Abstract
The aim of this article was to assess the associations of serum 25-hydroxyvitamin D [25(OH)D] and daily sun exposure time with myopia in Korean adults.
This study is based on the Korea National Health and Nutrition Examination Survey (KNHANES) of Korean adults in 2010–2012; multiple logistic regression analyses were performed to examine the associations of serum 25(OH)D levels and daily sun exposure time with myopia, defined as spherical equivalent ≤–0.5D, after adjustment for age, sex, household income, body mass index (BMI), exercise, intraocular pressure (IOP), and education level. Also, multiple linear regression analyses were performed to examine the relationship between serum 25(OH)D levels with spherical equivalent after adjustment for daily sun exposure time in addition to the confounding factors above.
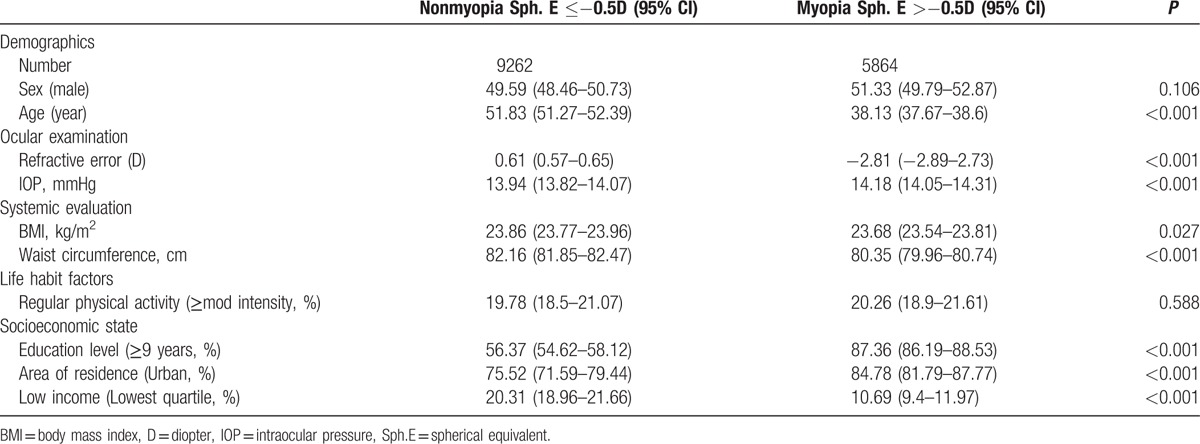
Between the nonmyopic and myopic groups, spherical equivalent, age, IOP, BMI, waist circumference, education level, household income, and area of residence differed significantly (all P < 0.05). Compared with subjects with daily sun exposure time <2 hour, subjects with sun exposure time ≥2 to <5 hour, and those with sun exposure time ≥5 hour had significantly less myopia (P < 0.001). In addition, compared with subjects were categorized into quartiles of serum 25(OH)D, the higher quartiles had gradually lower prevalences of myopia after adjustment for confounding factors (P < 0.001). In multiple linear regression analyses, spherical equivalent was significantly associated with serum 25(OH)D concentration after adjustment for confounding factors (P = 0.002).
Low serum 25(OH)D levels and shorter daily sun exposure time may be independently associated with a high prevalence of myopia in Korean adults. These data suggest a direct role for vitamin D in the development of myopia.
Keywords: KNHANES, myopia, outdoor acitivity, vitamin D
1. Introduction
Myopia is one of the most common ocular disorders worldwide,[1] and the myopic population in Southeast Asia has grown significantly in recent years.[2–6] The costs of examinations and surgical correction of myopia are significant, and this disorder has been associated with other pathological eye conditions, such as macular and retinal degeneration, foveoschisis, and rhegmatogenous retinal detachment.[7–9] Although the cause of myopia is as yet unclear, genetic and environmental factors may contribute to its development.[10–15] Some recent studies suggested that increasing time spent outdoors may prevent the development of myopia,[14–17] although the results of some epidemiological studies oppose this theory.[18,19]
One possible risk factor for myopia that has received attention is vitamin D, which is cutaneously synthesized during exposure to sunlight. The 25-hydroxyvitamin D [25(OH)D] is an essential hormone that controls calcium and bone metabolism.[20,21] It is now clear that 25(OH)D has diverse effects on the immune system, cardiovascular system, obesity, diabetes, and oncogenesis.[22–28] The relationship between 25(OH)D and several ocular diseases, such as myopia, age-related macular degeneration, diabetic retinopathy, uveitis, and glaucoma, is also under investigation.[29–38] Regarding myopia, several previous studies have found that low hydroxyvitamin D is associated with a high prevalence of myopia in young populations.[36,37,39] However, there is controversy as to whether 25(OH)D is an independent factor for progression of myopia.[38–40] To date, the possible independent associations of serum vitamin D levels and sunlight exposure time with myopia have not been sufficiently evaluated.
Compared with the United States and Canada, Korea has a lower mean 25(OH)D level and a higher prevalence of vitamin D insufficiency. A population study revealed that 25(OH)D deficiency (<20 ng/mL) was present in 69.5% of male and 83.1% of female workers aged 20 to 65 years.[41] This may be related to the high latitude (34°–38° N) of Korea, and reduced levels of time spent outdoors (owing to high educational pressure and indoor working conditions).[41–43] Considering the high prevalence of myopia and deficiency of vitamin D in the Korean population, it would be useful to understand the associations of vitamin D deficiency and daily sun exposure time with myopia in this population. Thus, in this cross sectional study, we investigated the relationship between serum 25(OH)D and daily sun exposure time and the prevalence of myopia in Korean adults.
