Abstract
Systemic lupus erythematosus (SLE) is a multisystemic autoimmune disease. The vitamin D receptor (VDR) gene is a candidate gene for susceptibility to autoimmune disorders. To date, only a few studies concerned the association of the VDR gene polymorphisms with childhood-onset SLE.
In this study, we aimed to investigate the BsmI polymorphisms in the VDR gene, for the first time in Egyptian children and adolescents with SLE, to determine whether this polymorphism could be a marker of susceptibility to or severity of SLE and we also measured the serum level of 25-hydroxyvitamin D (25[OH] D) to assess its relation to such polymorphism.
This was a case–control study including 100 patients with SLE and matched with age, sex, and ethnicity and 100 healthy controls. All subjects were genotyped for the VDR gene BsmI polymorphism by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), whereas the serum 25(OH) D levels were measured by enzyme-linked immunosorbent assay method.
Compared to the contros subjects, the VDR BsmI BB genotype and B allele were overrepresented among SLE patients (odda ratio [OR]: 5.5; 95% confidence interval [CI]: 1.9–15.9; P = 0.002 and OR: 1.84; 95% CI: 1.21–2.80; P = 0.003; respectively). We found a significant association between VDR BsmI BB genotype with lupus nephritis (OR: 6.8; 95% CI: 1.18–50.5; P = 0.001). However, we did not observe any significant association of studied polymorphisms with other clinical manifestations, laboratory profiles of SLE, or disease activity score. Our data revealed no association between VDR BsmI genotypes or alleles and serum 25-hydroxyvitamin D levels among studied patients with SLE (all P > 0.05).
We demonstrate for the first time, to the best of our knowledge, that the VDR BsmI gene polymorphisms may contribute to susceptibility to SLE in Egyptian children and adolescents. Moreover, we found that the BB genotype constituted a risk factor for the development of nephropathy among studied patients with SLE. However, we did not find any significant association of the VDR BsmI gene variants with other clinical manifestations, laboratory profiles of SLE, disease activity index score, or serum 25-hydroxyvitamin D levels.
Keywords: adolescents, children, gene polymorphisms, systemic lupus erythematosus, vitamin D receptors
1. Introduction
Systemic lupus erythematosus (SLE) is one of the most relevant worldwide autoimmune disorders with a broad spectrum of autoimmune phenomena, clinical symptoms, and unknown etiology[1] SLE diverse presentations range from arthritis and malar rash through anemia and thrombocytopenia to nephritis, serositis, seizures, and psychosis[2] Complex interactions between environmental, genetic, and hormonal factors have been linked with the phenotype and progression of SLE[3] One such environmental factor is vitamin D, a steroid hormone recognized as essential for bone and mineral homeostasis. Sun exposure is the major source of vitamin D, but it is also available in some foods and supplements. Recently, the function of vitamin D has been further studied to include this molecule as a pleiotropic regulator of human physiology, as playing a pivotal role in cardioprotection, cancer chemoprevention, and immune system modulation[4] Several epidemiological studies have revealed that vitamin D deficiency could contribute to the risk of autoimmune diseases like SLE.[5,6]
1, 25-dihydroxyvitamin D3 (calcitriol), the most active natural vitamin D metabolite, mediates its biological effects through the vitamin D receptors (VDRs) located at the nuclei of the target tissues[7] In addition to cells in the gut and bone, the presence of VDRs on immune cells suggests that vitamin D also has modulating effects on both the innate and adaptive immunity[8] At the molecular level, 1, 25-dihydroxyvitamin D3 inhibits the accumulation of mRNA for interleukin-2, interferon-γ, and granulocyte-macrophage colony-stimulating factor. At the cellular level, the hormone interferes with T helper cell function, reducing its induction of immunoglobulin production by B cells[5] In addition, calcitriol inhibits the differentiation and maturation of dendritic cells and promotes their apoptosis, thus preventing their transformation into antigen-presenting cells leading to suppression of dendritic cell-dependent T-cell activation[5] These suppressive immunologic properties have raised a great deal of interest in a possible role of vitamin D in SLE pathogenesis.
Previous studies have reported a high prevalence of vitamin D insufficiency and deficiency in patients with SLE[9,10] Furthermore, low vitamin D levels were associated with higher disease activity in Egyptian, Malaysian, European, Israeli, and Chinese cohorts[11–14] Despite these studies, the association of vitamin D levels with SLE does not constitute proof of cause. One way to clarify the issue of causation involves a genetic approach. The VDR gene is located on chromosome 12q and contains >470 single-nucleotide polymorphisms, some of which modulate 1, 25-dihydroxyvitamin D3 uptake[15] Four major polymorphisms (BsmI, ApaI, TaqI, and FokI) have been described, although their influences on VDR function are still unknown.[16]
We therefore designed this study to investigate the BsmI polymorphisms in the VDR gene, for the first time in Egyptian children and adolescents with SLE, to determine whether this polymorphism could be a marker of susceptibility to or severity of SLE and we also measured the serum level of 25-hydroxyvitamin D (25[OH] D) to assess its relation to such polymorphism.
