 0–1 compared to symptomatic individuals), respectively.
Results
Basic characteristics of the outbreaks
From April 1, 2005 to November 30, 2013, 2768 ILI emergency public health incidents in China were reported in EPRS. Following the exclusion of incidents with insufficient patient numbers (n = 30), unexamined pathogens, non-influenza viruses, and untyped influenza viruses, a total of 1267 influenza emergency public health incidents were applicable for the following analysis. Of these incidents, 654 incidents without patient data, with incomplete data, or with logical errors were also excluded. The remaining 613 outbreaks were used as the final data in this study. Of these 613 incidents, 138 were A (H3N2) subtype, 68 were A (H1N1) pdm09 subtype, 31 were A (H1N1) subtype, 350 were type B, and 26 were mixed infections (A+B: 6, H1N1+B: 3, H1N1+H3N2: 3, H1N1pdm+A: 1, H1N1pdm+ H1N1: 1, H1N1pdm+H3N2: 1, H1N1pdm+Seasonal: 5, H3N2+B: 6). The two datasets locations of the original 2768 and 613 final outbreaks, despite after tremendous filtering, still resembled to each other.
The period between disease onset and recovery
In the distributions of the disease course, the longest disease course was 12 days, and the shortest was 1 day; the mean was 4.27 (±1.98 SD), coefficient γwas 0.2342.
Asymptomatic infection ratio
The distributions of p of the various subtypes are shown in Table 1. The mean values of p of the subtypes ranged from 0.09–0.15, with a minimum of 0 and a maximum of 0.94. The non-parametric Kruskal-Wallis analysis indicated no significant differences in p among the subtypes (χ2 = 3.117, P = 0.538). (Figure B in S2 File)
Table 1. Asymptomatic infection ratio distribution of the different influenza virus subtypes.
| Subtype | N | Mean | Std. Deviation | Std. Error | 95% CI for Mean | Min | Max | |
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| A (H3N2) | 138 | 0.14 | 0.28 | 0.02 | 0.09 | 0.19 | 0.00 | 0.90 |
| A (H1N1) pdm | 68 | 0.09 | 0.24 | 0.03 | 0.03 | 0.15 | 0.00 | 0.89 |
| A (H1N1) | 31 | 0.16 | 0.28 | 0.05 | 0.05 | 0.26 | 0.00 | 0.89 |
| B | 350 | 0.15 | 0.27 | 0.01 | 0.12 | 0.18 | 0.00 | 0.94 |
| Mixed | 26 | 0.12 | 0.26 | 0.05 | 0.01 | 0.22 | 0.00 | 0.85 |
| Total | 613 | 0.14 | 0.27 | 0.01 | 0.12 | 0.16 | 0.00 | 0.94 |
Rt of different subtypes
The Rt values of the subtypes are shown in both Figure C in S2 File and Table 2. The mean Rt values of influenza A (H3N2), A (H1N1) pdm, A (H1N1), influenza B, and the mixed types were 8.46, 9.33, 7.63, 7.79, and 10.03, respectively. The homogeneity of variance test resulted in a Levene value of 1.243 (P = 0.291). Therefore, the variances of Rt of the subtypes were considered homogeneous. Moreover, an analysis of variance was used for the analysis among the subtypes, resulting in F = 2.051 and P = 0.086. Therefore, differences in Rt between the subtypes were considered no statistical significance.
