Abstract
Purpose
Antiplatelet and/or anticoagulant medication use is common. Abstinence a week before surgery may still result in altered hemostasis. The study aim was to report on perioperative antiplatelet and anticoagulant use in thyroidectomy and parathyroidectomy patients, and to determine the association with postoperative hematoma (POH) rates.
Methods
Retrospective review of a prospective endocrine surgery database was performed. Procedure extent was defined as unilateral, bilateral, or extensive. Antiplatelets were categorized as none, 325 mg aspirin (ASA), <325 mg ASA, clopidogrel, or other. Anticoagulants were categorized as none, oral, or injectable.
Results
A total of 4514 patients were identified. POH developed in 22 patients (0.5 %). Rates were similar between age, gender, and reoperative status. POH were seven times more common after thyroidectomy (0.8 vs. 0.1 %, p < 0.01). Unilateral procedures had lower POH rates than bilateral or extensive (0.1 vs. 0.9 vs. 0.8 %, p < 0.01). POH rates in patients receiving 325 mg ASA (0.8 %) or clopidogrel (2.2 %) were much higher than patients not receiving antiplatelets (0.5 %) or receiving <325 mg ASA (0.1 %, p = 0.04). Oral anticoagulants (2.2 %) and injectable anticoagulants (10.7 %) had much higher POH rates than patients not receiving anticoagulants (0.4 %, p < 0.01). Target organ, patient gender, procedure extent, antiplatelet use, and anticoagulant use were included on logistic regression to determine association with POH. Bilateral procedures, thyroidectomy, clopidogrel, oral, and injectable anticoagulants were all independently associated with POH.
Conclusions
POH occur more frequently after thyroidectomy and during bilateral procedures. Patients requiring clopidogrel or any anticoagulant coverage are at much higher risk for POH. These higher-risk patients should be considered for observation to ensure prompt POH recognition and intervention.
Endocrine surgery practices are increasingly moving toward outpatient and/or ambulatory surgery.1–6 Performance in an outpatient setting is associated with lower charges, providing potential cost savings.2,7 Not all surgeons have embraced this approach. Concern for postoperative hematoma (POH) and symptomatic hypocalcemia motivates some to observe patients. Both issues are argued as a reason to keep patients overnight after a thyroid or parathyroid procedure.8,9 While patient comorbidities or the extent of surgery required may make outpatient surgery a less viable option for some, specific data regarding risk factors for POH are sparse.10,11 If concern for POH is the main reason for avoidance of same-day surgery, better data are needed to help clarify which patients may in fact be high risk for POH and thus justify observation.
One potential patient factor that may influence concern for POH is the use of anticoagulant and/or antiplatelet medications. Use and indications for these medications are expanding in our aging population.12 These medications are designed to prevent platelet aggregation or clot formation, both of which are crucial for hemostasis after any operation. For cervical procedures, POH can lead to acute airway obstruction by either direct compression or through venous congestion resulting in significant airway edema. However, the indications for antiplatelet and anticoagulant use can be equally critical in preventing embolic or thrombotic events which may lead to stroke, heart attack, or pulmonary embolism.13 Surgeons must work closely with the primary care providers and other specialists involved in the care of the patient to weigh the risk of continued antiplatelet and/or anticoagulant coverage through the perioperative period against the risk of interruption. While the risk of continued therapy has been explored in other surgical specialties, the impact during thyroid and parathyroid procedures is unknown.11,14–19
We sought to address the issue of POH formation after thyroid and parathyroid procedures and to explore the potential association with the use of antiplatelet and/or anticoagulant medications. The study aim was to report on perioperative antiplatelet and anticoagulant use in thyroidectomy and parathyroidectomy patients and to determine the association with POH rates. The study hypothesis was that the use of anticoagulant and/or antiplatelet medications in the perioperative period increases rates of POH after thyroid and parathyroid procedures.
METHODS
An institutional review board—approved prospective endocrine surgery database was queried to identify all patients undergoing cervical procedures for parathyroid and thyroid disease. Patient demographic information was noted. Records were reviewed to determine if a POH was formed according to attending surgeon documentation, to determine the timing of the development, and to learn whether operative intervention was required.
Patients meeting the inclusion criteria were classified as follows. Primary reason for surgery was either parathyroid or thyroid in nature. Extent of operation was unilateral (lobectomy or unilateral parathyroid exploration), bilateral (bilateral parathyroid exploration, total thyroidectomy, completion thyroidectomy), or extensive (any neck dissection, total parathyroidectomy, parathyroidectomy with autotransplant, thymectomy). Reoperative neck was any previous anterior neck procedure. For those patients who had thyroid and parathyroid procedures combined, they were classified on the basis of the extent of dissection required; if parathyroid and thyroid lobe were ipsilateral, patients were considered to be undergoing a unilateral procedure, whereas contralateral procedures were considered bilateral.
