Abstract
Background:
At present, there are a lot of research about the effect of Alprostadil on preventing contrast-induced nephropathy for percutaneous coronary intervention (PCI) in diabetic patients, but the clinical efficacy is not consistent, so we conduct this study and therefore determine the dominant strategy for the treatment of PCI in diabetic patients based on the best evidence currently.
Methods:
An electronic database search was conducted in MEDLINE, Embase, Cochrane library, CBM, CNKI, VIP, and WanFang to retrieve randomized controlled trial (RCT) comparing Alprostadil versus hydration on preventing CIN for PCI in diabetic patients. Reference lists of relevant articles were also screened manually to retrieve additional ones. Two investigators independently assessed the eligibility of retrieved articles using predefined inclusion and exclusion criteria. All characteristics as well as outcome variables including incidence of CIN, blood urea nitrogen (BUN), cystatin C (CysC), glomerular filtration rate (GFR), serum creatinine (Scr), serum beta 2-microspheres (β2-MG) presented in each included study were extracted. Heterogeneity was thought to be significant when I2 > 50%. All of the meta-analytic procedures were performed by using Review Manager software, version 5.3.
Results:
Finally, data from 8 articles including 969 patients were included into this meta-analysis, among them, 487 patients in the experience group, and 482 patients in the control group. Meta analysis showed that the incidence of CIN in the experimental group was significantly lower than that in the control group (OR = 0.28,95%CI[0.18,0.42]). The incidence of adverse reactions in the experimental group was significantly lower than that in the control group (OR = 0.46,95%CI[0.24,0.85]). The BUN of 24 hours, 48 hours, and 72 hours in the experimental group were significantly lower than that of control group (MD = –0.77, 95%CI [−1.22, –0.32]; MD = –1.38, 95%CI [−1.83,–0.92]; MD = –2.43, 95%CI [−2.68,–2.19], respectively). The CysC of 24 hours, 48 hours, and 72 hours in the experimental group were significantly lower than that of control group (MD = –0.30, 95%CI [−0.40, –0.21]; MD = –0.54, 95%CI [−0.68,–0.41]; MD = –0.49, 95%CI [−0.63, –0.35], respectively). The GFR of 24 hours, 48 hours, and 72 hours in the experimental group were significantly higher than that of control group (MD = 7.86, 95%CI [4.44, 11.29], MD = 18.23, 95%CI [13.76,22.69], MD = 12.81, 95%CI [8.51,17.11], respectively). The Scr of 24 hours, 48 hours, and 72 hours in the experimental group were significantly lower than that of control group (MD = –9.09, 95%CI [−12.67, –5.51], MD = –19.14, 95%CI [−23.61, –14.66], MD = –6.50, 95%CI [−8.29, –4.71], respectively). The β2-MG of 24 hours, 48 hours, and 72 hours in the experimental group were significantly lower than that of control group (MD = –0.12, 95%CI [−0.27, 0.03], MD = –0.55, 95%CI [−0.71, –0.39], MD = –0.50, 95%CI [−0.60, –0.39], respectively).
Conclusion:
Our result suggested that comparing with conventional Hydration, Alprostadil can significantly reduce the incidence of CIN, adverse reaction, and protect renal function in PCI in diabetic patients. Due to the limitations of the quality and quantity of the articles, this conclusion still needs further research to confirm.
Keywords: Alprostadil, contrast-induced nephropathy, meta-analysis, percutaneous coronary intervention, diabetic
1. Introduction
Percutaneous coronary intervention (PCI)[1–4] is one of the important means in the treatment of coronary atherosclerotic heart disease. With the progress of technology, the accumulation of experience, and the development of interventional therapy apparatus, the PCI treatment is becoming more and more complex.[5–8] Therefore, the dose of contrast agent in PCI is increasing, and renal dysfunction led to by contrast agent is increasing year by year. Contrast-induced nephropathy (CIN)[9,10] is an acute renal function injury caused by the iodine contrast agent. CIN not only prolong hospitalization time and increased health care costs, but also increase in-hospital mortality, and have a relationship with long-term adverse events (such as death, stroke, dialysis, and adverse cardiovascular events). This study shows that the incidence of adverse events in patients with CIN was significantly higher than that in patients without CIN. One year follow-up study of 294 patients by Solomon[11] showed that the incidence of adverse events in patients with CIN was 2 times than that of the control group.