Table 2. Distributions of Rt of the variables.
| Variable | N | Mean | Std. Deviation | Std. Error | 95% CI for Mean | Min | Max | |
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Subtype | ||||||||
| A (H3N2) | 138 | 8.46 | 5.88 | 0.50 | 7.47 | 9.45 | 1.17 | 37.80 |
| A (H1N1) pdm | 68 | 9.33 | 5.48 | 0.66 | 8.00 | 10.66 | 1.18 | 32.59 |
| A (H1N1) | 31 | 7.63 | 4.20 | 0.75 | 6.09 | 9.17 | 2.64 | 22.53 |
| B | 350 | 7.79 | 5.24 | 0.28 | 7.24 | 8.34 | 1.19 | 43.64 |
| Mixed | 26 | 10.03 | 8.44 | 1.66 | 6.62 | 13.44 | 3.21 | 43.96 |
| Region | ||||||||
| Northern | 129 | 7.64 | 5.52 | 0.49 | 6.68 | 8.61 | 1.45 | 43.96 |
| Southern | 484 | 8.35 | 5.56 | 0.25 | 7.85 | 8.84 | 1.17 | 43.64 |
| Venue | ||||||||
| Northern Region | ||||||||
| Kindergarten | 1 | 4.39 | NA | NA | NA | NA | 4.39 | 4.39 |
| Primary school | 79 | 8.19 | 6.64 | 0.75 | 6.7 | 9.67 | 1.45 | 43.96 |
| Primary + middle school | 2 | 4.61 | 0.69 | 0.49 | -1.62 | 10.84 | 4.12 | 5.1 |
| Middle + high school | 37 | 6.92 | 2.97 | 0.49 | 5.93 | 7.91 | 2.35 | 14.28 |
| Technical secondary school | 2 | 7.13 | 0.62 | 0.44 | 1.54 | 12.72 | 6.69 | 7.57 |
| College | 2 | 6.6 | 6.62 | 4.68 | -52.87 | 66.07 | 1.92 | 11.28 |
| Other school | 3 | 5.29 | 1.48 | 0.85 | 1.62 | 8.96 | 3.98 | 6.89 |
| Work place | 2 | 9.24 | 1.22 | 0.87 | -1.76 | 20.23 | 8.37 | 10.1 |
| Community | 1 | 7.66 | NA | NA | NA | NA | 7.66 | 7.66 |
| Southern Region | ||||||||
| Kindergarten | 4 | 6.33 | 3.94 | 1.97 | 0.06 | 12.6 | 2.6 | 11.67 |
| Primary school | 265 | 8.4 | 5.9 | 0.36 | 7.69 | 9.11 | 1.17 | 43.64 |
| Primary + middle school | 3 | 7.39 | 2.79 | 1.61 | 0.47 | 14.31 | 4.58 | 10.15 |
| Middle + high school | 154 | 7.83 | 4.45 | 0.36 | 7.12 | 8.54 | 1.18 | 27.92 |
| Technical secondary school | 6 | 11.31 | 5.5 | 2.24 | 5.54 | 17.08 | 6.51 | 21.34 |
| College | 4 | 8.14 | 2.54 | 1.27 | 4.11 | 12.19 | 6.03 | 11.83 |
| Other school | 13 | 10.5 | 7.85 | 2.18 | 5.75 | 15.25 | 3.35 | 32.59 |
| Work place | 25 | 9.43 | 7.21 | 1.44 | 6.45 | 12.4 | 2.61 | 31.76 |
| Community | 10 | 8.78 | 5.98 | 1.89 | 4.5 | 13.05 | 3.07 | 22.53 |
| Population | ||||||||
| Northern Region | ||||||||
| 0~ | 74 | 7.9 | 5.19 | 0.6 | 6.7 | 9.11 | 2.35 | 32.53 |