Medical records were reviewed to determine the use of antiplatelet and/or anticoagulant medications during the perioperative period. General practice is to have oral antiplatelet and/or anticoagulant medications held for 5–7 days before surgery, with variable timing for resuming therapy after surgery. Decisions regarding bridging is individualized and are made on the basis of the recommendations of the primary care provider and/or cardiologist. Use in the perioperative period was defined as within 2 weeks of surgery in order to assess the potential impact on operative hemostasis even when held appropriately, and to assess the impact on late hematoma formation when resumed in the immediate postoperative period. Antiplatelet medications were classified as none, less than full-dose aspirin (ASA < 325 mg), full-dose ASA (≥325 mg), or clopidogrel (Plavix, Sanofi-Aventis). If the patient received both ASA and clopidogrel, the patient was classified as receiving clopidogrel. Anticoagulant medications were classified as none, oral [warfarin (Coumadin, Bristol-Meyers Squibb), dabigatran (Pradaxa, Boehringer Ingelheim Pharmaceuticals)], or injectable [heparin drip, enoxaparin (Lovenox, Sanofi-Aventis), dalteparin (Fragmin, Pfizer)]. Patients who normally received oral anticoagulants but who were bridged for surgery with injectable anticoagulants were coded as receiving injectable anticoagulation. Chemoprophylaxis for deep vein thrombosis is not performed at the study institution. We noted whether patients were receiving antiplatelet or anticoagulant medications, receiving only one, or receiving both.
Statistical analysis was performed by IBM SPSS Statistics 21 (IBM SPSS, Chicago, IL, USA). A Kaplan–Meier curve was created to demonstrate the timeline between the initial operation and reoperation for POH. χ2 test, unpaired t test, and the Kruskal–Wallis test were used as appropriate for univariate analysis. Binary logistic regression was performed to determine the odds ratio (OR) for hematoma formation. Reference values for logistic regression were set as no medication (when comparing the different antiplatelet or anticoagulants), unilateral procedures, and male gender. A p value of ≤0.05 was defined as significant. Data are expressed as number (percent), mean ± standard error of the mean (SEM), or OR (95 % confidence interval), unless otherwise stated.
RESULTS
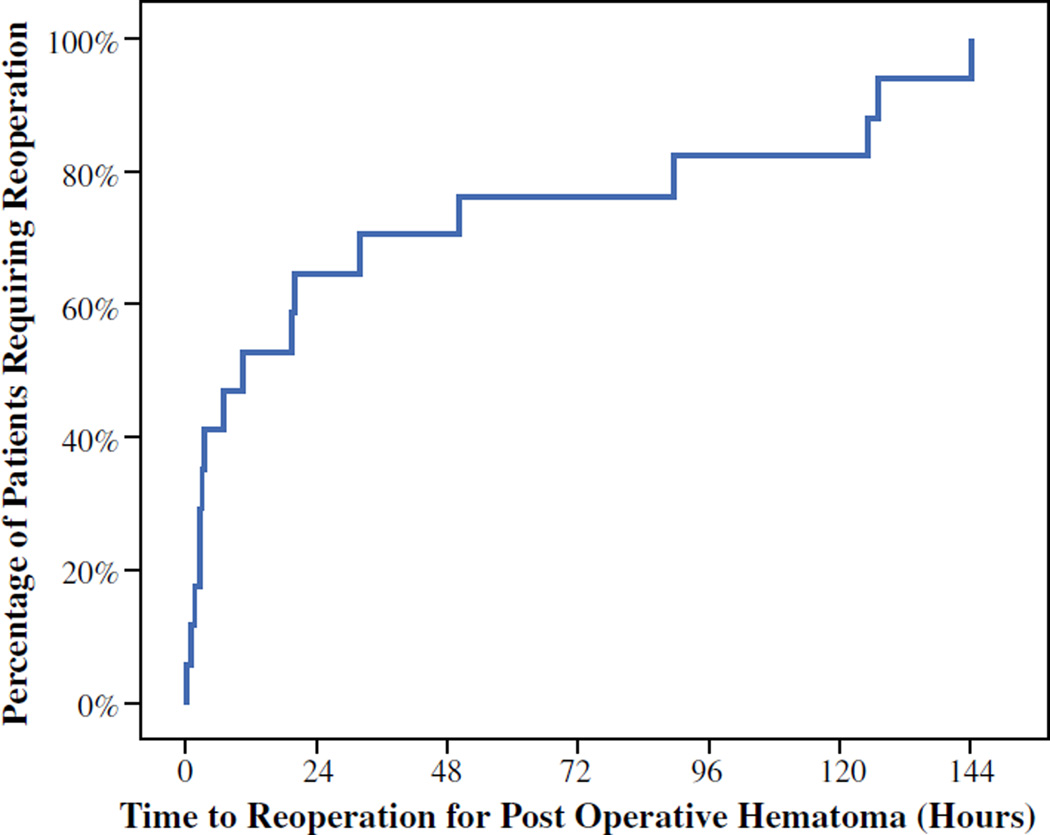
Between 1994 and 2013, a total of 4514 patients underwent a thyroid and/or parathyroid procedure and met the study’s inclusion criteria. Mean patient age was 53 ± 0.2 years. Female patients comprised 77 % of the study population. A total of 2395 patients (53 %) underwent thyroid surgery, and 2119 (47 %) underwent parathyroid surgery. A unilateral procedure was performed in 2332 (52 %) patients, 2147 (47 %) underwent a bilateral procedure, and 36 (1 %) underwent an extensive procedure. Antiplatelet medications were provided to 1131 patients (25.1 %), while anticoagulants were provided to only 167 patients (3.7 %). Less than full-dose ASA was the most common antiplatelet medication provided to patients (n = 670, 15 %), followed by ≥325 mg ASA (n = 372, 8 %) and clopidogrel (n = 89, 2 %). Oral anticoagulation was provided to 139 patients (3.1 %), and only 28 patients (0.6 %) were provided injectable anticoagulants in the perioperative period. POH were noted in 22 patients (0.5 %), with 17 patients requiring operative intervention and 5 patients undergoing successful, nonoperative management. Time from initial operation to need for reoperation for those 17 patients was noted (Fig. 1). POH development within the 4- or 6-h observation period was 42 %, while 50 % of POH occurred within 7 h of surgery. A majority (65 %) of the operative POH occurred within the first 24 h of surgery, with only 35 % occurring beyond the 24-h mark. There were no 30-day mortalities noted.