2. Methods
This was a prospective case–control study performed in Zagazig University Hospitals, Pediatrics and Rheumatology Departments, and outpatient clinics in the same hospitals from May 2013 to April 2016. This study was approved by the ethical committee of Zagazig University, Egypt, and written informed consent from parents was provided in accordance with the Declaration of Helsinki.
One hundred unrelated patients with definite SLE diagnosed according to the 1997and 2012 revised American College of Rheumatology (ACR) criteria for SLE[17] were recruited sequentially in this study. The mean age of patients with lupus was 11.5 years (range: 8–18 years), and median disease duration was 30 months.
2.1. Exclusion criteria
Patients with associated liver diseases, malabsorption, and patients received vitamin D, or calcium supplementations during the past three months and those who met criteria for other autoimmune diseases. All patients who were on dialysis were also excluded.
One hundred unrelated healthy children, of comparable age and sex, who attended our Pediatric Department for preoperative evaluation for elective surgery, were enrolled as control group. Patients and controls belonged to the same ethnic group: African whites. All patients and controls included were subjected to proper history-taking, and thorough clinical examination.
Clinical manifestations of SLE included the presence of malar rash, photosensitivity, discoid rash, mucosal ulcers, arthritis, nephritis, serositis, and neurological disease (defined as seizures, lupus headache, or psychosis). The laboratory evaluation included the presence of hematological disorders (thrombocytopenia, leukopenia, lymphopenia, or hemolytic anemia), positive antinuclear antibody (ANA), or other autoantibodies such as antibody to double-stranded DNA antigen (anti-dsDNA).
Disease activity was assessed using the SLE Disease Activity Index (SLEDAI)[18] at the time of the study visit (with scores <3 representing low, 3–10 moderate, and >10 high disease activity). Renal disease was defined by histopathological pattern on renal biopsy according to the WHO classification system[19] Patient demographics and medication use were also collected.
2.2. Blood sampling
For all subjects, blood samples were collected at the time of medical consultation, between 9 am and 11 am, after an overnight fasting and divided into 2 portions: 2 mL of whole blood was collected into tubes containing ethylenediaminetetraacetic acid, for genomic DNA extraction. Sera was separated immediately from remaining part of the samples and stored at −20°C till the time of analysis.
2.3. Estimation of serum 25(OH)D levels
Serum 25(OH) D levels were measured using a commercial ELISA kit according to the manufacturer's instructions (Immunodiagnostic Systems Inc., Fountain Hills, AZ). Vitamin D deficiency was considered as levels of 25(OH) D <30 ng/mL and severe deficiency for levels <10 ng/mL.[20]
2.4. Genomic DNA extraction
Genomic DNA was extracted from peripheral white blood cells using a genomic DNA extraction kit (Puregene Blood Kit, Gentra, Valencia, CA) according to the manufacturer's protocol. DNA quantification was done using an Eppendorf Bio Photometer (Hauppauge, NY). DNA was stored at −20°C before genotyping.
2.5. Genotyping of VDR gene polymorphisms
All subjects were genotyped for the BsmI polymorphism in intron 8 of the VDR gene by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) methodology. An 825-base-pair region was amplified by using the sense primer 5′-CAACCAAGA CTACAAGTACCGCGTCAGTGA-3′ and the antisense primer 5′AACCAGCGGG AAGAGGTCAAGGG-3′ as previously described.[21]
2.6. Statistical analysis
The VDR BsmI genotype and allele frequencies in SLE patients and controls were tested for Hardy–Weinberg equilibrium. The χ2 test was used to determine differences in the frequencies of the different VDR BsmI genotypes between SLE patients and healthy controls. The odds ratio (OR) and 95% confidence interval (95% CI) were calculated for disease susceptibility in relation to the studied VDR gene variants. Logistic regression analyses were performed to evaluate possible associations between VDR BsmI gene variants and clinical manifestations of SLE, disease activity score, and serum 25(OH)D level. The Student t test and analysis of variance were used to compare numeric variables within groups, depending on the distribution of the data. P value <0.05 was considered to be statistically significant. All data were analyzed using Statistical Package for Social Sciences version 22.0 (IBM Corp., Armonk, NY) and the Epi Info statistical software (version 6.2, World Health Organization, Geneva, Switzerland).