CIN or acute renal injury induced by the contrast agent is an important complication after application of iodinated contrast. CIN is generally defined as the renal function was damaged within 3 days after the injection of contrast agent, and the level of Scr was increased 0.5 mg/dL (44.2 μmol/L) or 25% higher than the basal level.[12,13] The study have found that the incidence of CIN was 13.1% in PCI in diabetic patients, and the success rate of PCI was significantly decreased (72.8% vs 94.0%), the risk of Q-wave myocardial infarction was significantly increased (3.95% vs 0.9%), and mortality increased significantly during hospitalization (22.0% vs 1.45%). Also, long-term adverse events and mortality were also significantly increased, which has become one of the important issues of social concern.
Diabetes can do harm to the systemic vascular system so that the probability of coronary heart disease and renal dysfunction in diabetic patients is markedly increased. Clinical observation[14,15] have found that the risk of coronary heart disease in patients with diabetes will be enhanced by 2 to 4 times. At present, diabetes has been clearly pointed out as a risk factors for coronary heart disease, and 20% to 50% diabetic patients have a renal insufficiency. Therefore, patients with diabetes are in an increased risk of CIN after exposure to contrast agents.
At present, there are ununiform methods or guidelines for prevention and treatment of CIN. Current treatment methods including hydration, selection of contrast agents, drugs (such as statins, misoprostol, etc.), but all are not the most effective way and adequate measures. Especially for patients with coronary heart disease and diabetes, there is no effectual and unified approach. Hydration, contrast agent selection, drug application, blood sugar control, and many other ways are currently methods for prevent CIN. So far, there are a variety of programs for the treatment of hydration, but there is still no clear study of the type of liquid, time, frequency, and the dosage that should be used. The incidence of CIN is about 3.3% to 8%, and actively seeking ways to prevent and treat CIN has become the urgent task of the medical community.
In recent years, with the clinical study of drugs for prevention of CIN development, alprostadil attracts more and more attention.[16] Alprostadil has extensive physiological and pharmacological effects, including vasodilation, inhibits platelet aggregation, promote erythrocyte deformation, thus improving microcirculation. Research[17,18] has also shown that alprostadil can stabilize the cell membrane, lysosomes and reduce the release of harmful cytokines, increasing renal blood flow, reducing the damage of the contrast agent to the blood vessel. Therefore, alprostadil plays an important role in the prevention of coronary heart disease in patients with diabetes mellitus in the process of PCI. But there is no system article that evaluates the effect of Alprostadil on preventing contrast-induced nephropathy for percutaneous coronary intervention in diabetic patients. Our study uses meta-analysis to evaluate Alprostadil on preventing CIN for PCI in diabetic patients, to provide a reference for clinical.
2. Methods
2.1. Literature search and screening
A systematic article search using PubMed was conducted by 2 independent psychiatrists (Z-LY and H-LL). If there were an inconsistent selection and lack of agreement, another senior psychiatrist (W-QG) made the final judgment and decision. The search was performed using the keywords “(Alprostadil) and (coronary Heart Disease) and (diabetes) and (Contrast-induced nephropathy)” for all articles available till August 18, 2016. Only articles written in English and Chinese were chosen. Initially, all articles meeting these inclusion criteria were collected, and the titles and abstracts were screened by Z-LY,H-LL, and W-RD to determine whether they were potentially eligible for inclusion in this meta-analysis. When there was disagreement on eligibility, we reached agreement through consensus. All reports that were not related to the topics of augmentation of Alprostadil for patients with coronary heart disease and diabetes were excluded. We then screened all the selected articles using the following inclusion criteria: (1) articles discussing comparisons of the treatment effect in coronary heart disease and diabetes patients treated with Alprostadil augmented with hydration with/without placebo; (2) and articles on clinical trials in humans. (3) All patients were in line with the diagnostic criteria for diabetes. (4) PCI was performed in all patients. (5) General information (age, gender, disease type, and so on) of the experimental group and the control group was not statistically significant. The exclusion criteria were (1) case reports or series; (2) nonclinical trials; (3) animal experiment. (4) Unable to extract data from the literature. In addition, investigating the quality of clinical trials included in the current meta-analysis. We used the Cochrane risk of bias assessment tools to evaluate the quality of clinical trials. All analyses were based on previous published studies; thus, no ethical approval and patient consent are required.