2. Subjects, materials, and methods
2.1. Study population
This study was performed with data from the Korea National Health and Nutrition Examination Survey (KNHANES) V 2010–2012. KNHANES is a cross-sectional, nationally representative sample of the Korean non-institutionalized civilian population aged 1 year or older that uses a rolling sampling design incorporating a complex, stratified, multistage probability sample method. KNHANES is organized regularly by the Korean Ministry of Health and Welfare to monitor the health and nutritional status of the South Korean population. KNHANES is comprised of a health interview survey, a health examination survey—including ophthalmic examination—and a nutrition survey. In this study, individuals aged 20 years or older who participated in the ophthalmologic survey were selected. In total, 25,534 subjects were included in KNHANES from 2010 to 2012, among whom a total of 23,239 completed the ophthalmologic survey. Of these 23,239 subjects, those who had a history of ocular surgery, were younger than 20 years, or had missing values were excluded.
In total, 15,126 subjects were finally selected as the study population. KNHANES V was conducted according to the Declaration of Helsinki, and was carried out by specially trained interviewers or examiners who were not provided with any previous information about the participants. All participants signed an informed consent form. This study was approved by the Institutional Review Board of the Catholic University of Korea.
2.2. Measurements
In the health interview survey, trained interviewers recorded information on demographic status and health-related characteristics, including age, education, residence, and household income. Information about lifestyle characteristics, including smoking, alcohol consumption, and exercise, were recorded using self-reported questionnaires. Residence was categorized as urban (8 major cities in South Korea: Seoul, Gyeonggi, Busan, Daegu, Incheon, Gwangju, Daejeon, Ulsan) or rural (8 other provinces in South Korea). Household income was divided into quartiles, after family income was adjusted for the number of family members. Participants were grouped in the low-income group if their household income was in the lowest quartile. Subjects’ education level was categorized into 4 groups by highest education achieved: elementary school, middle school, high school, and university. Subjects were categorized in the high education group if their highest education level was high school or university. Smoking status was categorized into 3 groups: nonsmokers, current smokers, and exsmokers. Alcohol consumption status was also classified into 3 groups: nondrinker, mild-to-moderate drinker (<30.0 g/day of alcohol) and heavy drinker (≥30.0 g/day of alcohol). Regular exercise was categorized as “yes” when participants were engaged in moderate-intensity physical activity (eg, carrying light loads, cycling at a regular pace, playing tennis) for at least 20 minutes at a time on at least 3 occasions per week. Data on current sunlight exposure time were categorized according to sun exposure time, as <2 hours; ≥2 hours to <5 hours; or ≥5 hours.
Body measurements of all participants were recorded during the health examination. Height was measured to the nearest 0.1 cm using a portable stadiometer (SECA 225; SECA Deutschland, Hamburg, Germany) while the participants were standing barefoot. Weight was measured to the nearest 0.1 kg using an electronic scale (GL–6000–20; CAS KOREA, Seoul, Korea) while the participants wore a lightweight gown. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2). Waist circumference (WC) was measured after normal expiration to the nearest 0.1 cm using a measuring tape (SECA 200; SECA Deutschland), at the mid-level between the lower margin of the ribcage and the iliac crest at the mid-axillary line. Obesity was defined as a BMI ≥25 kg/m2.[44] Blood pressure (BP) was measured from the right arm using a standard mercury sphygmomanometer (Baumanometer; WA Baum Co, Copiague, NY) after 5 minutes of rest in a sitting position. Systolic and diastolic BPs were measured 3 times at 30-second intervals, and the average of the last 2 values was used for analysis. Hypertension was defined as a systolic BP ≥140 mmHg, a diastolic BP ≥90 mmHg, or taking antihypertensive medications.
Serum 25(OH)D levels were assayed according to an agreed protocol. Overnight fasting blood samples were collected from each participant during the survey, and the level of serum 25(OH)D was measured by radioimmunoassay (DiaSorin, Stillwater, MN) using a gamma counter (1470 Wizard; Perkin Elmer, Turku, Finland). The KNHANES study participants were evaluated by the serum 25(OH)D Standardization Program, so the measurement of serum 25(OH)D was standardized to the recently developed National Institute of Standards and Technology-Ghent University reference procedure.[45] Subjects were categorized into quartiles (Q) of the vitamin D and with cutoff values.
Ophthalmic examination procedures were stratified according to age group, and autorefraction and visual acuity testing of participants aged 5 years and older were performed. Participants over 19 years of age underwent full ophthalmological examinations. All ophthalmologic examinations were performed using a slit-lamp (Haag-Streit model BQ-900; Haag-Streit AG, Köniz, Switzerland) by ophthalmologists. The participants underwent ophthalmologic interviews, visual acuity measurements, slit-lamp examinations of the anterior segment, refraction checks, and intraocular pressure (IOP) measurements. Refraction was measured 3 times without cycloplegia using an autorefractor-keratometer (KR-8800®, Topcon, Tokyo, Japan) by ophthalmology residents or ophthalmologists. Refraction measurements were converted into spherical equivalents, calculated as the spherical value plus half of the astigmatic value. Myopia was defined by spherical equivalent of −0.50 diopters (D) or more. Mild myopia was defined as >–3.0 D, moderate myopia was defined as ≤–3.0 D, and high myopia was defined as ≤−6.0 D. IOP was measured once per eye with Goldmann applanation tonometry during the slit-lamp examination. A digital nonmydriatic retinal camera and a Nikon D–80 digital camera (Nikon Inc., Tokyo, Japan) were used to obtain fundus images from all participants without dilating the pupil. Refractive error was defined based on the right eye.