| 1000~ | 25 | 7.34 | 3.61 | 0.72 | 5.85 | 8.83 | 2.86 | 16.89 |
| 2000~ | 30 | 7.26 | 7.45 | 1.36 | 4.48 | 10.04 | 1.45 | 43.96 |
| Southern Region | ||||||||
| 0~ | 216 | 9.55 | 6.6 | 0.45 | 8.66 | 10.43 | 1.19 | 43.64 |
| 1000~ | 141 | 7.23 | 4.42 | 0.37 | 6.5 | 7.97 | 1.48 | 32.53 |
| 2000~ | 127 | 7.54 | 4.25 | 0.38 | 6.8 | 8.29 | 1.17 | 27.62 |
| Year | ||||||||
| Northern Region | ||||||||
| 2005 | 2 | 10.29 | 6.47 | 3.87 | -38.82 | 59.39 | 6.42 | 14.15 |
| 2006 | 46 | 6.7 | 3.46 | 0.51 | 5.68 | 7.73 | 2.35 | 17.4 |
| 2007 | 23 | 5.96 | 2.42 | 0.51 | 4.91 | 7.01 | 2.85 | 11.54 |
| 2008 | 3 | 11.24 | 8.08 | 4.66 | -8.82 | 31.31 | 4.85 | 20.32 |
| 2009 | 39 | 8.77 | 7.96 | 1.27 | 6.19 | 11.35 | 1.92 | 43.96 |
| 2010 | 4 | 9.18 | 7.88 | 3.94 | -3.36 | 21.72 | 1.45 | 19.74 |
| 2011 | 2 | 8.41 | 4.06 | 2.87 | -28.06 | 44.88 | 5.54 | 11.28 |
| 2012 | 8 | 8.2 | 4.66 | 1.65 | 4.3 | 12.09 | 3.26 | 18.07 |
| 2013 | 2 | 12.63 | 6.02 | 4.26 | -41.5 | 66.76 | 8.37 | 16.89 |
| Southern Region | ||||||||
| 2005 | 28 | 5.96 | 2.96 | 0.56 | 4.82 | 7.11 | 1.19 | 14.06 |
| 2006 | 100 | 7.64 | 4.9 | 0.49 | 6.67 | 8.62 | 2.26 | 32.53 |
| 2007 | 44 | 9.49 | 7.74 | 1.17 | 7.14 | 11.85 | 1.48 | 39.66 |
| 2008 | 29 | 5.51 | 2.74 | 0.51 | 4.47 | 6.55 | 2.15 | 16.78 |
| 2009 | 175 | 9.09 | 5.45 | 0.41 | 8.28 | 9.9 | 1.17 | 37.8 |
| 2010 | 12 | 9.35 | 5.33 | 1.54 | 5.97 | 12.74 | 4.12 | 18.55 |
| 2011 | 35 | 9.8 | 7.89 | 1.33 | 7.09 | 12.51 | 1.18 | 43.64 |
| 2012 | 30 | 7.7 | 4.06 | 0.74 | 6.19 | 9.22 | 1.88 | 17.22 |
| 2013 | 31 | 8.19 | 5.13 | 0.92 | 6.31 | 10.07 | 3.32 | 30.43 |
| Season | ||||||||
| Northern Region | ||||||||
| 1 | 30 | 7.82 | 4.93 | 0.9 | 5.97 | 9.66 | 1.45 | 20.32 |
| 2 | 71 | 6.89 | 4.58 | 0.54 | 5.8 | 7.97 | 2.35 | 32.53 |
| 3 | 7 | 7.47 | 3.57 | 1.35 | 4.17 | 10.77 | 1.92 | 12.68 |
| 4 | 21 | 10.01 | 8.63 | 1.88 | 6.09 | 13.94 | 2.44 | 43.96 |
| Southern Region | ||||||||
| 1 | 145 | 7.45 | 4.73 | 0.39 | 6.67 | 8.22 | 1.18 | 32.53 |
| 2 | 148 | 8.42 | 5.47 | 0.45 | 7.53 | 9.31 | 1.17 | 39.66 |
| 3 | 88 | 9.76 | 6.36 | 0.68 | 8.41 | 11.1 | 1.57 | 37.8 |
| 4 | 103 | 8.31 | 5.88 | 0.58 | 7.16 | 9.46 | 1.19 | 43.64 |
| Total | 613 | 8.20 | 5.56 | 0.22 | 7.76 | 8.64 | 1.17 | 43.96 |
NA, not available.
