Abstract
Rationale:
Vemurafenib, an inhibitor of mutated B-rapidly accelerated fibrosarcoma, is frequently used in the treatment of melanoma and Erdheim–Chester disease (ECD) patients. Inflammatory adverse effects have been increasingly reported after vemurafenib treatment.
Patient concerns and diagnose:
We report 6 cases of vemurafenib-associated vasculitis, of whom a personal case of a 75-year-old man with history of ECD who developed purpura and rapidly progressive pauci-immune glomerulonephritis during treatment with vemurafenib.
Intervention:
In the 5 others cases from the literature, all patients presented skin vasculitis, and with joint involvement in 60% of them. Vemurafenib treatment was stopped (n = 3), continued at reduced doses (n = 1), or continued at the same dose (n = 2).
Outcomes:
Three patients (50%) received corticosteroids combined with cyclophosphamide (n = 1), and all achieved remission of vasculitis. One patient experienced vasculitis relapse after vemurafenib therapy was restarted.
Lessons:
Systemic vasculitis is a rare vemurafenib-associated adverse event that may be life-threatening.
Keywords: case report, histiocytosis, immunotherapy, melanoma, vasculitis, vemurafenib
1. Introduction
Vemurafenib (PLX4032) is an inhibitor of mutated B-rapidly accelerated fibrosarcoma (BRAF), especially BRAFV600E.[1] Metastatic melanoma patients treated with vemurafenib showed a response rate of 48% and improved survival.[2] Erdheim–Chester disease (ECD) is a rare non-Langherans cell hystiocytosis where BRAFV600E mutation is frequently found.[3,4] ECD patients with BRAFV600E presented good response when treated with vemurafenib.[5,6]
Serious adverse effects (SAEs) were reported in 8% of patients treated with vemurafenib for metastatic melanoma.[2] SAEs were also reported in 94% of ECD or Langherans cell histocytiosis patients treated with vemurafenib.[6] Main side effects included skin (rash, squamous cell carcinoma, hyperkeratosis, alopecia) and gut involvement (nausea, diarrhea), cytopenia, and arthralgia.[2] More recently, inflammatory adverse effects like panniculitis have been reported.[7] Very few cases of vemurafenib-associated vasculitis have been reported.[8–10] Vasculitis may be life-threatening depending on which organ is involved.
Herein, we report a patient with ECD with vemurafenib-associated severe systemic vasculitis. We reviewed the literature and analyzed 5 additional cases of vemurafenib-associated vasculitis.
2. Methods
The patient reported was followed in Internal Medicine and Clinical Immunology Department at Pitié-Salpêtrière University Hospital, Paris, France. Demographic, medical history, laboratory, imaging, histology, treatment, and follow-up data were extracted from medical records. Patient gave his informed consent.
2.1. Literature review
MEDLINE (National Library of Medicine, Bethesda, MD) search was performed until April 2016 using [vemurafenib] or [BRAF inhibitor] and [vasculitis]. Five cases were identified and analyzed.
