Abstract
The aim of this study was to determine the effects of nanocarbon particles in combination with meticulous capsular dissection on enhancing the identification and protecting the function of parathyroid glands in thyroid cancer surgery.
The data of 97 patients with papillary thyroid tumors diagnosed and treated at the Second Affiliated Hospital, Harbin Medical University between January 2014 and February 2015 were reviewed. Data regarding the sex, age, calcium and parathyroid hormone (PTH) levels, tumor size, multifocality, T stage, and extrathyroid invasion were collected. The incidence of surgeries in which the parathyroid glands were cut mistakenly, the concentration of serum calcium and parathyroid hormone before surgery (baseline) and after surgery on days 1, 3, and 7, and 1 and 6 months in the patients of the two groups (the nanocarbon and control groups) were analyzed.
Fifty-two patients underwent meticulous capsular dissection combined with nanocarbon treatment (nanocarbon group), and 45 underwent meticulous capsular dissection alone (control group). The nanocarbon group showed a significantly higher total and average number of revealed parathyroid glands (average number is the mean for different individuals have different number) and a lower incidence of the parathyroid glands being mistakenly cut, in addition to a lower level of hypoparathyroidism than control group following surgery (P < 0.05). Serum calcium and PTH levels were significantly lower in patients from both groups after surgery on days 1, 3, and 7 and after 1 month, compared with the preoperative levels (P < 0.05). Compared with the control group, the serum calcium and PTH levels were significantly higher in the nanocarbon group after surgery on days 1, 3, 7, than in the control group.
Treatment with nanocarbon in combination with meticulous capsular dissection can significantly facilitate the identification of the parathyroid in thyroid cancer surgery, reduce the risk of mistakenly cutting the parathyroid, and reduce the incidence of postoperative hypoparathyroidism.
Keywords: meticulous capsular dissection, nanocarbon, parathyroid, total thyroidectomy
1. Introduction
Thyroid cancer is the most common endocrine malignancy, with increasing incidence in most countries,[1–3] and papillary thyroid tumors (PTCs) account for 80% to 90% of all thyroid malignancies.[4,5] Currently, total thyroidectomy and central lymph node dissection are the preferred treatments for thyroid cancer. However, during a total thyroidectomy, the hypoparathyroidism due to parathyroid damage caused by surgery remains problematic for the surgeon. There are intensive staggered blood vessels in area of thyroid bed, and blood supply for parathyroid often varies. Moreover, the unique embryonic development of the parathyroid glands means that its relative anatomical position is not fixed. In particular, the dissecting scope of the inferior parathyroid glands varies largely. In surgery for thyroid cancer, in the event that a larger and broader range of dissection on the lymph nodes is required, factors including physiological anatomy and embryonic development can result in the parathyroid being easily damaged, or even mistakenly cut, thus causing postoperative hypoparathyroidism.[6]
An injection of a suspension of carbon nanoparticles (CNs) comprises nanosized carbon particles with an average diameter of 150 nm. CNs have a characteristically high degree of lymphatic system tropism, tracing speed, rate of dyeing black, and a high color contrast with the surrounding tissue.[7] The latest research confirms that nanocarbon can be used to identify the parathyroid in thyroid surgery.[8] In addition, the application of the meticulous capsular dissection technique in a total thyroidectomy can also reduce the possibility of complications following parathyroid injury.[9] Therefore, in the present study, we investigated the enhanced identification ability and functional protective role of nanocarbon particles combined with meticulous capsular dissection on the parathyroid glands during surgical treatment for thyroid cancer.