Rt in southern and northern regions of China
Of the 613 incidents, 129 from the northern regions in China exhibited a mean Rt of 7.64 (±5.52), and the remaining 484 incidents from the southern regions exhibited a mean Rt of 8.35 (±5.56). The t-test indicated that the difference in the Rt value between the northern and southern regions was not statistically significant (t = -1.278, P = 0.202; Table 2).
Rt in different provinces
Distributions of Rt in different provinces are shown in Table A in S2 File. There were 613 outbreaks distributed in 26 provinces in Mainland China (Hong Kong SAR, Macao SAR, and Taiwan were not included). Of these provinces, Shanxi, Shaanxi, and Hainan provinces exhibited the largest Rt values of 13.07, 13.07, and 10.60, respectively. The autonomous regions of Inner Mongolia, Ningxia and Shanghai city exhibited the smallest Rt values of 5.15, 5.73, and 5.80, respectively. Non-parametric Kruskal-Wallis analysis indicated significant differences in the Rt values among the provinces (χ2 = 48.358, P = 0.003).
Rt at different locations
Of the 613 incidents, primary (344 incidents) and secondary (191 incidents) schools accounted for 87.28% of the total incidents. The homogeneity of variance test resulted in a Levene value of 1.373 (P = 0.205). Therefore, the variances in Rt of the subtypes were considered homogeneous, and analysis of variance was used for the analysis among the subtypes. Analysis of variance resulted in F = 0.866 and P = 0.545, indicating that the differences in Rt among the subtypes were not statistically significant (Table 2). A total of 129 and 484 outbreaks took place in northern and southern China respectively. Although there was a difference in the number of outbreaks, there was no significant difference in Rt for different regions (Northern: F = 0.379 and P = 0.930 by analysis of variance, Southern: F = 0.823 and P = 0.583).
Rt in various populations
As shown in Table 2, of the 613 incidents, 290 incidents with an affected number of less than 1000 were designated “0~ group”. Similarly, 166 incidents with an affected number of 1000–1999 and the remaining 157 incidents with an affected number of ≥2000 were designated “1000~ group” and “2000~ group”, respectively. The homogeneity of variance test resulted in a Levene value of 6.556 (P = 0.002), indicating that the variances of Rt at the various population levels were heterogeneous. Following mixed sorting of Rt, we conducted a homogeneity of variance test for the Rt order. The result indicated that the Levene value was 0.032 (P = 0.969), suggesting that the variances of the Rt order at the various population levels were homogeneous, and analysis of variance was conducted. The analysis of variance resulted in a difference between the population levels that was statistically significant (F = 6.972, P = 0.001). Bonferroni paired comparisons indicated that the differences between the “0~ group” and “1000~ group” and between the “0~ group” and “2000~ group” were statistically significant and that the difference between the “1000~ group” and “2000~ group” was not statistically significant. In the northern region, again there was no significant difference in Rt for different population size (F = 0.190, P = 0.827). In contrast, it was for the southern part (F = 9.457, P = 0.000). Post-hoc analysis with Bonferroni adjustments showed that cities with small population (0~ group) had significantly higher Rt than their counterparts.
Rt at different times of occurrence
As shown in Table 2, the epidemics were prevalent every year, with the most incidents occurring in 2009 (214), followed by 2006 (146), whereas 2010 had the least number of incidents (16). The non-parametric Kruskal-Wallis analysis resulted in differences in Rt between the different years that were statistically significant (χ2 = 27.955, P = 0.000). The 2009 flu pandemic appeared to strike more in the southern region, because analysis of variance showed that there was no significant difference in Rt for different years (F = 1.121, P = 0.354) while there was for the southern part between 2008 and 2009 (F = 2.897, P = 0.004).