FIG. 1.
Time from initial surgery to reoperation in patients with postoperative hematoma
To see if there was any difference in practice patterns contained within the data set over time, procedures were categorized by year of operation. The first half of the series spanned from 1994 to 2003 and contained 956 patients (21 %), while the latter half spanned from 2004 to 2013 and included 3558 patients (79 %). During these two time periods, the nature of the procedures performed shifted from being mostly thyroid during the first half (78 % thyroidectomy and 22 % parathyroidectomy) to more evenly divided between thyroid and parathyroid (46 and 54 %, respectively, p < 0.001). The hematoma rates were equivalent between the two time periods (0.3 vs. 0.5 %, p = 0.60).
Parathyroid Versus Thyroid Patient Populations
Initial analysis looked at patients undergoing thyroid surgery compared to parathyroid surgery (Table 1). Parathyroid patients were older (59 ± 0.3 years) than thyroid patients (49 ± 0.3 years, p < 0.001). However, female predominance was equivalent between both populations (p = 0.37). Parathyroid procedures were more often unilateral (60 %) versus bilateral (40 %), while thyroid procedures were more often bilateral (55 %) versus unilateral (44 %, p < 0.001). Parathyroid patients were more likely to have had previous neck surgery (14 vs. 9 %, p < 0.001). Antiplatelet receipt differed between the thyroid and parathyroid patients (p < 0.001). Anticoagulant receipt also differed, with more parathyroid patients receiving oral anticoagulation (4 vs. 2 %, p < 0.001), although use of injectable therapy was similar (0.5 vs. 0.8 %). Parathyroid patients required some form of antiplatelet and/or anticoagulant more often than thyroid patients (p < 0.001). Despite this greater receipt of anticoagulants and antiplatelet medications, parathyroid patients still had lower rates of POH than thyroid patients (0.1 vs. 0.8 %, p < 0.001).
TABLE 1.
Demographics of patients who underwent thyroid-related operation versus parathyroid-related operation
| Characteristic | Parathyroid | Thyroid | p |
|---|---|---|---|
| No. of patients | 2119 | 2395 | |
| Age (years) | 59 ± 0.3 | 49 ± 0.3 | <0.001 |
| Female | 77 % | 78 % | 0.37 |
| Extent of operation | |||
| Unilateral | 1279 (60 %) | 1046 (44 %) | <0.001 |
| Bilateral | 824 (39 %) | 1243 (52 %) | |
| Extensive | 16 (0.8 %) | 106 (4 %) | |
| Reoperative neck | 293 (14 %) | 206 (9 %) | <0.001 |
| Antiplatelet use | |||
| None | 1426 (67 %) | 1957 (82 %) | <0.001 |
| <325 mg aspirin | 460 (22 %) | 210 (9 %) | |
| ≥325 mg aspirin | 181 (8 %) | 191 (8 %) | |
| Clopidogrel | 52 (2 %) | 37 (2 %) | |
| Anticoagulant use | |||
| None | 2021 (95 %) | 2326 (97 %) | <0.001 |
| Oral | 87 (4 %) | 52 (2 %) | |
| Injectable | 11 (0.5 %) | 17 (0.8 %) | |
| Use of either medication type | |||
| None | 1359 (64 %) | 1903 (79 %) | <0.001 |
| One | 729 (34 %) | 480 (20 %) | |
| Both | 31 (1.5 %) | 12 (0.5 %) | |
| Hematoma rate | 2 (0.1 %) | 20 (0.8 %) | <0.001 |
Data are presented as mean ± SEM or as n (%)
Predictors of POH
Further univariate analysis was performed comparing patients who developed POH to those who did not (Table 2). Patient age (p = 0.44) and gender (p = 0.61) were equivalent, as was previous neck surgery (p = 0.73). POH rate was much higher in thyroid patients. Patients undergoing bilateral or extensive surgery had higher rates of POH (0.9 and 0.8 %, respectively), in contrast to unilateral procedures (0.1 %, p = 0.002). Rates of POH in patients receiving <325 mg ASA or not receiving any form of antiplatelet medication (0.1 and 0.5 %, respectively) were low, while patients receiving ≥325 mg ASA (0.8 %) and clopidogrel (2.2 %) had higher rates of POH (p = 0.045). Patients not receiving any form of anticoagulation had low POH rates (0.4 %), but oral anticoagulation increased POH rates 5.5-fold (2.2 %) and injectable anticoagulant increased POH rates 26.8-fold (10.7 %, p < 0.001). As expected, use of a single agent (either antiplatelet or anticoagulant) resulted in a slight increase in POH rate (0.8 %), whereas use of both increased POH over sevenfold (2.3 %, p = 0.033).