2. Methods
2.1. Patients
Ninety-seven patients with PTC who underwent thyroidectomy during the period between January 2014 and February 2015 in the department of Second Affiliated Hospital in Harbin Medical University were recruited for this study. All patients met the inclusion criteria, which were as follows: diagnosed with papillary thyroid carcinoma by fine needle aspiration cytology (FNAC) using color ultrasound equipment, no lymph node metastasis in the central and lateral neck as detected by ultrasound and contrast-enhanced computed tomography (CT); no history of thyroid disease and not currently receiving thyroid-related medications; no history of Graves’ disease; preoperative serum calcium and parathyroid hormone (PTH) levels within in the normal range; based on levels of preoperative serum anti-thyroglobulin antibody (TGAb) and serum anti-thyroid peroxidase antibody (TPOA) and pathological diagnosis, cases with chronic lymphocytic thyroiditis were excluded; a total thyroidectomy and preventative ipsilateral central lymph node dissection, in addition to a postoperative follow-up period of at least 6 months. Central neck lymph node dissection[10] refers to the dissection of all the lymph adipose tissue and the prelaryngeal lymph node to the front and side of the trachea, and laryngeal recurrent nerve areas. The specific dissection rage includes all the lymph adipose tissue in the region below the thyroid cartilage, above the sternal notch, and the medial area of the common carotid artery. The relevant data were collected, including demographic features (such as sex and age), clinical features (calcium and PTH levels, tumor size), and pathological features (multifocality, T stage, extrathyroid invasion). In this study, all patients and their families were fully aware of the treatment process, and informed consent was obtained from all participants. The retrospective study was approved by Ethics Committee of the Second Affiliated Hospital in Harbin Medical University (No. 2015-research-077).
2.2. Experimental grouping and pathological diagnosis
During the surgery, the parathyroid and the related blood supply were preserved by the meticulous capsular dissection method. Among all the patients, 52 who underwent surgery and treatment with CNs were termed the nanocarbon group, and the remaining 45 cases who did not receive treatment with CNs formed the control group. All patients were confirmed to have PTC by preoperative FNAC and postoperative pathological diagnosis, and histological specimens were independently reviewed by 2 blinded pathologists. The clinical pathological classification was performed according to the (2010) American Joint Committee (AJCC) on Cancer (seventh edition).[11] All surgeries were performed by the same team of doctors, and an accidental cut of the parathyroid was ascertained by the presence of parathyroid tissue in the resected specimens.
2.3. Calcium and PTH monitoring
PTH and serum calcium concentration were determined using radioimmunoassay and biochemical methods, respectively, in all patients before surgery (baseline) and after surgery on days 1, 3, 7, and at 1 and 6 months. All patients were routinely supplemented with calcium through intravenous injection, and dose adjustment of calcium and appropriate amounts of vitamin D were administered based on clinical symptoms. One month after surgery, patients with hypocalcemia or hypoparathyroidism were considered temporary. If the symptoms were not alleviated after 6 months postoperative, it could be seen as permanent. Hypocalcemia and hypoparathyroidism were diagnosed according to the American Thyroid Surgery Society 2009 guideline.[12]
2.4. Application of nanocarbon
CNs was applied in the form of a standard CN suspension injection (1 mL: 50 mg). The suspension does not enter the blood circulation and has no toxic side effects on the human body.[8] The nanocarbon suspension comprises nanosized carbon particles with an average diameter of 150 nm. The cells gap between capillary endothelial cells is 20 to 50 nm, and the capillary lymphatic endothelial cell gap is 120 to 500 nm with a hypoplasia of the basement membrane. Thus, nanocarbon is unable to enter the blood vessels when it is injected into the thyroid tissue, and it will rapidly enter lymphatic vessels or the lymphatic capillaries through macrophage phagocytosis, and be retained in the lymph nodes. Furthermore, the thyroid and lymph in their drainage areas are stained in black in surgery.[8] However, the parathyroid glands do not stain black, and thus, the black-stained thyroid and lymph nodes can be identified and distinguished easily. After cutting along the white cervical line, the thyroid gland can be identified. Carefully removing the false fibrous capsule from the thyroid gland and revealing the gland near the isthmus. Excessive freed gland can cause damage to the surrounding lymphatic network and influence the effects of CNs after injection.
