Abstract
Recent studies have paid much attention on the safety of bevacizumab as adjuvant chemotherapy for metastatic colorectal cancer. The aim of this meta-analysis was to study the efficacy and safety of bevacizumab in combination with irinotecan, bolus followed by infusional 5-fluorouracil, and leucovorin (FOLFIRI) and, irinotecan, bolus fluorouracil, leucovorin (IFL) for patients with metastatic colorectal cancer (mCRC).
An electronic search of related trials was conducted from PubMed, EMBASE, Cochrane Library databases. Risk ratio (RRs) and its 95% confidence intervals (95% CIs) were calculated by using either DerSimonian–Laird method or Mantel–Haenszel method according to the heterogeneity of included articles. The risk of mortality, therapeutic efficacy, and adverse effect were meta-analyzed.
In total, 6 RCTs including 2165 participants (1109 in the treatment group, 1056 in the control group) were included in this meta-analysis. Compared with FOLFIRI-panitumumab/cetuximab, the bevacizumab addition significantly reduced the complete response (CR) rate (RR [95%CI] = 0.31[0.11, 0.89], P = 0.03) and the risk of grade 3/4 adverse event (RR [95%CI] = 0.89[0.80, 0.98], P = 0.01). Compared with FOLFIRI and IFL alone, the addition of bevacizumb significantly increased the partial response (PR) and objective response (OR) rates. Compared with IFL alone, the addition of bevacizumb significantly reduced the mortality risk of PFS (RR [95%CI] = 0.53[0.42, 0.66], P < 0.00001) and OS (RR[95%CI] = 0.70[0.60, 0.82], P < 0.00001), but increased the risk of adverse events (RR[95%CI] = 1.14[1.06, 1.21], P = 0.0002).
Combination chemotherapy of bevacizumab plus FOLFIRI or IFL had a relative high efficacy and acceptable safety for treatment of mCRC.
Keywords: bevacizumab, FOLFIRI, IFL, metastatic colorectal cancer (mCRC), meta-analysis
1. Introduction
Colorectal cancer is one of the most important causes of cancer deaths worldwide.[1] It has been reported that about half of the patients with colorectal cancer develop inoperable metastases.[2] Even, among patients who underwent curative surgery, the mortality rate remained high due to relapse of this metastatic disease.[3] Therefore, an effective therapeutic regimen for the treatment of metastatic colorectal cancer (mCRC) is urgently needed.
Bevacizumabis a humanized monoclonal antibody targeted against vascular endothelial growth factor (VEGF). Previous studies have shown that the monoclonal antibody against VEGF significantly suppressed the growth of tumor xenografts.[4,5] Therefore, the humanized monoclonal antibody bevacizumab has been extensively evaluated for treatment of various cancers.[6,7] In recent years, bevacizumab has been introduced for treatment of mCRC aimed to prolong the patients’ survival. The addition of bevacizumab to fluorouracil plus leucovorin increased the median time to disease progression, improved the response rate, as well as the survival duration in patients with mCRC.[8]
However, recent studies have paid much attention on the safety of bevacizumab. As we know, VEGF plays multiple roles in several physiologic processes. Thus, its inhibition could cause potentially serious consequences,[9] such as hemorrhage,[10] hypertension,[11] arterial thrombosis.[12] Several meta-analyses have evaluated the efficacy and safety of bevacizumabin the treatment of human cancers. For example, Huang et al[13] found that, compared with chemotherapy alone, the addition of bevacizumab significantly increased the risk of fatal adverse events among patients with special tumor types. Qi and his colleagues[9] suggested that bevacizumab treatment was associated with the risk of infectious events in cancer patients, and the risks may vary with concomitant drugs. However, Ahmadizar et al[14] suggested that though bevacizumab was associated with increased risks of adverse drug reactions such as bleeding and hypertension, both the fatigue and anemia of cancer patients were improved by the use of bevacizumab.
Clinical data have shown that bevacizumab improved the survival rate of patients with mCRC, when combined with different fluorouracil regimens (infusions and bolus), such as irinotecan, bolus followed by infusional5-fluorouracil, and leucovorin (FOLFIRI) and irinotecan, bolus 5-fluorouracil, leucovorin (IFL). With respect to the safety and efficacy of bevacizumab on mCRC patients, a recent meta-analysis has been conducted.[15] However, the authors of this study (1) did not explain the efficacy and safety of bevacizumab plus FOLFIRI or IFL on treatment of mCRC, (2) did not report the effect of bevacizumab on complete response rate and treatment discontinued by bevacizumab toxicity; (3) just focused on the first-line treatment. Therefore, we performed this meta-analysis to study the effectiveness of bevacizumab (Be)-containing FOLFIRI and IFL regimens for mCRC patients.