3. Results
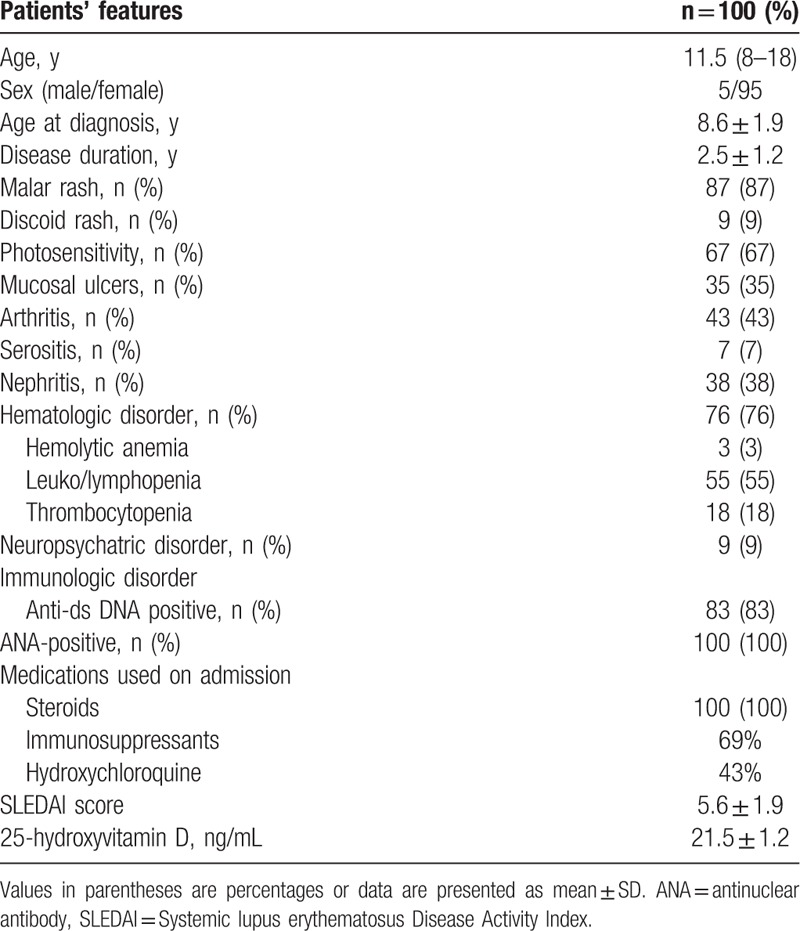
Our study included 100 patients with SLE, their age ranged from 8 to 18 years (median 11.5 years), and their mean age at diagnosis was 8.6 ± 1.9 years with median disease duration 30 months. Of these patients, 95 were females (95%) and 5 were males (5%). The control group were age-, sex-, and ethnicity-matched to patients with SLE (P > 0.05). The clinical manifestations and laboratory findings of SLE patients according to revised ACR criteria for classification of SLE[17] are shown in Table 1. The main clinical manifestations they had were malar rash (87%), photosensitivity (67%), mucosal ulcers (35%), arthritis (43%), nephritis (38%), hematologic abnormalities (76%), neurologic involvements (9%), serositis (7%), and immunologic disorders (83%). On admission, all patients were receiving steroids, 69% were on immunosuppressant, and 43% were on hydroxychloroquine. The mean SLEDAI score of patients was 5.6 ± 1.9 (Table 1).
Table 1.
Baseline demographic, clinical, and laboratory features of patients with systemic lupus erythematosus.
Distribution of VDR BsmI genotypes, alleles, and serum 25(OH)D levels in patients with SLE and controls are summarized in Table 2. Both groups were in Hardy–Weinberg equilibrium, with no significant χ2 values for the observed and expected genotype frequencies.
Table 2.
Distribution of the VDR BsmI genotypes, alleles, and serum 25-hydroxyvitamin D in patients with systemic lupus erythematosus and controls.
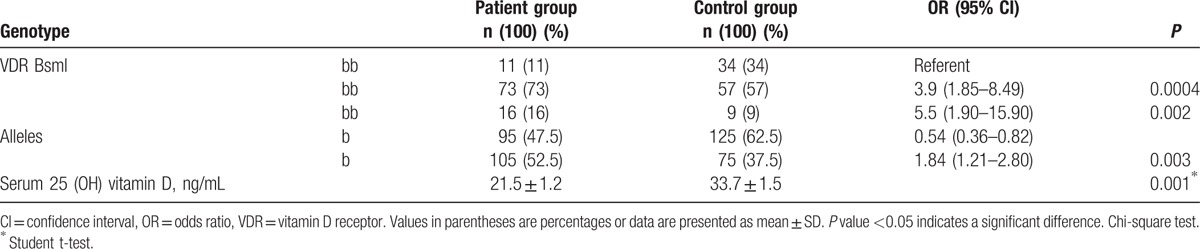
The VDR BsmI genotype distribution differed between patients with SLE and healthy controls. The BB and Bb genotypes were overrepresented among SLE patients, compared with healthy controls (16% vs. 9% for BB genotype; and 73% vs. 57% for Bb genotype, P < 0.01, respectively; Table 2). The risk of SLE was significantly higher among patients carrying the VDR BsmI BB or Bb genotypes than patients carrying the bb genotype (OR: 5.5; 95% CI: 1.9–15.9; P = 0.002 and OR: 3.9; 95% CI: 1.85–8.49; P = 0.0004; respectively); Table 2.
Of note, we found a significant increase in the frequency of the B allele among SLE patients (52.5%, OR: 1.84; 95% CI: 1.21–2.80; P = 0.003), where a concomitant significant decrease in the frequency of the b allele was observed compared to the control group (47.5%, OR: 0.54; 95% CI: 0.36–0.82); Table 2.