The integrity of fibrous capsule of thyroid glands should be maintained. Destruction of the fibrous capsule of thyroid glands can cause a leakage of nanocarbon after injection, and result in the entire operative field will be stain blacked, which will render it impossible to distinguish the parathyroid gland and increase surgical risk. In this study, the nanocarbon was provided by Chongqing Laimei Medicine (0.5 mL, National Drug Approval No. H20073246). One injection of 0.2 mL nanocarbon were administered with a fine needle in the lower 1/3 of ventral surface to each of the bilateral glands, and the injection depth was approximately within the upper third of the glands. Too deep or shallow an injection can also cause the extravasation of nanocarbon, which can result in a blacked surgical field, and increase the risk of surgery. All the above factors are important when performing the injection. Thus, the injection requires meticulous attention. The needle must avoid the tumor, and the surgeon must proceed to inject slowly if no blood is found upon pumpback. Increasing the pressure on the injection site for 5 minutes with a gauze after injection and waiting for the full staining of nanocarbon in the gland (Fig. 1A), identifying the parathyroid glands by negative visualization (Fig. 1B), and identifying lymph nodes by positive visualization (Fig. 1C).
Figure 1.
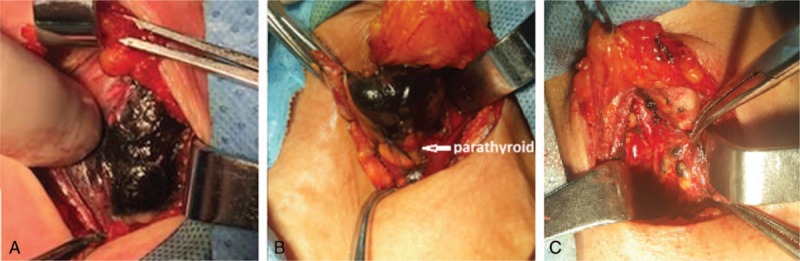
Staining and identification of the parathyroid glands using nanocarbon. (A) Parathyroid glands were stained with nanocarbon for 5 minutes; (B) parathyroid glands were identified by negative visualization of black staining; (C) lymph nodes were identified by positive visualization of black staining.
2.5. Meticulous capsular dissection
In 2009, Thompson[13] first proposed capsular dissection in thyroid surgery, which refers to the isolation and ligation of the vascular, performed near the capsular of glands. Retention of the tissues from the artery to the capsular of thyroid results in the preservation of greater blood supply to the parathyroid. The major factor underlying the meticulous capsular dissection technique is based on the fact that, when the thyroid is removed, all surgery should be close to the capsular to manage the thyroid vessels.[8]
2.6. Statistical analysis
Statistical analyses were carried out using SPSS13.0 (version 13.01S; Beijing Stats Data Mining Co. Ltd, Beijing, China). Data were presented as the mean ± s.d. or as percentages, as appropriate. Independent samples t test and χ2 tests were used to compare continuous and categorical variables, respectively. All P values are two-tailed, and a P value <0.05 was considered significant for all statistical analyses in this study.
3. Results
3.1. Characteristics of subjects in the two groups