2.2. Data extraction and quality assessment
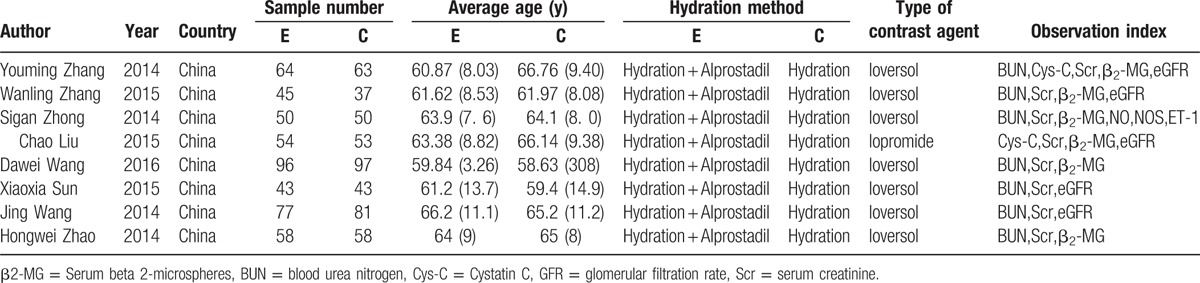
The following information was extracted from each eligible study (when available): the names of the first author; year of publication; countries where the study was conducted; participants; sample size; number of cases; and sample size; incidence of CIN; incidence of adverse reactions; BUN, Cys C, GFR, Scr, β2-MG. The adjusted RRs were extracted in case studies provided both crude and adjusted RRs. If a study reported >1 multivariable-adjusted effect estimate, we chose the result fully adjusted for potential confounding variables.
2.3. Statistical analysis
All of the meta-analytic procedures were conducted by using Review Manager software, version 5.3. Two-tailed P values < 0.05 were regarded as statistically significant. We used Q statistics, their related P values, and the I-square statistic to investigate the heterogeneity of each study. I-square statistic is a quantitative measure describing the percentage of total variation due to heterogeneity. The extracted I-square statistic value was utilized to assess the heterogeneity of each variable across studies.[19] According to the Cochrane Handbook, between-study heterogeneity of variables is thought to be substantial when the I-square range from 50% to 90%.Therefore, an I-square of < 50% is considered acceptable in this systematic review. If the research results were not statistically different, the fixed effect model was used for meta-analysis. If there is statistical heterogeneity among the research results, the heterogeneity of the sources of heterogeneity is further analyzed. After excluding the obvious clinical heterogeneity, the random effects model was utilized to analyze the Meta. In addition, we investigated publication bias through funnel plots.[20,21] The meta-analytic procedure fulfills the criteria of Preferred Reporting Items for systematic reviews and Meta-Analyses (PRISMA) compliant.
3. Results
3.1. Study selection and characteristics
Initially, 502 articles were found by the search of electronic databases and references of relevant articles. By screening titles and abstracts, 258 apparently irrelevant articles were first excluded. Then, the full texts of remainders were downloaded to assess in detail. Eventually, data from 8 articles including 969 patients were included into this meta-analysis, among them, 487 patients were in the experience group, and 482 patients in the control group. The flow diagram of study selection is shown in Fig. 1. The basic information of each included literature can be seen in Table 1.
Figure 1.
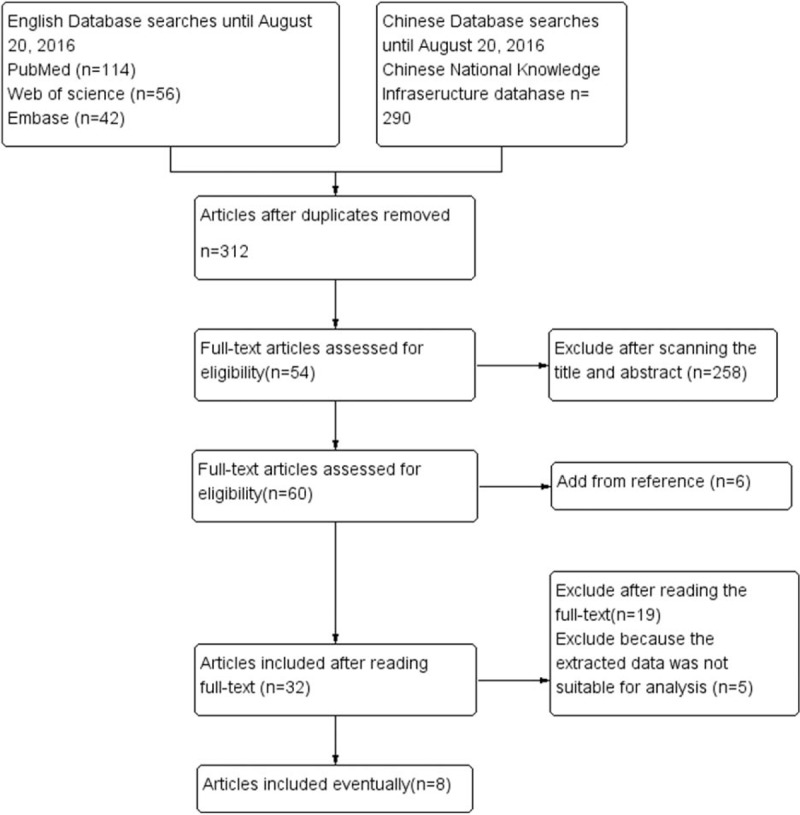
Flow chart for study selection.
