Abstract
Host immune responses play a key role in promoting bone resorption in periodontitis via RANKL-dependent osteoclastogenesis. Both membrane-bound RANKL (mRANKL) expressed on lymphocytes and soluble RANKL (sRANKL) are found in periodontal lesions. However, the underlying mechanism and cellular source of sRANKL release and its biological role in periodontitis are unclear. Tumor necrosis factor-α-converting enzyme (TACE) is reported to cleave 1) precursor TNF-α with release of mature, soluble TNF-α (sTNF-α) and 2) mRANKL with release of sRANKL. Both sTNF-α and sRANKL are found in the periodontitis lesion, leading to the hypothesis that TACE expressed on lymphocytes is engaged in RANKL shedding and that the resulting sRANKL induces osteoclastogenesis. In the present study, upon stimulating peripheral blood lymphocytes (PBLs) with mitogens in vitro, RANKL expression, sRANKL secretion, and TACE expression were all upregulated. Among the four putative mRANKL sheddases examined in neutralization assays, TACE was the only functional sheddase able to cleave mRANKL expressed on PBL. Moreover, PBL culture supernatant stimulated with mitogens in the presence of anti-TACE-antibody or anti-RANKL-antibody showed a marked reduction of osteoclastogenesis from osteoclast precursors, indicating that TACE-mediated sRANKL may possess sufficient osteoclastogenic activity. According to double-color confocal microscopy, B cells expressed a more pronounced level of RANKL and TACE expression than T cells or monocytes in periodontally diseased gingiva. Conditioned medium of patients’ gingival lymphocyte culture increased in vitro osteoclastogenic activity, which was suppressed by the addition of anti-TACE-antibody and anti-RANKL-antibody. Therefore, TACE-mediated cleavage of sRANKL from activated lymphocytes, especially B cells, can promote osteoclastogenesis in periodontitis.
Introduction
Recent studies demonstrated that host immune responses induced by polymicrobial infection play a key role in promoting bone resorption in periodontitis (1–3). Abundant lymphocytes are found in a dense mononuclear inflammatory infiltrate in inflamed gingival and periodontal tissues. In periodontal lesions, these B and T lymphocytes express receptor activator of NF-κB (RANK) ligand (RANKL) in response to bacterial infection (4). Stimulation of RANKL expression in these lymphocytes then induces osteoclastogenesis (4). During homeostatic bone remodeling, osteoclastic activity is triggered via RANKL bound on the surface of osteoblasts, which activates RANK bound on the surface of osteoclasts. Therefore, membrane-bound RANKL (mRANKL) is an essential osteoclastogenic factor (5), and, as noted, mRANKL-RANK signaling induces osteoclastogenesis with cell-cell contact between osteoclast and osteoblast/stromal cells in the physiological context (6). Because osteoprotegerin (OPG) is a soluble decoy receptor for RANKL and inhibits osteoclastogenesis (7), the balance between RANKL and OPG contributes to normal regulation of bone resorption (8). However, some evidence suggests that soluble RANKL (sRANKL) can be generated from the cleavage of mRANKL by enzymes, such as tumor necrosis factor-α-converting enzyme (TACE), to induce osteoclastogenesis (9). It is true that sRANKL can play a role in bone resorption as a soluble factor (cytokine) during bone remodeling (10). Other enzymes, such as matrix metalloproteinase (MMP)-7, −14, and a disintegrin and metalloproteinase domain-containing protein (ADAM)-10, have also been proposed as potential RANKL sheddases (11, 12). Both mRANKL and sRANKL were detected in the lytic lesion of periodontitis, and T and B cells are the major cellular source of mRANKL (4), but the mechanisms underlying the generation of sRANKL, as well as its functional role, have not been established.
Clinical studies reported that sRANKL is found in the gingival crevicular fluid (GCF) from periodontitis patients and that the level of sRANKL in GCF is positively correlated with the severity of periodontitis (13), as well as inflammatory biomarkers detected in GCF (4). Not surprisingly, a decreased concentration of OPG was correlated with increased periodontitis (13). In addition, the RANKL:OPG ratio favored the RANKL side with the progression of periodontitis, both at the mRNA (14–16) and protein levels (17). Lymphocytes are suggested to be a potential source of sRANKL in periodontitis (18). Furthermore, elevated levels of sRANKL were detected in the GCF of periodontitis patients who had other systemic diseases, such as rheumatoid arthritis or type-II diabetes, both known to exacerbate periodontitis (19, 20). Notwithstanding these reports, no study has ever addressed if sRANKL secreted within the periodontitis lesion can functionally induce osteoclastogenesis.
On the other hand, it is reported that the level of TACE in the GCF of periodontitis patients is elevated compared to healthy subjects (21) and that TACE production from T cells is upregulated by stimulation with the periodontal pathogen Porphyromonas gingivalis (P. gingivalis) (22). In addition to sRANKL, tumor necrosis factor-α (TNF-α), which is thought to be another osteoclastogenic cytokine (23–25), is produced by TACE-mediated shedding. More specifically, TNF-α is produced as a type II transmembrane protein that is physiologically cleaved by membrane-bound TACE (26–29). Many studies have reported that the level of TNF-α increases in the gingival tissues, as well as GCF, of patients with periodontitis (30–34). In terms of the effects of TNF-α on periodontal bone loss, the administration of TNF-soluble receptor that neutralizes TNF-α produced in the periodontitis lesion can suppress not only inflammation, but also pathogenic bone resorption of periodontitis induced in the non-human primate model of periodontitis (35). Taken together, it was hypothesized that sRANKL and TNF-α cleaved from activated lymphocytes by TACE might play an important and synergistic role as soluble remote osteoclastogenic factors in periodontitis. To test this hypothesis, the expression patterns of TACE in the inflammatory infiltrates of periodontally inflamed tissues were monitored by fluorescent immunochemistry. Then, conditioned medium was prepared from the lymphocytes isolated from patients with periodontitis in the presence or absence of anti-TACE-antibody, and the production of sRANKL and TNF-α, as well as the effects of in vitro osteoclastogenesis induction by the conditioned medium, were examined. In brief, the results from this study indicated that TACE-mediated cleavage of sRANKL from activated lymphocytes, especially B cells, can promote osteoclastogenesis in periodontitis.
Materials and Methods
Peripheral blood lymphocytes (PBL)
Human peripheral blood was obtained from normal, healthy adult male donors. The cells were collected by gradient centrifugation (1.077 g/mL; Lympholyte-H, Cedarlane, Ontario, Canada) and resuspended in Dulbecco’s modified Eagle’s Medium (D-MEM; Invitrogen; Carlsbad, CA) containing 10% (v/v) fetal bovine serum (Invitrogen; Carlsbad, CA) supplemented with antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin). All cells were cultured at 37°C in a 5% CO2 incubator. The protocol for these experiments, including PBL, as well as gingival mononuclear cells (GMC, see below), was reviewed and approved by the Institutional Review Board of the Forsyth Institute, and informed consent was obtained from all volunteers.
Culture of gingival mononuclear cells (GMC) isolated from human gingival tissue samples
Human gingival tissue samples were obtained from chronic periodontitis patients at the Boston University School of Dental Medicine under written informed consent. The protocols used in this experiment were approved by the Institutional Review Board. Characteristics of the study samples are shown in Table I. Excised gingival tissue samples during periodontal surgery were divided into two pieces. Small samples of gingival tissue were fixed with 4% paraformaldehyde in PBS, embedded into OCT compound (Sakura Finetek, Torrance, CA), and serial frozen sections (8μm thick) were prepared for immunofluorescent analysis of TACE expression. The remainder of the gingival tissue samples was weighed, cut into pieces, and digested by type IV collagenase solution (36). After 5 washes with PBS, cells were separated by density gradient centrifugation, and the lymphocyte layer was considered as human gingival mononuclear cells (GMC). The same amount of human GMC was cultured for 5 days with or without anti-TACE antibody (2.5μg/ml, rat IgG) (37, 38), and culture supernatants were collected and used as conditioned medium. In some experiments, RNA extraction, or immunodetection of TACE, CD3, and CD20 with flow cytometry analyses, were performed on day-0.
Table I.
Characteristics of patients with periodontitis
| No. of samples | 21 |
| Age median (range) | 46 (32 – 68) |
| Sex, no. of men/no. of women | 14/7 |
| Pocket depth mean (range) | 5.7 mm (4–10 mm) |
| Diagnosis | Chronic periodontitis |
Stimulation of PBL
PBL samples were stimulated with 50 ng/ml of Phorbol 12-myristate 13-acetate (PMA), 1 μg/ml of ionomycin, and 10 μg/ml of LPS extracted from E. coli serotype O55:B5 (all from Sigma, St. Louis, MO) following the published method (39) with some modifications. After stimulation, cell culture supernatant was collected, and total RNA was extracted. In some experiments, specific neutralizing antibodies for potential RANKL sheddases were applied to the culture to examine the participation of sheddases in RANKL and TNF-α cleavage. The following antibodies were used in our experiments: rabbit IgG anti-TACE antibody (Santa Cruz Biotechnology; Santa Cruz, CA), goat IgG anti-MMP-7 antibody (R&D Systems; Minneapolis, MN), mouse IgG anti-ADAM-10 antibody (R&D Systems), and mouse IgG anti-MMP-14 antibody (EMD; Madison, WI). In some experiments, exogenous recombinant TACE (R&D Systems) was added into the culture to examine the participation of TACE in RANKL and TNF-α cleavage.
RNA extraction and reverse transcription
In some experiments, RNA was extracted using E.Z.N.A. Total RNA Midi Kit (Omega Bio-Tek, Norcross, GA) according to the manufacturer’s instructions. After measuring the concentration of RNA, an equal amount of RNA was reverse transcribed with qScript (Quanta, Gaithersburg, MD) according to the manufacturer’s instructions.
Semi-quantitative reverse transcription (semi-RT-qPCR) for potential RANKL sheddases
Semi-RT-qPCR was performed in a final volume of 25 μl of Phire™ Hot Start II DNA Polymerase (New England Biolabs; Ipswich, MA). The sense and antisense primers used in these experiments are shown in Table II. The reaction mixtures contained 0.5 μl of Phire DNA polymerase, 5 μl of 5× reaction buffer, 200 μM of dNTPs and 500nM of each primer. The PCR products underwent electrophoresis and were visualized by SYBR® Safe DNA Gel Stain (Invitrogen; Carlsbad, CA) with UV-light illumination. The relative intensities of the gel bands were measured by ImageJ (http://rsb.info.nih.gov/ij/).