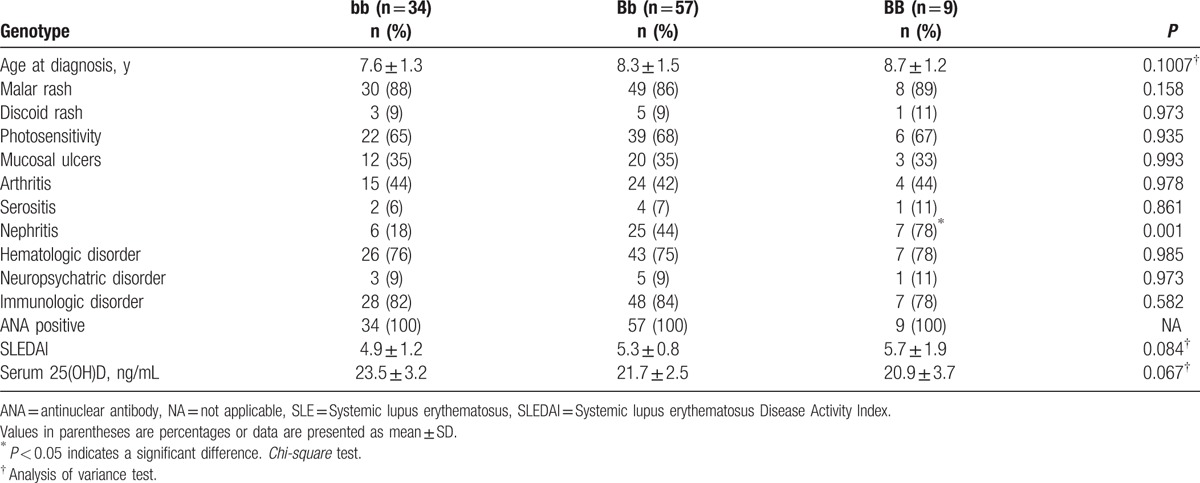
We found a significant association between VDR BsmI BB genotype with lupus nephritis (OR: 6.8; 95% CI: 1.18–50.5; P = 0.001; Table 3). However, we did not observe any significant association of studied polymorphisms with other clinical manifestations of SLE or disease activity score (all P > 0.05). No significant association was evident between VDR BsmI gene variants and the presence of ANA or anti-dsDNA (Table 3).
Table 3.
Association of the VDR BsmI genotypes with clinical, laboratory features, and serum 25-hydroxyvitamin D levels in patients with SLE.
Our data showed that 57 (57%) of studied SLE patients were vitamin D-deficient with 17% being severely deficient; meanwhile, 43 (43%) patients had normal vitamin D level. However, 21 (21%) of the control group were vitamin D-deficient and 79 (79%) had normal serum vitamin D level (P < 0.01; Fig. 1). The risk for vitamin D deficiency was 4.9-fold higher in SLE patients than in healthy controls (OR = 4.9; 95% CI: 2.56–9.78, P < 0.01; Fig. 1).
Figure 1.
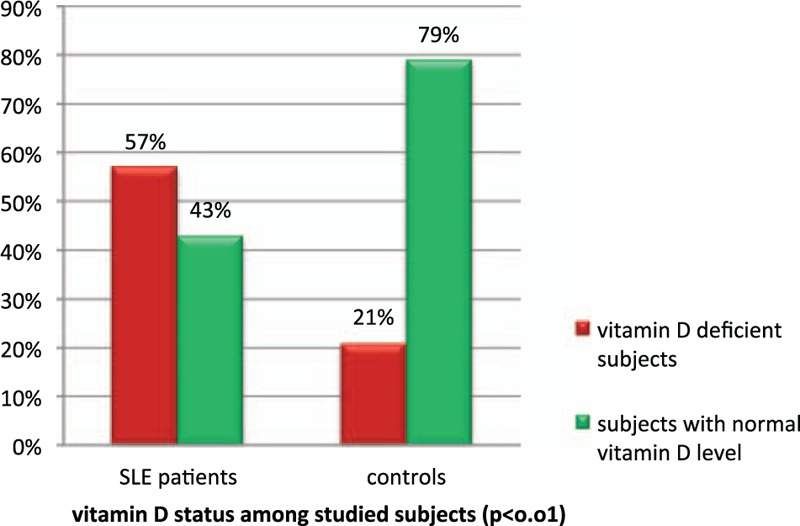
Vitamin D status among studied subjects.
As expected, we found that patients with SLE had significantly lower mean serum 25(OH)D levels than did healthy controls (21.5 ± 1.2 vs. 33.7 ± 1.5; P < 0.01 respectively; Table 2). However, we could not find any significant association between VDR BsmI genotypes or alleles and serum 25(OH)D levels among studied patients with SLE (all P > 0.05; Table 3).
4. Discussion
SLE is a multisystemic autoimmune disease linked to loss of immune tolerance to self-antigens and the production of a diversity of autoantibodies.[22] Childhood-onset SLE is known to confer a worse prognosis than adult disease in terms of disease activity, organ damage, and mortality.[23] Many of the observed immunological aberrations in SLE such as defective T-cell regulation of B-cell function, overproduction of autoantibodies, and reduced phagocytic clearance of immune complexes are opposite to the actions of vitamin D.[24] Experimental work confirmed that administration of vitamin D ameliorates inflammation in animal models of autoimmune disorders including lupus.[25] The discovery that VDR is expressed on cells of the immune system, such as monocytes, dendritic cells, macrophages, and activated T cells,[26] suggests that functional polymorphisms in the VDR gene may modulate the immune system and an individual's susceptibility to developing SLE. To the best of our knowledge, this is the first evaluation of the association between the VDR BsmI gene polymorphisms and the susceptibility to SLE in Egyptian children and adolescents.