2. Materials and methods
2.1. Search strategy
The relevant studies about the effectiveness of FOLFIRI-Beor IFL-Beon treatment of mCRC have been searched from PubMed, EMBASE, and Cochrane Library databases. Last query was updated in March 2016. We also searched the references listed in the relevant literatures. The keywords used for search strategy were (“colorectal cancer” OR “rectal cancer” OR “rectum cancer” OR “rectal carcinoma”) AND (randomized OR randomised OR random∗ OR blind) AND ([{5-Fluorouracil OR Leucovorin} AND irinotecan] OR FOLFIRI) AND bevacizumab.
2.2. Inclusion and exclusion criteria
Studies included in this meta-analysis should meet the inclusion criteria, as follows: (1) the studies are designed as random control trials (RCTs); (2) patients in the treatment group received FOLFIRI-Be or IFL-Be, whereas patients in the control group were treated with FOLFIRI alone or IFL alone or plus by additional agents or interventions; (3) studies should evaluate the outcomes including complete response (CR), partial response (PR), objective response (OR), progression-free survival (PFS), overall survival (OS), grade3/4adverse events (based on the National Cancer Institute Common Toxicity Criteria, version 2) and treatment discontinued by drug toxicity.
We tried to avoid duplicated data by carefully examine the names of authors and their affiliated unit in each publication. We also excluded studies from not original articles such as comments, reviews, and conferences accounts.
2.3. Data extraction
Two investigators independently screened the literature according to the inclusion and exclusion criteria described above. They also extracted the following data from the eligible studies independently: the information of the literature (e.g., the name of the first author, the year of publication, and the countries) and patients (the gender, the sample size of the patients, the therapeutic regimen, and the outcomes). The quality of the included studies was assessed by the Cochrane risk of bias assessment tool.[16] Discrepancies between the 2 investigators were resolved by discussing with each other or communicating with the original authors via E-mail. The adverse events were graded by the Criteria for Adverse Events version 3.0.[17]
2.4. Statistical analysis
The meta-analysis was conducted to compare the efficacy and safety of bevacizumab versus combined chemotherapy on treatment of mCRCby the Review Manager 5.3 software.[18] The risk ratio (RR) and 95% CI were used to compare the 2 therapies. The effect size of PFS and OS was pooled through harzard ratio (HR) and it is 95% CI, whereas the effect size of the other outcomes was evaluated via the number of patients. The heterogeneity across studies was examined by Q statistic[19] and the I2 statistic. The Mantel–Haenszel method was selected for pooling the homogeneous outcomes when P ≥ 0.05 and I2 < 50%, and the DerSimonian–Laird method was applied for pooling heterogeneous outcomes when P < 0.05 and I2 ≥ 50%. Publication bias was examined by a funnel plot. P < 0.05 was considered as statistically significant.
2.5. Informed consent and ethical approval
Our present study was a meta-analysis and was based on the data of 7 articles published in PubMed, EMBASE, and Cochrane Library databases. Thus, ethical approval was not necessary.
3. Results
3.1. The characteristics of included literature
The flowchart of literature retrieval is shown in Fig. 1. A total of 716 studies (180 from PubMed, 420 from Embase, and 116 from Cochrane) were initially retrieved from the databases. After excluding 188 duplicates, the remaining 528 were screened for eligibility using titles and abstracts. We then excluded 506 articles because of obviously irrelevant topic (n = 454), reviews (n = 33), animal experiment (n = 10), and non-RCT (n = 9).The remaining 22 studies were full-text reviewed, and 15 studies were excluded from these 22 (12 studies did not refer to FOLFIRI or IFL, 3 studies included duplicated data). No additional studies were identified from manual retrieve. Finally, 7[11,20–25] articles were included in this meta-analysis. However, among the 7 included studies, 2 studies by Hurwitz[24] and Kbbinavar[25] reported the same patients; thus, we combined the data of these 2 studies into 1.
Figure 1.
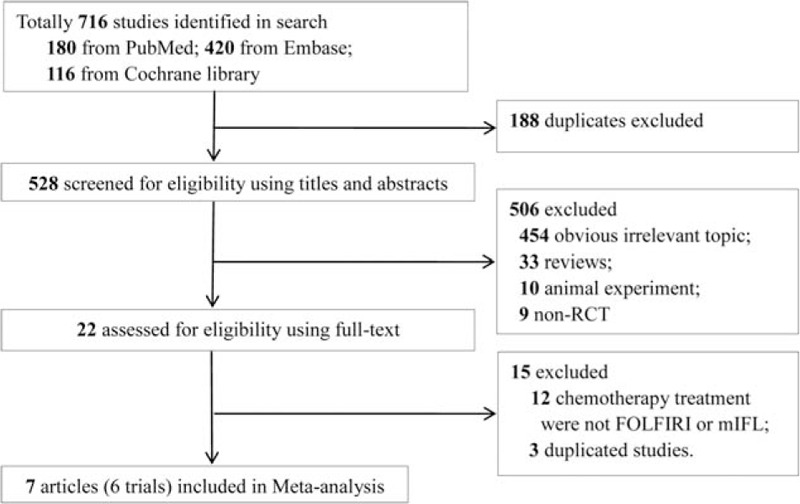
Literature search and study selection.
