Abstract
In this study, we evaluated the impact of preoperative high mobility group box 1 (HMGB1) on myocardial injury post-percutaneous coronary intervention.
We evaluated 302 consecutive patients who underwent percutaneous coronary intervention. They were divided into equal tertiles based on their preoperative HMGB1 levels. Creatine kinase-MB and troponin I levels were measured at baseline, 8- and 24-hours after the procedure, while clinical outcomes were followed up for 1 year.
The occurrence of post-procedural myocardial injury was significantly higher in the tertile comprising of patients with elevated HMGB1 levels. Moreover, these patients showed significantly higher post-procedural peak values of creatine kinase-MB and troponin I in comparison to patients with lower HMGB1 levels. Event-free survival was significantly associated with HMGB1 levels, with worst event-free survival in patients with elevated HMGB1 levels.
Elevated preoperative HMGB1 was a predictor of myocardial injury after percutaneous coronary intervention, and was associated with the worst clinical outcomes during 1-year follow up.
Keywords: creatine kinase-MB, high mobility group box1, myocardial injury, percutaneous coronary intervention, troponin I
1. Introduction
Percutaneous coronary intervention (PCI) is an effective method for treating coronary artery disease. However, 5% to 30% of patients undergoing PCI show elevated creatine kinase-myocardial band (CK-MB) and cardiac troponin I (cTnI), indicating post-procedural myocardial injury (PMI).[1] Relationship of elevated levels of both CK-MB and cTnI with short- and long-term outcomes has been established by multiple studies.[2–5] While several factors that affect CK-MB or cTnI elevation post-PCI, including age, acute coronary syndrome, and coronary thrombogenicity, have also been associated with PMI; the inflammatory response could also be an important contributing factor.[6–8]
In recent years, high mobility group box-1 (HMGB1), the ubiquitous DNA-binding protein has been identified for its involvement in sepsis and inflammatory reactions, hence, emerging as a potentially useful prognostic parameter.[9] HMGB1 could also enhance the inflammatory response leading to deterioration of cardiac function and ventricular remodeling. In this regard, HMGB1, also known as amphoterin protein, is a critical mediator for acute experimental ischemic injury and predictor of the myocardial infarction outcome.[10–12]
However, the utility of HMGB1 as a predictor, in myocardial injury post-PCI has not been previously evaluated. In this study, we sought to evaluate the impact of preoperative HMGB1 on myocardial injury and studied the long-term clinical outcomes in patients who underwent PCI.
2. Methods
2.1. Patient population and study design
This study protocol was conducted in accordance with the Helsinki declaration, and approved by Institutional Ethical Committee. Three hundred forty three consecutive patients who underwent PCI in China National Petroleum Corporation Central Hospital from January 2013 to March 2014 were enrolled in this study. All the participants provided written informed consents. Exclusion criteria included: active or chronic inflammatory or autoimmune diseases, myocarditis or alcohol abuse in last 6 months, liver and kidney dysfunction, heart failure (left ventricular ejection fraction <40%), active cancer treatment, and significant cognitive impairment, acute myocardial infarction (MI) (<1 month), or any disease resulting in CK-MB or cTnI increased. After considering these criteria, the study finally included 302 patients.
We obtained the baseline clinical data prospectively. The baseline clinical data included medical history, cardiovascular risk factors (including diabetes, hypertension, dyslipidemia, etc.), and left ventricular ejection fraction (LVEF). We obtained LVEF from echocardiography reports. The therapy was asked to take at least for ≥5 days on chronic aspirin (100 mg/day) and clopidogrel (75 mg/day) or at least 12 hours before PCI on 600 mg clopidogrel loading. After stent implantation, aspirin (100 mg/day) and clopidogrel (75 mg/day) were given at least for 12 months. Venous blood samples for the measurement of CK-MB and cTnI were obtained from all patients before operation, and at 8 and 24 hours after the operation. Measurements of CK-MB and cTnI were obtained using the Access 2 Immunoch emiluminometric assay (Beckman Coulter, CA). HMGB1 measurement was performed immediately before surgery, using ELISA (Shino-Test Corp., Kanagawa, Japan, distributed by IBL, Hamburg, Germany) according to the manufacturer's instructions.