Table II.
Primer sets for conventional RT-PCR (Human)
| Gene | (Gene bank no.) | Direction | Primer sequence |
|---|---|---|---|
| TACE | (NM_003183) | Forward | GCTGACCTGGTTACAACTCAT |
| Reverse | AACCAGGACAGACCCAACGAT | ||
| β-actin | (NM_001101) | Forward | ATGAAGATCCTCACCGAGCGCGGCTACAGC |
| Reverse | CACTGTGTTGGCGTACAGGTCTTTGC | ||
| ADAM10 | (NM_001110.2) | Forward | CACGAAGTTGGACATAACTTTGGATCCCCA |
| Reverse | GCCATTACATATTCCTTCCCTTGCACAGTC | ||
| MMP-7 | (NM_002423.3) | Forward | TTAGAAGCCAAACTCAAGGAGAT |
| Reverse | CCATAGGTTGGATACATCACTGC | ||
| MMP-14 | (NM_004995.2) | Forward | TCTTTGCCGAGGGCTTCCATGG |
| Reverse | TCACCCGCCAGAACCAGCGC |
Quantitative PCR (qPCR) for RANKL and TACE expression
Quantitative PCR was performed in a final volume of 25 μl of Phire Hot Start II DNA polymerase containing EvaGreen dye (Phenix Research; Candler, NC) using an iCycler instrument (Bio-Rad Laboratories, Benicia, CA). The sense and antisense primers used in these experiments are shown in Table III. Fold changes of the gene of interest were calculated using the ΔΔCt method and either β-actin (for human transcripts) (40) or GAPDH (for mouse transcripts) (41) as a reference (42).
Table III.
Primer sets for real-time RT-PCR
| Species | Gene | (Gene bank no.) | Direction | Primer sequence |
|---|---|---|---|---|
| Human | TACE | (NM_003183) | Forward | CGCATTCTCAAGTCTCCACA |
| Reverse | CACATCCCAAGCATCCTTTT | |||
| RANKL | (NM_003701) | Forward | ATCGTTGGATCACAGCACATC | |
| Reverse | AGACTCACTTTATGGGAACCAGA | |||
| TNF-α | (NM_000594) | Forward | ATGAGCACTGAAAGCATGATCC | |
| Reverse | GAGGGCTGATTAGAGAGAGGTC | |||
| β-actin | (NM_001101) | Forward | CTGGAACGGTGAAGGTGACA | |
| Reverse | AAGGGACTTCCTGTAACAATGCA | |||
|
| ||||
| Mouse | Atp6v0d2 | (NM_175406) | Forward | GAAGCTGTCAACATTGCAGA |
| Reverse | TCACCGTGATCCTTGCAGAAT | |||
| c-Src | (NM_001025395) | Forward | CCAGGCTGAGGAGTGGTACT | |
| Reverse | CAGCTTGCGGATCTTGTAGT | |||
| DC-STAMP | (NM_029422) | Forward | TGGAAGTTCACTTGAAACTACGTG | |
| Reverse | CTCGGTTTCCCGTCAGCCTCTCTC | |||
| Integrin β3 | (NM_016780) | Forward | GGCCTTCGTGGACAAGCCTG | |
| Reverse | CGGGACACCTGGTCGGTTAG | |||
| vATPase | (AB022322) | Forward | TCCAACACAGCCTCCTACTT | |
| Reverse | ACAGCAAAGGCAGCAAAC | |||
| GAPDH | (NM_008084) | Forward | CTCCCACTCTTCCACCTTCG | |
| Reverse | TTGCTGTAGCCGTATTCATT | |||
Flow cytometry for the detection of mRANKL and putative RANKL sheddases expressed on plasma membrane of human PBL
The lymphocytes were harvested and examined for the expression of mRANKL and potential RANKL sheddases on plasma membrane by flow cytometry. Briefly, cells were preincubated in 1% BSA in PBS for 30min, followed by incubation with primary antibody. The primary antibodies used in our experiments were as follows: rabbit IgG anti-RANKL antibody (43), rabbit IgG anti-TACE antibody, goat IgG anti-MMP-7 antibody, mouse IgG anti-ADAM-10 antibody, and mouse IgG anti-MMP-14 antibody. Isotype-matched control IgG was used for control. After washing, cells were incubated with FITC-conjugated anti-rabbit IgG, anti-goat IgG, or anti-mouse IgG antibody (Jackson Immuno Research Laboratories, West Grove, PA). Flow cytometry analyses were carried out to monitor fluorescence of FITC intensity by using a FACSAreaIII flow cytometer (BD Biosciences, San Jose, CA) or EPICS ALTRA flow cytometer (Beckman Coulter). Cells on the lymphocyte gate, as defined by forward and side scatter characteristics, were investigated.
Measurement of sRANKL and TNF-α concentration in culture supernatant
After cultivation, culture supernatant of PBL was collected, and the concentration of sRANKL and TNF-α was measured by ELISA (Peprotech, Rocky Hill, NJ) according to the manufacturer’s instructions.
Osteoclastogenesis assay
Osteoclastogenesis assay using RAW 264.7 cells (ATCC, TIB-71) was performed using filtered (pore size = 0.2 μm) culture supernatant of PBL and human GMC. Briefly, RAW 264.7 cells (103 cells/well) were cultured with 50% supernatant in a 96-well plate. 100 ng/ml of sRANKL (Peprotech) or 10 ng/ml of TNF-α (Peprotech) were used as positive control. In some experiments, rabbit IgG anti-RANKL antibody, OPG-Fc (a kind gift from Dr. Colin Dunstan, Amgen Inc., Thousand Oaks CA), or goat IgG anti-TNF-α antibody (Peprotech) was applied. After cultivation, RNAs were extracted in some experiments to detect osteoclast differentiation marker gene transcripts by RT-PCR, or cells were stained for tartrate-resistant acid phosphatase (TRAP) using Acid Phosphatase kit (Sigma-Aldrich) according to the manufacturer’s instructions. Dark red multinucleated cells (over 3 nuclei) were counted as osteoclasts.
Pit formation assays
To evaluate resorption activity of TRAP-positive multinucleated cells differentiated from RAW 264.7 cells, a resorption assay was performed using OsteoAssay™ Human Bone Plate (Corning, Corning, NY) or osteologic discs (BD Biosciences). Briefly, RAW 264.7 cells (103 cells/well) were plated on the wells of each plate or disc with 50% human GMC supernatant and cultured for 6 days. Recombinant sRANKL (100 ng/ml) was used as a positive control. In some experiments, anti-RANKL antibody (43) was added to the culture. After cultivation, cells were removed with bleach solution, and plates or discs were washed with distilled water, followed by air drying. Images were scanned with an Epson V500 scanner (Epson America, Long Beach, CA), and the percentage of resorbed area was calculated with ImageJ.
Immunofluorescent analysis of human inflamed gingival tissue samples
Immunofluorescent analyses of human inflamed gingival tissue samples were performed using frozen sections. Briefly, sections were washed with PBS, blocked with 5% skim milk in PBS, and incubated with primary antibodies in 1% skim milk in PBS-T. The primary antibodies used in our experiments were anti-TACE antibody (rabbit IgG) (Santa Cruz), anti-CD3 Ab (mouse IgG) (BD Biosciences), or anti-CD20 Ab (mouse IgG) (BD Biosciences). After washing, cells were incubated with Dylight-488-conjugated anti-rabbit IgG (Jackson Immuno Research Laboratories) and Phycoerythrin (PE)-conjugated anti-mouse IgG (BD Biosciences), followed by thorough washing and mounting with antifade mounting media (0.2% n-propyl gallate (Sigma) in PBS-glycerol). Immunofluorescence was observed with a fluorescent microscope (Olympus FSX100, Olympus, Center Valley, PA). Leakage of Dylight-488 (green) fluorescent into the red channel (575–650 nm) was eliminated by adjusting sensitivity and exposure time.
Flow cytometry analysis of human inflamed gingival tissue samples
In some experiments, freshly prepared human GMC were examined for the expression of TACE, CD3, and CD20 on plasma membrane by flow cytometry. Briefly, cells were resuspended in 2% FBS in PBS containing primary antibody. The primary antibodies used in our experiments were anti-TACE Ab (rabbit IgG), anti-CD3 Ab (mouse IgG), or anti-CD20 Ab (mouse IgG). After washing, cells were incubated with Dylight 488-conjugated anti-rabbit IgG Ab and PE-conjugated anti-mouse IgG Ab. Flow cytometry analyses were carried out to monitor fluorescence of Dylight 488 and PE intensity using a Flow cytometer. Cells on the lymphocyte gate, as defined by forward and side scatter characteristics, were investigated.
Measurement of sRANKL and TNF-α concentration in human GMC supernatant
After 5 days of culture with or without anti-TACE antibody, culture supernatant of human GMC was collected and filtered (ϕ 0.2μm, Pall Corporation, Ann Arbor, MI). Concentrations of sRANKL and TNF-α in human GMC supernatant were measured with a commercially available ELISA kit (Peprotech) according to the manufacturer’s instructions. The remaining supernatant was used for osteoclastogenesis assays.
Statistical Analysis
Multiple comparisons were performed by one-way analysis of variance (ANOVA) with Tukey’s post hoc test. p < 0.05 was considered statistically significant. All data are presented as mean ± standard deviation (SD). All data are representative of at least 3 independent experiments.