2.2. Patient
We report a 75-year-old man with a 7-year history of ECD admitted in our department. He had a past medical history with arterial hypertension, type 2 diabetes, ST-elevation myocardial infarction (STEMI) without chronic heart failure, and nonalcoholic steatohepatitis. ECD was diagnosed in 2009 on a biopsy sample of perirenal fibrosis, showing foamy CD68+ CD1a− histiocytes. Immunohistochemistry analysis detected the presence of BRAFV600E mutation. Thoracoabdominal computed tomography (CT) scan showed a right pleural effusion, mesenteric panniculitis, right upper femoral metaphysis osteosclerosis, and perirenal fibrosis with hairy kidney sign. Brain magnetic resonance imaging (MRI) showed a left retro-orbital mass. Pegylated interferon α (PEG-IFN) treatment was started in October 2009. Because of disease progression, PEG-IFN was stopped in September 2010 and a recombinant human interleukin (IL)-1 receptor antagonist treatment was initiated. This treatment permitted disease control, but was stopped in September 2013 because of skin adverse effects. Our patient was treated with infliximab from September 2013 to January 2014. This treatment was stopped due to disease progression. A targeted therapy with BRAFV600E inhibitor (vemurafenib) was initiated in January 2014 at 480 mg/d. In September 2014, the dose was reduced to 240 mg/d because of cholestasis. In February 2015, the treatment dose was resumed to 480 mg. This treatment permitted disease control. In February 2016, our patient was admitted to our department for annual disease re-evaluation. Clinical evaluation showed leg edema, infiltrated purpura on the legs, and bilateral lung crackles. Biological explorations found acute kidney injury with serum creatinine level of 225 μmol/l and C-reactive protein (CRP) was elevated at 16 mg/l. Kidney ultrasound showed no renal dilatation. Urine sediment analysis showed microscopic hematuria (47.104 red blood cells/ml). Urine biological explorations found a glomerular proteinuria, with urine protein-to-creatinine ratio of 0.25 g/mmol and albumin-to-creatinine ratio of 0.2 g/mmol. Immune explorations found no complement consumption, no antineutrophil cytoplasmic antibody (ANCA), no cryoglobulins and rheumatoid factor, and no anti-glomerular basement membrane antibody (anti-GBM). Antinuclear antibodies (ANAs) were positive at a 1:320 dilution, with no anti-DNA (ELISA) and no anti-extractable nuclear antigen (ENA). Kidney function