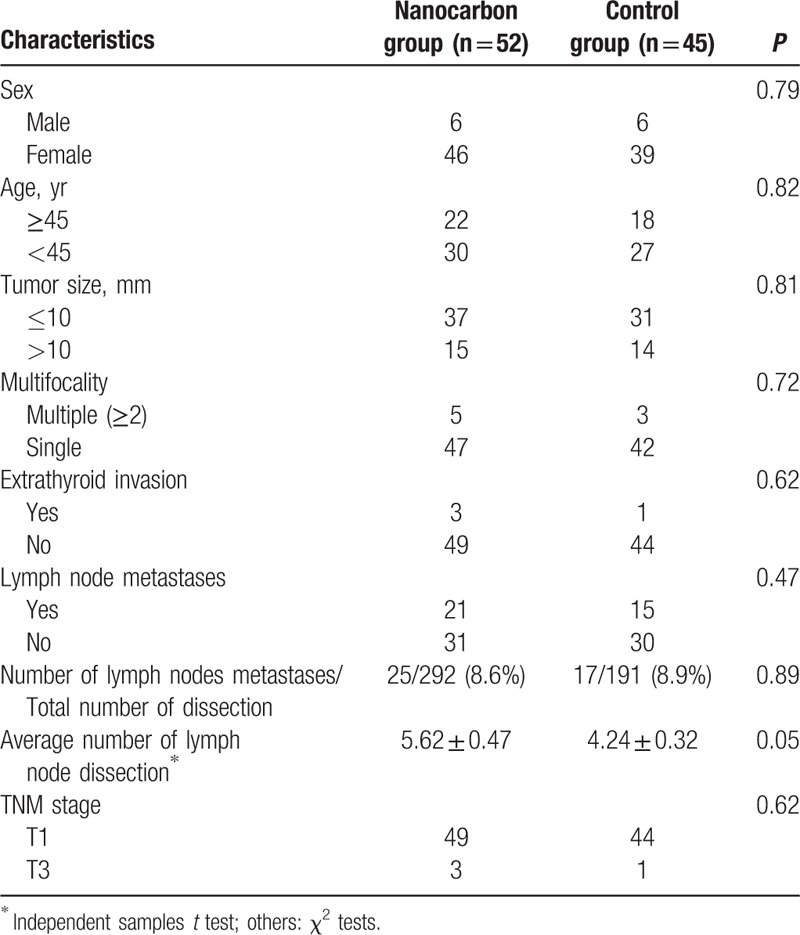
During the period between January 2014 and February 2015, 97 patients with PTC were identified and were included in the present study. The mean ages were 45.2 ± 5.8 years (range, 20–58) and 42 ± 4.3 years (range, 17–55) in the nanocarbon and control groups, respectively. The mean size of the tumors was 9.3 ± 4.6 mm (range, 2–40) and 9.1 ± 5.1 mm (range, 2–38) in the nanocarbon and control groups, respectively. There were no significant differences in the sex, age, tumor size, multifocality, extrathyroid invasion, rate of number of lymph nodes metastases and total number of dissections, average number of lymph node dissections, and Tumor node metastasis stage between the two groups (P = NS) (Table 1).
Table 1.
Clinicopathological characteristics of patients.
3.2. Parathyroid of patients after operation
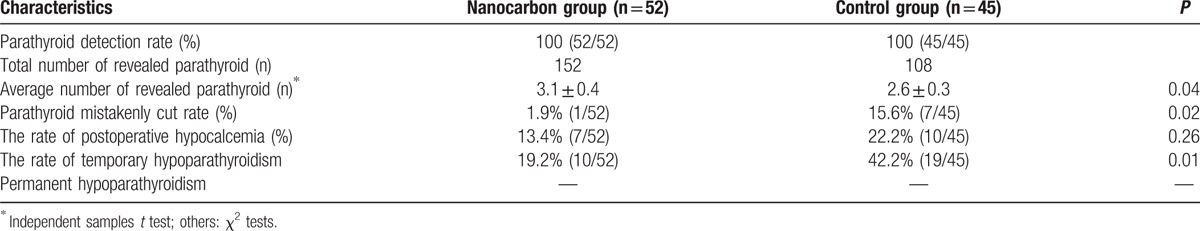
As shown in Table 2, the parathyroid could be detected in all patients of both groups. The nanocarbon group displayed a significantly higher total and average number of revealed parathyroid, and lower incidence of parathyroid that had been mistakenly cut, and temporary hypoparathyroidism, than control group (P < 0.05). The hypoparathyroidism rate was significantly lower than that in control group. There was no permanent hypoparathyroidism for patients in both groups.
Table 2.
Parathyroid and serum calcium of patients after the operation.
3.3. Serum calcium and PTH levels of patients in 2 groups before and after operation
As shown in Fig. 2, serum calcium and PTH levels were significantly lower in both groups 1, 3, and 7 days after surgery compared with preoperative levels (P < 0.05), and the PTH levels were still significantly lower at 1 month after surgery than before (P < 0.05). Compared with the control group, the postoperative PTH levels decreased less in the nanocarbon group, and the serum calcium and PTH levels were significantly higher in nanocarbon group 1, 3 and 7 days after surgery compared with the control group (P < 0.05). There was no significant difference in the rate of postoperative hypocalcemia between the 2 groups, and the rates were 13.4% and 22.2%, respectively, for the nanocarbon and control groups. After 6 months, the serum calcium levels had returned to normal. Changes in the blood calcium levels in patients were affected by the changes of postoperative PTH. Although calcium was administered after surgery in patients, the alteration calcium concentration was still affected by the PTH levels. Furthermore, with the supplementation of calcium through the veins, postoperative serum calcium concentration levels in the nanocarbon group were returned to approximately normal levels more rapidly compared with the control group.