Results
Activated PBL upregulated both RANKL mRNA and mRANKL expression
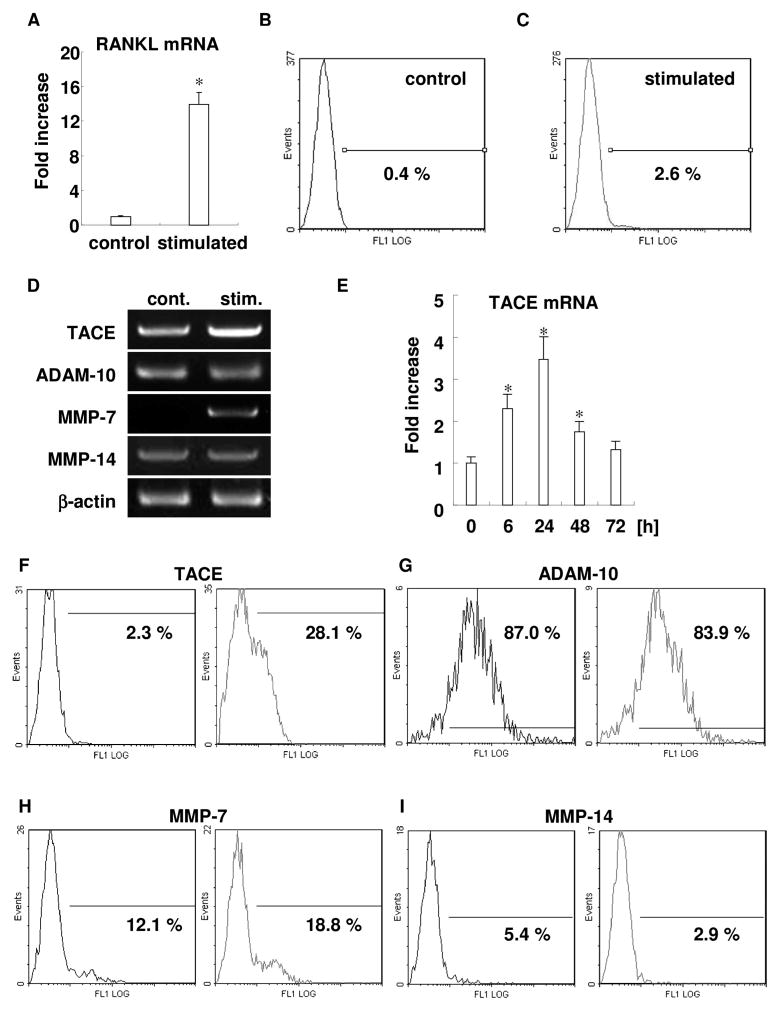
To clarify the mechanism of sRANKL upregulation in inflamed gingival tissue, human PBL samples were stimulated in vitro, with the mixture of PMA, ionomycin and LPS, and the expression of RANKL mRNA and mRANKL was examined. Stimulated PBL showed prominent upregulation of RANKL mRNA (Fig. 1A). Flow cytometry revealed mRANKL upregulation on plasma membrane of activated PBL (Fig. 1B and C). Among the whole leukocyte population, including neutrophils and lymphocytes, 2.6% of cells expressed TACE after stimulation with the mixture of mitogens, whereas control unstimulated leukocytes did not express TACE.
Figure 1. Activated PBLs upregulated RANKL, and expressed potential RANKL sheddases, including TACE.
RANKL mRNA expression of both stimulated and unstimulatred PBLs was compared by real-time RT-PCR. Column and bar indicate mean + SD (n=3/group) (Fig. 1A). mRANKL expression on plasma membrane of PBLs was examined by flow cytometry. Representative data of unstimulated PBLs (Fig. 1B) and stimulated PBLs (Fig. 1C) are shown. Cells on the lymphocyte gate, as defined by forward and side scatter characteristics, were investigated. Percentages in the figure indicate mRANKL-positive cells in the population (0.4 and 2.6%, respectively).
RNA expressions of putative RANKL sheddases were examined in PBLs stimulated with or without mitogen (PMA, ionomycin and LPS) for 24h by semi-quantitative RT-PCR (Fig. 1D). Representative data of three independent experiments for TACE, ADAM-10, MMP-7, MMP-14, and β-actin are respectively shown from top to bottom. Relative band intensities of each gene normalized by β-actin to the control are shown on the right side (TACE: 2.4, ADAM-10: 1.0, MMP-7: 6.4, MMP-14: 1.0, respectively). Time course change of TACE mRNA expression measured by real-time RT-PCR (Fig. 1E). RNA samples from unstimulated or stimulated PBLs were extracted at 0, 6, 24, 48, and 72 h, reverse transcribed, and the expression of TACE mRNA expression was analyzed by real-time RT-PCR. All experiments were performed in triplicate wells for each condition, and mean + SD is shown in the graph. Representative data of three independent experiments is shown (* p<0.05 versus control). Protein expression of each of putative RANKL sheddase was examined by flow cytometry at 48h. Representative data of TACE (Fig. 1F), ADAM-10 (Fig. 1G), MMP-7 (Fig. 1H), and MMP-14 (Fig. 1I) are shown. Each panel shows control (left side histogram) and stimulated condition (right side histogram). Cells on the lymphocyte gate, as determined by forward and side scatter characteristics, were investigated. Values in the figure indicate the percentage of positive cells in each population using the same threshold.
PBLs express potential RANKL sheddases, including TACE
RT-PCR analysis revealed that PBLs express potential RANKL sheddases, such as TACE, ADAM-10, MMP-7, and MMP-14 (Fig. 1D). ADAM-10 was highly constitutively expressed, whereas MMP-14 was weakly expressed. However, both TACE and MMP-7 were upregulated by stimulation (2.4- and 6.4-fold, respectively). Real-time RT-PCR analysis for TACE mRNA expression revealed time-course upregulation until 24h with a peak response at 24h, and gradual decrease after that (Fig. 1E). Next, the level of protein expression of each potential RANKL sheddase was examined by flow cytometry after 48h (Fig. 1F–I). Real-time RT-PCR showed results similar to those of TACE and MMP-7 upregulated by stimulation (Fig. 1F and H). ADAM-10 was constitutively highly expressed (Fig. 1G). MMP-14 expression was decreased by stimulation (Fig. 1I).
TACE is the most important proteolytic enzyme with RANKL sheddase property
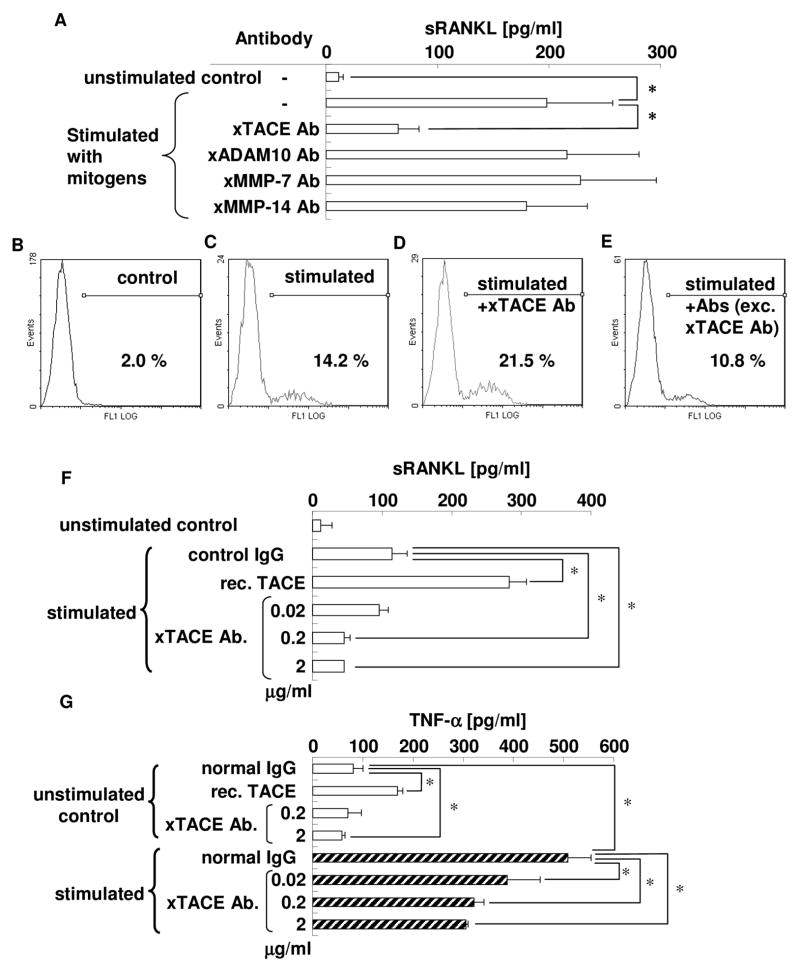
To determine the functionally active sheddase(s) for RANKL, as expressed on PBLs, inhibition experiments of RANKL shedding using specific antibodies for each potential RANKL sheddase were performed (Fig. 2A). Anti-TACE antibody, but no other antibodies, such as anti-ADAM-10, anti-MMP-7, or anti-MMP-14 antibody, could reduce sRANKL concentration in culture supernatant of activated PBLs. To confirm the inhibition of sRANKL cleavage from plasma membrane by antibody, flow cytometry for mRANKL was performed on the mitogen-stimulated lymphocytes. Among the mononuclear cell population, anti-TACE antibody was found to increase mRANKL expression (21.5%) in mitogen-stimulated PBLs compared to mRANKL expression (14.2%) which received no antibody (Fig. 2C and D), indicating that TACE could participate in RANKL cleavage from the plasma membrane of PBLs. When sRANKL was tested in the presence of anti-TACE antibody, but absence of anti-ADAM-10, anti-MMP-7, and anti-MMP-14 antibodies, the suppression of RANKL production from stimulated PBLs was determined by ELISA. Flow cytometry for mRANKL showed that the addition of anti-ADAM-10, anti-MMP-7, and anti-MMP-14 antibodies could not increase mRANKL expression by stimulated PBLs (Fig. 2E). Thus, among four putative sheddases, these results confirm that only TACE expressed on PBLs is engaged in the shedding of mRANKL to generate sRANKL.
Figure 2. TACE is the most important proteolytic enzyme with RANKL sheddase properties in activated PBLs.
PBLs were stimulated with PMA (50 ng/ml), ionomycin (1 μg/ml), and LPS (10 μg/ml) in the presence or absence of specific neutralizing antibodies for each potential RANKL sheddase, followed by collection of culture supernatant. Subsequently, sRANKL concentrations were measured by ELISA in triplicate wells for each group (Fig. 2A) * p<0.05. mRANKL expression on plasma membrane of PBLs was examined by flow cytometry. Cells on the lymphocyte gate, as defined by forward and side scatter characteristics, were investigated. Representative data of unstimulated PBLs (Fig. 2B), stimulated PBLs (Fig. 2C), PBLs stimulated in the presence of anti-TACE specific antibody (Fig. 2D), and PBLs stimulated in the presence of antibodies, except anti-TACE antibody (combination of anti-ADAM-10 antibody, anti-MMP-7 antibody, and anti-MMP-14 antibody) (Fig. 2E), are shown. Percentages in the figure indicate mRANKL-positive cells in the population (2.0, 14.2, 21.5, and 10.8%, respectively).