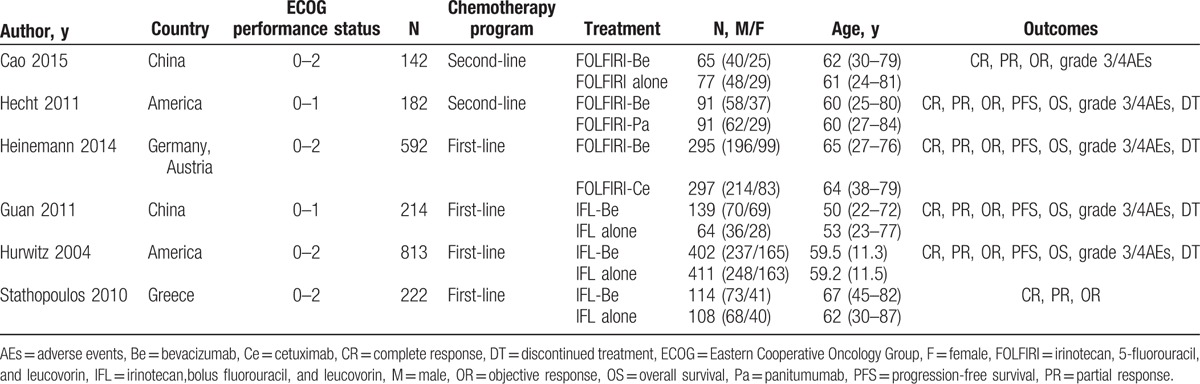
The characteristics of the included studies are shown in Table 1. A total of 6 RCTs, including 2165 participants (1109 treated, 1056 control), were included in this meta-analysis. These literatures were published between 2004 and 2015, and distributed in China, America, and some European countries (Austria, Greece, and Germany). The patients with mCRC had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2. Patients reported by Cao et al[20] and Hecht et al[22] had received second-line treatment, but patients reported in the other studies had received the first-line treatment. With regard to the therapeutic methods, 1 study referred to the treatment of FOLFIRI-Be versus FOLFIRI alone,[20] 2 referred to the treatment of FOLFIRI-Be versus others (FOLFIRI-panitumumab, FOLFIRI-cetuximab),[22,23] the others compared the treatment of IFL-Be versus IFL alone.[11,21,24]
Table 1.
Characteristics of the included articles.
3.2. Quality assessment
The quality of the included studies was assessed by the Cochrane risk of bias assessment tool (Fig. 2). The levels of performance bias and detection bias were both high because of the open-label design of all the included studies. Two studies had a high selection bias because they introduced allocation concealment method.[23,24] In general, all the included studies were suitable for meta-analysis with relatively high quality.
Figure 2.
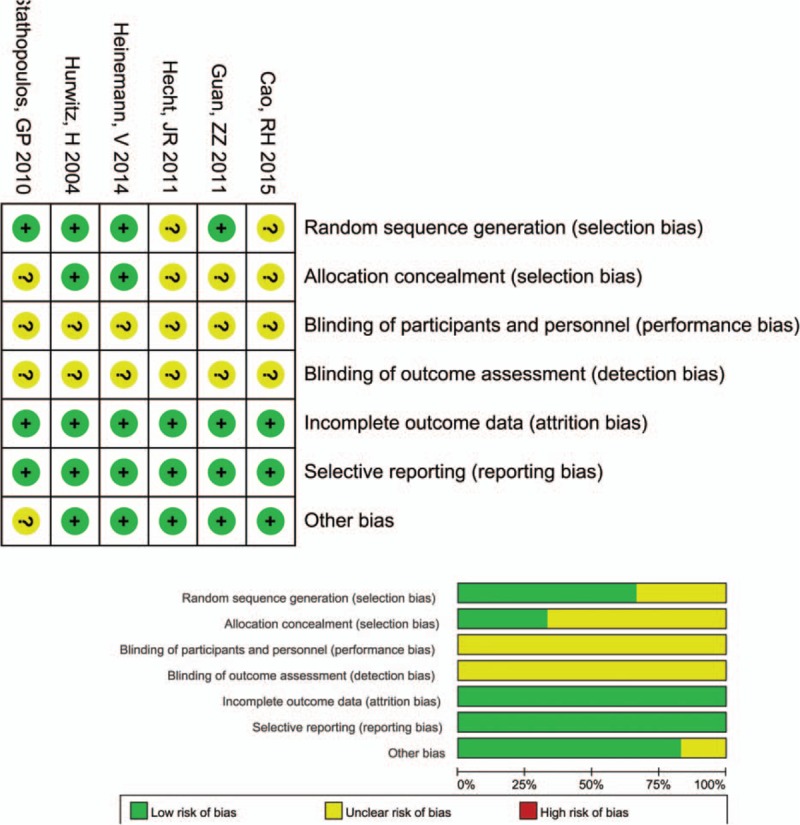
Risk bias of included studies.