2.3. Statistical analysis
Statistical analyses were conducted with the survey procedure of the SAS software package (ver. 9.3; SAS Institute Inc, Cary, NC), using sampling weights of KNHANES, to acquire nationally representative prevalence estimates. All the analyses performed in this study were adjusted for survey year to minimize the variation among survey years. Data are presented as means ± SE for continuous variables or as proportions (SE) for categorical variables. General and clinical characteristics of subjects were compared according to the presence of myopia. Variables with skewed distributions were analyzed after logarithmic transformation. Simple and multiple linear regression analyses were used to examine the associations of serum 25(OH)D concentrations and daily sun exposure time with spherical equivalent. Multiple logistic regression analysis was used to estimate the magnitude of the association of serum 25(OH)D level and average daily sun exposure time with myopia (spherical equivalent ≤–0.5 D). The odds ratio (OR) was calculated after adjusting for surveyed year, age, and sex in Model 1. Model 2 adjusted for age, sex, household income, BMI, life habitat factors, and education level, and additionally adjusting for serum 25(OH)D levels (for the association between sun exposure and myopia) and average time of daily sun exposure (for the association between serum 25[OH]D level and myopia), respectively. Adjusted ORs for serum 25(OH)D level and average time of daily sun exposure in subjects with myopia were calculated. A P value <0.05 was considered statistically significant.
3. Results
Among the study participants, 61.2% had myopia (−0.5 D or more myopic). Table 1 shows the general and clinical characteristics of the subjects according to the presence of myopia. The spherical equivalent of refractive error of the myopic group (−2.81 ± 0.04 D) was significantly different from that of nonmyopic group (0.61 ± 0.02 D; P < 0.001). Age, IOP, BMI, WC, education level, household income, and area of residence were significantly different between the nonmyopic and myopic groups (all P < 0.05).
Table 1.
Clinical characteristics of study subjects according to the presence of myopia in Korean adults (n = 15,126).
In the multiple linear regression analysis for the association between serum 25(OH)D concentration and refractive error (Table 2), refractive error was significantly associated with serum 25(OH)D concentration after adjusting for age and sex (P < 0.001), which was maintained after additional adjustment for BMI, smoking, drinking, education, income, exercise, IOP, and daily sun exposure time (P = 0.002).
Table 2.
Multiple linear regression analysis for the association between serum 25(OH)D concentrations and refractive error (D) in Korean adults.
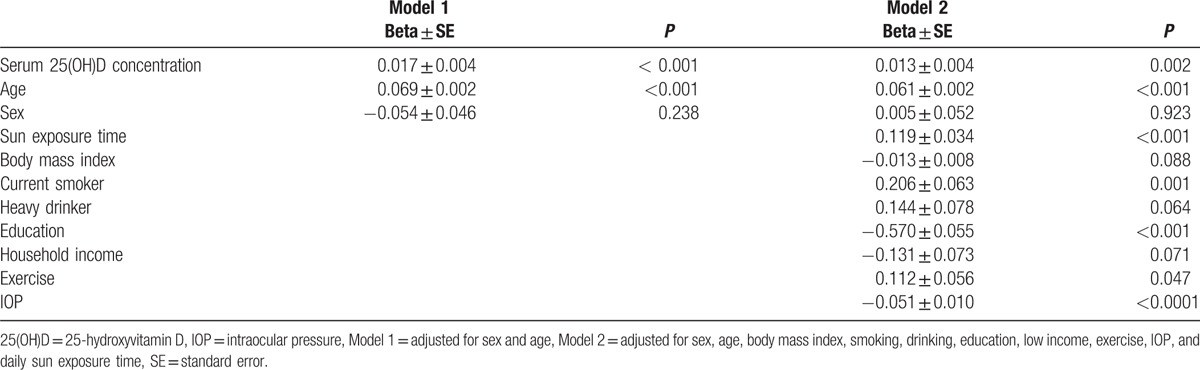
Figure 1 shows the distribution of daily sun exposure time and serum 25(OH)D concentration according to the severity of myopia. The more myopic subjects had less sun exposure. In contrast, subjects with emmetropia or lower myopia had a tendency to receive more sun exposure (Fig. 1A). Likewise, the higher myopia subjects had lower levels of serum 25(OH)D. Emmetropia or less myopic subjects tended to have higher levels of serum 25(OH)D (Fig. 1B).
Figure 1.
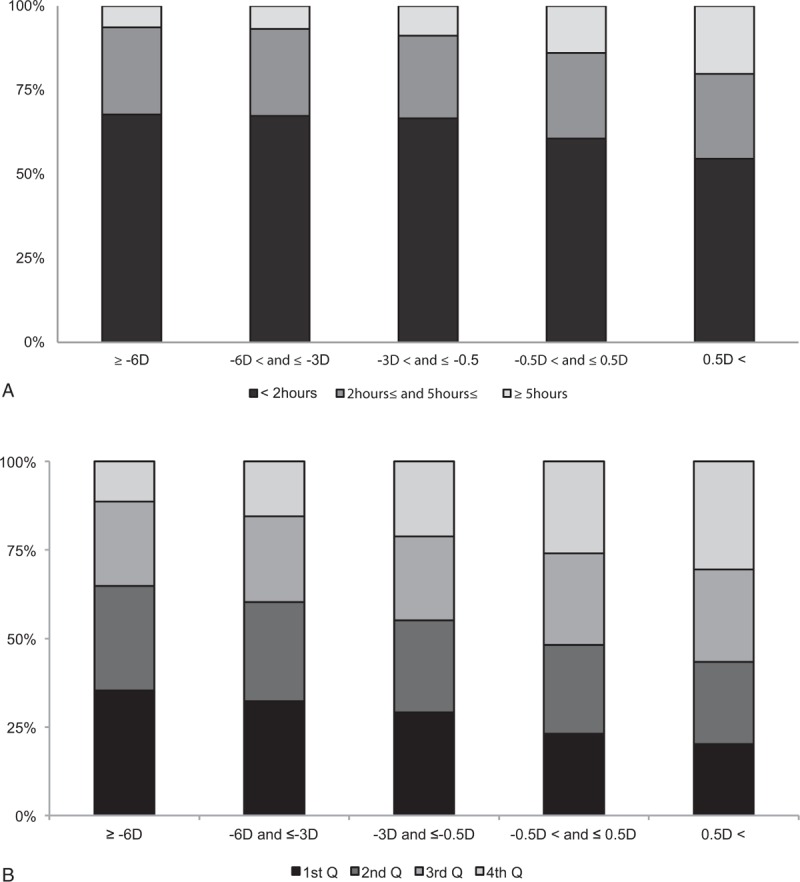
Distribution of daily sun exposure time and serum 25-hydroxyvitamin D [25(OH)D] level according to severity of myopia. (A) The more myopic subjects had less sun exposure time. In contrast, subjects with emmetropia or lower myopia tended to have more sun exposure time. (B) Likewise, the higher myopia subjects had lower levels of serum 25(OH)D. Subjects with emmetropia or lower myopia tended to have higher levels of serum 25(OH)D. The quartile(Q) cutoff values of 25(OH)D (ng/mL) were 1st Q less than 13.2, 2nd Q 13.2 to <16.72, 3rd Q 16.72 to <20.93, and 4th Q greater ≥20.93. D, diopter.