TABLE 2.
Demographics of patients according to development of postoperative hematoma
| Characteristic | No hematoma | Hematoma | p |
|---|---|---|---|
| No. of patients | 4492 | 22 | |
| Age (years) | 54 ± 0.2 | 55 ± 4.2 | 0.44 |
| Patient gender | |||
| Female | 99.5 % | 0.5 % | 0.61 |
| Male | 99.4 % | 0.6 % | |
| Target organ | |||
| Thyroid | 2375 (99.2 %) | 20 (0.8 %) | <0.001 |
| Parathyroid | 2117 (99.9 %) | 2 (0.1 %) | |
| Extent of operation | |||
| Unilateral | 2322 (99.9 %) | 3 (0.1 %) | 0.002 |
| Bilateral | 2049 (99.1 %) | 18 (0.9 %) | |
| Extensive | 121 (99.2 %) | 1 (0.8 %) | |
| Reoperative neck | 496 (99.4 %) | 3 (0.6 %) | 0.73 |
| Antiplatelet use | |||
| None | 3367 (99.5 %) | 16 (0.5 %) | 0.045 |
| <325 mg aspirin | 669 (99.9 %) | 1 (0.1 %) | |
| ≥325 mg aspirin | 369 (99.2 %) | 3 (0.8 %) | |
| Clopidogrel | 87 (97.8 %) | 2 (2.2 %) | |
| Anticoagulant use | |||
| None | 4331 (99.6 %) | 16 (0.4 %) | <0.001 |
| Oral | 136 (97.8 %) | 3 (2.2 %) | |
| Injectable | 25 (89.3 %) | 3 (10.7 %) | |
| Use of either medication type | |||
| None | 3251 (99.7 %) | 11 (0.3 %) | 0.033 |
| One | 1199 (99.2 %) | 10 (0.8 %) | |
| Both | 42 (97.7 %) | 1 (2.3 %) |
Data are expressed as mean ± SEM or as n (%)
Patient gender, type of procedure, extent of procedure, antiplatelet use, and anticoagulant use were all used for binary logistic regression to determine which factors were independently associated with the formation of POH (Table 3). With this multivariate analysis, female gender, extensive procedures, and the use of ASA regardless of dose did not result in an increased likelihood of POH formation. However, thyroid procedures conveyed a 7.9 (1.8–35) increased odds of POH, and bilateral procedures conveyed a 4.9 (1.42–17.0) increased odds of POH. Clopidogrel use was associated with a 5.6 (1.15–27.13) increased odds of POH. Oral (OR 7.51, 2.00–28.24) and injectable anticoagulation (29.45, 7.46–116.37) were both independently associated with significantly increased likelihood of POH.
TABLE 3.
Multivariate analysis of factors independently associated with postoperative hematoma formation
| Characteristic | Odds ratio (95 % confidence interval) | p |
|---|---|---|
| Female gender | 0.92 (0.34–2.47) | 0.86 |
| Thyroid procedure | 7.92 (1.78–35.16) | 0.007 |
| Extent of procedure | ||
| Bilateral | 4.92 (1.42–17.00) | 0.012 |
| Extensive | 4.27 (0.43–42.28) | 0.21 |
| Antiplatelet use | ||
| <325 mg aspirin | 0.43 (0.06–3.38) | 0.43 |
| ≥325 mg aspirin | 2.75 (0.76–9.92) | 0.12 |
| Clopidogrel | 5.60 (1.15–27.13) | 0.033 |
| Anticoagulant use | ||
| Oral | 7.51 (2.00–28.24) | 0.003 |
| Injectable | 29.45 (7.46–116.37) | <0.001 |
Additional analysis of the thyroidectomy only (Tables 4, 5) and parathyroidectomy only (Table 6) are provided. A narrative on these results is provided as the Online Supplement.
TABLE 4.