Table 1.
Characteristics of studies included in meta-analysis.
3.2. Literature quality evaluation
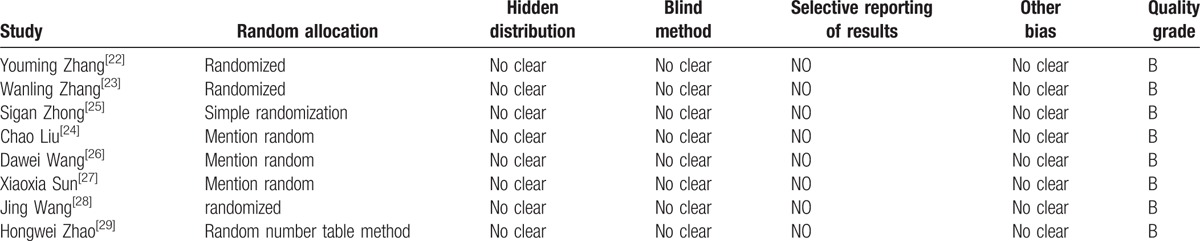
Of the 8 articles,[22–29] 1 article[25] using a simple random method, 1 article[29] using a random number table method, and the other 6 articles[22–24,26–28] only refer to the random method, but not the specific description. The distribution of the 8 articles is not clear; the implementation method of the 8 articles is not clear; the selective reporting bias and other bias of the 8 articles were not clear. Literature quality score was shown in Table 2.
Table 2.
Assessment of methodological quality of included studies.
3.3. Incidence of contrast-induced nephropathy
Seven studies evaluating the effect of alprostadil on CIN in patients with diabetes mellitus complicated with coronary heart disease after PCI. Meta-analysis showed that I2 = 0%, P = 0.49, the heterogeneity was small, so using a fixed effect model. Meta-analysis (fixed effect model) showed that Alprostadil can significantly reduce the incidence of CIN in coronary heart disease patients complicated with diabetes after PCI [OR = 0.28, 95%CI (0.18, 0.42), P < 0.00001], as shown in Fig. 2.
Figure 2.
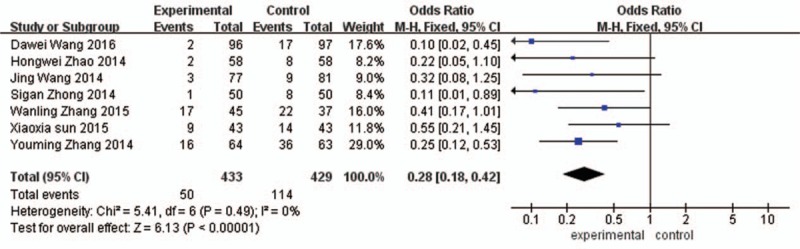
Alprostadil prevent the incidence of contrast-induced nephropathy of coronary heart disease and diabetes in patients after coronary angiography.
3.4. Incidence of adverse reactions
Two studies evaluating the incidence of adverse reactions of alprostadil on CIN in patients with diabetes mellitus complicated with coronary heart disease after PCI. Meta-analysis showed that I2 = 6%, P = 0.30, the heterogeneity was small, so using a fixed effect model. Meta-analysis (fixed effect model) results showed that Alprostadil can significantly reduce the incidence of adverse reactions of coronary heart disease patients complicated with diabetes after PCI (OR = 0.46, 95%CI [0.24, 0.85], P < 0.00001]. As shown in Fig. 3.
Figure 3.
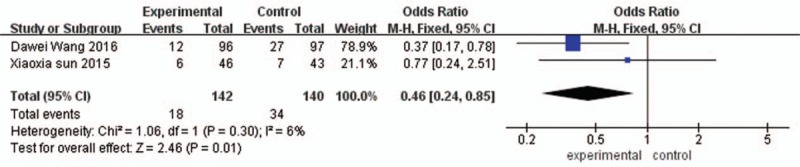
The incidence of adverse reactions in the experimental group and the control group.