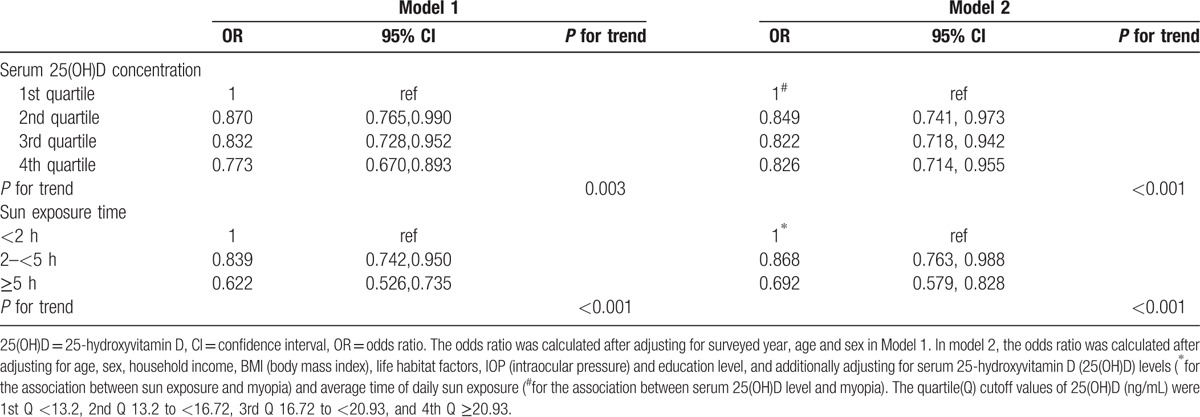
Results of the multiple logistic regression analyses for the association of serum 25(OH)D concentration and daily sun exposure time with myopia are shown in Table 3. Compared with subjects were categorized into lowest quartile of serum 25(OH)D, subjects with the higher quartiles had gradually lower prevalence of myopia after adjusting for age and sex (P = 0.003), which was maintained after additional adjustment for household income, BMI, exercise, education level, IOP, and daily sun exposure time (P < 0.001, Model 2). In addition, compared with subjects with daily sun exposure time <2 hours, subjects with sun exposure times of ≥2 to <5 hours, and those with sun exposure times of ≥5 hours, had a significantly lower prevalence of myopia after adjusting for age and sex (P < 0.001), which was maintained after adjusting for household income, BMI, exercise, education level, IOP, and serum 25(OH)D level (P < 0.001, Model 2).
Table 3.
Multiple logistic regression analysis for the association of myopia with sun exposure time and serum (25(OH)D) levels.
Serum 25(OH)D levels according to the severity of myopia are shown in Figure 2. The serum 25(OH) levels decreased significantly according to the severity of myopia after adjustment for survey year, age, sex, household income, BMI, exercise, education level, and daily sun exposure time (P for trend <0.001).
Figure 2.
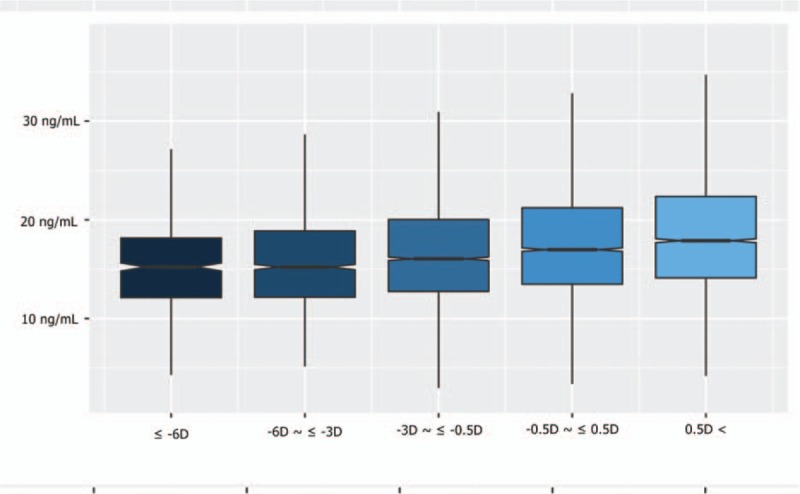
The box and whisker plots of level of serum 25-hydroxyvitamin D [25(OH)D] according to the severity of myopia. The “box” is the interquartile range (IQR, 25th and 75th percentiles) with a notch to show the median. The whiskers show the 10th and 90th percentiles. Serum 25(OH) levels are shown according to the severity of myopia after adjustment for surveyed year, age, sex, household income, body mass index (BMI), exercise, education level, intraocular pressure (IOP), and daily sun exposure time (P for trend <0.001). D, diopter.
4. Discussion
In this study, we found that serum vitamin D levels and daily sun exposure time had independent associations with myopia after adjusting for confounding factors (Tables 2 and 3).