In order to confirm the functionality of anti-TACE antibody in neutralizing the TACE activity, PBLs were stimulated with PMA (50 ng/ml), ionomycin (1 μg/ml), and LPS (10 μg/ml) in the presence or absence of recombinant TACE (0.7 μg/ml) or anti-TACE antibody (0.02, 0.2, or 2 μg/ml). The collected culture supernatants were subjected to ELISA for the measurement of sRANKL (Fig. 2F) and TNF-α concentrations (Fig. 2G) in triplicate wells for each group. * p<0.05
Activation of PBLs results in the release of sRANKL and TNF-α in a TACE-dependent manner
Next, we examined the function of TACE in shedding of sRANKL and TNF-α from stimulated PBLs (Fig. 2F and G). Inhibition experiments using anti-TACE antibody clearly showed that blockade of TACE activity decreased the concentrations of sRANKL and TNF-α in the culture supernatant of PBLs in a dose-dependent manner. In contrast, the addition of recombinant TACE to the culture significantly increased both sRANKL and TNF-α release from plasma membranes. These results indicate that TACE expressed on PBLs facilitates the shedding of both mRANKL and membrane-bound TNF-α.
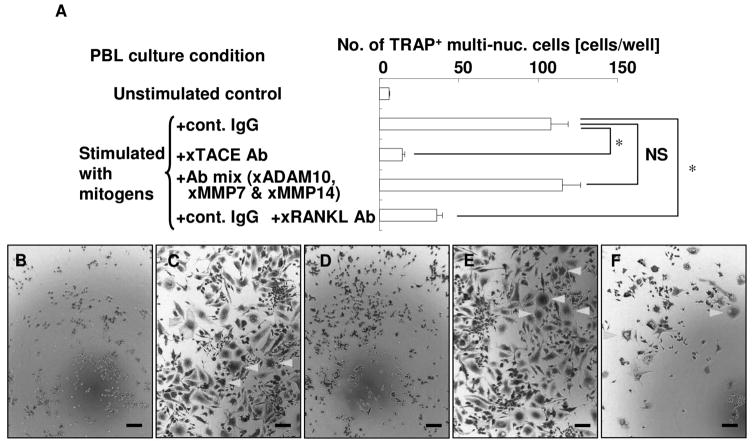
Culture supernatant of activated PBLs has osteoclastogenic activity
To investigate whether culture supernatant of activated PBLs has osteoclastogenic activity, osteoclastogenesis assays were performed (Fig. 3). Culture supernatant of activated PBLs induced numerous TRAP-positive multinucleated cells (Fig. 3C). However, the number of TRAP-positive multinucleated cells from supernatant of PBLs treated with anti-TACE antibody was dramatically reduced (Fig. 3D). These data are consistent with the decrease in the concentrations of sRANKL and TNF-α in the supernatant of PBLs activated in the presence of anti-TACE antibody (Fig. 2). Supernatant of PBLs treated with antibodies, except anti-TACE antibody (i.e., the combination of anti-ADAM-10 antibody, anti-MMP-7 antibody, and anti-MMP-14 antibody), also induced TRAP-positive multinucleated cells in an amount equal to that observed in the supernatant of stimulated PBLs (Fig. 3E). To confirm whether this osteoclastogenic activity of supernatant of stimulated PBLs is RANKL-dependent or not, anti-RANKL neutralizing antibody was applied to the culture, and the blockade of sRANKL significantly inhibited osteoclastogenesis (Fig. 3F).
Figure 3. Culture supernatant of activated PBLs has osteoclastogenic effect.
PBLs were stimulated in the presence of control IgG, anti-TACE antibody, or antibodies, except anti-TACE antibody (combination of anti-ADAM-10 antibody, anti-MMP-7 antibody, and anti-MMP-14 antibody). Then culture supernatants were collected. Subsequently, RAW 264.7 cells were co-cultured with 50% of each PBL culture supernatant. In the same condition, anti-RANKL antibody was administered to block sRANKL-dependent osteoclastogenesis. After cultivation, TRAP staining was performed, and the number of TRAP-positive multinucleated cells in the well was counted. All experiments were performed in triplicate wells for each condition. Representative data of three independent experiments is shown * p<0.05; NS not significant.
Representative photographs of TRAP staining (Fig. 3B–F). RAW 264.7 cells cultured with supernatant of unstimulated PBLs (Fig. 3B), stimulated PBLs (Fig. 3C), PBLs stimulated in the presence of anti-TACE antibody (Fig. 3D), PBLs stimulated in the presence of antibodies, except anti-TACE antibody (combination of anti-ADAM-10 antibody, anti-MMP-7 antibody, and anti-MMP-14 antibody) (Fig. 3E), and stimulated PBLs plus anti-RANKL antibody (Fig. 3F) are shown. Bar = 100 μm.
TACE expression is induced in human GMC
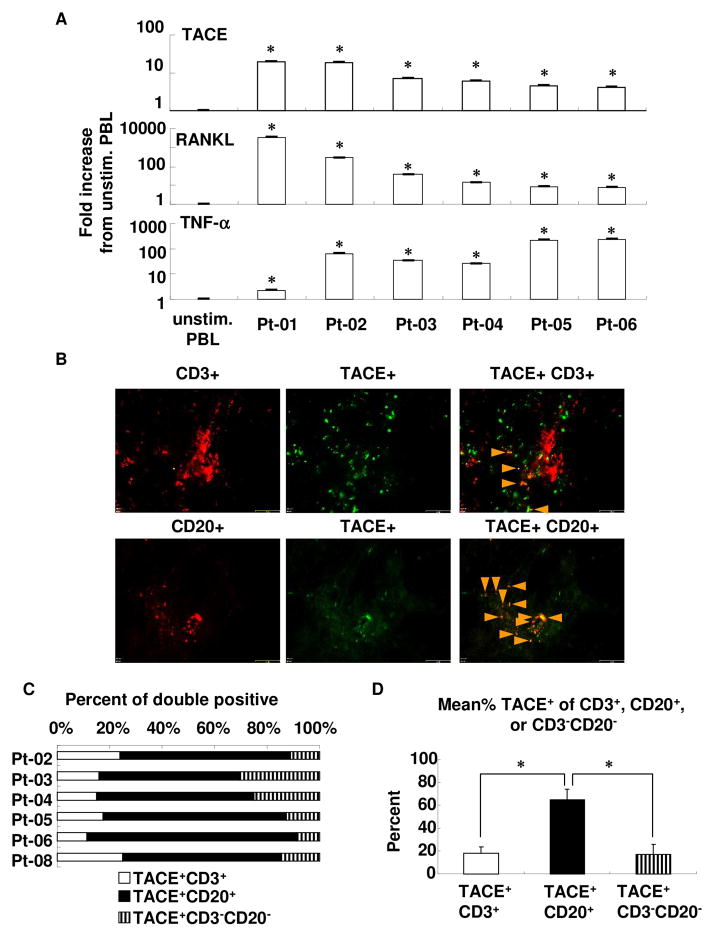
To clarify whether TACE is expressed in freshly prepared human GMCs from periodontitis patient samples, we first examined the level of RNA expression of TACE by real-time RT-PCR (Fig. 4A). Compared to unstimulated PBLs from healthy donors, all six human GMC samples isolated from different patients showed prominently elevated TACE mRNA expression compared to control unstimulated PBL. These induction levels are almost at the same level as that seen in PMA-, ionomycin- and LPS-stimulated PBLs (stimulated PBLs: 9.2-fold; human GMCs: 4.0- to 19.7-fold; data not shown in the figure). Expressions of RANKL and TNF-α mRNA were also upregulated in human GMCs.
Figure 4. Expression of TACE, RANKL, and TNF-α in human gingival tissue from periodontitis patients.
(A) RNA was extracted from freshly prepared human GMCs, reverse transcribed, and cDNA was then used for real-time RT-PCR detection of above transcripts. Fold increase of the expression against unstimulated human PBLs was calculated, as mentioned in the Materials and Methods. Results are expressed as mean ± SD. *: p < 0.01 versus unstimulated PBLs.
(B) Immunofluorescent analysis of human gingival tissue samples. From each patient, three sections at intervals of about 100 μm were examined. Photographs of green and red fluorescence were taken under the same exposure conditions between samples, and merged representative photographs are shown. Bar: 65 μm. Red: CD3+ or CD20+, Green: TACE+, Orange (merged): TACE+CD3+ or TACE+CD20+.
(C) Percentage of TACE+ population in CD3+, CD20+, and CD3−CD20− cells in human inflamed gingival tissue samples. In each sample, the percentage of TACE+CD3+ and TACE+CD20+ double positives or TACE+CD3−CD20− was calculated with the area of each fluorescence band.
(D) Mean percentage of TACE+ of CD3+, CD20+, or CD3−CD20−. The mean value of percentage of TACE+ population in CD3+, CD20+, and CD3−CD20− cells was calculated from the value shown in Fig. 4C of 6 patients. Results are expressed as mean ± SD. * p < 0.05
Next, the expression pattern of TACE protein in the inflamed human gingival tissue samples was examined using frozen sections. Immunofluorescent analyses for TACE revealed extensive TACE expression in the gingival tissue of periodontitis patients (Fig. 4B). Double-staining for both TACE and CD3 (T cell marker) or TACE and CD20 (B cell marker) revealed that TACE-expressing cells are dominated by lymphocytes, especially B-cells (TACE+CD3+: 18%; TACE+CD20+: 65%; and TACE+CD3-CD20-: 17%, Fig. 4C and D).
To further confirm the expression of TACE in lymphocytes in gingival tissue of periodontitis patients, we examined TACE expression in freshly prepared human GMCs by flow cytometry. Judging from the number of positive cells, the mean percentage of TACE+CD20+ double-positive cells among CD20+ cells was significantly higher than TACE+CD3+ double-positive cells among CD3+ cells (data not shown). In addition, all patients showed a higher percentage of TACE+CD20+ than TACE+CD3+ by intra-patient comparison (data not shown). In sum, the data from clinical sample analyses showed that the predominant TACE-bearing cells in inflamed gingival tissue are B cells.