3.3. Meta-analysis for therapeutic efficacy
The outcomes of therapeutic efficiency (CR, PR, and OR) analyzed based on 3 therapeutic regimens (FOLFIRI-Be vs others; FOLFIRI-Be vs FOLFIRI alone; IFL-Be vs IFL alone) are presented in Fig. 3.
Figure 3.
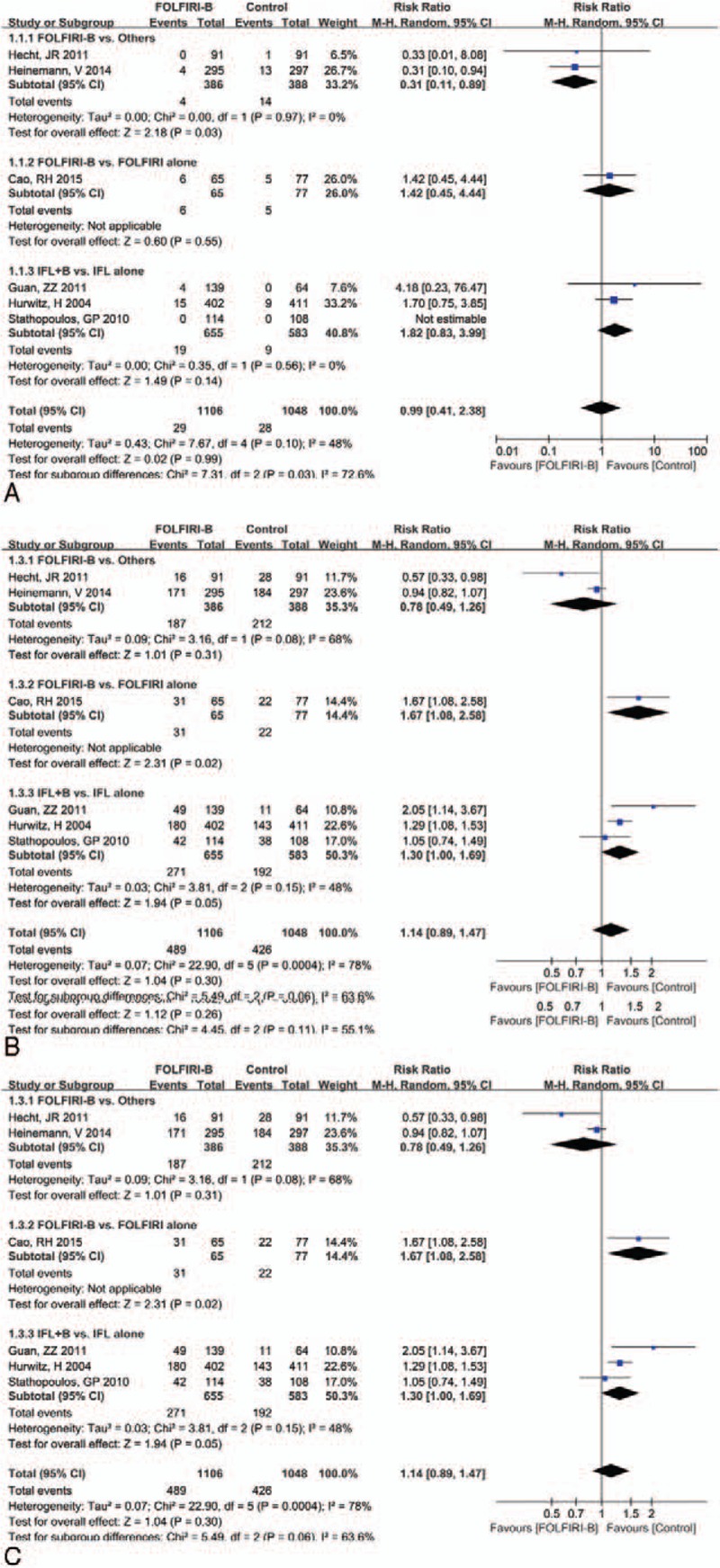
Forest plot of therapeutic efficiency for the addition of bevacizumab to FOLFIRI or IFL: (A) complete response, (B) partial response, (C) objective response. FOLFIRI = 5-fluorouracil, and leucovorin, IFL = fluorouracil, leucovorin.