Patient population was divided into 3 groups in accordance with tertiles of HMGB1 at baseline as follows: tertile 1: HMGB1 of <5.52 ng/mL in 101 patients; tertile 2: HMGB1 of 5.53 ng/mL to 9.76 ng/mL in 101 patients; tertile 3: HMGB1 of >9.77 ng/mL in 100 patients.
2.2. Endpoints and definitions
The primary endpoint was the occurrence of MI after surgery, defined by post-procedural elevation of CK-MB or cTnI above the normal limits and mean peak values of CK-MB and cTnI after procedure. Normal limits were ≤4 ng/mL for CK-MB and ≤0.04 ng/mL for cTnI, as measured by our local laboratory. Secondary endpoint was obtained by a clinic visit or by telephonic interview which were the composite rate of adverse cardiac vascular disease events, including cardiac related death, MI, and repeated revascularization. Follow-up in this study was 1 year. According the universal definition, MI was defined as the occurrence of ischemic syndrome and elevation of cTnI above 5× the normal limits within 48 hours post-PCI; beyond 48 hours, MI was defined as an increase of cTnI above the normal limits.[13]
2.3. Statistical analysis
All values were presented as the mean ± SD or percentage. Variables that were not normally distributed, were expressed as median (interquartile range). Continuous variables were tested for normal distribution using Student's t test or one-way analysis of variance (ANOVA) as appropriate, while Kruskal–Wallis test or Mann–Whitney U test was used for non-normal distribution. Comparisons of categorical variables were performed using chi-square test followed by Bonferroni's correction. Event-free survival was generated by the Kaplan–Meier method, and the log-rank test was used to evaluate the significance of differences between groups. Hazard ratios (HRs) and 95% confidence intervals (CIs) were assessed by using Cox proportional hazards regression models. A P value <0.05 was considered statistically significant. All P values were derived from two-sided significance tests. All statistical analyses were performed using SPSS version 13.0 software (SPSS, Chicago, IL).
3. Results
3.1. Baseline characteristics
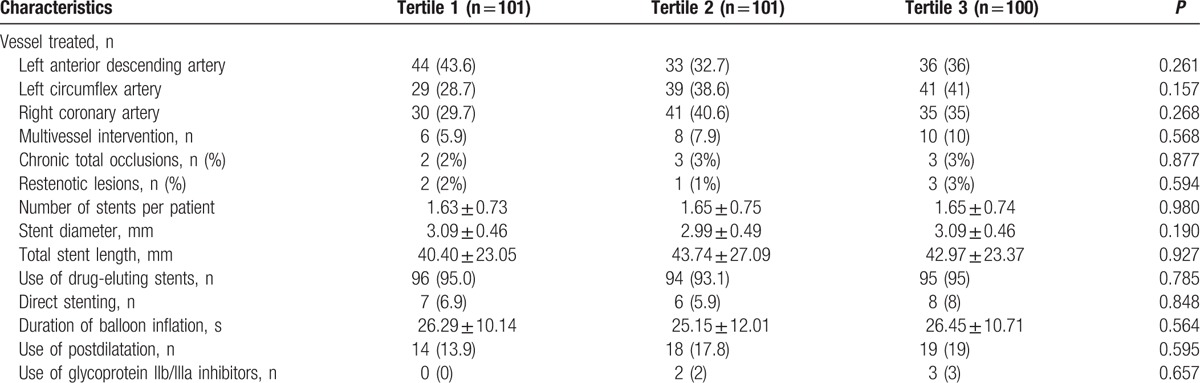
Clinical, laboratory, and procedural data of patients are presented in (Table 1). However, there was a significant association between increased tertiles of HMGB1 and C-reactive protein (CRP). Coronary anatomy, procedural characteristics, diameter, and length of implanted stents were also similar between the three groups (Table 2). Procedural success was achieved in all patients, and none of the patients showed any incidence of reflow phenomenon or significant side branch closure during the procedure.
Table 1.
Patient baseline characteristics.