TACE plays a role in sRANKL and TNF-α release from human GMC
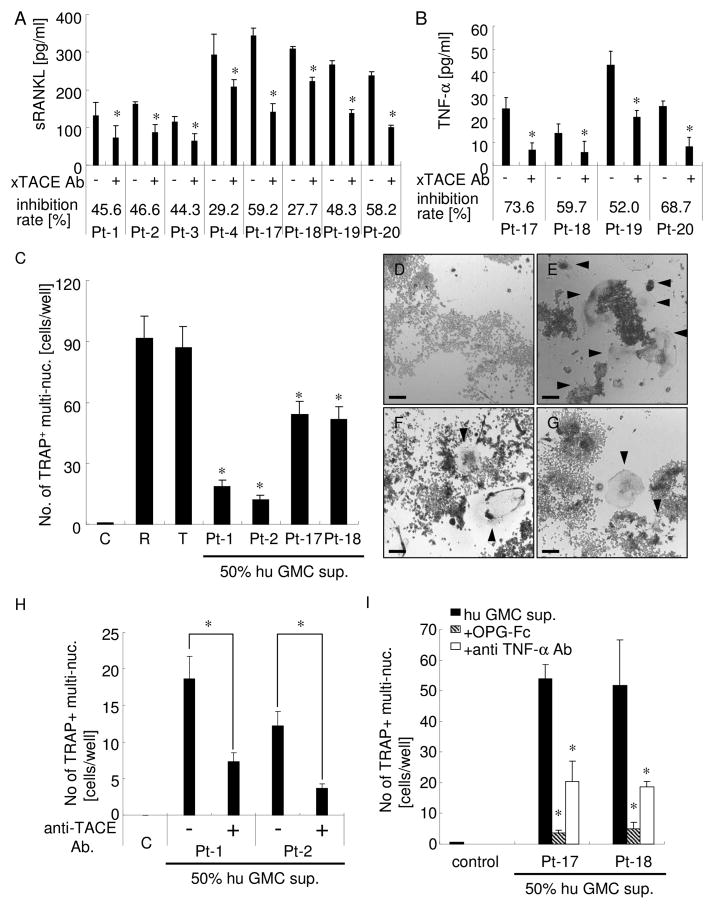
To examine the role of TACE in osteoclastogenic cytokine release from human GMCs, isolated human GMCs were cultured with or without anti-TACE antibody (2.5 μg/ml; sufficiently efficient concentration, as determined by the experiments shown in Fig. 2). In all cases, when anti-TACE antibody was added to human GMC culture, the sRANKL concentration in culture supernatants was diminished (8/8 patient samples, inhibition rate: 28 – 59 % ; Fig. 5A). The concentration of TNF-α was also diminished (4/4 patient samples, inhibition rate: 52 – 74 %; Fig. 5B). These data suggest that TACE plays a role in sRANKL and TNF-α release from human GMCs and that sRANKL and TNF-α released from activated lymphocytes can activate osteoclasts at distant sites.
Figure 5. Human GMC supernatant exhibited sRANKL/TNF-α-mediated osteoclastogenic activity.
sRANKL (Fig. 5A) and TNF-α (Fig. 5B) concentration in the culture supernatant of human GMCs. Eight (for sRANKL; Fig. 5A) or four (for TNF-α; Fig. 5B) human GMCs prepared from gingival tissue samples were cultured with or without anti-TACE antibody. In each GMC sample, the same number of cells was plated into each well. sRANKL and TNF-α concentrations in the culture supernatant were measured by ELISA. Inhibition rates of cytokine release were calculated as ((without antibody)-(with antibody) / (without antibody)). All experiments were performed in triplicate wells for each group. Results are expressed as mean ± SD. * p < 0.05: intraGMC (between with and without anti-TACE antibody).
(C) Osteoclastogenic activity of human GMC supernatants was confirmed by co-culture of RAW 264.7 cells with 50% human GMC supernatant. Four human GMC supernatants were analyzed. C: control culture media; R: RANKL (100 ng/ml); T: TNF-α (10 ng/ml). All experiments were performed in triplicate wells for each group. Results are expressed as mean ± SD. * p < 0.05 versus control.
(D–G) Representative photographs of TRAP staining of RAW 264.7 cells cultured with control (Fig. 5D), RANKL (100ng/ml, Fig. 5E), TNF-α (10ng/ml, Fig. 5F), and 50% human GMC supernatant (Fig. 5G). Bar: 100μm. Arrowhead shows TRAP-positive multinucleated cells.
(H) Anti-TACE antibody treatment during human GMC culture greatly reduced osteoclastogenic activity of human GMC supernatant. RAW 264.7 cells were co-cultured with 50% human GMC supernatant from the culture with or without anti-TACE antibody. All experiments were performed in triplicate wells for each condition. Results are expressed as mean ± SD. * p < 0.05: between groups.
(I) Osteoclastogenic activity of human GMC supernatant was sRANKL- and TNF-α-dependent. RAW 264.7 cells were co-cultured with 50% human GMC supernatant with OPG-Fc or anti-TNF-α antibody. All experiments were performed in triplicate wells for each group. Results are expressed as mean ± SD. * p < 0.05 vs. human GMC supernatant only.
Human GMC conditioned medium induced the formation of TRAP-positive multinucleated cells
Next, we examined whether conditioned medium of human GMC culture has osteoclastogenic activity, as applied to RAW264.7 cells (Fig. 5C–I). Four out of four GMC supernatants had osteoclastogenic activity based on the number of TRAP+ multinucleated cells, albeit in relatively low numbers when compared to 100 ng/ml RANKL or 10 ng/ml TNF-α stimulation. These data suggest that human GMC supernatant contains active osteoclastogenic cytokines, possibly sRANKL and TNF-α. To confirm that sRANKL or/and TNF-α released from human GMCs by TACE sheddase is (are) responsible for the osteoclastogenic activity present in conditioned medium of human GMCs, we performed two types of inhibition experiments: 1) blockade of TACE activity during ex vivo culture of human GMCs (Fig. 5H) and 2) neutralization of sRANKL and TNF-α during osteoclastogenesis assay (Fig. 5I). As shown in Figure 2, blockade of TACE activity during ex vivo culture of human GMCs greatly diminished both sRANKL and TNF-α release, and, as expected, the number of TRAP+ multinucleated cells induced by human GMC supernatant was significantly reduced. Addition of OPG-Fc or anti-TNF-α antibody into the osteoclastogenesis assay also inhibited human GMC supernatant-dependent formation of TRAP+ multinucleated cells. These data provide secondary support that sRANKL and TNF-α released from human GMCs by TACE sheddase activity can activate osteoclasts at distant sites.
TRAP-positive multinucleated cells differentiated by human GMC conditioned medium had characteristics of functional osteoclasts
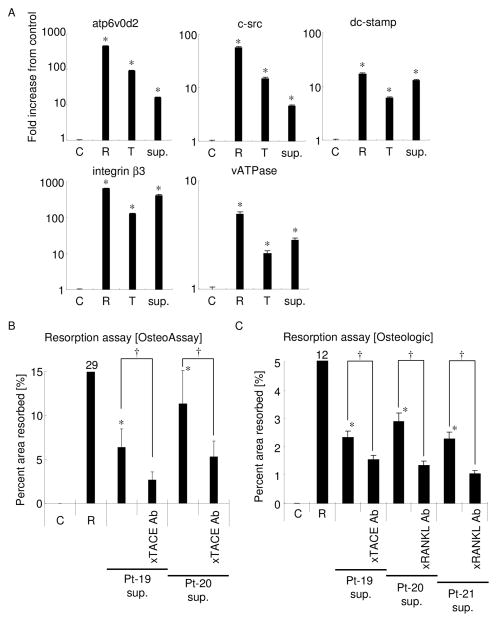
In some cases, TRAP-positive multinucleated cells were lacking in other fundamental characteristics of functional osteoclasts, especially under the condition of TNF-α-only stimulation (24). To examine other functional characteristics of differentiated osteoclasts induced by conditioned medium of human GMCs, the expression of osteoclast differentiation and functional marker genes in RAW264.7 cells was monitored by real-time RT-PCR (Fig. 6A). More specifically, RAW264.7 cells incubated with human GMC conditioned medium induced high level of expressions for both ATP6v0d2 and c-Src genes compared to the controls without stimulation, while such elevated levels of ATP6v0d2 and c-Src genes induced by human GMC conditioned medium were relatively lower than those induced by recombinant sRANKL or TNF-α. However, stimulation of RAW264.7 cells with human GMC conditioned medium induced remarkable gene expressions for DC-STAMP, integrin β3, and vATPase comparable to that of sRANKL, or even higher than that of TNF-α. These data suggest that human GMC supernatant contains the soluble factor that can induce osteoclastogenesis in a manner equal to that of RANKL or TNF-α.
Figure 6. Osteoclast differentiation marker gene expression and resorption activity induced by human GMC supernatants.
(A) RNAs from RAW 264.7 cells cultured with normal culture media (C), sRANKL (R; 100 ng/ml), TNF-α (T; 10 ng/ml), or 50% human GMC conditioned medium (sup.) were extracted after incubation for 5 days. The isolated RNAs were then reverse transcribed, and five osteoclast marker gene transcripts (ATP6v0d2, c-src, DC-STAMP, integrin β3, and vATPase) were detected by real-time RT-PCR. GAPDH was used as internal control. Fold increase from control in each transcript is shown. All experiments were performed in triplicate wells for each group. Results are expressed as mean ± SD. * p<0.001 vs. control. RAW 264.7 cells were co-cultured on OsteoAssay plate (Fig. 6B) or Osteologic plate (Fig. 6C) with 50% human GMC conditioned medium harvested from GMC culture with or without anti-TACE antibody. In some conditions, anti-RANKL antibody was added to block sRANKL activity (Fig. 6C). Three human GMC supernatants were analyzed. C: cultured with normal culture media; R: cultured with sRANKL (100 ng/ml). All experiments were performed in triplicate wells for each group. Results are expressed as mean ± SD. *: p<0.05 vs. control. †: p<0.05 between groups.