At present, recommended methods for preventing myopia that are supported by strong evidence are time outdoors, bifocal lenses, and a drop of atropine.[17,46–49] Bifocal lenses may slow the progression of myopia by reducing accommodative efforts, whereas the use of atropine not only inhibits accommodation but also has biochemical effects on the retina and remodeling of the sclera.[50–54] Studies on outdoor activities are still controversial: some indicate no association between myopia and outdoor activities,[18,55] but in others reports there is a strong association between myopia and outdoor activities.[17,56–58]
One theory regarding the protective effect of outdoor activity is that it may be mediated by the effects of light on retinal dopamine: this theory is partly supported by animal experiments.[57,59,60] Another theory is that 25(OH)D produced by outdoor activities may reduce the progression of myopia.[39] We designed the present study to assess whether 25(OH)D independently affected the prevalence of myopia and showed that serum 25(OH)D levels can influence myopia prevalence independently, regardless of sun exposure time. Some researchers suggest that a deficiency in 25(OH)D affects myopia development. One hypothesis posits that lower 25(OH)D levels induce alterations in intracellular calcium and impaired contraction and relaxation of the ciliary muscles, thereby leading to myopia development.[61,62] Another hypothesis is that 25(OH)D may be involved in a retinoscleral signaling pathway.[63] Retinoic acid is known to be a factor in the retinoscleral signal for eyeball lengthening.[36,64,65] Retinoic acid can act after both retinoic acid receptors and the 25(OH)D receptor heterodimerize with retinoid X receptors.[66] In the former study, low serum 25(OH)D levels were significantly associated with myopia, especially high myopia in adolescents in Korea.[36] The population in the previous study was in their growth period and myopia was progressing; most studies seeking the cause of myopia are targeted at this age group,[3,10,15,18,40,47] whereas the present study targeted a large adult population in which ocular growth was complete. In this regard, 25(OH)D and sun exposure levels may have different mechanisms in the prevalence of myopia and exert independent effects on myopia.
We also analyzed any association of myopia with sex, age, socioeconomic status, place of living, education level, physical activity, and IOP, because age, education level, income level, place of living, and IOP showed significant differences according to the presence of myopia (Table 1). Concerning the associations of serum 25(OH)D level and sun exposure time with myopia, we adjusted for these confounding factors. First, the age of the myopic population was younger than that of the nonmyopic population, and an analysis by age also showed that the younger population had more myopia (Table 1). This result may be associated with the recent increase in the myopic population in Southeast Asia, a phenomenon that could be explained by genetic factors.[67–69] However, most epidemiological studies of myopia suggest an interaction of genetic susceptibility and environmental factors that predisposes myopia development.[70–73] It is thought that, in accordance with the industrialization and urbanization process, younger urban populations tend to spend more time indoors and live nearer to their place of work. The prevalence of myopia also differed significantly according to education level (Table 1). We suggest that those with a higher level of education may spend more time indoors, both during their education and after graduation. Such life habits may result in less sun exposure and more myopia. Additionally, IOP showed significantly higher values in the myopic group (Table 1). There are many recent studies regarding an association between myopia progression and IOP, almost all of which indicate a significant effect of IOP on myopia progression.[63,74–78]
Most epidemiologic studies have measured the level of 25(OH)D, which is primary circulating form of vitamin D, for investigation of association with myopia.[36,38–40] But the active form of vitamin D involved in calcium homeostasis is 1,25(OH)D acting on bone, intestine, and kidney.[79–80] Some studies found in receptor polymorphism of 1,25(OH)D in myopic population.[61,81] But it is still unclear that how 1,25 (OH)D affects myopic progression.[82]
The present study had some limitations. First, we used a cross-sectional study design to investigate the association of 25(OH)D with myopia in the target population, in which refractive errors become stable. Therefore, the association discovered here may not imply a causal relationship. To investigate the definite causal relationship of serum 25(OH)D level with myopic progression, cohort study designs targeting population in the process of ocular growth seem to be necessary. Second, refractive status was considered as manifest refraction. Because we did not use cycloplegics, there is a possibility of transient accommodative error.[83] However, we tried to reduce the potential for error by having subjects wear corrective glasses. We did not check axial length, corneal thickness, or family history of myopia. Therefore, there is a possibility that the myopia was of another type than axial myopia. Myopia is associated not only with environmental factors but also with genetic factors. Data adjusting for family history may be helpful in identifying the cause of myopia. And there is potential of bias in that we use self-reported information of life habitat including physical activity. There are some controversies about variation of serum 25(OH)D level by season,[84–86] and the adjustment of seasonal variation is needed for accurate assessment.[87] But KNHANES does not provide information about the seasons on blood samples examination, so we could not adjust for seasonal variations in serum 25(OH)D level.
In this study, the emmetropia group showed the highest level of 25(OH)D, whereas the hyperopic group showed lower values than the emmetropia group (Fig. 2). To date, no reported study has examined the association of hyperopia and 25(OH)D level; we suggest that understanding this association is necessary.
In conclusion, this study demonstrated that low serum 25(OH)D levels and shorter daily sun exposure times may be independently associated with a high prevalence of myopia in Korean adults. These data suggest a direct role of vitamin D as a risk factor for myopia.
Acknowledgments
The authors thank the Epidemiologic Survey Committee of the Korean Ophthalmologic Society for conducting examination in the KNHANES and supplying for this study. The authors also thank Kyungdo Han, a biostatics specialist, for statistical analysis support.
Footnotes
Abbreviations: 25(OH)D = 25-hydroxyvitamin D, BMI = body mass index, BP = blood pressure, D = diopter, h = hour, IQR = interquartile range, KNHANES = Korea National Health and Nutrition Examination Survey (KNHANES), OR = odds ratio, Q = quartile, SE = standard error, Sph. E = spherical equivalent.
No author has a financial and proprietary interest in any material or method mentioned.
Funding: Supported by the National Research Foundation of Korea Grant funded by the Korean government (MSIP) (No. NRF- 2016R1C1B10112/87).
The authors report no conflicts of interest.