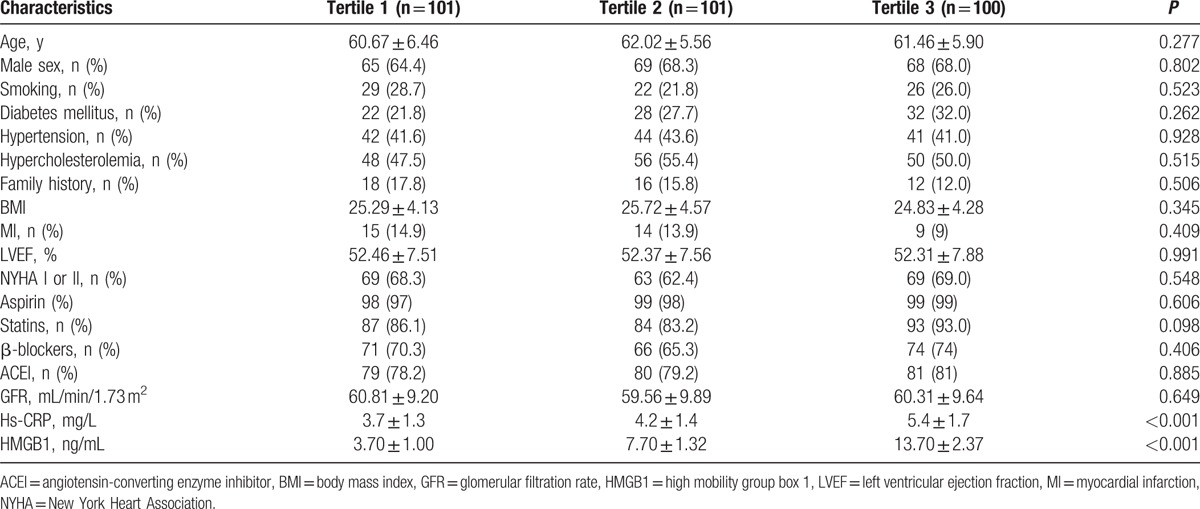
Table 2.
Procedural features in different group.
3.2. Post-procedural elevation of CK-MB and cTnI
The occurrence of PMI was significantly increased across the tertiles of HMGB1. Similar pre-procedural levels of markers CK-MB and cTnI were determined amongst the three tertiles. CK-MB elevation was observed in 28 (28.0%) patients of tertile 3, 16 (15.8%) patients of tertile 2, and 10 (9.9%) patients of tertile 1 (P = 0.037 and 0.001, respectively). Also, elevated cTnI levels were observed in 30 (30%) patients in tertile 3, 18 (17.8%) in tertile 2, and 9 (8.9%) in tertile 1 (P = 0.043 and P < 0.001, respectively) (Fig. 1). Post-procedural peak values of CK-MB were significantly higher for patients in tertile 3 (4.2 [1.8–11.0] ng/mL) than patients in tertile 2 (3.0 [1.5–8.5] ng/mL, P = 0.042) and tertile 1 (1.5 [1.0–3.1] ng/mL, P = 0.001). Post-procedural cTnI levels were significantly higher in tertile 3 patients (0.030 [0.010–0.148] ng/mL) than those in tertile 2 (0.020 [0.000–0.085] ng/mL, P = 0.030) and tertile 1 (0.020 [0.000–0.040] ng/mL, P < 0.001) (Fig. 2).
Figure 1.
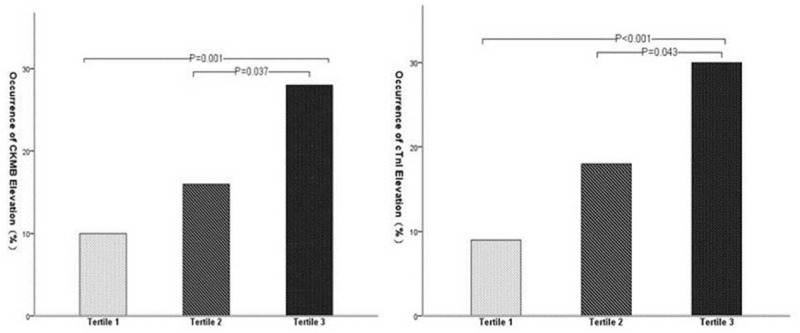
Occurrence of post-procedural increase of creatine kinase-myocardial band (CK-MB) and cardiac troponin I (cTnI).