In addition, we finally assessed whether human GMC-stimulated TRAP+ multinucleated cells had resorption activity using two types of synthetic biomimetic substrate-coated culture plates, Osteologic and OsteoAssay (Fig. 6B and C). In both cases, RAW 264.7 cells stimulated with 50% human GMC supernatant resorbed a certain level of surface area, albeit quite low when compared to 100ng/ml of RANKL stimulation. As expected, inhibition of TACE activity during GMC culture by addition of anti-TACE neutralizing antibody resulted in diminished resorption activity, whereas addition of anti-RANKL antibody into the culture supernatant of GMCs also showed decreased resorption activity, suggesting that TACE could suppress the generation of sRANKL in the GMC culture.
Discussion
TACE is the most important proteolytic enzyme with RANKL sheddase properties in activated lymphocytes
In the present work, sRANKL cleavage was hypothesized to act as an important soluble bone destruction factor in periodontitis. Therefore, we first examined the cleavage mechanisms of RANKL. Importantly, one well-known RANKL sheddase, tumor necrosis factor-α-converting enzyme (TACE), was previously found to release TNF-α from cells (44). Later, TACE was discovered to be upregulated in arthritic cartilage, but not normal cartilage (45), and secretion of TNF-α from synovial tissue was deemed a contributing factor for the bone destruction in rheumatoid arthritis (46). Other reports have suggested a relationship between TACE and endotoxin shock (47), induction and development of stroke (48), and periodontitis (21). On the other hand, it is reported that TACE expressed on T cells can cleave RANKL (9), while other enzymes, such as ADAM-10, MMP-7, or MMP-14, expressed on cancer cells, as well as osteoblasts, could cleave sRANKL from plasma membranes (11, 12, 49). However, no study has thus far addressed RANKL shedding from B cells or monocytes. Therefore, in order to discover the enzyme responsible for RANKL cleavage from activated lymphocytes, including T cells, B cells and monocytes, the expression of potential RANKL shedding enzymes in PBL was evaluated by PCR and flow cytometry. As a result, MMP-7 and TACE were found to be upregulated in response to stimulation with mitogens, while ADAM-10 was constitutively vigorously expressed, and MMP-14 was downregulated. An inhibition experiment using a panel of specific neutralizing antibodies for these four putative sheddases was then carried out. Among the four different putative sheddases tested, TACE was clearly identified as the key proteolytic enzyme with RANKL-cleaving property, essentially because specific anti-TACE antibody, but no other antibodies tested, resulted in significant reduction of sRANKL generation.
On the other hand, there are several reports that showed contradictory results to our findings. Lynch et al. reported that MMP-7 could cleave mRANKL into sRANKL in prostate cancer cells and that such cleaved sRANKL was able to maintain osteoclastogenic potency (11, 50). Among our results, no data emerged to corroborate this outcome. However, this could have been the result of differences between cancer cells and lymphocytes or the expression intensity of MMP-7. In addition, Alla et al. reported that the human leukocyte elastase secreted by polymorphonuclear leukocytes could act as a natural inactivator of MMP-7 (51), indicating that MMP-7 expressed on lymphocytes might be inactivated steadily by leukocyte elastase. Like MMP-7, constitutively expressed ADAM-10 did not participate in sRANKL cleavage from activated PBLs, contrary to Hikita’s report (12) showing that shedding of mRANKL expressed on osteoblasts by ADAM10 and MMP14 down-regulates osteoclastogenesis in the in vitro co-culture of osteoblasts and bone marrow macrophages. In fact, the cleavage activity of all four putative sheddases, including TACE, ADAM10, MMP7 and MMP14, was confirmed by the neutralizing effect of four respective antibodies reacting with activated PBLs in the presence of Mca-KPLGL-Dpa-AR-NH2 Fluorogenic Peptide Substrate (R&D Systems, Minneapolis, MN) (data not shown). Since we used mitogens for the stimulation of PBLs, as explained below, the manner of PBL activation might have also affected the outcomes.
Ex vivo stimulation of PBL with mitogens
To perform ex vivo stimulation of PBLs, we used a combination of PMA, ionomycin, and LPS to stimulate both populations of T and B cells in PBLs, following the method reported by Yanaba et al. (39) with some modification. PMA+ionomycin could induce intracellular calcium influx and protein kinase C activation in T cells and replace T cell receptor signals (52). On the other hand, LPS is a potent stimulant for B cells and monocytes (53–55). Indeed, the combination of PMA+ionomycin was more potent than LPS alone for stimulating a nylon wool-enriched T cell population (data not shown). On the other hand, LPS was more potent than PMA+ionomycin for stimulating an enriched B cell population by the EasySep™ Human B Cell Enrichment Kit (StemCell Technologies, Vancouver, BC, Canada (data not shown)). Stimulation of nylon wool-enriched T cell population by anti-CD3/CD28 antibody induced a response similar to that induced by the combination of PMA, ionomycin, and LPS (data not shown). We presumed that the combination of these three reagents could stimulate both T and B cells, as well as monocytes, in a manner similar to the physiological context or periodontal lesion where bacteria-derived LPS, as well as a number of immunostimulatory agents, are released.
Expression profiles and functional roles of TACE in human periodontal tissue
Using human clinical samples of periodontally diseased tissue, B cells were discovered to be the major cellular source of TACE in conjunction with their mRANKL expression, which contributes to the secretion of sRANKL. Thus, this study, for the first time, demonstrated that TACE expressed on activated lymphocytes, especially B cells, plays a pivotal role in the shedding of mRANKL to generate sRANKL in the context of periodontitis. In support of our findings, Bostanci et al. reported that TACE concentrations in GCF were higher in persons with periodontitis than in healthy persons (21). However, the cellular source and the pathophysiological roles of elevated TACE have been largely unknown. To this end, we examined the localization of TACE expression, type of TACE-bearing cells, and function of TACE relative to sRANKL and TNF-α release in inflamed gingival tissue by using human gingival tissue samples obtained from chronic periodontitis patients. Among the gingival tissue samples collected in this study, cellular infiltrates were dominated by T cells (about 70%, as examined by flow cytometry, data not shown), followed by B cells (about 15%) and monocytes (about 10%). Nonetheless, TACE-positive cells in the cellular infiltrates of inflamed gingival tissue were mainly represented by B cells (about 65%), followed by T cells (about 18%) and monocytes (about 17%), indicating that B cells may be more actively engaged in periodontal bone loss than T cells or monocytes. The result were obtained from present experiments were limited to in vitro and ex vivo assays; nevertheless, all outcomes clearly indicated that osteoclastogenic cytokines, in particular sRANKL and TNF-α, are released from lymphocytes by TACE-mediated cleavage and can activate osteoclast precursors to induce osteoclastogenesis from a site distant from the alveolar bone surface in the periodontitis lesion. On the other hand, a study reported that recombinant TACE can promote the production of RANKL from human osteoblast cell line (MG63) in vitro (56), suggesting that osteoblasts may also be engaged in secretion of sRANKL by means of TACE-mediated cleaving of mRANKL expressed on the osteoblasts. Further studies are required to elucidate molecular and cellular mechanisms underlying the production of sRANKL in the context of periodontitis.
Soluble RANKL (sRANKL) cleaved from activated lymphocytes may be more functionally engaged in osteoclastogenesis than mRANKL in periodontitis
The proximity of inflammatory infiltrate to bone surface is a significant factor in the osteoclastic resorption of alveolar bone (57). However, even without direct contact to osteoclasts, infiltrating lymphocytes distant from osteoclasts on the alveolar bone surface could still regulate osteoclastogenesis based on their release of soluble factors. Indeed, our results revealed that activated PBLs could produce abundant sRANKL and TNF-α in a TACE-dependent manner. It is well known that sRANKL can play a key role in bone resorption as a soluble remote factor in the remodeling of bone (10), indicating that sRANKL cleaved from activated lymphocytes might induce osteoclastogenesis from distant sites in periodontitis. Very interestingly, most in vitro experiments for osteoclastogenesis assays utilize recombinant sRANKL, but not recombinant mRANKL (58), suggesting the biological relevance of sRANKL in generating mature functional osteoclasts. Since the binding affinity of RANKL to OPG is much higher than its binding affinity to RANK (59), sRANKL could be neutralized by OPG in the context of healthy bone tissue where abundant OPG is present in the site. On the other hand, in the inflamed periodontium, a lower concentration of OPG compared with that in healthy periodontium was reported (13), demonstrating that neutralization of RANKL by OPG in inflamed gingival or periodontal tissue is not sufficient to suppress the osteoclastogenic action elicited by locally produced RANKL. Furthermore, OPG is mainly produced by mesenchymal cells, such as, osteoblasts, fibroblasts and stromal cells (60, 61). Therefore, it is plausible that OPG from mesenchymal cells cannot sufficiently neutralize the sRANKL produced by lymphocytes constantly infiltrating into the inflamed gingival issue which can remotely reach RANK-expressing osteoclasts. To support this premise, Kanamaru et al. reported that the expression of mRANKL on T cells is strictly limited by the constant shedding by TACE and that most RANKL protein released by T cells is active in the soluble form after shedding (62). The latter study also showed that the addition of KB-R8301, a metalloprotease inhibitor, could inhibit RANKL shedding effects in T cells. Pharmacological information that would support KB-R8301 as a chemical inhibitor of TACE is absent (62), but it is assumed that KB-R8301 blocks TACE activity. Taken together, this line of evidence supports TACE-mediated sRANKL secretion by lymphocytes is actively engaged in osteoclastogenesis in periodontally diseased gingival tissue.