References
- 1.Saw SM, Katz J, Schein OD, et al. Epidemiology of myopia. Epidemiol Rev 1996; 18:175–187. [DOI] [PubMed] [Google Scholar]
- 2.Wong TY, Foster PJ, Hee J, et al. Prevalence and risk factors for refractive errors in adult Chinese in Singapore. Invest Ophthalmol Vis Sci 2000; 41:2486–2494. [PubMed] [Google Scholar]
- 3.Lin LL, Shih YF, Hsiao CK, et al. Epidemiologic study of the prevalence and severity of myopia among schoolchildren in Taiwan in 2000. J Formos Med Assoc 2001; 100:684–691. [PubMed] [Google Scholar]
- 4.Shimizu N, Nomura H, Ando F, et al. Refractive errors and factors associated with myopia in an adult Japanese population. Jpn J Ophthalmol 2003; 47:6–12. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Jee D, Kwon JW, et al. Prevalence and risk factors for myopia in a rural Korean population. Invest Ophthalmol Vis Sci 2013; 54:5466–5471. [DOI] [PubMed] [Google Scholar]
- 6.Lv L, Zhang Z. Pattern of myopia progression in Chinese medical students: a two-year follow-up study. Graefes Arch Clin Exp Ophthalmol 2013; 251:163–168. [DOI] [PubMed] [Google Scholar]
- 7.Zheng YF, Pan CW, Chay J, et al. The economic cost of myopia in adults aged over 40 years in Singapore. Invest Ophthalmol Vis Sci 2013; 54:7532–7537. [DOI] [PubMed] [Google Scholar]
- 8.Saw SM, Gazzard G, Shih-Yen EC, et al. Myopia and associated pathological complications. Ophthalmic Physiol Opt 2005; 25:381–391. [DOI] [PubMed] [Google Scholar]
- 9.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet 2012; 379:1739–1748. [DOI] [PubMed] [Google Scholar]
- 10.Cuellar-Partida G, Lu Y, Kho PF, et al. Assessing the genetic predisposition of education on myopia: A Mendelian Randomization Study. Genet Epidemiol 2016; 40:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li YT, Xie MK, Wu J. Association between ocular axial length-related genes and high myopia in a Han Chinese population. Ophthalmologica 2016; 235:57–60. [DOI] [PubMed] [Google Scholar]
- 12.Khan AO, Aldahmesh MA, Alkuraya FS. Clinical characterization of LRPAP1-related pediatric high myopia. Ophthalmology 2016; 123:434–435. [DOI] [PubMed] [Google Scholar]
- 13.Saw SM, Carkeet A, Chia KS, et al. Component dependent risk factors for ocular parameters in Singapore Chinese children. Ophthalmology 2002; 109:2065–2071. [DOI] [PubMed] [Google Scholar]
- 14.Lee YY, Lo CT, Sheu SJ, et al. What factors are associated with myopia in young adults? A survey study in Taiwan Military Conscripts. Invest Ophthalmol Vis Sci 2013; 54:1026–1033. [DOI] [PubMed] [Google Scholar]
- 15.Guo Y, Liu LJ, Xu L, et al. Outdoor activity and myopia among primary students in rural and urban regions of Beijing. Ophthalmology 2013; 120:277–283. [DOI] [PubMed] [Google Scholar]
- 16.Jones LA, Sinnott LT, Mutti DO, et al. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci 2007; 48:3524–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherwin JC, Reacher MH, Keogh RH, et al. The association between time spent outdoors and myopia in children and adolescents: a systematic review and meta-analysis. Ophthalmology 2012; 119:2141–2151. [DOI] [PubMed] [Google Scholar]
- 18.Jones-Jordan LA, Sinnott LT, Cotter SA, et al. Time outdoors, visual activity, and myopia progression in juvenile-onset myopes. Invest Ophthalmol Vis Sci 2012; 53:7169–7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saw SM, Nieto FJ, Katz J, et al. Factors related to the progression of myopia in Singaporean children. Optom Vis Sci 2000; 77:549–554. [DOI] [PubMed] [Google Scholar]
- 20.Masuyama R. [Bone and Nutrition. Vitamin D independent calcium absorption]. Clin Calcium 2015; 25:1023–1028. [PubMed] [Google Scholar]
- 21.Thacher TD, Fischer PR, Pettifor JM. Vitamin D treatment in calcium-deficiency rickets: a randomised controlled trial. Arch Dis Child 2014; 99:807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SH, Oh MK, Namgung R, et al. Prevalence of 25-hydroxyvitamin D deficiency in Korean adolescents: association with age, season and parental vitamin D status. Public Health Nutr 2014; 17:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shui IM, Mondul AM, Lindstrom S, et al. Circulating vitamin D, vitamin D-related genetic variation, and risk of fatal prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer 2015; 121:1949–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peelen E, Knippenberg S, Muris AH, et al. Effects of vitamin D on the peripheral adaptive immune system: a review. Autoimmun Rev 2011; 10:733–743. [DOI] [PubMed] [Google Scholar]
- 25.Antico A, Tozzoli R, Giavarina D, et al. Hypovitaminosis D as predisposing factor for atrophic type A gastritis: a case-control study and review of the literature on the interaction of Vitamin D with the immune system. Clin Rev Allergy Immunol 2012; 42:355–364. [DOI] [PubMed] [Google Scholar]
- 26.Pereira-Santos M, Costa PR, Assis AM, et al. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev 2015; 16:341–349. [DOI] [PubMed] [Google Scholar]
- 27.Harinarayan CV. Vitamin D and diabetes mellitus. Hormones (Athens) 2014; 13:163–181. [DOI] [PubMed] [Google Scholar]
- 28.Zittermann A. Vitamin D and cardiovascular disease. Anticancer Res 2014; 34:4641–4648. [PubMed] [Google Scholar]
- 29.Goncalves A, Milea D, Gohier P, et al. Serum vitamin D status is associated with the presence but not the severity of primary open angle glaucoma. Maturitas 2015; 81:470–474. [DOI] [PubMed] [Google Scholar]
- 30.He R, Shen J, Liu F, et al. Vitamin D deficiency increases the risk of retinopathy in Chinese patients with type 2 diabetes. Diabet Med 2014; 31:1657–1664. [DOI] [PubMed] [Google Scholar]