Figure 2.
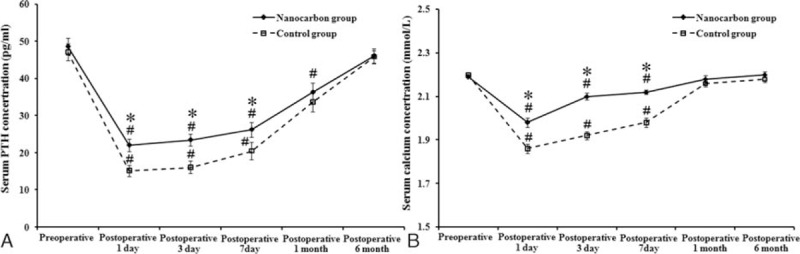
Serum calcium and PTH levels of patients in the 2 groups before and after surgery. (A) Calcium and (B) PTH levels; #P < 0.05 in comparison with preoperative levels in both groups; ∗P < 0.05, in comparison with the control group.
4. Discussion
The present study showed that staining with nanocarbon combined with meticulous capsular dissection in surgery for thyroid cancer could reduce the possibility of mistakenly cutting the parathyroid and the hypoparathyroidism rate, in addition to make the PTH and calcium levels restored more rapidly.
Giordano et al,[14] indicated that the incidence rates of temporary and permanent hypoparathyroidism after surgery in patients who underwent total thyroidectomy were 27.7% and 6.3%, respectively. The reason is mainly due to the different condition of parathyroid contusion, mistakenly cut and the blood supply. In this study, the data of patients were collected and analyzed until 6 months after surgery, during which the incidence rates of parathyroid dysfunction were 19.2% and 42.2% in the nanocarbon and control groups, respectively. Considering that the mechanism of postoperative parathyroid function can gradually recovered, the results of this study were in accordance with the 14% to 60% temporary hypoparathyroidism rates reported in previous studied after total thyroidectomy.[12,15–19]
Usually, in patients with thyroid cancer complicated with chronic lymphocytic thyroiditis, the texture of the thyroid is tough with a granular or nodular surface. In the area surrounding the thyroid, more reactive hyperplasia of lymph nodes could be found, and even lead to the adhesions of gland with surrounding tissue.[20] This can increase the difficulty of administering a nanocarbon injection in surgery, reduce the dispersion of nanocarbon in the gland, and is also not conducive to parathyroid identification. Our study found that the average number of detected parathyroid glands was significantly higher in patients undergoing treatment with the nanocarbon injection compared with those who did not receive the injection. Therefore, results suggested that the protective effect of nanocarbon to the parathyroid is due to significantly facilitating the identification of the parathyroid, thereby enabling the surgeon has the target in the process of refinement surgery. Ultimately, the aim of achieving superior protection of the parathyroid blood supply is accomplished, and the retention rate of the parathyroid is increased, while the incidence rate of postoperative hypoparathyroidism is minimized. The incidence of mistaken cutting of the parathyroid in the present study confirmed the protective effect of nanocarbon, with an incidence of 1.9% (1/52) in patients in the nanocarbon group, which was significantly lower than that in the control group (15.6% [7/45]).