Soluble osteoclastogenesis promotion factor, TNF-α, produced from activated immune cells by TACE-mediated shedding may facilitate a synergism with sRANKL in upregulation of osteoclastogenesis
In addition to sRANKL, TNF-α cleaved from activated lymphocytes by TACE is thought of as another osteoclastogenic cytokine (23–25). In the present study, osteoclastogenesis assay using RAW264.7 cells revealed that the conditioned medium from gingival mononuclear cells (GMC) isolated from patients with periodontitis showed osteoclastogenic potency from both sRANKL and TNF-α. While OPG-Fc greatly reduced the number of osteoclasts induced by the conditioned medium of GMCs, it could not completely inhibit osteoclastogenesis. Meanwhile, anti-TNF-α-antibody by itself showed 60–70% inhibition of osteoclastogenesis induced by the same conditioned medium. These results suggested that TNF-α might induce osteoclastogenesis independent of RANKL. Indeed, as shown in Fig. 5C, TNF-α alone could induce TRAP+ osteoclasts from RAW264.7 cells, while expression levels of osteoclast differentiation and functional marker genes induced by TNF-α were all significantly lower than those induced by sRANKL (Fig. 6A), suggesting that TNF-α is less potent in induction of osteoclastogenesis than sRANKL. This notion of direct TNF-α potency has been argued by many investigators. For example, Li et al. reported that TNF-α may represent an alternative compensatory pathway for the local formation of TRAP- and cathepsin K-positive osteoclasts in RANK−/− mice (23). Kobayashi et al. reported that TRAP-positive osteoclasts were formed when bone marrow macrophages were cultured with mouse TNF-α in the presence of M-CSF (24). In the latter study, they also reported that the addition of OPG could not inhibit TNF-α-dependent osteoclastogenesis, suggesting that TNF-α could stimulate osteoclastogenesis by direct stimulation of osteoclast precursors, not indirectly by RANKL. Others have demonstrated that TNF-α-mediated osteoclastogenesis arises from some indirect effects, such as upregulation of RANKL production from osteoblast/stromal cells (63), which then enhances osteoclast precursor expression of RANK and sensitivity to RANKL (64). It has also been reported that RANKL prompts TNF-α expression in a circuitous manner through osteoclast precursors (65, 66). Using TNF-α transgenic crossed with RANK−/− congenic mice, Li et al. further reported that TNF-α might only be able to induce a population of preosteoclasts (67). Systemic administration of TNF-α antagonist not only suppressed inflammation, but also inhibited periodontal bone loss induced in non-human primates (35). These data, together with our results, suggest a potential synergistic effect of sRANKL and TNF-α on the upregulation of osteoclastogenesis in inflamed periodontal tissue.
In sum, it was demonstrated that sRANKL and TNF-α cleaved from activated TACE-bearing lymphocytes, especially B cells, might play an important role as soluble remote osteoclastogenic factors in periodontitis. For this reason, inhibition of RANKL- and TNF-α-shedding might lead to a therapeutic approach against the pathogenic bone resorption that occurs in periodontitis, and TACE might be a novel therapeutic target.
Acknowledgments
We thank Daniel Nguyen for flow cytometry support and Mikihito Kajiya and Justine Dobeck for technical support.
Source of supports: This research was supported in part by NIH Grants RO1 DE-03420, RO1 DE-018499, RO1 DE-019917, R56 DE-023807 and RO1 DE025255 and T32 DE 7327-12 from NIDCR, Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (23689081, 25670841, and 15K11376), and German Society of Periodontology #2-RST-2013.
Abbreviations used in this article
- ADAM
A Disintegrin and Metalloproteinase domain-containing protein
- GCF
gingival crevicular fluid
- GMC
gingival mononuclear cells
- MMP
matrix metalloproteinase
- TACE
TNF-α converting enzyme
- PBL
peripheral blood lymphocyte
- PMA
Phorbol 12-myristate 13-acetate
Footnotes
Disclosures
The authors have no financial conflicts interest.
References
- 1.Taubman MA, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. Journal of periodontology. 2005;76:2033–2041. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- 2.Teng YT, Nguyen H, Gao X, Kong YY, Gorczynski RM, Singh B, Ellen RP, Penninger JM. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. The Journal of clinical investigation. 2000;106:R59–67. doi: 10.1172/jci10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker PJ, Garneau J, Howe L, Roopenian DC. T-cell contributions to alveolar bone loss in response to oral infection with Porphyromonas gingivalis. Acta odontologica Scandinavica. 2001;59:222–225. doi: 10.1080/00016350152509247. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CW, Izumi Y, Taubman MA. B and T Lymphocytes Are the Primary Sources of RANKL in the Bone Resorptive Lesion of Periodontal Disease. Am J Pathol. 2006;169:987–998. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Goto M, Mochizuki SI, Tsuda E, Morinaga T, Udagawa N, Takahashi N, Suda T, Higashio K. A novel molecular mechanism modulating osteoclast differentiation and function. Bone. 1999;25:109–113. doi: 10.1016/s8756-3282(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 7.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 8.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2000;15:2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 9.Lum L, Wong BR, Josien R, Becherer JD, Erdjument-Bromage H, Schlondorff J, Tempst P, Choi Y, Blobel CP. Evidence for a role of a tumor necrosis factor-alpha (TNF-alpha)-converting enzyme-like protease in shedding of TRANCE, a TNF family member involved in osteoclastogenesis and dendritic cell survival. J Biol Chem. 1999;274:13613–13618. doi: 10.1074/jbc.274.19.13613. [DOI] [PubMed] [Google Scholar]
- 10.Mizuno A, Kanno T, Hoshi M, Shibata O, Yano K, Fujise N, Kinosaki M, Yamaguchi K, Tsuda E, Murakami A, Yasuda H, Higashio K. Transgenic mice overexpressing soluble osteoclast differentiation factor (sODF) exhibit severe osteoporosis. Journal of bone and mineral metabolism. 2002;20:337–344. doi: 10.1007/s007740200049. [DOI] [PubMed] [Google Scholar]
- 11.Lynch CC, Hikosaka A, Acuff HB, Martin MD, Kawai N, Singh RK, Vargo-Gogola TC, Begtrup JL, Peterson TE, Fingleton B, Shirai T, Matrisian LM, Futakuchi M. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer cell. 2005;7:485–496. doi: 10.1016/j.ccr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Hikita A, Yana I, Wakeyama H, Nakamura M, Kadono Y, Oshima Y, Nakamura K, Seiki M, Tanaka S. Negative regulation of osteoclastogenesis by ectodomain shedding of receptor activator of NF-kappaB ligand. The Journal of biological chemistry. 2006;281:36846–36855. doi: 10.1074/jbc.M606656200. [DOI] [PubMed] [Google Scholar]
- 13.Mogi M, Otogoto J, Ota N, Togari A. Differential expression of RANKL and osteoprotegerin in gingival crevicular fluid of patients with periodontitis. Journal of dental research. 2004;83:166–169. doi: 10.1177/154405910408300216. [DOI] [PubMed] [Google Scholar]
- 14.Liu D, Xu JK, Figliomeni L, Huang L, Pavlos NJ, Rogers M, Tan A, Price P, Zheng MH. Expression of RANKL and OPG mRNA in periodontal disease: possible involvement in bone destruction. International journal of molecular medicine. 2003;11:17–21. doi: 10.3892/ijmm.11.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Bostanci N, Ilgenli T, Emingil G, Afacan B, Han B, Toz H, Berdeli A, Atilla G, McKay IJ, Hughes FJ, Belibasakis GN. Differential expression of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin mRNA in periodontal diseases. Journal of periodontal research. 2007;42:287–293. doi: 10.1111/j.1600-0765.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 16.Wara-aswapati N, Surarit R, Chayasadom A, Boch JA, Pitiphat W. RANKL upregulation associated with periodontitis and Porphyromonas gingivalis. Journal of periodontology. 2007;78:1062–1069. doi: 10.1902/jop.2007.060398. [DOI] [PubMed] [Google Scholar]
- 17.Bostanci N, Ilgenli T, Emingil G, Afacan B, Han B, Toz H, Atilla G, Hughes FJ, Belibasakis GN. Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: implications of their relative ratio. Journal of clinical periodontology. 2007;34:370–376. doi: 10.1111/j.1600-051X.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 18.Vernal R, Dutzan N, Hernandez M, Chandia S, Puente J, Leon R, Garcia L, Del Valle I, Silva A, Gamonal J. High expression levels of receptor activator of nuclear factor-kappa B ligand associated with human chronic periodontitis are mainly secreted by CD4+ T lymphocytes. Journal of periodontology. 2006;77:1772–1780. doi: 10.1902/jop.2006.050376. [DOI] [PubMed] [Google Scholar]
- 19.Gumus P, Buduneli E, Biyikoglu B, Aksu K, Sarac F, Nile C, Lappin D, Buduneli N. Gingival crevicular fluid, serum levels of receptor activator of nuclear factor-kappaB ligand, osteoprotegerin, and interleukin-17 in patients with rheumatoid arthritis and osteoporosis and with periodontal disease. Journal of periodontology. 2013;84:1627–1637. doi: 10.1902/jop.2013.120595. [DOI] [PubMed] [Google Scholar]
- 20.Santos VR, Lima JA, Goncalves TE, Bastos MF, Figueiredo LC, Shibli JA, Duarte PM. Receptor activator of nuclear factor-kappa B ligand/osteoprotegerin ratio in sites of chronic periodontitis of subjects with poorly and well-controlled type 2 diabetes. Journal of periodontology. 2010;81:1455–1465. doi: 10.1902/jop.2010.100125. [DOI] [PubMed] [Google Scholar]
- 21.Bostanci N, Emingil G, Afacan B, Han B, Ilgenli T, Atilla G, Hughes FJ, Belibasakis GN. Tumor necrosis factor-alpha-converting enzyme (TACE) levels in periodontal diseases. Journal of dental research. 2008;87:273–277. doi: 10.1177/154405910808700311. [DOI] [PubMed] [Google Scholar]