- 31.Gungor A, Ates O, Bilen H, et al. Retinal nerve fiber layer thickness in early-stage diabetic retinopathy with vitamin D deficiency. Invest Ophthalmol Vis Sci 2015; 56:6433–6437. [DOI] [PubMed] [Google Scholar]
- 32.Millen AE, Meyers KJ, Liu Z, et al. Association between vitamin D status and age-related macular degeneration by genetic risk. JAMA Ophthalmol 2015; 133:1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itty S, Day S, Lyles KW, et al. Vitamin D deficiency in neovascular versus nonneovascular age-related macular degeneration. Retina 2014; 34:1779–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinwender G, Lindner E, Weger M, et al. Association between polymorphism of the vitamin D metabolism gene CYP27B1 and HLA-B27-associated uveitis. Is a state of relative immunodeficiency pathogenic in HLA B27-positive uveitis? PLoS One 2013; 8:e62244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khabbazi A, Rashtchizadeh N, Ghorbanihaghjo A, et al. The status of serum vitamin D in patients with active Behcet's disease compared with controls. Int J Rheum Dis 2014; 17:430–434. [DOI] [PubMed] [Google Scholar]
- 36.Choi JA, Han K, Park YM, et al. Low serum 25-hydroxyvitamin D is associated with myopia in Korean adolescents. Invest Ophthalmol Vis Sci 2014; 55:2041–2047. [DOI] [PubMed] [Google Scholar]
- 37.Reins RY, McDermott AM. Vitamin D: Implications for ocular disease and therapeutic potential. Exp Eye Res 2015; 134:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yazar S, Hewitt AW, Black LJ, et al. Myopia is associated with lower vitamin D status in young adults. Invest Ophthalmol Vis Sci 2014; 55:4552–4559. [DOI] [PubMed] [Google Scholar]
- 39.Guggenheim JA, Williams C, Northstone K, et al. Does vitamin D mediate the protective effects of time outdoors on myopia? Findings from a prospective birth cohort. Invest Ophthalmol Vis Sci 2014; 55:8550–8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mutti DO, Marks AR. Blood levels of vitamin D in teens and young adults with myopia. Optom Vis Sci 2011; 88:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeong H, Hong S, Heo Y, et al. Vitamin D status and associated occupational factors in Korean wage workers: data from the 5th Korea national health and nutrition examination survey (KNHANES 2010–2012). Ann Occup Environ Med 2014; 26:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi HS, Oh HJ, Choi H, et al. Vitamin D insufficiency in Korea--a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab 2011; 96:643–651. [DOI] [PubMed] [Google Scholar]
- 43.Lee YA, Kim HY, Hong H, et al. Risk factors for low vitamin D status in Korean adolescents: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008-2009. Public Health Nutr 2014; 17:764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Misra A. Ethnic-specific criteria for classification of body mass index: a perspective for Asian Indians and American Diabetes Association Position Statement. Diabetes Technol Ther 2015; 17:667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sempos CT, Vesper HW, Phinney KW, et al. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl 2012; 243:32–40. [DOI] [PubMed] [Google Scholar]
- 46.He M, Xiang F, Zeng Y, et al. Effect of time spent outdoors at school on the development of myopia among children in China: A randomized clinical trial. Jama 2015; 314:1142–1148. [DOI] [PubMed] [Google Scholar]
- 47.Walline JJ, Lindsley K, Vedula SS, et al. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev 2011; Cd004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Repka MX. Prevention of myopia in children. Jama 2015; 314:1137–1139. [DOI] [PubMed] [Google Scholar]
- 49.Wu PC, Tsai CL, Wu HL, et al. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology 2013; 120:1080–1085. [DOI] [PubMed] [Google Scholar]
- 50.Cheng D, Woo GC, Drobe B, et al. Effect of bifocal and prismatic bifocal spectacles on myopia progression in children: three-year results of a randomized clinical trial. JAMA Ophthalmol 2014; 132:258–264. [DOI] [PubMed] [Google Scholar]
- 51.Cheng D, Woo GC, Schmid KL. Bifocal lens control of myopic progression in children. Clin Exp Optom 2011; 94:24–32. [DOI] [PubMed] [Google Scholar]
- 52.Berntsen DA, Mutti DO, Zadnik K. The effect of bifocal add on accommodative lag in myopic children with high accommodative lag. Invest Ophthalmol Vis Sci 2010; 51:6104–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loh KL, Lu Q, Tan D, et al. Risk factors for progressive myopia in the atropine therapy for myopia study. Am J Ophthalmol 2015; 159:945–949. [DOI] [PubMed] [Google Scholar]
- 54.Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology 2012; 119:347–354. [DOI] [PubMed] [Google Scholar]
- 55.Chua SY, Ikram MK, Tan CS, et al. Relative contribution of risk factors for early-onset myopia in young Asian children. Invest Ophthalmol Vis Sci 2015; 56:8101–8107. [DOI] [PubMed] [Google Scholar]
- 56.Dirani M, Tong L, Gazzard G, et al. Outdoor activity and myopia in Singapore teenage children. Br J Ophthalmol 2009; 93:997–1000. [DOI] [PubMed] [Google Scholar]
- 57.Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology 2008; 115:1279–1285. [DOI] [PubMed] [Google Scholar]
- 58.Wu PC, Tsai CL, Hu CH, et al. Effects of outdoor activities on myopia among rural school children in Taiwan. Ophthalmic Epidemiol 2010; 17:338–342. [DOI] [PubMed] [Google Scholar]
- 59.Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res 2013; 114:106–119. [DOI] [PubMed] [Google Scholar]