All 613 incidents were distributed across all four seasons, with more incidents in the second season than those in the first or fourth seasons where the first to the fourth seasons are the same as spring, summer, fall and winter. The third season had the least number of incidents (Table 2). The homogeneity of variance test resulted in a Levene value of 1.915 (P = 0.126). Therefore, the variances of Rt in the various seasons were homogenous, and analysis of variance was used to analyze the subtypes. The analysis of variance resulted in differences in Rt between the different seasons that were statistically significant (F = 3.306, P = 0.020). The Bonferroni paired comparison resulted in a statistically significant difference between the first and third seasons, whereas the differences between the remaining seasons were not statistically significant. Influenza seasonality were not found to result in different Rt in the northern region (F = 1.777, P = 0.155). In contrast, in the southern counterpart, there was lower Rt in the first season and higher Rt in the third season.
Sensitivity analysis
Among the 10 randomly selected outbreaks, two located in Fujian province, the others located in Inner Mongolia, Gansu, Jiangxi, Guangdong, Zhejiang, Jiangsu, Guangxi, Hubei, respectively. Four of them were occurred in the year 2006, three in 2007, one in 2008, one in 2009 and one in 2013. According to the results of the sensitivity analysis, we found that κ and S0 can only slightly impact Rt (see Tables 3 and 4). The larger S0 is, the smaller pR would be. The results of curve estimation showed that the 10 functions could depicted the relationship between S0 and pR. But according to the formula of the functions, the fuction Inverse (pR = 1.344+3841.5/S0) should be the best one to estimate, of which the coefficient of determination R2 = 0.776, P = 0.001. For the outbreak which has a relative larger S0 (e.g. outbreak ID = 25), Rt would be stable after 50S0, but along with the decreasing of S0, Rt would be stable after 100S0 (e.g. outbreak ID = 215).
Table 3. Estimated Rt value based on different κ in 10 randomly selected outbreaks in China.
| Outbreak ID | Province | Year | Rt | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| κ = 0.9 | κ = 0.8 | κ = 0.7 | κ = 0.6 | κ = 0.5 | κ = 0.4 | κ = 0.3 | κ = 0.2 | κ = 0.1 | |||
| 1 | Inner Mongolia | 2007 | 3.88 | 3.89 | 3.91 | 3.93 | 3.97 | 4.00 | 4.04 | 4.10 | 4.16 |
| 13 | Fujian | 2007 | 6.96 | 6.97 | 6.97 | 6.97 | 6.98 | 7.00 | 7.03 | 7.07 | 7.12 |
| 25 | Gansu | 2007 | 5.24 | 5.27 | 5.30 | 5.33 | 5.38 | 5.43 | 5.49 | 5.57 | 5.66 |
| 103 | Fujian | 2008 | 4.83 | 4.84 | 4.86 | 4.87 | 4.89 | 4.92 | 4.96 | 5.01 | 5.06 |
| 215 | Jiangxi | 2006 | 6.82 | 6.82 | 6.83 | 6.84 | 6.87 | 6.89 | 6.93 | 6.99 | 7.05 |
| 245 | Guangdong | 2006 | 6.13 | 6.13 | 6.14 | 6.15 | 6.17 | 6.19 | 6.22 | 6.27 | 6.33 |
| 251 | Zhejiang | 2006 | 2.56 | 2.57 | 2.59 | 2.61 | 2.63 | 2.66 | 2.69 | 2.73 | 2.78 |
| 275 | Jiangsu | 2006 | 7.06 | 7.06 | 7.08 | 7.11 | 7.14 | 7.18 | 7.24 | 7.30 | 7.39 |
| 475 | Guangxi | 2013 | 6.34 | 6.34 | 6.33 | 6.33 | 6.33 | 6.34 | 6.35 | 6.37 | 6.41 |
| 533 | Hubei | 2009 | 6.24 | 6.24 | 6.25 | 6.25 | 6.27 | 6.29 | 6.32 | 6.36 | 6.42 |
Table 4. Estimated Rt value based on different S0 in 10 randomly selected outbreaks in China.