The total effect size for CR, PR, and OR were RR (95% CI) = 1.01 (0.61, 1.69),P = 0.99; RR (95%CI) = 1.14 (0.91, 1.43), P = 0.26; and RR (95%CI) = 1.14 (0.89, 1.47), P = 0.30, respectively. Subgroup analysis showed that the addition of bevacizumabto FOLFIRI was significantly associated with increase in PR (RR = 1.74, 95% CI = 1.04, 2.93, P = 0.04)and OR (RR = 1.67, 95% CI = 1.08, 1.58, P = 0.02) compared with the treatment of FOLFIRI alone. Besides, the addition of bevacizumab toIFL also significantly increased the PR rate (RR = 1.26, 95% CI = 1.01, 1.57, P = 0.04) in comparison with IFL alone. However, a significant decrease in the CR rate was noted in patients who receivedFOLFIRI-Be compared with those who received others (FOLFIRI-panitumumab or FOLFIRI-cetuximab) (RR = 0.31, 95% CI = 0.11, 0.89, P = 0.03).
3.4. Meta-analysis for risk of mortality
The outcomes of PFS and OS analyzed based on 2 subgroups (FOLFIRI-Be vs others; IFL-Be vs IFL alone) are presented in Fig. 4. The overall effects for PFS and OS were RR (95%CI) = 0.70 (0.49, 0.99), P = 0.04, and RR (95%CI) = 0.87 (0.61, 1.24), P = 0.43. There was no significant difference for PFS (RR = 0.95, 95% CI = 0.80, 1.13, P = 0.55) and OS (RR = 0.95, 95% CI = 0.80, 0.99, P = 1.13) between FOLFIRI-Be and FOLFIRI-panitumumab/cetuximab. However, a marked advantage in OS (RR = 0.53, 95% CI = 0.42, 0.66, P < 0.00001) and PFS (RR = 0.70, 95% CI = 0.60, 0.82, P < 0.00001) was noted for patients in the IFL-Be group compared with the IFL group. These results indicated that the addition of bevacizumab to IFL significantly reduced the risk of mortality of mCRC patients.
Figure 4.
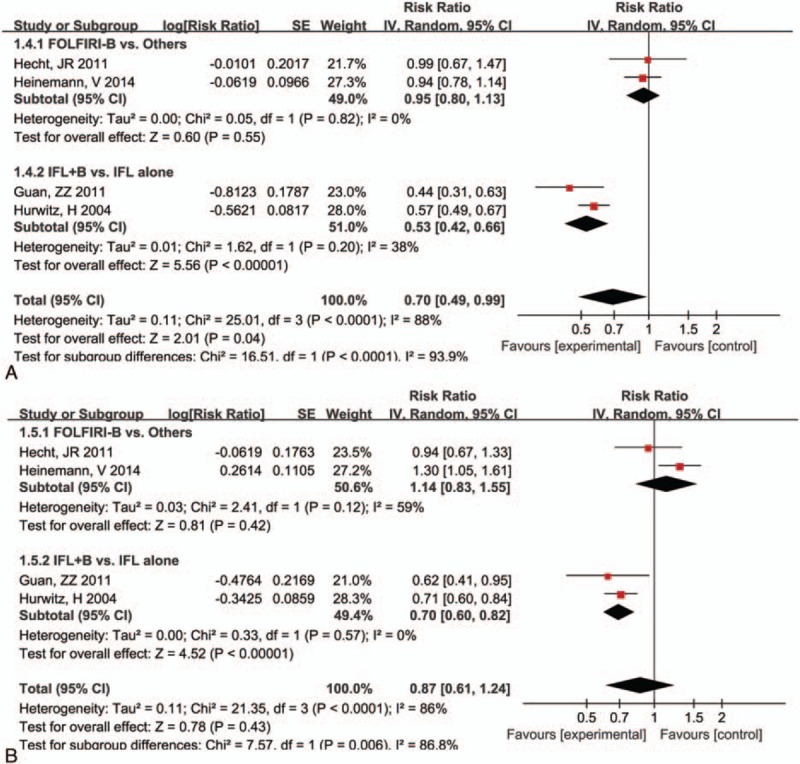
Forest plot of mortality risk for the addition of bevacizumab to FOLFIRI or IFL: (A) progression-free survival, (B) overall survival. FOLFIRI = 5-fluorouracil, and leucovorin, IFL = fluorouracil, leucovorin.