In the present study, we found a significant difference in the genotype and allele frequencies of the VDR BsmI polymorphisms between patients with SLE and the control group. The BB genotype and B allele were overrepresented in patients with SLE compared to the control group. In addition, we observed that homozygous individuals with the BB genotypes had a 5.5-fold higher risk for developing SLE, thus revealing that patients were more susceptible to SLE. Moreover, we found that the BB genotype constituted a risk factor for the development of nephropathy among studied patients with SLE (OR: 6.8; P < 0.01). However, we did not find any significant association of the VDR BsmI gene variants with other clinical manifestations, laboratory profiles of SLE, or disease activity index score. Studies concerning the association of VDR BsmI gene polymorphism with SLE in children and adolescents are limited; conflicting results are often inferred from adult studies.
Our results confirm and extend the previous findings of Emerah et al[27] who studied the VDR gene SNPs on genomic DNAs of 107 Egyptian women with SLE, compared to 129 ethnicity-matched controls. The authors reported that carrying the BB genotype or at least carrying one B allele were associated with the risk of developing SLE in Egyptian women. They suggested a significant association of VDR polymorphisms with lupus nephritis. However, we did not find any association between VDR BsmI gene variants and SLE activity scores as they did which may be explained by the younger age group and shorter disease duration among our studied SLE patients.
A recent meta-analysis of 4 previous studies in Asian populations reported a positive association between BsmI VDR polymorphisms and susceptibility to SLE.[28] Huang et al[29] observed that Chinese patients with SLE exhibited significantly higher frequencies of the VDR BsmI B allele and BB genotype than did control subjects. However, they did not detect any associations of VDR genotype with the clinical, laboratory profiles, or lupus nephritis. In a Han Chinese population, Luo et al[30] confirmed that the frequencies of the VDR BsmI B allele, but not BB genotype, were significantly more common in their SLE patients group than in their control group. The authors added that the VDR B allele was associated with the development of nephritis and the downregulation of VDR mRNA expression, thus influencing the immunomodulatory function of VDR. Ozaki et al[31] reported BsmI polymorphisms in 58 Japanese SLE patients; their findings revealed that the BB genotype might trigger the development of lupus and the bb genotype was associated with lupus nephritis. Carvalho et al[32] suggested a possible role of VDR gene polymorphisms in Portuguese patients with SLE as a positive association was found between VDR gene variants and lupus severity (chronic damage).
By contrast, Abbasi et al[33] reported that the distribution of the VDR BsmI genotypes and alleles did not differ significantly between patients with SLE and healthy subjects living in northeastern Iran. This research added that no association was detected between the VDR BsmI genotype and organ involvement or laboratory profiles, SLE disease activity index, and SLE damage score. Another similar study by Sakulpipatsin et al[34] did not find any association between VDR gene BsmI polymorphisms and susceptibility to SLE in 101 Thai female patients with lupus compared to 194 healthy control subjects. Mostowska et al[35] demonstrated no association of the FokI, BsmI, ApaI, and TaqI polymorphisms with an individual's susceptibility to SLE in a Polish population. Monticielo et al[36] indicated that the BsmI and FokI VDR polymorphisms were not risk factors for SLE, and vitamin D deficiency seems not to have a direct effect on clinical or laboratory expressions of disease in a Brazilian-European cohort with SLE.
Discrepancies between previously published studies and ours might be explained by the differences in age group; study design or geographic/ethnicity, or by gene–gene or gene–environmental interactions. The genetic predisposition to SLE could be polygenic, with many variants in multiple gene loci, playing an important role.
The VDR gene polymorphisms could lead to significant receptor dysfunction, which may affect calcium metabolism, cell proliferation, and the immune response.[37] Thus, genetic variation on the VDR gene can modify the immunomodulatory action of vitamin D and may have an effect on the clinical manifestations of lupus.[38] However, it is possible that the VDR BsmI SNP may not be functional by itself, but may be in linkage disequilibrium with other functional mutations as several mutations occurring in this intronic region have been associated with various autoimmune diseases.[28] In other words, this polymorphism may either have a direct effect on transcription or may represent linkage disequilibrium with another yet-to-be-identified marker. Further measurements of serum 25-hydroxyvitamin D levels and genotyping of other VDR gene polymorphisms (such as ApaI, TaqI, and FokI) should be examined in children and adolescents with SLE to confirm our findings in different populations.