- 22.Bostanci N, Reddi D, Rangarajan M, Curtis MA, Belibasakis GN. Porphyromonas gingivalis stimulates TACE production by T cells. Oral microbiology and immunology. 2009;24:146–151. doi: 10.1111/j.1399-302X.2008.00488.x. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, Kaufman S, Juan SC, Sun Y, Tarpley J, Martin L, Christensen K, McCabe J, Kostenuik P, Hsu H, Fletcher F, Dunstan CR, Lacey DL, Boyle WJ. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. The Journal of biological chemistry. 2000;275:4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- 26.Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Becherer JD, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 27.Overall CM, Blobel CP. In search of partners: linking extracellular proteases to substrates. Nature reviews Molecular cell biology. 2007;8:245–257. doi: 10.1038/nrm2120. [DOI] [PubMed] [Google Scholar]
- 28.Black RA. Tumor necrosis factor-alpha converting enzyme. The international journal of biochemistry & cell biology. 2002;34:1–5. doi: 10.1016/s1357-2725(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 29.Murphy G, Murthy A, Khokha R. Clipping, shedding and RIPping keep immunity on cue. Trends in immunology. 2008;29:75–82. doi: 10.1016/j.it.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Champagne CM, Buchanan W, Reddy MS, Preisser JS, Beck JD, Offenbacher S. Potential for gingival crevice fluid measures as predictors of risk for periodontal diseases. Periodontology 2000. 2003;31:167–180. doi: 10.1034/j.1600-0757.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- 31.Ballini A, Cantore S, Farronato D, Cirulli N, Inchingolo F, Papa F, Malcangi G, Inchingolo AD, Dipalma G, Sardaro N, Lippolis R, Santacroce L, Coscia MF, Pettini F, De Vito D, Scacco S. PERIODONTAL DISEASE AND BONE PATHOGENESIS: THE CROSSTALK BETWEEN CYTOKINES AND PORPHYROMONAS GINGIVALIS. Journal of biological regulators and homeostatic agents. 2015;29:273–281. [PubMed] [Google Scholar]
- 32.Graves DT. The potential role of chemokines and inflammatory cytokines in periodontal disease progression. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1999;28:482–490. doi: 10.1086/515178. [DOI] [PubMed] [Google Scholar]
- 33.Javed F, Ahmed HB, Mikami T, Almas K, Romanos GE, Al-Hezaimi K. Cytokine profile in the gingival crevicular fluid of rheumatoid arthritis patients with chronic periodontitis. Journal of investigative and clinical dentistry. 2014;5:1–8. doi: 10.1111/jicd.12066. [DOI] [PubMed] [Google Scholar]
- 34.Rossomando EF, White L. A novel method for the detection of TNF-alpha in gingival crevicular fluid. Journal of periodontology. 1993;64:445–449. [PubMed] [Google Scholar]
- 35.Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. Journal of immunology (Baltimore, Md : 1950) 1998;160:403–409. [PubMed] [Google Scholar]
- 36.Stoufi ED, Taubman MA, Ebersole JL, Smith DJ. Preparation and characterization of human gingival cells. Journal of periodontal research. 1987;22:144–149. doi: 10.1111/j.1600-0765.1987.tb01554.x. [DOI] [PubMed] [Google Scholar]
- 37.Kanzaki H, Han X, Lin X, Kawai T, Taubman M. Is RANKL shedding involved in immune cell-mediated osteoclastogenesis? In: Sasano T, Suzuki O, editors. Interface Oral Health Science 2009. Springer; Japan: 2010. pp. 403–405. [Google Scholar]
- 38.Kanzaki H, Han X, Asami Y, Suzuki M, Kawai T, Taubman M. Inhibition of T-Cell-Mediated and Infection-Induced Periodontal Bone Resorption by TACE Blockade. In: Sasaki K, Suzuki O, Takahashi N, editors. Interface Oral Health Science 2011. Springer; Japan: 2012. pp. 173–175. [Google Scholar]
- 39.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. Journal of immunology (Baltimore, Md : 1950) 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeichi O, Haber J, Kawai T, Smith DJ, Moro I, Taubman MA. Cytokine profiles of T-lymphocytes from gingival tissues with pathological pocketing. Journal of dental research. 2000;79:1548–1555. doi: 10.1177/00220345000790080401. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi T, Movila A, Kataoka S, Wisitrasameewong W, Ruiz Torruella M, Murakoshi M, Murakami S, Kawai T. Proinflammatory M1 macrophages inhibit RANKL-induced osteoclastogenesis. Infection and immunity. 2016 doi: 10.1128/IAI.00461-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin X, Han X, Kawai T, Taubman MA. Antibody to receptor activator of NF-kappaB ligand ameliorates T cell-mediated periodontal bone resorption. Infection and immunity. 2011;79:911–917. doi: 10.1128/IAI.00944-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 45.Patel IR, Attur MG, Patel RN, Stuchin SA, Abagyan RA, Abramson SB, Amin AR. TNF-alpha convertase enzyme from human arthritis-affected cartilage: isolation of cDNA by differential display, expression of the active enzyme, and regulation of TNF-alpha. Journal of immunology (Baltimore, Md : 1950) 1998;160:4570–4579. [PubMed] [Google Scholar]
- 46.Ohta S, Harigai M, Tanaka M, Kawaguchi Y, Sugiura T, Takagi K, Fukasawa C, Hara M, Kamatani N. Tumor necrosis factor-alpha (TNF-alpha) converting enzyme contributes to production of TNF-alpha in synovial tissues from patients with rheumatoid arthritis. The Journal of rheumatology. 2001;28:1756–1763. [PubMed] [Google Scholar]
- 47.Horiuchi K, Kimura T, Miyamoto T, Takaishi H, Okada Y, Toyama Y, Blobel CP. Cutting edge: TNF-alpha-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. Journal of immunology (Baltimore, Md : 1950) 2007;179:2686–2689. doi: 10.4049/jimmunol.179.5.2686. [DOI] [PubMed] [Google Scholar]
- 48.Lovering F, Zhang Y. Therapeutic potential of TACE inhibitors in stroke. Current drug targets CNS and neurological disorders. 2005;4:161–168. doi: 10.2174/1568007053544147. [DOI] [PubMed] [Google Scholar]
- 49.Hikita A, Tanaka S. Ectodomain shedding of receptor activator of NF-kappaB ligand. Advances in experimental medicine and biology. 2007;602:15–21. doi: 10.1007/978-0-387-72009-8_2. [DOI] [PubMed] [Google Scholar]
- 50.Thiolloy S, Halpern J, Holt GE, Schwartz HS, Mundy GR, Matrisian LM, Lynch CC. Osteoclast-derived matrix metalloproteinase-7, but not matrix metalloproteinase-9, contributes to tumor-induced osteolysis. Cancer research. 2009;69:6747–6755. doi: 10.1158/0008-5472.CAN-08-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alla V, Kashyap A, Gregor S, Theobald M, Heid H, Galle PR, Strand D, Strand S. Human leukocyte elastase counteracts matrix metalloproteinase-7 induced apoptosis resistance of tumor cells. Cancer letters. 2008;268:331–339. doi: 10.1016/j.canlet.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Takahama Y, Nakauchi H. Phorbol ester and calcium ionophore can replace TCR signals that induce positive selection of CD4 T cells. Journal of immunology (Baltimore, Md : 1950) 1996;157:1508–1513. [PubMed] [Google Scholar]
- 53.Mosier DE, Subbarao B. Thymus-independent antigens: complexity of B-lymphocyte activation revealed. Immunology today. 1982;3:217–222. doi: 10.1016/0167-5699(82)90095-0. [DOI] [PubMed] [Google Scholar]
- 54.Sweet MJ, Hume DA. Endotoxin signal transduction in macrophages. Journal of leukocyte biology. 1996;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 55.Venkataraman C, Shankar G, Sen G, Bondada S. Bacterial lipopolysaccharide induced B cell activation is mediated via a phosphatidylinositol 3-kinase dependent signaling pathway. Immunology letters. 1999;69:233–238. doi: 10.1016/s0165-2478(99)00068-1. [DOI] [PubMed] [Google Scholar]
- 56.Lee JH, Choi YJ, Heo SH, Lee JM, Cho JY. Tumor necrosis factor-alpha converting enzyme (TACE) increases RANKL expression in osteoblasts and serves as a potential biomarker of periodontitis. BMB reports. 2011;44:473–477. doi: 10.5483/BMBRep.2011.44.7.473. [DOI] [PubMed] [Google Scholar]
- 57.Rowe DJ, Bradley LS. Quantitative analyses of osteoclasts, bone loss and inflammation in human periodontal disease. Journal of periodontal research. 1981;16:13–19. doi: 10.1111/j.1600-0765.1981.tb00945.x. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi N, Udagawa N, Kobayashi Y, Suda T. Generation of osteoclasts in vitro, and assay of osteoclast activity. Methods in molecular medicine. 2007;135:285–301. doi: 10.1007/978-1-59745-401-8_18. [DOI] [PubMed] [Google Scholar]
- 59.Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Yano K, Morinaga T, Higashio K. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochemical and biophysical research communications. 1998;253:395–400. doi: 10.1006/bbrc.1998.9788. [DOI] [PubMed] [Google Scholar]
- 60.Saidenberg Kermanac’h N, Bessis N, Cohen-Solal M, De Vernejoul MC, Boissier MC. Osteoprotegerin and inflammation. European cytokine network. 2002;13:144–153. [PubMed] [Google Scholar]
- 61.Aubin JE, Bonnelye E. Osteoprotegerin and its ligand: A new paradigm for regulation of osteoclastogenesis and bone resorption. Medscape women’s health. 2000;5:5. [PubMed] [Google Scholar]
- 62.Kanamaru F, Iwai H, Ikeda T, Nakajima A, Ishikawa I, Azuma M. Expression of membrane-bound and soluble receptor activator of NF-kappaB ligand (RANKL) in human T cells. Immunology letters. 2004;94:239–246. doi: 10.1016/j.imlet.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 63.Abu-Amer Y, Abbas S, Hirayama T. TNF receptor type 1 regulates RANK ligand expression by stromal cells and modulates osteoclastogenesis. Journal of cellular biochemistry. 2004;93:980–989. doi: 10.1002/jcb.20197. [DOI] [PubMed] [Google Scholar]
- 64.Komine M, Kukita A, Kukita T, Ogata Y, Hotokebuchi T, Kohashi O. Tumor necrosis factor-alpha cooperates with receptor activator of nuclear factor kappaB ligand in generation of osteoclasts in stromal cell-depleted rat bone marrow cell culture. Bone. 2001;28:474–483. doi: 10.1016/s8756-3282(01)00420-3. [DOI] [PubMed] [Google Scholar]
- 65.Zou W, Hakim I, Tschoep K, Endres S, Bar-Shavit Z. Tumor necrosis factor-alpha mediates RANK ligand stimulation of osteoclast differentiation by an autocrine mechanism. Journal of cellular biochemistry. 2001;83:70–83. doi: 10.1002/jcb.1202. [DOI] [PubMed] [Google Scholar]
- 66.Zou W, Amcheslavsky A, Takeshita S, Drissi H, Bar-Shavit Z. TNF-alpha expression is transcriptionally regulated by RANK ligand. Journal of cellular physiology. 2005;202:371–378. doi: 10.1002/jcp.20127. [DOI] [PubMed] [Google Scholar]
- 67.Li P, Schwarz EM, O’Keefe RJ, Ma L, Boyce BF, Xing L. RANK signaling is not required for TNFalpha-mediated increase in CD11(hi) osteoclast precursors but is essential for mature osteoclast formation in TNFalpha-mediated inflammatory arthritis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2004;19:207–213. doi: 10.1359/JBMR.0301233. [DOI] [PubMed] [Google Scholar]