Figure 2.
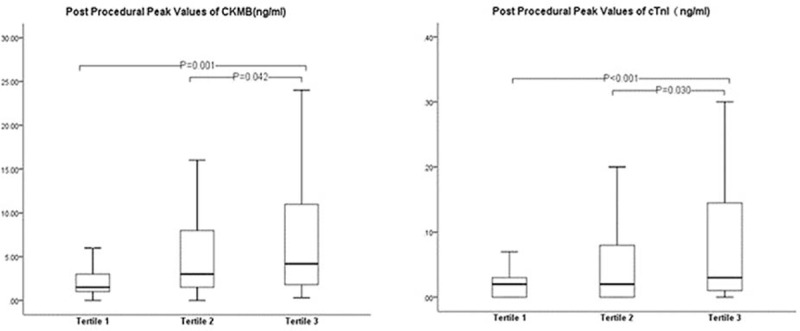
Post-procedural peak values of creatine kinase-myocardial band (CK-MB) and cardiac troponin I (cTnI).
3.3. 1-year follow-up of clinical outcomes
All the patients were clinically followed-up after 1-year. The combined endpoint occurred in 18 (18.0%) patients of tertile 3, 10 (9.9%) patients in tertile 2, and 7 (6.9%) in tertile 1. While cardiac death occurred in a single patient of tertile 3, the same was not observed for patients in tertile 2 or 1. MI was observed in 10 patients of tertile 3, 7 patients of tertile 2, and 4 patients of tertile 1. Revascularization was required in 7 patients of tertile 3, 3 patients of tertile 2, and 3 patients in tertile 1. Event-free survival was significantly associated with HMGB1 tertiles, with worse rates in tertile 3 patients than in tertile 2 and tertile 1 patients (HR 1.66; 95% CI: 1.08–2.55; Log Rank P = 0.046) (Fig. 3).
Figure 3.
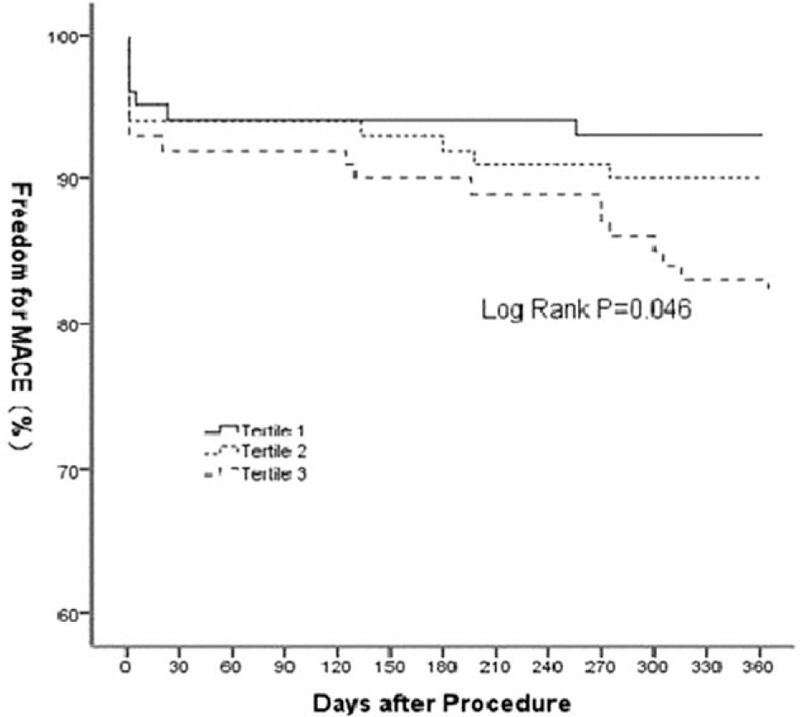
1-year outcome amongst the three groups using the combined endpoint.
4. Discussion
In the present study, we demonstrate elevated preoperative HMGB1 as a predictor of myocardial injury after percutaneous coronary intervention. We also observed its association with the worst clinical outcomes during the 1-year follow up.