worsened in few days to a serum creatinine level of 410 μmol/l. Kidney biopsy sample analysis showed 13 glomeruli with normal permeability (Fig. 1). One glomerulus presented a crescent, compatible with extracapillary proliferative glomerulonephritis. Immufluorescent analysis showed pauci-immune glomerulonephritis (ie, no immunoglobulin deposit, no C1q deposit, no light chains deposit, and some C3 deposits). Thoracoabdominal CT scan and brain MRI findings showed no signs of ECD evolution. We retained the diagnosis of vemurafenib-associated vasculitis with skin and kidney involvement. Vemurafenib was stopped. Our patient was treated with high-dose glucocorticosteroids (methyprednisolone pulse of 1 g/d during 3 days) and cyclophosphamide (0.5 g/m2), monthly during 2 months. Glucocorticosteroids were pursued with prednisone orally at 1 mg/kg/d. The outcome was favorable with quick renal function improvement (Fig. 1).
Figure 1.
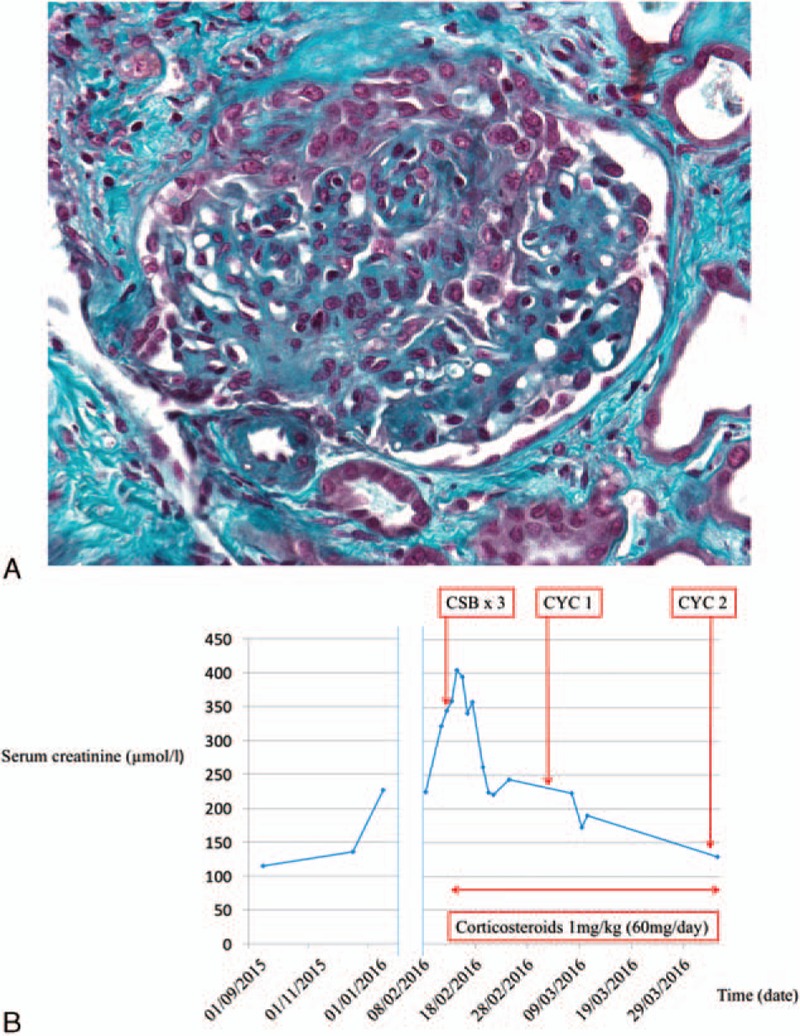
Vemurafenib-associated pauci-immune glomerulonephritis. A, Kidney biopsy reveals extracapillary crescent (Trichrome stain; original magnification: ×400). B, Course of kidney function (ie, serum creatinine level). We observed a dramatic improvement within days after corticosteroid therapy combined with cyclophosphamide. CSB = corticosteroids bolus, CYC = cyclophosphamide.
2.3. Literature review
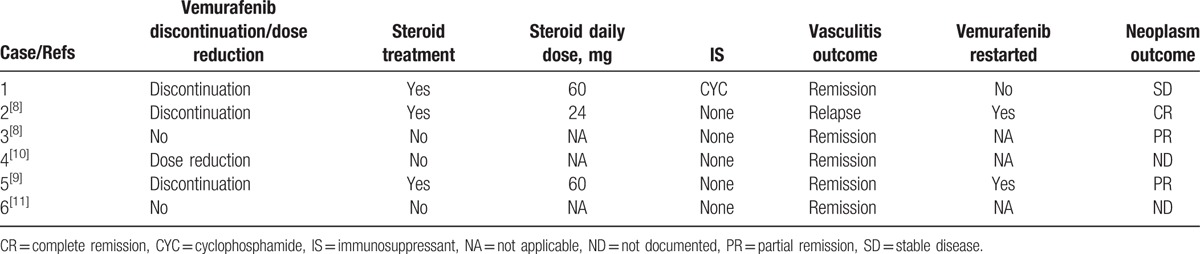
To our knowledge, there are only 5 additional reported cases of vemurafenib-associated vasculitis (Table 1).[8–11] Apart from our patient, all 5 other patients received vemurafenib as treatment for metastatic melanoma. Median age was 47 (29.8; 58.5) years and sex ratio was 1. Vasculitis appeared 15.5 (9.5; 232.8) days after treatment initiation. Three patients (60%) described arthralgia associated with skin vasculitis. All patients presented biological inflammatory syndrome with elevated CRP. Elevated ANA titers were detected in 3 cases. Vasculitis was biopsy-proven in every case. Vemurafenib treatment was stopped in 3 cases, continued with reduced doses in 1 case, and continued at the same dose in 2 cases (Table 2). Three patients (60%) received steroids as vasculitis treatment. In 1 case, vasculitis relapsed after vemurafenib therapy was restarted. No progressive neoplasm was reported after vasculitis treatment.
Table 1.
Demographics, clinical and biological presentations, and histopathology of patients with vemurafenib-associated vasculitis.