3.5. Meta-analysis for adverse effect
The comparison of grade 3/4 adverse events and treatment discontinued by drug toxicity based on 3 therapeutic regimens (FOLFIRI-Be vs others; FOLFIRI-Be vs FOLFIRI alone; IFL-Be vs IFL alone) are presented in Fig. 5. Results showed that the grade 3/4 adverse events were much less in the FOLFIRI-Be than that in the FOLFIRI-panitumumab/cetuximab group (RR = 0.89, 95% CI = 0.80, 0.98, P = 0.01). Significantly more grade 3/4 adverse event was found in bevacizumab plus IFL group than that in the IFL alone group (RR = 1.14, 95% CI = 1.06, 1.21, P = 0.0002). With regard to the discontinued treatment, there were no significant differences between the 3 pairwise groups (P > 0.05).
Figure 5.
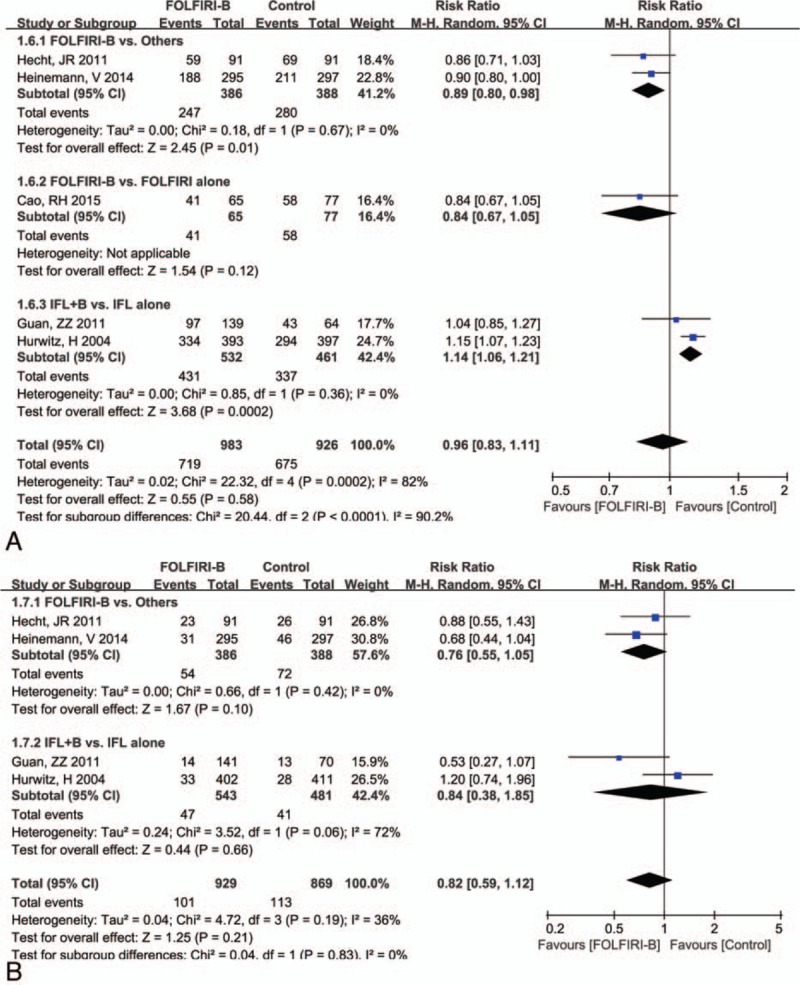
Forest plot of adverse effect for the addition of bevacizumab to FOLFIRI or IFL: (A) grade 3/4 side events, (B) treatment discontinued by drug toxicity. FOLFIRI = 5-fluorouracil, and leucovorin, IFL = fluorouracil, leucovorin.
4. Discussion
This meta-analysis evaluated the feasibility of administering bevacizumab in combination with 2 different fluorouracil regimens (infusions and bolus) for therapy of mCRC. Results showed that compared with FOLFIRI-panitumumab/cetuximab, the addition of bevacizumab significantly reduced the CR rate; however, the numbers of grade 3/4 adverse event were also significantly reduced. In comparison with FOLFIRI or IFL alone, the addition of bevacizumab to the 2 regimens showed a better efficacy and a reduced mortality risk, but a higher risk of adverse event. These results indicated that compared with the other concomitant drugs, bevacizumab treatment is associated with low efficacy but also low risk of adverse events. Reversely, compared with the chemotherapy (FOLFIRI or IFL) alone, bevacizumab treatment is associated with high efficacy, low risk of mortality, but high risk of adverse events.