In an experimental murine lupus model, mice received 1, 25-dihydroxyvitamin D3 supplementation, did not develop skin lesions or alopecia, and had less proteinuria[39] In vitro studies demonstrated that 1, 25-dihydroxyvitamin D3 inhibits proliferation and induces apoptosis of activated B lymphocytes isolated from patients with SLE.[40] In addition, plasma cell differentiation and immunoglobulin secretion were lower in the presence of 1, 25-dihydroxyvitamin D3. Dendritic cells are antigen-presenting cells critical to lupus pathogenesis. 1, 25-dihydroxyvitamin D3 inhibits interferon-induced dendritic cell differentiation and maturation, maintaining them in an immature phase with lower secretion of IL-12, actions that could have specific relevance in SLE[41] 1, 25-dihydroxyvitamin D3 treated dendritic cells also have attenuated T-cell allostimulatory and chemotactic capacity.[42]
Low levels of vitamin D have been hypothesized to be a risk factor for the development of autoimmune disorders and persistence of disease activity.[43] Several epidemiological studies have reported a high prevalence of vitamin D insufficiency and deficiency among adults with SLE.[9,10]
In line with previous observations, this study demonstrates that vitamin D deficiency in our population was observed in 57% of all SLE patients with 17% being severely deficient despite the fact that our population resides in areas with plenty of sunny days. As expected, we found that patients with SLE had significantly lower mean serum 25(OH)D levels than did healthy controls. However, we could not find any significant association between VDR BsmI genotypes or alleles and serum 25(OH)D levels among studied patients with SLE. These results were concordant with those of Hamza et al[11] who confirmed that low 25(OH) vitamin D status was frequent among Egyptian lupus patients, as 73.30% of their cases had low 25(OH) vitamin D levels, with 60% being insufficient and 13.30% being deficient. In Saudi Arabia, a strikingly higher prevalence of low 25(OH) vitamin D status was detected in a study by Damanhouri,[44] wherein 89.7% of their SLE patients had deficient levels and 9.1% had insufficient levels. These findings are consistent with an earlier report by Wright et al[45] who studied vitamin D status in children and adolescents with SLE between 5 and 21 years of age compared to 207 healthy controls. Severe vitamin D deficiency was observed in a significantly higher proportion of subjects with SLE (36.8% vs. 9.2%, P < .001). They also found a significant inverse relationship between disease activity index and levels of 25(OH) D, particularly when it was <20 ng/mL. A pilot study performed in Rio Grande do Sul, involving 68 patients with SLE, reported levels of 25(OH) D <20 ng/mL in 20% of cases.[46] Borba et al reported that there is an increased prevalence of vitamin D deficiency in Brazilian patients with SLE, especially those with the active disease.[47]
By contrast, one study reported that vitamin D status was not associated with SLE or disease activity index scores with the majority of participants being white (98%) and their SLEDAI scores being <3 (81%).[48]
Children with SLE may be at high risk for vitamin D deficiency as a result of photosensitivity, with consequent sun avoidance and sunscreen use because sun exposure may exacerbate SLE. This practice further limits cutaneous vitamin D synthesis. In addition, chronic use of medications prescribed for SLE management may interfere with vitamin D metabolism. Physical inactivity secondary to fatigue or pain may also diminish sun exposure. Vitamin D autoantibodies were observed in SLE, but likely do not contribute to deficiency.[49] Whether these low vitamin D levels play a role in the pathogenesis of SLE or a consequence of disease activity must be investigated further. It remains unclear whether vitamin D deficiency confers an increased likelihood to progress to a full autoimmune disorder in a genetically susceptible individual or whether vitamin D deficiency is permissive for increased immunologic activation and disease activity or both. Nontoxic immune modulatory modalities like vitamin D supplementation would be a welcome addition to the current agents used to manage autoimmune disorders including lupus.
However, the small sample size was one of our limitations in this study; we suggest that multicenter approaches may be necessary to attain larger sample size. A lack of detailed dietary intake and sun exposure data among studied patients was another limitation in our study.
5. Conclusion
We demonstrate for the first time, to the best of our knowledge, that the VDR BsmI gene polymorphisms may contribute to susceptibility to SLE in Egyptian children and adolescents. Moreover, we found that the BB genotype constituted a risk factor for the development of nephropathy among studied patients with SLE. However, we did not find any significant association of the VDR BsmI gene variants with other clinical manifestations, laboratory profiles of SLE, disease activity index score, or serum 25(OH)D levels.
Finally, further studies and more genetic information on ethnicities from different parts of the world might reveal additional insights into the association of BsmI gene polymorphism in VDR with SLE, and elucidate the mechanism underlying the role of this locus in susceptibility to SLE.
Acknowledgments
The authors thank the staff of Pediatric Department and Outpatient Clinics in Zagazig University Children's Hospital for their collaboration in sampling as well as our patients who participated in the study.
Footnotes
Abbreviations: CI = confidence interval, EEG = Electroencephalography, ELISA = enzyme-linked immunosorbent assay, FS = febrile seizure, IL-1 = interleukin-1, IL-1Ra = interleukin-1 receptor antagonist, IL-6 = interleukin-6, OR = odds ratio, PCR = polymerase chain reaction, RFLP = restriction fragment length polymorphism, SNPs = single nucleotide polymorphisms, TNFa = tumor necrosis factor- a.
Authors’ contributions: SFA designed the study, and submitted the manuscript; MANA, AAE, and EAB conceived of the study and coordinated the sample collection and data analysis; MAAF and MEH wrote the discussion; YFA and NIA critically revised the manuscript and approve final version; HHG, RMN, LMK, NMA, and GMA performed laboratory analysis and genotyping; DSF and MIAH reviewed the results, and helped to draft the manuscript; SFA and HMA participated in the design of the study and performed the statistical analysis; MAN and ARA performed final revisions in response to reviewers’ comments; All authors read and approved all the manuscript.
No funds were available for the current research
The authors declare that they have no conflicts of interest.