| Outbreak ID | S0 | pR (%) | Rt | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S0 | 5S0 | 10S0 | 20S0 | 50S0 | 100S0 | 1000S0 | |||
| 103 | 295 | 13.18 | 4.89 | 4.35 | 4.3 | 4.27 | 4.26 | 4.25 | 4.25 |
| 215 | 508 | 8.96 | 6.87 | 6.35 | 6.3 | 6.27 | 6.26 | 6.25 | 6.25 |
| 275 | 595 | 11.73 | 7.14 | 6.44 | 6.37 | 6.34 | 6.32 | 6.31 | 6.31 |
| 533 | 747 | 5.02 | 6.27 | 6.01 | 5.98 | 5.97 | 5.96 | 5.96 | 5.95 |
| 1 | 930 | 6.9 | 3.97 | 3.74 | 3.71 | 3.7 | 3.7 | 3.69 | 3.69 |
| 13 | 957 | 3.92 | 6.98 | 6.76 | 6.73 | 6.72 | 6.71 | 6.71 | 6.71 |
| 475 | 2441 | 1.04 | 6.33 | 6.27 | 6.27 | 6.26 | 6.26 | 6.26 | 6.26 |
| 245 | 2848 | 1.49 | 6.17 | 6.1 | 6.09 | 6.09 | 6.08 | 6.08 | 6.08 |
| 251 | 3079 | 1.57 | 2.63 | 2.6 | 2.59 | 2.59 | 2.59 | 2.59 | 2.59 |
| 25 | 3624 | 5.19 | 5.38 | 5.15 | 5.12 | 5.11 | 5.1 | 5.1 | 5.1 |
Our model was sensitive to the parameters ω, ω', and k; however, the value that we set in our model led to a similar prevalence as the mean value of sensitivity analysis based on the constructed models (Figure D Panels A-C in S2 File). The model is not sensitive to the parameter γ', and the prevalence was highly similar to the mean, mean—SD, mean + SD, or our set value (Figure D Panel D in S2 File).
Discussion
This study is the first to systematically analyze the influenza outbreaks in Mainland China by ODE modeling.
We estimated the proportion of the asymptomatic in influenza outbreaks, as well as the basic reproduction numbers under different groups of influenza virus subtypes, northern and southern regions, provinces, sites, size of the population affected, years and seasons. The data provide China and also the rest of the world a reference to understand characteristics of transmission and develop prevention and control strategies.
The existence of asymptomatic cases significantly challenges the prevention and control of influenza. Some studies have shown that the p value of influenza can exceed 75%, and the difference between seasonal and pandemic influenza was not significant [2]. But recently, Leung et al [18] did a systematic review and found that estimates of the p based on outbreak investigations and household transmission studies appeared to provide more homogeneity in estimates of the p, with most point estimates in the range 4%–28% and a pooled mean of 16% (95% CI: 13–19). Our study indicated that the p value of the influenza virus can reach a maximum of 94% and exhibited a mean of 14% (95% CI: 12%–16%) during an outbreak, which was similar to the finding of Leung et al [18]; however, most outbreaks did not include asymptomatic infections. Different subtypes appeared to follow similar transmission pattern and this information can be exploited to strategically allocate resources in future prevention and control management. Our estimated p were different from what has been found in other studies, likely due to that the source of our data is from large-scale prevalence data from schools. We only included the incident with more than or equal to 30 cases of ILI per week, which intrinsically leads to lower proportion of asymptomatic individuals.
According to the definition of Rt, influenza spreads more rapidly with a larger Rt, resulting in greater challenges in the prevention and control of an epidemic. The results of this study indicated that during an influenza outbreak, the transmission capacity of the influenza virus was relatively high; the mean Rt reached 8.20 (95% CI: 7.76–8.64), whereas Rt of the total population is approximately 1.2–2.3 [11, 14, 19]. This difference may be related to the high population density and high population contact level in schools, collective organizations, closed locations, and certain small villages. Our estimated Rt were also different from what has been found in other studies, likely due to the inclusion criteria aforementioned, which means that the data we collected would be super spreading event and this intrinsically leads to higher Rt. Indeed, a study in Taiwan also showed that Rt estimated from influenza outbreaks in schools can be up to 19 [16].