Demographics of thyroid patients according to development of postoperative hematoma
| Characteristic | No hematoma | Hematoma | p |
|---|---|---|---|
| No. of patients | 2375 | 20 | |
| Age (years) | 48.8 ± 0.3 | 54.6 ± 4.6 | 0.11 |
| Patient gender | |||
| Female | 99.2 % | 0.8 % | 0.79 |
| Male | 99.1 % | 0.9 % | |
| Extent of operation | |||
| Unilateral | 1044 (99.8 %) | 2 (0.2 %) | <0.01 |
| Bilateral | 1226 (98.6 %) | 17 (1.4 %) | |
| Extensive | 105 (99.1 %) | 1 (0.9 %) | |
| Reoperative neck | 203 (98.5 %) | 3 (1.5 %) | 0.41 |
| Antiplatelet use | |||
| None | 1943 (99.3 %) | 14 (0.7 %) | 0.01 |
| <325 mg aspirin | 209 (99.5 %) | 1 (0.5 %) | |
| ≥325 mg aspirin | 188 (98.4 %) | 3 (1.6 %) | |
| Clopidogrel | 35 (94.6 %) | 2 (5.4 %) | |
| Anticoagulant use | |||
| None | 2311 (99.4 %) | 15 (0.6 %) | <0.001 |
| Oral | 50 (96.2 %) | 2 (3.8 %) | |
| Injectable | 14 (82.4 %) | 3 (17.6 %) | |
| Use of either medication type | |||
| None | 1893 (99.8 %) | 10 (0.5 %) | <0.001 |
| One | 471 (98.1 %) | 9 (1.9 %) | |
| Both | 11 (91.7 %) | 1 (8.3 %) |
Data are expressed as mean ± SEM or as n (%)
TABLE 5.
Multivariate analysis of factors independently associated with postoperative hematoma formation in patients undergoing thyroid surgery
| Characteristic | Odds ratio (95 % confidence interval) | p |
|---|---|---|
| Female gender | 0.95 (0.35–3.04) | 0.95 |
| Extent of procedure | ||
| Bilateral | 6.65 (1.51–29.3) | 0.01 |
| Extensive | 5.89 (0.52–66.4) | 0.15 |
| Antiplatelet use | ||
| <325 mg aspirin | 0.59 (0.07–4.71) | 0.60 |
| ≥325 mg aspirin | 3.44 (0.93–12.60) | 0.06 |
| Clopidogrel | 8.28 (1.65–41.50) | 0.01 |
| Anticoagulant use | ||
| Oral | 5.36 (1.08–26.51) | 0.04 |
| Injectable | 34.05 (8.27–140.23) | <0.001 |
TABLE 6.
Demographics of parathyroid patients according to development of postoperative hematoma
| Characteristic | No hematoma | Hematoma | p |
|---|---|---|---|
| No. of patients | 2117 | 2 | |
| Age (years) | 59.3 ± 0.3 | 61.5 ± 6.5 | 0.82 |
| Patient gender | |||
| Female | 99.8 % | 0.2 % | 0.41 |
| Male | 99.9 % | 0.1 % | |
| Extent of operation | |||
| Unilateral | 1278 (99.9 %) | 1 (0.1 %) | 0.94 |
| Bilateral | 823 (99.9 %) | 1 (0.1 %) | |
| Extensive | 16 (100 %) | 0 | |
| Reoperative neck | 293 (100 %) | 0 | 1.0 |
| Antiplatelet use | |||
| None | 1424 (99.9 %) | 2 (0.1 %) | 0.81 |
| <325 mg aspirin | 460 (100 %) | 0 | |
| ≥325 mg aspirin | 181 (100 %) | 0 | |
| Clopidogrel | 52 (100 %) | 0 | |
| Anticoagulant use | |||
| None | 2020 (100 %) | 1 (0 %) | <0.01 |
| Oral | 86 (98.9 %) | 1 (1.1 %) | |
| Injectable | 11 | 0 | |
| Use of either medication type | |||
| None | 1358 (99.9 %) | 1 (0.1 %) | 0.89 |
| One | 728 (99.9 %) | 1 (0.1 %) | |
| Both | 31 (100 %) | 0 |
Data are expressed as mean ± SEM or as n (%)
DISCUSSION
In this single institution study, spanning 19 years, we noted an overall POH rate of 0.5 %, but only 0.4 % required operative intervention. Within this sample of over 4500 patients, 25 % of the population used antiplatelets and 3.7 % used anticoagulants. This is the only large cohort study to date that includes the use of anticoagulant and/or antiplatelet medications as a variable to elucidate potential association to POH formation. It provides helpful information by way of ORs for POH formation, which can foster the development of guidelines specific to thyroid and parathyroid procedures weighing the risk–benefit ratios of continuation versus interruption of antiplatelet and/or antiplatelet therapy.
The overall POH rate described herein of 0.5 % is along the lower side of previously reported POH rates in the literature.9,18,20–23 Abbas et al. noted a POH rate of 0.8 % in their combined thyroidectomy and/or parathyroidectomy population, with parathyroidectomy having a higher POH rate (1.1 vs. 0.7 %).20 Burkey et al. reviewed over 13,000 patients with a POH rate of only 0.3 %.18 However, analysis into factors associated with POH was performed in a 1-to-1 case–control fashion, limiting the conclusions. Lang et al. found a POH rate of 0.7 % that required operative intervention, with an additional 0.6 % with POH not requiring reoperation.22 The Scandinavian experience focused on thyroidectomy only, with a POH rate of 2.1 % associated with older age and male gender.21 An additional European series found a POH rate of 1.7 % in thyroidectomy patients.9 The authors made mention of anticoagulant and antiplatelet management that required discontinuation of the medications for at least 10 days before surgery.