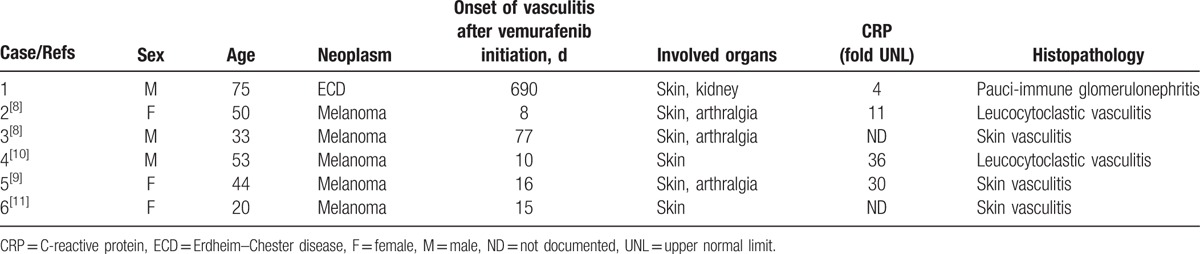
Table 2.
Vasculitis management and outcomes.
3. Discussion
Since BRAF inhibitors have entered the field of neoplasm therapy, inflammatory adverse effects have been increasingly reported.[9–15] These adverse effects were mainly skin reactions like panniculitis. Skin vasculitis has already been described in 5 patients treated with vemurafenib.[8–11] Severe systemic vasculitis, including rapidly progressive kidney glomerulonephritis, has never been described to our knowledge.
Other vasculitis-reported cases involved mainly skin and joints. In these cases, vasculitis appeared early after vemurafenib treatment initiation, within the first month. In 1 case, vasculitis appeared 11 weeks after treatment initiation.[8] In our case, serum creatinine level began to increase 20 months after vemurafenib treatment initiation. A delay of more than 1 year of BRAF inhibitor treatment has already been described in a patient who developed panniculitis.[8]
Acute kidney injury has already been described during vemurafenib treatment.[16] However, the diagnosis was an acute immunoallergic interstitial nephritis and there was no evidence of vasculitis.
Vemurafenib-associated vasculitis seems to be very rare. Thus, there is no consensus for vasculitis management. For some reported patients, vasculitis regressed only with vemurafenib dose reduction. For other patients, a treatment with steroids has been necessary. In patients treated with vemurafenib for metastatic melanoma, vemurafenib could be continued or restarted after vasculitis management. This is very important for neoplasm control and patient survival. However, restarting vemurafenib treatment may induce vasculitis relapse.[8] In our case, vemurafenib has been stopped because kidney vasculitis might induce kidney failure and require extrarenal replacement therapy. For our patient, vemurafenib benefit might not be superior to the risk of kidney failure.
The pathogenesis of BRAF inhibitor associated vasculitis is not clear. It has been shown that RAF inhibitors inhibit extracellular signal-regulated kinase (ERK) signaling in cells with mutant BRAF, but unexpectedly enhance signaling in cells with wild-type BRAF.[17] It explains why incidence of some neoplasms, like cutaneous squamous cell carcinomas, is more important in vemurafenib-treated patients. Furthermore, it has been shown that stimulation of neutrophils with lipopolysaccharide (LPS) resulted in the activation of ERK and p38 mitogen-activated protein kinase (MAPK).[18] MAPK pathway activation was associated with neutrophil migration and may explain inflammatory reactions.
It could be hypothesized that BRAFV600E inhibitors like vemurafenib could paradoxically activate BRAF wild-type and RAS (RAt Sarcoma) pathway in other cells and induce vasculitis or other inflammatory reactions.
In conclusion, inflammatory disorders associated with BRAF inhibitors are increasingly reported. Vasculitis is a rare vemurafenib-induced adverse event that may be life-threatening. Kidney involvement might lead to kidney failure. Continuing or restarting vemurafenib therapy may induce vasculitis relapse.