According to these results, transmission capacity differences between the subtypes were not significant, indicating that outbreaks caused by seasonal influenza A (H3N2), A (H1N1), and A (H1N1) pdm09 viruses may be handled using similar prevention and control measures and do not require different treatments. This finding provides important scientific basis for the future prevention and control of influenza outbreaks in China. This result also quantitatively supported the decision by Chinese health administrative departments to adjust influenza A (H1N1) pdm09 from Category B to Category C and to combine influenza A (H1N1) pdm09 with current influenza management [20].
In spite of extensive data collection and analyses, this study carried limitations. First, we only included the incident with more than or equal to 30 cases of ILI per week. Second, data uncertainty such as reporting errors and under-reporting could lead to estimation errors of varying size.
Our study also indicated that there were no statistically significant differences in influenza transmission capacity during an outbreak between northern and southern regions in China or different location types in which incidents occurred. Although the difference between provinces was significant, this difference may arise from the small or zero numbers of influenza outbreaks reported by certain provinces. Notably, influenza transmission capacity is related to various population levels. The difference between the numbers of affected people was significant between the “0~ group” and “1000~ group” and between the “0~ group” and “2000~ group”. If the sample was less than 1000 people, the influenza transmission capacity was higher than a sample of ≥1000 people, indicating that schools, prisons, and factories with smaller populations have relatively higher prevention and control measures during an influenza outbreak and should apply earlier and more substantial prevention and control measures. Additionally, the influenza transmission capacity exhibits seasonality. The difference between the first and the third seasons was statistically significant, whereas the difference between the remaining seasons was not statistically significant. Transmission capacity in the third season was stronger than that in the first season. Therefore, future prevention and control measures for outbreaks should be conducted throughout the year in Mainland China, particularly during the third season. Furthermore, our study provides important prevention and control indicators for other countries.
Supporting Information
(PDF)
This file contains the following: Figure A. Temporal distribution of new influenza cases in a school in Mainland China, October 2009. Figure B. Asymptomatic infection ratio of various influenza virus subtypes. Figures 2-A to 2-F show the distribution of asymptomatic infection ratios of all of the subtypes of influenza (total, A (H3N2), A (H1N1) pdm, A (H1N1), B, and mixed subtypes, respectively). Figure C. Histogram of which means that the data we collected would be super spreading event of the influenza virus subtypes. Figures 3-A to 3-F show the distribution of Rt of all of the subtypes of influenza (total, A (H3N2), A (H1N1) pdm, A (H1N1), B, and mixed subtypes, respectively). Figure D. Results of the sensitivity analysis of the SEIAR model. Figures 4-A to 4-D show the results of the sensitivity analysis of κ, ω, ω', andγ', respectively. Table A. Distribution of Rt in various provinces.