With these limited data on this rare complication, the debate is raised regarding the length of observation after thyroidectomy or parathyroidectomy. While some advocate for routine same-day surgery, others are more cautious, preferring to be more selective for same-day surgery cases and requiring a period of observation before dismissal.6,9,18,23,24 Recommended time periods of observation vary and also differ with procedure (lobectomy vs. total).4,6,9,18,24 Recommendations are based on the observations within large, retrospective studies and the timing of POH formation. However, additional variables also need to be considered, as all studies represent the work of high-volume thyroid surgeons and thyroid centers. Low-volume surgeons are known to have much higher rates of complications compared to their high-volume counterparts, and therefore a more conservative period of observation should be considered in these instances.25,26 Additionally, surgical technique has evolved over time to include the use of new technology and pharmacologic agents, although in the data presented here, no difference was noted in POH rates between the first and latter half of the study period.27–32 Ultimately, this leaves the duration of observation period up to the individual surgeon, to be based on experience, comfort level, and extent of surgery. Our data demonstrate that 42 % of POH occur within the first 4 h, and 50 % occur within 7 h of surgery.
The impact of antiplatelet and anticoagulant therapies on postoperative hemostasis is not equal, as found in this study. Antiplatelet therapies are often provided to prevent in-stent thrombosis in patients who have undergone previous percutaneous coronary or other vascular intervention, and they are less problematic for many surgical procedures.14,16,33,34 Anticoagulant therapy poses a much larger problem. We have demonstrated POH rates of 2.2 and 10.7 %, respectively, for those patients receiving particular antiplatelet or anticoagulant therapies, which is significantly higher than the overall POH rate of 0.5 %. This is consistent with increased rates of postoperative bleeding complications seen with other surgical procedures.14–16,35–37 Our data provide objective rates of potential bleeding complications for patients undergoing thyroid and/or parathyroid surgery. The additional subgroup analysis based on the target organ (thyroid vs. parathyroid) demonstrates a greater influence on POH after thyroidectomy than with parathyroidectomy, even when bilateral parathyroid explorations are performed (data provided in the Online Supplement). Our cardiology and internal medical colleagues managing the antiplatelet and anticoagulant therapies can utilize these data to assist in developing an individualized plan for management in the perioperative period that weighs both the risks and benefits of both continuation of therapy and interruption of therapy.
This study has inherent limitations due to the retrospective nature of its design. However, given the rare nature of this complication, it is difficult to study POHs in a prospective fashion. No formal protocol was in place regarding the alteration of patient use of antiplatelet and anticoagulant therapy, in part because of the lack of guidelines regarding the risks and benefits of hemorrhagic versus embolic/thrombotic complications. Records on patients early in the study period may not have included all information regarding both the use of antiplatelets or anticoagulants; nor may they have information on hematomas that did not require operative management. The use of antiplatelets and/or anticoagulants was defined for the purposes of this study within the 2-week perioperative period, which may actually underestimate the true effects of antiplatelet or anticoagulant therapy when they are at full therapeutic levels. However, we thought it was imperative to define these patients as such to ensure adequate representation of how patients are often managed with respect to their anticoagulation and antiplatelet therapy, and how that potentially affects operative outcome. It is unclear how additional variables thought to also influence the development of POH may interact and influence POH rates.10,24
CONCLUSIONS
POH after thyroid and parathyroid surgery remains a rare complication, occurring in only 0.5 % over the 19-year study period. Antiplatelet medications are commonly used by patients undergoing thyroid and parathyroidectomy, and anticoagulant use is seen in less than 4 % of the study population. Despite their infrequent use, anticoagulants have a strong association with increased rates of hematoma formation in both parathyroid and thyroid surgery, even if stopped 5–7 days before surgery. Injectable anticoagulants in particular increase the odds of hematoma formation 29-fold. For these reasons, patients receiving anticoagulation therapy should be individually considered for the risk of thrombotic event versus the risk of POH, with an appropriate perioperative plan for anticoagulation formulated accordingly. This may also alter the treatment plan for the thyroid or parathyroid pathology; depending on the underlying pathology, the operative risk–benefit ratio of the procedure may not outweigh the risk–benefit ratio of alteration of the anticoagulation. Patients with multiple identifiable risk factors for hematoma should be considered for a period of observation after thyroid or parathyroid surgery.
Supplementary Material
Footnotes
Presented as an oral presentation at the ACS Surgical Forum, American College of Surgeons Clinical Congress, San Francisco, CA, October 2014.
Electronic supplementary material The online version of this article (doi:10.1245/s10434-016-5241-0) contains supplementary material, which is available to authorized users.