In this study, in patients from both groups, the PTH concentrations were significantly lower than the preoperative levels at each detection point after surgery, and the change in calcium levels were closely related to those of PTH levels, which could be observed by calcium and PTH curves. All patients were routinely supplied with calcium through intravenous injections, with the dose adjusted based on the clinical symptoms after surgery, and vitamin D was also supplied as appropriate. However, we found that patients who received the CN injection combined with meticulous capsular dissection demonstrated small changes in trends of PTH and calcium levels postoperatively following thyroid cancer surgery. The results suggested that the improvement of the skills of the surgeon combined with the reasonable application of nanocarbon, would provide a reliable choice for better and more comprehensive protection of the parathyroid in thyroid surgery. In addition, compared with the control group, patients in the nanocarbon group showed significantly higher PTH and calcium levels on postoperative days 1, 3, and 7, which further demonstrated that nanocarbon can significantly increase the recognition of the parathyroid intraoperatively, and achieve better protection of the parathyroid blood supply, and thus reduce the postoperative incidence rate of hypoparathyroidism. Moreover, all patients in this study were supplied with calcium after surgery; however, the patients in the nanocarbon group recovered to a normal level with respect to serum calcium levels more rapidly, which might be related to the superior protection of parathyroid function.[21] Notable, the PTH and calcium levels of the 2 groups were not significantly different after 1 month. Six months after surgery, there were no significant difference in the PTH and serum calcium levels for the patients in the 2 groups, compared with preoperative levels. Therefore, the results suggested that effect preventing the loss of parathyroid function by nanocarbon is evident in the early postoperative period, and the specific time period is 1 month.
We found that the rate of lymph node metastasis in patients with PTC was 37.1%, which was consistent with the rate of 21% to 90% reported in previous studies.[22–24] Interestingly, the average number of lymph node dissection in the experimental group was only slightly higher than that in the control group (5.62 vs. 4.24, respectively) (P = 0.052). Moreover, we did not observe significant differences in lymph node metastasis rates (40.2% vs. 33.3%) and lymph node positive rate (8.6% vs. 8.9%) between the two groups (P > 0.05). This was distinctly different with the viewpoint in the application of nanocarbon could increase the numbers of lymph node dissection and transfer rate.[25] In this study, the total number of lymph node dissections in the experimental group was higher than that of the control group. This may be due to the application of nanocarbon, which caused the enhancement of certain smaller lymph nodes or those located in the occult position. However, whether the more lymph node dissections in the experimental group belonged to the metastasis or requires removal is yet to be further studied. In addition, the nanocarbon is only a visualization developer, not all lymph nodes of the thyroid will be able to be visualized following the administration of nanocarbon. Therefore, based on the visualization, it is inappropriate to use selective lymph node dissection to replace lymphatic tissue dissection in the standard area, as this will lead to the omission of lymph nodes and thus affect the lymph node that requires removal.
In this study, there are certain limitations. First, the sample size should have been larger. Secondly, because of the characteristic rapid recovery after surgery and earlier discharge of patients with thyroid disease, the time in 1-month review period could not be further increased (such as increase 2 times in first and second week). Therefore, earlier, more precise time-points in terms of reducing parathyroid function loss in patients using nanocarbon injection could not be accurately determined. Finally, the effects of nanocarbon on the visualization of cervical lymph nodes bilaterally should be assessed in future studies, which will render the effects of nanocarbon more comprehensive during thyroid cancer surgery.
5. Conclusions
In summary, the results of this study indicate that nanocarbon can significantly enhance the identification of the parathyroid in thyroid cancer surgery, and reduce the risk of mistakenly cutting the parathyroid. Combined with meticulous capsular dissection, nanocarbon has a good protective effect on the thyroid blood supply, and reduces the incidence of postoperative hypoparathyroidism. Furthermore, the effect of nanocarbon on the loss of parathyroid function after surgery is demonstrated in the early postoperative period, and the specific time-period is within 1 month after surgery. Although nanocarbon facilitates lymph node visualization, it was not able significantly improve the number of lymph nodes dissected or metastasis in thyroid cancer.
Footnotes
Abbreviations: AJCC = American Joint Committee, CNs = carbon nanoparticles, FNAC = fine needle aspiration cytology, PTC = papillary thyroid tumor, PTH = parathyroid hormone, TGAb = thyroglobulin antibody, TPOA = peroxidase antibody.