The bevacizumab-induced high risk of adverse events might be associated with its inhibitory effect on the VEGF. The VEGF can affect the host response to infections by influencing the function of immune tumor cells presented in the tumor microenvironment.[26,27] Besides, the VEGF receptors were reported to be involved in regulating fibroblasts in the tumor stroma.[28] Rafii et al[29] reported that inhibition of VEGF receptors could inhibit the hematopoietic stem-cell cycling, which will related to the risk of myelosuppression. A previous meta-analysis indicated that the addition of bevacizumab significantly increased the risk of hypertension by 6.2%,[15] whereas no significant differences were found in grade 3–4 proteinuria and bleeding. In our present meta-analysis, the adverse events were graded. Data of our meta-analysis showed that the addition of bevacizumab to different therapy regimes resulted in different risk of adverse events. Therefore, we thought that different concomitant drugs with chemotherapy may exert different functions on adverse events. Our finding confirmed the previous study by Qi et al[9] who also found significant difference in risk of side effect with bevacizumab among different chemotherapeutics. The reason might be explained that treatment with bevacizumab, in combination with FOLFIRI or IFL, caused more toxic effects than other concomitant drugs.
Compared with IFL alone, we found that the addition of bevacizumab to this regimen showed a much longer PFS and OS, but a higher risk of adverse event. It is possible that patients with longer PFS or OS have more time or chance to develop side effect than those with shorter PFS or OS.[30] However, all the included studies in our meta-analysis did not evaluate the association of this potential bias caused by prolonged time and the risk of adverse effects. Therefore, the relationship between them needs more studies to uncover.
First-line treatment is considered to be the most important phase for therapy of cancers. However, it was reported that ∼70% to 80% of the first-line treatment patients with mCRC were exposed to the later lines of therapy.[23] Therefore, the later lines including the second-line treatment is also important for mCRC. Both first-line and second-line chemotherapy programs were reported in our study, suggesting that our study evaluated the efficacy and safety of bevacizumab comprehensively. Besides, the studies included in our meta-analysis were all RCTs with relatively high quality. Therefore, the results of our meta-analysis were creditable.
Besides the sample size, our study also has limitations that we should point out. First, the number of included studies of this meta-analysis was limited, and thus, we cannot evaluate if there was publication bias among included studies. Second, in the case of selection criteria, the detailed therapeutic processes varied among studies, which might contribute to the heterogeneity of the results and is likely to produce effects on the final outcome.
In conclusion, our results showed that compared with FOLFIRI-panitumumab/cetuximab, bevacizumab addition induced a lower CR rate and lower risk of adverse event; and compared with FOLFIRI or IFL alone, the addition of bevacizumab to the 2 regimens induced a better efficacy and a reduced mortality risk, but a higher risk of adverse events. We concluded that combination chemotherapy containing bevacizumab plus FOLFIRI or IFL had a relative high efficacy with acceptable safety.
Acknowledgments
The authors would like to express their warmest gratitude to Prof Senxiang Yan, for his instructive suggestions on the writing of this thesis. At the same time, they gratefully appreciate their families’ support.
Footnotes
Abbreviations: CIs = confidence intervals, CR = complete response, ECOG = Eastern Cooperative Oncology Group, FOLFIRI = 5-fluorouracil, and leucovorin, HR = harzard ratio, IFL = fluorouracil, leucovorin, mCRC = metastatic colorectal cancer, OR = objective response, OS = overall survival, PFS = progression-free survival, PR = partial response, RCTs = random control trials, RRs = risk ratio, VEGF = vascular endothelial growth factor.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer Statistics, 2010. CA Cancer J Clin 2012; 62:10–29. [DOI] [PubMed] [Google Scholar]
- 2.Cutsem EV, Nordlinger B, Adam R, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer 2006; 42:2212–2221. [DOI] [PubMed] [Google Scholar]
- 3.Obrand DI, Gordon PH. Incidence and patterns of recurrence following curative resection for colorectal carcinoma. Dis Colon Rectum 1997; 40:15–24. [DOI] [PubMed] [Google Scholar]
- 4.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993; 362:841–844. [DOI] [PubMed] [Google Scholar]
- 5.Asano M, Yukita A, Matsumoto T, et al. An anti-human VEGF monoclonal antibody, MV833, that exhibits potent anti-tumor activity in vivo. Hybridoma 1998; 17:185–190. [DOI] [PubMed] [Google Scholar]