References
- 1.De Azevêdo Silva J, Addobbati C, Sandrin-Garcia P, et al. Systemic lupus erythematosus: old and new susceptibility genes versus clinical manifestations. Curr Genom 2014; 15:52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chai HC, Phipps ME, Chua KH. Genetic risk factors of systemic lupus erythematosus in the Malaysian population: a minireview. Clin Dev Immunol 2012; 2012:963730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jönsen A, Bengtsson AA, Nived O, et al. Gene-environment interactions in the aetiology of systemic lupus erythematosus. Autoimmunity 2007; 40:613–617. [DOI] [PubMed] [Google Scholar]
- 4.Maruotti N, Cantatore FP. Vitamin D and the immune system. J Rheumatol 2010; 37:491–495. [DOI] [PubMed] [Google Scholar]
- 5.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Annals of the Rheumatic Diseases 2007; 66:1137–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoenfeld N, Amital H, Shoenfeld Y. The effect of melanism and vitamin D synthesis on the incidence of autoimmune disease. Nat Clin Pract Rheumatol 2009; 5:99–105. [DOI] [PubMed] [Google Scholar]
- 7.Pike JW, Meyer MB. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3). Endocrinol Metab Clin North Am 2010; 39:255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am 2010; 39:365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szodoray P, Tarr T, Bazso A, et al. The immunopathological role of vitamin D in patients with SLE: data from a single centre registry in Hungary. Scand J Rheumatol 2011; 40:122–126. [DOI] [PubMed] [Google Scholar]
- 10.Wu PW, Rhew EY, Dyer AR, et al. 25-hydroxyvitamin D and cardiovascular risk factors in women with systemic lupus erythematosus. Arthritis Rheum 2009; 61:1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamza RT, Awwad KS, Ali MK, et al. Reduced serum concentrations of 25-hydroxy vitamin D in Egyptian patients with systemic lupus erythematosus: relation to disease activity. Med Sci Monit 2011; 17:CR711–CR718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeap SS, Othman AZ, Zain AA, et al. Vitamin D levels: its relationship to bone mineral density response and disease activity in premenopausal Malaysian systemic lupus erythematosus patients on corticosteroids. Int J Rheum Dis 2012; 15:17–24. [DOI] [PubMed] [Google Scholar]
- 13.Amital H, Szekanecz Z, Szücs G, et al. Serum concentrations of 25-OH vitamin D in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: is it time to routinely supplement patients with SLE with vitamin D? Ann Rheum Dis 2010; 69:1155–1157. [DOI] [PubMed] [Google Scholar]
- 14.Mok CC, Birmingham DJ, Leung HW, et al. D levels in Chinese patients with systemic lupus erythematosus: relationship with disease activity, vascular risk factors and atherosclerosis. Rheumatology (Oxford) 2012; 51:644–652. [DOI] [PubMed] [Google Scholar]
- 15.McCullough ML, Bostick RM, Mayo TL. Vitamin D gene pathway polymorphisms and risk of colorectal, breast, and prostate cancer. Annu Rev Nutr 2009; 29:111–132. [DOI] [PubMed] [Google Scholar]
- 16.Uitterlinden AG, Fang Y, Van Meurs JB, et al. Genetics and biology of vitamin D receptor polymorphisms. Gene 2004; 338:143–156. [DOI] [PubMed] [Google Scholar]
- 17.Petri M, Orbai AM, Alarco’n GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012; 64:2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI: a disease activity index for lupus patients: the Committee on Prognosis Studies in SLE. Arthritis Rheum 1992; 35:630–640. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz MM. The pathology of lupus nephritis. Semin Nephrol 2007; 27:22–34. [DOI] [PubMed] [Google Scholar]
- 20.Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006; 84:18–28. [DOI] [PubMed] [Google Scholar]
- 21.Luo XY, Wu LJ, Chen L, et al. The association of vitamin D receptor gene ApaI and BsmI polymorphism with systemic lupus erythematosus. Zhonghua Nei Ke Za Zhi 2012; 51:131–135. [PubMed] [Google Scholar]
- 22.Choi J, Kim ST, Craft J. The pathogenesis of systemic lupus erythematosus – an update. Curr Opin Immunol 2012; 24:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamphuis S, Silverman ED. Prevalence and burden of pediatric onset systemic lupus erythematosus. Nat Rev Rheum 2010; 6:538–546. [DOI] [PubMed] [Google Scholar]
- 24.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med 2008; 358:929–939. [DOI] [PubMed] [Google Scholar]
- 25.Koizumi T, Nakao Y, Matsui T, et al. Effects of corticosteroid and 1,24R-dihydroxy-vitamin D3 administration on lymphoproliferation and autoimmune disease in MRL/MP-lpr/lpr mice. Int Arch Allergy Appl Immunol 1985; 77:396–404. [DOI] [PubMed] [Google Scholar]
- 26.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3)receptor in the immune system. Arch Biochem Biophys 2000; 374:334–338. [DOI] [PubMed] [Google Scholar]