There are no conflicts of interest.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006; 295:2164–2167. [DOI] [PubMed] [Google Scholar]
- 2.Shi CL, Sun Y, Ding C, et al. Correlation between the BRAF V600E mutation status and the clinicopathologic features of papillary thyroid carcinoma. Genet Mol Res 2015; 14:7377–7385. [DOI] [PubMed] [Google Scholar]
- 3.Pellegriti G, Frasca F, Regalbuto C, et al. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013; 2013:965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Udelsman R, Chen H. The current management of thyroid cancer. Adv Surg 1999; 33:1–27. [PubMed] [Google Scholar]
- 5.Paterson IC, Greenlee R, Adams Jones D. Thyroid cancer in Wales, 1985–1996: a cancer registry-based study. Clin Oncol (R Coll Radiol) 1999; 44:245–251. [DOI] [PubMed] [Google Scholar]
- 6.Mohebati A, Shaha AR. Anatomy of thyroid and parathyroid glands and neurovascular relations. Clin Anat 2012; 25:19–31. [DOI] [PubMed] [Google Scholar]
- 7.Yang F, Jin C, Yang D, et al. Magnetic functionalised carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment. Eur J Cancer 2011; 47:1873–1882. [DOI] [PubMed] [Google Scholar]
- 8.Zhu J, Tian W, Xu Z, et al. Expert consensus statement on parathyroid protection in thyroidectomy. Ann Transl Med 2015; 3:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang ZH, Cai YC, Chen J, et al. Application of meticulous capsular dissection technique in total thyroidectomy. Chin Arch Gen Surg 2014; 8:8–10. [Google Scholar]
- 10.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016; 26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stratmann M, Sekulla C, Dralle H, et al. Current TNM system of the UICC/AJCC: the prognostic significance for differentiated thyroid carcinoma. Chirurg 2012; 83:646–651. [DOI] [PubMed] [Google Scholar]
- 12.Cooper DS, Doherty GM, Haugen BR, et al. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Thyroid 2009; 19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 13.Thompson LD. Parathyroid carcinoma. Ear Nose Throat J 2009; 88:722–724. [PubMed] [Google Scholar]
- 14.Giordano D, Valcavi R, Thompson GB, et al. Complications of central neck dissection in patients with papillary thyroid carcinoma: results of a study on 1087 patients and review of the literature. Thyroid 2012; 22:911–917. [DOI] [PubMed] [Google Scholar]
- 15.Lee YS, Kim SW, Kim SW, et al. Extent of routine central lymph node dissection with small papillary thyroid carcinoma. World J Surg 2007; 31:1954–1959. [DOI] [PubMed] [Google Scholar]
- 16.Sywak M, Cornford L, Roach P, et al. Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid cancer. Surgery 2006; 140:1000–1005. [DOI] [PubMed] [Google Scholar]
- 17.Pereira JA, Jimeno J, Miquel J, et al. Nodal yield, morbidity, and recurrence after central neck dissection for papillary thyroid carcinoma. Surgery 2005; 138:1095–1101. [DOI] [PubMed] [Google Scholar]
- 18.Ito Y, Tomoda C, Uruno T, et al. Clinical significance of metastasis to the central compartment from papillary microcarcinoma of the thyroid. World J Surg 2006; 30:91–99. [DOI] [PubMed] [Google Scholar]
- 19.Goropoulos A, Karamoshos K, Christodoulou A, et al. Value of the cervical compartments in the surgical treatment of papillary thyroid carcinoma. World J Surg 2004; 28:1275–1281. [DOI] [PubMed] [Google Scholar]
- 20.Li XY, Guo Y, Zhong DR, et al. Diagnosis and treatment of chronic lymphocytic thyroiditis coexistent with thyroid malignancy (In Chinese). Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2006; 28:410–414. [PubMed] [Google Scholar]
- 21.Sekhar S, Nayak UK, Suhasini D, et al. Parathyroid hormone as a marker for predicting the severity of hypocalcaemia following parathyroidectomy. Ind J Otolaryngol Head Neck Surg 2015; 67:407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grebe SK, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am 1996; 5:43–63. [PubMed] [Google Scholar]
- 23.Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med 1993; 328:553–559. [DOI] [PubMed] [Google Scholar]
- 24.Scheumann GF, Gimm O, Wegener G, et al. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J Surg 1994; 18:559–567. [DOI] [PubMed] [Google Scholar]
- 25.Hao RT, Chen J, Zhao LH, et al. Sentinel lymph node biopsy using carbon nanoparticles for Chinese patients with papillary thyroid microcarcinoma. Eur J Surg Oncol 2012; 38:718–724. [DOI] [PubMed] [Google Scholar]