- 6.Seiwert T, Haraf D, Cohen E, et al. Phase I study of bevacizumab added to fluorouracil- and hydroxyurea-based concomitant chemoradiotherapy for poor-prognosis head and neck cancer. J Clin Oncol 2008; 26:1732–1741. [DOI] [PubMed] [Google Scholar]
- 7.Okita NT, Esaki T, Baba E, et al. A multicenter phase II study of the stop-and-go modified FOLFOX6 with bevacizumab for first-line treatment of patients with metastatic colorectal cancer. Invest New Drugs 2012; 30:2026–2031. [DOI] [PubMed] [Google Scholar]
- 8.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 2003; 21:60–65. [DOI] [PubMed] [Google Scholar]
- 9.Qi WX, Fu S, Zhang Q, et al. Bevacizumab increases the risk of infections in cancer patients: A systematic review and pooled analysis of 41 randomized controlled trials. Crit Rev Oncol Hematol 2015; 94:323–336. [DOI] [PubMed] [Google Scholar]
- 10.Hapani S, Sher A, Chu D, et al. Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology 2010; 79:27–38. [DOI] [PubMed] [Google Scholar]
- 11.Stathopoulos G, Batziou C, Trafalis D, et al. Treatment of colorectal cancer with and without bevacizumab: a phase III study. Oncology 2010; 78:376–381. [DOI] [PubMed] [Google Scholar]
- 12.Schutz F, Je Y, Azzi G, et al. Bevacizumab increases the risk of arterial ischemia: a large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol 2011; 22:1404–1412. [DOI] [PubMed] [Google Scholar]
- 13.Huang H, Zheng Y, Zhu J, et al. An updated meta-analysis of fatal adverse events caused by bevacizumab therapy in cancer patients. PLos One 2014; 9:e89960–e90060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmadizar F, Onland-Moret NC, Boer AD, et al. Efficacy and safety assessment of the addition of bevacizumab to adjuvant therapy agents in cancer patients: a systematic review and meta-analysis of randomized controlled trials. PLos One 2015; 10:6104–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loupakis F, Bria E, Vaccaro V, et al. Magnitude of benefit of the addition of bevacizumab to first-line chemotherapy for metastatic colorectal cancer: meta-analysis of randomized clinical trials. J Exp Clin Cancer Res 2010; 29:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins, Julian PT, Sally G, eds. Cochrane handbook for systematic reviews of interventions. Vol. 5 Chichester: Wiley-Blackwell, 2008. [Google Scholar]
- 17.Williams J, Chen Y, Rubin P, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003; 13:176–181. [DOI] [PubMed] [Google Scholar]
- 18.RTC Collaboration. Review Manager (RevMan). 5.3[J]. 2014. [Google Scholar]
- 19.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Int Med 1997; 127:820–826. [DOI] [PubMed] [Google Scholar]
- 20.Cao R, Zhang S, Ma D, et al. A multi-center randomized phase II clinical study of bevacizumab plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) compared with FOLFIRI alone as second-line treatment for Chinese patients with metastatic colorectal cancer. Med Oncol 2015; 32:1–5. [DOI] [PubMed] [Google Scholar]
- 21.Guan Z-Z, Xu J-M, Luo R-C, et al. Efficacy and safety of bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: a randomized phase III ARTIST trial. Chin J Cancer 2011; 30:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hecht JR, Cohn A, Dakhil S, et al. SPIRITT: a randomized, multicenter, phase II study of panitumumab with FOLFIRI and bevacizumab with FOLFIRI as second-line treatment in patients with unresectable wild type KRAS metastatic colorectal cancer. Clin Colorectal Cancer 2015; 14:72–80. [DOI] [PubMed] [Google Scholar]
- 23.Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15:1065–1075. [DOI] [PubMed] [Google Scholar]
- 24.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 25.Kabbinavar F, Irl C, Zurlo A, et al. Bevacizumab improves the overall and progression-free survival of patients with metastatic colorectal cancer treated with 5-fluorouracil-based regimens irrespective of baseline risk. Oncology 2008; 75:215–223. [DOI] [PubMed] [Google Scholar]
- 26.Hansen W, Hutzler M, Abel S, et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J Exp Med 2012; 209:2001–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer 2013; 13:871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yaqoob U, Cao S, Shergill U, et al. Neuropilin-1 stimulates tumor growth by increasing fibronectin fibril assembly in the tumor microenvironment. Cancer Res 2012; 72:4047–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rafii S, Avecilla S, Shmelkov S, et al. Angiogenic factors reconstitute hematopoiesis by recruiting stem cells from bone marrow microenvironment. Ann N Y Acad Sci 2003; 996:49–60. [DOI] [PubMed] [Google Scholar]
- 30.Minor DR. Risk of venous thromboembolism with bevacizumab in cancer patients. J Am Med Assoc 2009; 300:2277–2285. [DOI] [PubMed] [Google Scholar]