- 27.Emerah AA, El-Shal AS. Role of vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D level in Egyptian female patients with systemic lupus erythematosus. Mol Biol Rep 2013; 40:6151–6162. [DOI] [PubMed] [Google Scholar]
- 28.Lee YH, Bae SC, Choi SJ, et al. Song GG Associations between vitamin D receptor polymorphisms and susceptibility to rheumatoid arthritis and systemic lupus erythematosus: a meta-analysis. Mol Biol Rep 2011; 38:3643–3651. [DOI] [PubMed] [Google Scholar]
- 29.Huang CM, Wu MC, Wu JY, et al. Association of vitamin D receptor gene BsmI polymorphisms in Chinese patients with systemic lupus erythematosus. Lupus 2002; 11:31–34. [DOI] [PubMed] [Google Scholar]
- 30.Luo XY, Yang MH, Wu FX, et al. Vitamin D receptor gene BsmI polymorphism B allele, but not BB genotype, is associated with systemic lupus erythematosus in a Han Chinese population. Lupus 2012; 21:53–59. [DOI] [PubMed] [Google Scholar]
- 31.Ozaki Y, Nomura S, Nagahama M, et al. Vitamin-D receptor genotype and renal disorder in Japanese patients with systemic lupus erythematosus. Nephron 2000; 85:86–91. [DOI] [PubMed] [Google Scholar]
- 32.Carvalho C, Marinho A, Leal B, et al. Association between vitamin D receptor (VDR)gene polymorphisms and systemic lupus erythematosus in Portuguese patients. Lupus 2015; 24:846–853. [DOI] [PubMed] [Google Scholar]
- 33.Abbasi M, Rezaieyazdi Z, Afshari JT, et al. Lack of association of vitamin D receptor gene BsmI polymorphisms in patients with systemic lupus erythematosus. Rheumatol Int 2010; 30:1537–1539. [DOI] [PubMed] [Google Scholar]
- 34.Sakulpipatsin W, Verasertniyom O, Nantiru KJ, et al. Janwityanujit S Vitamin D receptor gene Bsm1 polymorphisms in thai patients with systemic lupus erythematosus. Arthritis Res Ther 2006; 8:R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mostowska A, Lianeri M, Wudarski M, et al. Jagodzin'ski PP Vitamin D receptor gene BsmI, FokI, ApaI and TaqI polymorphisms and the risk of systemic lupus erythematosus. Mol Biol Rep 2013; 40:803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monticielo OA, Brenol JC, Chies JA, et al. The role of BsmI and FokI vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D in Brazilian patients with systemic lupus erythematosus. Lupus 2012; 21:43–52. [DOI] [PubMed] [Google Scholar]
- 37.Valdivielso JM, Fernandez E. Vitamin D receptor polymorphisms and diseases. Clin Chim Acta 2006; 371:1–12. [DOI] [PubMed] [Google Scholar]
- 38.Ritterhouse LL, Crowe SR, Niewold TB, et al. Vitamin D deficiency is associated with an increased autoimmune response in healthy individuals and in patients with systemic lupus erythematosus. Ann Rheum Dis 2011; 70:1569–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemire JM, Ince A, Takashima M. 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity 1992; 12:143–148. [DOI] [PubMed] [Google Scholar]
- 40.Chen S, Sims GP, Chen XX, et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 2007; 179:1634–1647. [DOI] [PubMed] [Google Scholar]
- 41.Griffin MD, Lutz W, Phan VA, et al. Dendritic cell modulation by 1alpha, 25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A 2001; 98:6800–6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gauzzi MC, Purificato C, Donato K, et al. Suppressive effect of 1alpha,25-dihydroxyvitamin D3 on type I IFNmediated monocyte differentiation into dendritic cells: impairment of functional activities and chemotaxis. J Immunol 2005; 174:270–276. [DOI] [PubMed] [Google Scholar]
- 43.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol 2008; 4:404–412. [DOI] [PubMed] [Google Scholar]
- 44.Damanhouri L. Vitamin D deficiency in Saudi patients with systemic lupus erythematosus. Saudi Med J 2009; 30:1291–1295. [PubMed] [Google Scholar]
- 45.Wright TB, Shults J, Leonard MB, et al. Hypovitaminosis D is associated with greater body mass index and disease activity in pediatric systemic lupus erythematosus. J Pediatr 2009; 155:260–265. [DOI] [PubMed] [Google Scholar]
- 46.Lora P, Scalco R, Furlanetto TW, Xavier RM. (2008) Vitamin D status in patients with systemic lupus erythematosus from southern Brazil, vol 46(Supplement: IFCC WorldLab Fortaleza 2008. Clinical Chemistry and Laboratory Medicine, Walter de Gruyter, New York, p 7. [Google Scholar]
- 47.Borba V, Vieira J, Kasamatsu T. Vitamin D deficiency in patients with active systemic lupus erythematosus. Osteoporos Int 2008; 20:427–433. [DOI] [PubMed] [Google Scholar]
- 48.Ruiz-Irastorza G, Egurbide MV, Olivares N, et al. Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatology (Oxford) 2008; 47:920–923. [DOI] [PubMed] [Google Scholar]
- 49.Carvalho JF, Blank M, Kiss E, et al. Anti-vitamin D, vitamin D in SLE: preliminary results. Ann N Y Acad Sci 2007; 1109:550–557. [DOI] [PubMed] [Google Scholar]