CONFLICT OF INTEREST The authors declare no conflict of interest.
REFERENCES
- 1.Wang TS, Pasieka JL, Carty SE. Techniques of parathyroid exploration at North American endocrine surgery fellowship programs: what the next generation is being taught. Am J Surg. 2014;207:527–532. doi: 10.1016/j.amjsurg.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Sun GH, DeMonner S, Davis MM. Epidemiological and economic trends in inpatient and outpatient thyroidectomy in the United States, 1996–2006. Thyroid. 2013;23:727–733. doi: 10.1089/thy.2012.0218. [DOI] [PubMed] [Google Scholar]
- 3.Clark N, Schneider DF, Vrabec S, Bauer PS, Chen H, Sippel RS. Increased efficiency of endocrine procedures performed in an ambulatory operating room. J Surg Res. 2013;184:200–203. doi: 10.1016/j.jss.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazeh H, Khan Q, Schneider DF, Schaefer S, Sippel RS, Chen H. Same-day thyroidectomy program: eligibility and safety evaluation. Surgery. 2012;152:1133–1141. doi: 10.1016/j.surg.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 5.Tuggle CT, Roman S, Udelsman R, Sosa JA. Same-day thyroidectomy: a review of practice patterns and outcomes for 1168 procedures in New York State. Ann Surg Oncol. 2011;18:1035–1040. doi: 10.1245/s10434-010-1398-0. [DOI] [PubMed] [Google Scholar]
- 6.Snyder SK, Hamid KS, Roberson CR, et al. Outpatient thyroidectomy is safe and reasonable: experience with more than 1,000 planned outpatient procedures. J Am Coll Surg. 2010;210:575–582. 582–584. doi: 10.1016/j.jamcollsurg.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 7.Marino M, Spencer H, Hohmann S, Bodenner D, Stack BC., Jr Costs of outpatient thyroid surgery from the University HealthSystem Consortium (UHC) database. Otolaryngol Head Neck Surg. 2014;150:762–769. doi: 10.1177/0194599814521583. [DOI] [PubMed] [Google Scholar]
- 8.Rutledge J, Siegel E, Belcher R, Bodenner D, Stack BC., Jr Barriers to same-day discharge of patients undergoing total and completion thyroidectomy. Otolaryngol Head Neck Surg. 2014;150:770–774. doi: 10.1177/0194599814521568. [DOI] [PubMed] [Google Scholar]
- 9.Promberger R, Ott J, Kober F, et al. Risk factors for postoperative bleeding after thyroid surgery. Br J Surg. 2012;99:373–379. doi: 10.1002/bjs.7824. [DOI] [PubMed] [Google Scholar]
- 10.Campbell MJ, McCoy KL, Shen WT, et al. A multi-institutional international study of risk factors for hematoma after thyroidectomy. Surgery. 2013;154:1283–1289. doi: 10.1016/j.surg.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Dehal A, Abbas A, Hussain F, Johna S. Risk factors for neck hematoma after thyroid or parathyroid surgery: ten-year analysis of the nationwide inpatient sample database. Perm J. 2015;19:22–28. doi: 10.7812/TPP/14-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert-Ebadi H, Le Gal G, Righini M. Use of anticoagulants in elderly patients: practical recommendations. Clin Interv Aging. 2009;4:165–177. doi: 10.2147/cia.s4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capodanno D, Angiolillo DJ. Management of antiplatelet therapy in patients with coronary artery disease requiring cardiac and noncardiac surgery. Circulation. 2013;128:2785–2798. doi: 10.1161/CIRCULATIONAHA.113.003675. [DOI] [PubMed] [Google Scholar]
- 14.Culkin DJ, Exaire EJ, Green D, et al. Anticoagulation and antiplatelet therapy in urological practice: ICUD/AUA review paper. J Urol. 2014;192:1026–1034. doi: 10.1016/j.juro.2014.04.103. [DOI] [PubMed] [Google Scholar]
- 15.Iqbal CW, Cima RR, Pemberton JH. Bleeding and thromboembolic outcomes for patients on oral anticoagulation undergoing elective colon and rectal abdominal operations. J Gastrointest Surg. 2011;15:2016–2022. doi: 10.1007/s11605-011-1611-x. [DOI] [PubMed] [Google Scholar]
- 16.Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17:103–107. doi: 10.1001/jamafacial.2014.1147. [DOI] [PubMed] [Google Scholar]
- 17.Torn M, Rosendaal FR. Oral anticoagulation in surgical procedures: risks and recommendations. Br J Haematol. 2003;123:676–682. doi: 10.1046/j.1365-2141.2003.04652.x. [DOI] [PubMed] [Google Scholar]
- 18.Burkey SH, van Heerden JA, Thompson GB, Grant CS, Schleck CD, Farley DR. Reexploration for symptomatic hematomas after cervical exploration. Surgery. 2001;130:914–920. doi: 10.1067/msy.2001.118384. [DOI] [PubMed] [Google Scholar]