HMGB1 binds to DNA in the nucleus resulting in stable nucleosome structure and is involved in regulating replication, DNA repair, and transcription.[14,15] Since HMGB1 is released by damaged or necrotic cells into the extracellular milieu, thereby mediating inflammatory responses, this intracellular protein is an important “necrotic marker” for the immune system.[16]
Association of HMGB1 with the occurrence, development, and prognosis of many cardiovascular diseases, has been recently supported by multiple studies. Research has shown that smooth muscle cells, foam cells, activated endothelial cells and platelets in the atherosclerotic plaque secrete HMGB1. This secretion raises the levels of HMGB1, and is highly correlated with monocyte-macrophage infiltration.[17,18] Yan et al[19] observed a significant correlation between coronary heart disease and levels of HMGB1 in the serum. Not surprisingly, patients with acute MI show increased HMGB1 levels, and these levels are furthermore associated with length of hospital stay, pump failure, and cardiac rupture.[20] Moreover, significant increase in HMGB1 levels is also observed in heart failure patients and is associated with its severity, making this protein an independent predictor of adverse cardiovascular events and heart transplantation.[21]
In this study, we showed for the first time that elevated preoperative HMGB1 levels increase the risk of myocardial injury post percutaneous coronary intervention. While the underlying mechanism is unclear, elevated HMGB1 levels could imply that the body is at a higher state of chronic inflammation. This could cause formation of coronary atherosclerotic plaque composed of high lipid content and large necrotic core, leading to formation of even more unstable plaques. During coronary artery intervention treatment, unstable plaques are more likely to rupture, releasing their contents, which could lead to distal microvascular thrombosis. This is corroborated by the study performed by Andrassy et al,[22] where they showed that elevated HMGB1 is significantly correlated with increased non-calcified plaque load. Furthermore, higher state of chronic inflammation may lead to endothelial dysfunction and microcirculation dysfunction. However, this proposed mechanism would require further investigation.
Through this study, our results provide evidence that patients with highest levels of HMGB1 exhibit the worst clinical outcomes during 1-year follow up. This could be partly since, elevated HMGB1 could cause increase in postoperative myocardial injury, thus affecting the prognosis. Porto et al[23] showed that HMGB1 induces increased migration of vascular smooth muscle cells and hyperplasia of endothelial cells, and this mechanism is the key reason of atherosclerotic plaque formation and in-stent restenosis. Altogether, this suggests that elevated HMGB1 could also increase the probability of unexpected revascularization.
4.1. Study limitations
This study had several limitations. Firstly, use of single blood sample did not allow assessment of the stability of HMGB1 in an individual over time. Secondly, post-procedural echocardiography examination was not performed in all patients, which prevented further analysis on left ventricular function caused by myocardial injury.
5. Conclusions
Elevated preoperative HMGB1 is a predictor of myocardial injury post-PCI, and is associated with the worst clinical outcomes during 1-year follow-up. Therefore, it may assist with risk stratification in patients undergoing PCI.
Footnotes
Abbreviations: CIs = confidence intervals, CK-MB = creatine kinase-myocardial band, CRP = C-reactive protein, cTnI = cardiac troponin I, HMGB1 = high mobility group box 1, HRs = hazard ratios, LVEF = left ventricular ejection fraction, MACE = major adverse cardiac events, MI = myocardial infarction, PCI = percutaneous coronary intervention, PMI = post-procedural myocardial injury.
Funding: None.
There are no conflicts of interest to declare.