(DOC)
Acknowledgments
We thank the all local Centers for Disease Control and Prevention for data report and field investigation. We thank Dr. Ross Ka-kit Leung, epidemiologist of Stanley Ho Centre for Emerging Infectious Diseases, The Chinese University of Hong Kong, for his critical reading and revision of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by China-U.S. Collaborative Program on Emerging and Re-emerging Infectious Disease. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization (WHO) (2015) A Manual for Estimating Disease Burden Associated With Seasonal Influenza. World Health Organization; Available: http://apps.who.int/iris/bitstream/10665/178801/1/9789241549301_eng.pdf?ua=1&ua=1 [Google Scholar]
- 2.Hayward AC, Fragaszy EB, Bermingham A, Wang L, Copas A, Edmunds WJ, et al. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med. 2014; 2(6):445–54. 10.1016/S2213-2600(14)70034-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y, Sugimoto JD, Halloran ME, Basta NE, Chao DL, Matrajt L, et al. The Transmissibility and Control of Pandemic. Influenza A (H1N1) virus. Science 2009; 326 (5953): 729–733. 10.1126/science.1177373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longini IM Jr, Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, Cummings DA, et al. Containing pandemic influenza at the source. Science 2005; 309 (5737): 1083–1087. 10.1126/science.1115717 [DOI] [PubMed] [Google Scholar]
- 5.Longini IM Jr, Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiol 2004; 159 (7): 623–633. [DOI] [PubMed] [Google Scholar]
- 6.Kermack W O, Mckendrick A G. A contribution to the mathematical theory of epidemics [M]. Proceedings of the Royal Society of London. 1927. [Google Scholar]
- 7.Stone L, Olinky R, Huppert A. Seasonal dynamics of recurrent epidemics. Nature 2007; 446:533–6. 10.1038/nature05638 [DOI] [PubMed] [Google Scholar]
- 8.Dushoff J, Plotkin JB, Levin SA, Earn DJ. Dynamical resonance can account for seasonality of influenza epidemics. PNAS 2004; 101:16195–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arino J, Brauer F, van den Driessche P, Watmough J, Wu J. Simple models for containment of a pandemic. J. R. Soc. Interface 2006; 3:453–7. 10.1098/rsif.2006.0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Health and Family Planning Commission of the People’s Republic of China. (2010) Influenza surveillance program of China (2010thedition). (in Chinese)
- 11.National Health and Family Planning Commission of the People’s Republic of China. (2011) Diagnosis and Treatment Guidelines for Influenza (2011thedition). (in Chinese)
- 12.Sertsou G, Wilson N, Baker M, Nelson P, Roberts MG. Key transmission parameters of an institutional outbreak during the 1918 influenza pandemic estimated by mathematical modeling. Theoretical Biology and Medical Modelling 2006, 3:38 10.1186/1742-4682-3-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tracht SM, Del Valle SY, Hyman JM. Mathematical Modeling of the Effectiveness of Facemasks in Reducing the Spread of Novel Influenza A(H1N1). PLoS ONE 5(2): e9018 10.1371/journal.pone.0009018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coburn BJ, Wagner BG, Blower S. Modeling influenza epidemics and pandemics: insights into the future of swine flu (H1N1). BMC Medicine 2009; 7:30 10.1186/1741-7015-7-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikolajczyk R, Krumkamp R, Bornemann R, Ahmad A, Schwehm M, Duerr HP. Influenza—Insights from Mathematical Modelling. Dtsch Arztebl Int 2009; 106(47): 777–82. 10.3238/arztebl.2009.0777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen SC, Liao CM. Modelling control measures to reduce the impact of pandemic influenza among schoolchildren. Epidemiol. Infect 2008; 136:1035–45. 10.1017/S0950268807009284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viral shedding and clinical illness in naturally acquired influenza virus infections, J. Infect. Dis 201: 1509–16(2010). 10.1086/652241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A systematic review and meta–analysis, Epidemiology 26, 862–872 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser C, Donnelly C, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 2009; 324(5934):1557–61. 10.1126/science.1176062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.http://www.chinapop.gov.cn/jkj/s3577/201311/f6ee56b5508a4295a8d552ca5f0f5edd.shtml
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
This file contains the following: Figure A. Temporal distribution of new influenza cases in a school in Mainland China, October 2009. Figure B. Asymptomatic infection ratio of various influenza virus subtypes. Figures 2-A to 2-F show the distribution of asymptomatic infection ratios of all of the subtypes of influenza (total, A (H3N2), A (H1N1) pdm, A (H1N1), B, and mixed subtypes, respectively). Figure C. Histogram of which means that the data we collected would be super spreading event of the influenza virus subtypes. Figures 3-A to 3-F show the distribution of Rt of all of the subtypes of influenza (total, A (H3N2), A (H1N1) pdm, A (H1N1), B, and mixed subtypes, respectively). Figure D. Results of the sensitivity analysis of the SEIAR model. Figures 4-A to 4-D show the results of the sensitivity analysis of κ, ω, ω', andγ', respectively. Table A. Distribution of Rt in various provinces.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.