- 19.Calo PG, Erdas E, Medas F, et al. Late bleeding after total thyroidectomy: report of two cases occurring 13 days after operation. Clin Med Insights Case Rep. 2013;6:165–170. doi: 10.4137/CCRep.S13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbas G, Dubner S, Heller KS. Re-operation for bleeding after thyroidectomy and parathyroidectomy. Head Neck. 2001;23:544–546. doi: 10.1002/hed.1076. [DOI] [PubMed] [Google Scholar]
- 21.Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3660 patients. Langenbecks Arch Surg. 2008;393:667–673. doi: 10.1007/s00423-008-0366-7. [DOI] [PubMed] [Google Scholar]
- 22.Lang BH, Yih PC, Lo CY. A review of risk factors and timing for postoperative hematoma after thyroidectomy: is outpatient thyroidectomy really safe? World J Surg. 2012;36:2497–2502. doi: 10.1007/s00268-012-1682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenbaum MA, Haridas M, McHenry CR. Life-threatening neck hematoma complicating thyroid and parathyroid surgery. Am J Surg. 2008;195:339–343. doi: 10.1016/j.amjsurg.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Li Z, Liu S, Wang X, Xu Z, Tang P. Risk factors for and occurrence of postoperative cervical hematoma after thyroid surgery: a single-institution study based on 5156 cases from the past 2 years. Head Neck. 2016;38:216–219. doi: 10.1002/hed.23868. [DOI] [PubMed] [Google Scholar]
- 25.Kandil E, Noureldine SI, Abbas A, Tufano RP. The impact of surgical volume on patient outcomes following thyroid surgery. Surgery. 2013;154:1346–1352. doi: 10.1016/j.surg.2013.04.068. [DOI] [PubMed] [Google Scholar]
- 26.Stavrakis AI, Ituarte PH, Ko CY, Yeh MW. Surgeon volume as a predictor of outcomes in inpatient and outpatient endocrine surgery. Surgery. 2007;142:887–899. doi: 10.1016/j.surg.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Musunuru S, Schaefer S, Chen H. The use of the LigaSure for hemostasis during thyroid lobectomy. Am J Surg. 2008;195:382–384. doi: 10.1016/j.amjsurg.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Zarebczan B, Mohanty D, Chen H. A comparison of the LigaSure and harmonic scalpel in thyroid surgery: a single institution review. Ann Surg Oncol. 2011;18:214–218. doi: 10.1245/s10434-010-1334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coiro S, Frattaroli FM, De Lucia F, et al. A comparison of the outcome using LigaSure small jaw and clamp-and-tie technique in thyroidectomy: a randomized single center study. Langenbecks Arch Surg. 2015;400:247–252. doi: 10.1007/s00423-014-1270-y. [DOI] [PubMed] [Google Scholar]
- 30.Hwang SO, Jung JH, Park HY, Kim WW. A prospective, randomized study between the Small Jaw® and the Harmonic Focus® in open thyroidectomy. Otolaryngol Head Neck Surg. 2014;150:943–948. doi: 10.1177/0194599814527730. [DOI] [PubMed] [Google Scholar]
- 31.Wright JD, Ananth CV, Lewin SN, et al. Patterns of use of hemostatic agents in patients undergoing major surgery. J Surg Res. 2014;186:458–466. doi: 10.1016/j.jss.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Docimo G, Tolone S, Ruggiero R, et al. Total thyroidectomy with harmonic scalpel combined to gelatin-thrombin matrix hemostatic agent: is it safe and effective? A single-center prospective study. Int J Surg. 2014;12(Suppl 1):S209–S212. doi: 10.1016/j.ijsu.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Mohr TS, Brouse SD. Perioperative management of antiplatelet agents. Orthopedics. 2012;35:687–691. doi: 10.3928/01477447-20120725-06. [DOI] [PubMed] [Google Scholar]
- 34.Chu EW, Telem DA, Chernoguz A, Divino CM. Assessing the risk of clopidogrel-related bleeding complications in patients undergoing inguinal herniorrhaphy. Hernia. 2011;15:31–35. doi: 10.1007/s10029-010-0732-6. [DOI] [PubMed] [Google Scholar]
- 35.Ercan M, Bostanci EB, Ozer I, et al. Postoperative hemorrhagic complications after elective laparoscopic cholecystectomy in patients receiving long-term anticoagulant therapy. Langenbecks Arch Surg. 2010;395:247–253. doi: 10.1007/s00423-009-0483-y. [DOI] [PubMed] [Google Scholar]
- 36.Piątek B, Piwnik M, Podgórski M, Strzelczyk J. Anticoagulants as a risk factor in patients operated on for abdominal hernia. Pol Przegl Chir. 2014;86:263–267. doi: 10.2478/pjs-2014-0047. [DOI] [PubMed] [Google Scholar]
- 37.Koc U, Bostanci EB, Karaman K, et al. Basic hemostatic parameters in patients with long-term oral anticoagulation undergoing cholecystectomy. J Laparoendosc Adv Surg Tech A. 2011;21:417–425. doi: 10.1089/lap.2010.0391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.