Footnotes
Abbreviations: ANAs = antinuclear antibodies, ANCA = antineutrophil cytoplasmic antibody, anti-ENA = antiextractable nuclear antigen, anti-GBM = anti-glomerular basement membrane antibody, CRP = C-reactive protein, CT = computed tomography, ECD = Erdheim–Chester disease, ERK = extracellular signal-regulated kinase, LPS = lipopolysaccharide, MAPK = p38 mitogen-activated protein kinase, MRI = magnetic resonance imaging, PEG-IFN = pegylated interferon α, SAE = serious adverse effect, STEMI = ST-elevation myocardial infarction.
The authors have no conflicts of interest to disclose.
References
- 1.Sala E, Mologni L, Truffa S, et al. BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells. Mol Cancer Res MCR 2008; 6:751–759. [DOI] [PubMed] [Google Scholar]
- 2.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond EL, Dagna L, Hyman DM, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood 2014; 124:483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haroche J, Charlotte F, Arnaud L, et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood 2012; 120:2700–2703. [DOI] [PubMed] [Google Scholar]
- 5.Haroche J, Cohen-Aubart F, Emile J-F, et al. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAF(V600E)-mutated Erdheim-Chester disease. J Clin Oncol 2015; 33:411–418. [DOI] [PubMed] [Google Scholar]
- 6.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 2015; 373:726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macdonald JB, Macdonald B, Golitz LE, et al. Cutaneous adverse effects of targeted therapies: part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol 2015; 72:221–236.[quiz 237–238]. [DOI] [PubMed] [Google Scholar]
- 8.Mössner R, Zimmer L, Berking C, et al. Erythema nodosum-like lesions during BRAF inhibitor therapy: report on 16 new cases and review of the literature. J Eur Acad Dermatol Venereol 2015; 29:1797–1806. [DOI] [PubMed] [Google Scholar]
- 9.Zimmer L, Livingstone E, Hillen U, et al. Panniculitis with arthralgia in patients with melanoma treated with selective BRAF inhibitors and its management. Arch Dermatol 2012; 148:357–361. [DOI] [PubMed] [Google Scholar]
- 10.Novoa RA, Honda K, Koon HB, et al. Vasculitis and panniculitis associated with vemurafenib. J Am Acad Dermatol 2012; 67:e271–e272. [DOI] [PubMed] [Google Scholar]
- 11.Chaminade A, Conte H, Jouary T, et al. BRAF inhibitors-induced panniculitis: a cutaneous side effect mimicking subcutaneous melanoma metastasis. J Eur Acad Dermatol Venereol 2015; 29:392–393. [DOI] [PubMed] [Google Scholar]
- 12.Choy B, Chou S, Anforth R, et al. Panniculitis in patients treated with BRAF inhibitors: a case series. Am J Dermatopathol 2014; 36:493–497. [DOI] [PubMed] [Google Scholar]
- 13.Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J 2013; 19:16. [PubMed] [Google Scholar]
- 14.Kim GH, Levy A, Compoginis G. Neutrophilic panniculitis developing after treatment of metastatic melanoma with vemurafenib. J Cutan Pathol 2013; 40:667–669. [DOI] [PubMed] [Google Scholar]
- 15.Monfort J-B, Pagès C, Schneider P, et al. Vemurafenib-induced neutrophilic panniculitis. Melanoma Res 2012; 22:399–401. [DOI] [PubMed] [Google Scholar]
- 16.Regnier-Rosencher E, Lazareth H, Gressier L, et al. Acute kidney injury in patients with severe rash on vemurafenib treatment for metastatic melanomas. Br J Dermatol 2013; 169:934–938. [DOI] [PubMed] [Google Scholar]
- 17.Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010; 464:427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aomatsu K, Kato T, Fujita H, et al. Toll-like receptor agonists stimulate human neutrophil migration via activation of mitogen-activated protein kinases. Immunology 2008; 123:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]