- 60.Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci 2010; 51:5247–5253. [DOI] [PubMed] [Google Scholar]
- 61.Annamaneni S, Bindu CH, Reddy KP, et al. Association of vitamin D receptor gene start codon (Fok1) polymorphism with high myopia. Oman J Ophthalmol 2011; 4:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lepple-Wienhues A, Stahl F, Willner U, et al. Endothelin-evoked contractions in bovine ciliary muscle and trabecular meshwork: interaction with calcium, nifedipine and nickel. Curr Eye Res 1991; 10:983–989. [DOI] [PubMed] [Google Scholar]
- 63.McBrien NA, Gentle A. Role of the sclera in the development and pathological complications of myopia. Prog Retin Eye Res 2003; 22:307–338. [DOI] [PubMed] [Google Scholar]
- 64.Kim RY, Stern WH. Retinoids and butyrate modulate fibroblast growth and contraction of collagen matrices. Invest Ophthalmol Vis Sci 1990; 31:1183–1186. [PubMed] [Google Scholar]
- 65.McFadden SA, Howlett MH, Mertz JR. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res 2004; 44:643–653. [DOI] [PubMed] [Google Scholar]
- 66.Tavera-Mendoza L, Wang TT, Lallemant B, et al. Convergence of vitamin D and retinoic acid signalling at a common hormone response element. EMBO Rep 2006; 7:180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshikawa M, Yamashiro K, Miyake M, et al. Comprehensive replication of the relationship between myopia-related genes and refractive errors in a large Japanese cohort. Invest Ophthalmol Vis Sci 2014; 55:7343–7354. [DOI] [PubMed] [Google Scholar]
- 68.Chen CD, Yu ZQ, Chen XL, et al. Evaluating the association between pathological myopia and SNPs in RASGRF1. ACTC1 and GJD2 genes at chromosome 15q14 and 15q25 in a Chinese population. Ophthalmic Genet 2015; 36:1–7. [DOI] [PubMed] [Google Scholar]
- 69.Li J, Jiang D, Xiao X, et al. Evaluation of 12 myopia-associated genes in Chinese patients with high myopia. Invest Ophthalmol Vis Sci 2015; 56:722–729. [DOI] [PubMed] [Google Scholar]
- 70.Verhoeven VJ, Buitendijk GH, Rivadeneira F, et al. Education influences the role of genetics in myopia. Eur J Epidemiol 2013; 28:973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams KM, Bertelsen G, Cumberland P, et al. Increasing prevalence of myopia in Europe and the impact of education. Ophthalmology 2015; 122:1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin Z, Gao TY, Vasudevan B, et al. Generational difference of refractive error and risk factors in the Handan Offspring Myopia Study. Invest Ophthalmol Vis Sci 2014; 55:5711–5717. [DOI] [PubMed] [Google Scholar]
- 73.Goldschmidt E, Jacobsen N. Genetic and environmental effects on myopia development and progression. Eye (Lond) 2014; 28:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeong da W, Kook MS, Lee KS, et al. Circadian pattern of intraocular pressure fluctuations in young myopic eyes with open-angle glaucoma. Invest Ophthalmol Vis Sci 2014; 55:2148–2156. [DOI] [PubMed] [Google Scholar]
- 75.Yang Y, Li Z, Wang N, et al. Intraocular pressure fluctuation in patients with primary open-angle glaucoma combined with high myopia. J Glaucoma 2014; 23:19–22. [DOI] [PubMed] [Google Scholar]
- 76.Yan L, Huibin L, Xuemin L. Accommodation-induced intraocular pressure changes in progressing myopes and emmetropes. Eye (Lond) 2014; 28:1334–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi JA, Han K, Park YM, et al. Age-related association of refractive error with intraocular pressure in the Korea National Health and Nutrition Examination Survey. PLoS One 2014; 9:e111879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang WJ, Wu JF, Hu YY, et al. Intraocular pressure and associated factors in children: the Shandong children eye study. Invest Ophthalmol Vis Sci 2014; 55:4128–4134. [DOI] [PubMed] [Google Scholar]
- 79.Lieben L, Carmeliet G, Masuyama R. Calcemic actions of vitamin D: effects on the intestine, kidney and bone. Best Pract Res Clin Endocrinol Metab 2011; 25:561–572. [DOI] [PubMed] [Google Scholar]
- 80.Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357:266–281. [DOI] [PubMed] [Google Scholar]
- 81.Mutti DO, Cooper ME, Dragan E, et al. Vitamin D receptor (VDR) and group-specific component (GC, vitamin D-binding protein) polymorphisms in myopia. Invest Ophthalmol Vis Sci 2011; 52:3818–3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tideman JW, Polling JR, Voortman T, et al. Low serum vitamin D is associated with axial length and risk of myopia in young children. Eur J Epidemiol 2016; 31:491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nayak BK, Ghose S, Singh JP. A comparison of cycloplegic and manifest refractions on the NR-1000F (an objective Auto Refractometer). Br J Ophthalmol 1987; 71:73–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park H, F PM, West AA, et al. Vitamin D metabolism varies among women in different reproductive states consuming the same intakes of vitamin D and related nutrients. J Nutr 2016; 146:1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Geng SS, Ma JQ, Liu SS, et al. Vitamin D Insufficiency and its association with biochemical and anthropometric variables of young children in Rural Southwestern China. Chin Med J (Engl) 2016; 129:1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith M. Seasonal, ethnic and gender variations in serum vitamin D3 levels in the local population of Peterborough. Bioscience Horizons 2010; 3:124–131. [Google Scholar]
- 87.Zhan Y, Samietz S, Holtfreter B, et al. Prospective study of serum 25-hydroxy vitamin D and tooth loss. J Dent Res 2014; 93:639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]