References
- 1.Califf RM, Abdelmeguid AE, Kuntz RE, et al. Myonecrosis after revascularization procedures. J Am Coll Cardiol 1998; 31:241–251. [DOI] [PubMed] [Google Scholar]
- 2.Ioannidis JP, Karvouni E, Katritsis DG. Mortality risk conferred by small elevations of creatine kinase-MB isoenzyme after percutaneous coronary intervention. J Am Coll Cardiol 2003; 42:1406–1411. [DOI] [PubMed] [Google Scholar]
- 3.Roe MT, Mahaffey KW, Kilaru R, et al. Creatine kinase-MB elevation after percutaneous coronary intervention predicts adverse outcomes in patients with acute coronary syndromes. Eur Heart J 2004; 25:313–321. [DOI] [PubMed] [Google Scholar]
- 4.Nienhuis MB, Ottervanger JP, Bilo HJ, et al. Prognostic value of troponin after elective percutaneous coronary intervention: a meta-analysis. Catheter Cardiovasc Interv 2008; 71:318–324. [DOI] [PubMed] [Google Scholar]
- 5.Testa L, Van Gaal WJ, Biondi Zoccai GG, et al. Myocardial infarction after percutaneous coronary intervention: a meta-analysis of troponin elevation applying the new universal definition. QJM 2009; 102:369–378. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann J. Peri-procedural myocardial injury: 2005 update. Eur Heart J 2005; 26:2493–2519. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt DL, Topol EJ. Does creatinine kinase-MB elevation after percutaneous coronary intervention predict outcomes in 2005? Periprocedural cardiac enzyme elevation predicts adverse outcomes. Circulation 2005; 112:906–915.discussion 923. [DOI] [PubMed] [Google Scholar]
- 8.Uetani T, Amano T, Kumagai S, et al. Intracoronary electrocardiogram recording with a bare-wire system: perioperative ST-segment elevation in the intracoronary electrocardiogram is associated with myocardial injury after elective coronary stent implantation. Jacc Cardiovasc Interv 2009; 2:127–135. [DOI] [PubMed] [Google Scholar]
- 9.Andersson U, Erlandsson-Harris H, Yang H, et al. HMGB1 as a DNA-binding cytokine. J Leukoc Biol 2002; 72:1084–1091. [PubMed] [Google Scholar]
- 10.Andrassy M, Volz HC, Igwe JC, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation 2008; 117:3216–3226. [DOI] [PubMed] [Google Scholar]
- 11.Andrassy M, Volz HC, Riedle N, et al. HMGB1 as a predictor of infarct transmurality and functional recovery in patients with myocardial infarction. J Intern Med 2011; 270:245–253. [DOI] [PubMed] [Google Scholar]
- 12.Yamada S, Yakabe K, Ishii J, et al. New high mobility group box 1 assay system. Clin Chim Acta 2006; 372:173–178. [DOI] [PubMed] [Google Scholar]
- 13.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012; 126:2020–2035. [DOI] [PubMed] [Google Scholar]
- 14.Nightingale K, Dimitrov S, Reeves R, et al. Evidence for a shared structural role for HMG1 and linker histones B4 and H1 in organizing chromatin. EMBO J 1996; 15:548–561. [PMC free article] [PubMed] [Google Scholar]
- 15.Lange SS, Mitchell DL, Vasquez KM. High mobility group protein B1 enhances DNA repair and chromatin modification after DNA damage. Proc Natl Acad Sci USA 2008; 105:10320–10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulloa L, Messmer D. High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev 2006; 17:189–201. [DOI] [PubMed] [Google Scholar]
- 17.de Souza AW, Westra J, Limburg PC, et al. HMGB1 in vascular diseases: Its role in vascular inflammation and atherosclerosis. Autoimmun Rev 2012; 11:909–917. [DOI] [PubMed] [Google Scholar]
- 18.Kalinina N, Agrotis A, Antropova Y, et al. Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines. Arterioscler Thromb Vasc Biol 2004; 24:2320–2325. [DOI] [PubMed] [Google Scholar]
- 19.Yan XX, Lu L, Peng WH, et al. Increased serum HMGB1 level is associated with coronary artery disease in nondiabetic and type 2 diabetic patients. Atherosclerosis 2009; 205:544–548. [DOI] [PubMed] [Google Scholar]
- 20.Kohno T, Anzai T, Naito K, et al. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovasc Res 2009; 81:565–573. [DOI] [PubMed] [Google Scholar]
- 21.Volz HC, Laohachewin D, Schellberg D, et al. HMGB1 is an independent predictor of death and heart transplantation in heart failure. Clin Res Cardiol 2012; 101:427–435. [DOI] [PubMed] [Google Scholar]
- 22.Andrassy M, Volz HC, Maack B, et al. HMGB1 is associated with atherosclerotic plaque composition and burden in patients with stable coronary artery disease. PloS ONE 2012; 7:e52081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porto A, Palumbo R, Pieroni M, et al. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. FASEB J 2006; 20:2565–2566. [DOI] [PubMed] [Google Scholar]