3.5. Blood urea nitrogen
BUN of 24 hours after operation: Comparing with the control group, BUN of 24 h in the experimental group was significantly decreased, the difference was statistically significant (MD = –0.77, 95%CI [−1.22, –0.32], P = 0.0007). BUN of 48 hours after operation: Comparing with the control group, BUN of 48 hours in the experimental group was significantly decreased, the difference was statistically significant (MD = –1.38, 95%CI [−1.83, –0.92], P < 0.00001). BUN of 72 hours after operation: Comparing with the control group, BUN of 72 hours in the experimental group was significantly decreased, the difference was statistically significant (MD = –2.43, 95%CI [−2.68, –2.19], P < 0.00001], as shown in Fig. 4.
Figure 4.
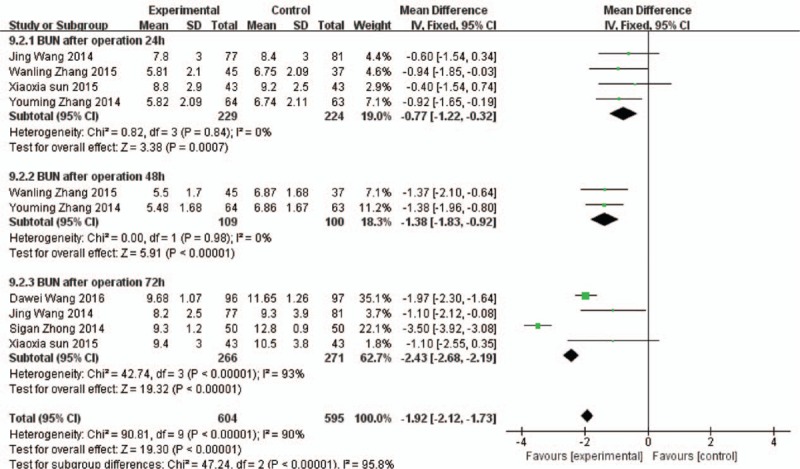
Comparison of 24 h, 48 h, and 72 h blood urea nitrogen between the experimental group and the control group.
3.6. Cystatin C
Cys C of 24 hours after operation: Comparing with the control group, Cys C of 24 hours in the experimental group was significantly decreased, the difference was statistically significant (MD = –0.30, 95%CI [−0.40, –0.21], P < 0.00001). Cys C of 48 hours after operation: Comparing with the control group, Cyst C of 48 hours in the experimental group was significantly decreased, the difference was statistically significant (MD = –0.54, 95%CI [−0.68, –0.41], P < 0.00001).
Cys C of 72 hours after operation: Comparing with the control group, Cys C of 72 hours in the experimental group was significantly decreased, the difference was statistically significant (MD = –0.49, 95%CI [−0.63, –0.35], P < 0.00001), as shown in Fig. 5.
Figure 5.
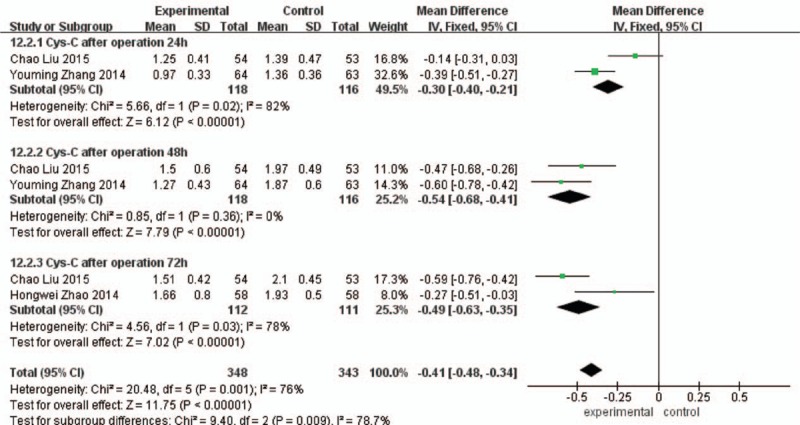
Comparison of 24 h, 48 h, and 72 h Cystatin C between the experimental group and the control group.
3.7. Glomerular filtration rate
GFR rate of 24 hours after the operation: Comparing with the control group, the GFR of 24 hours in the experimental group was significantly increased, the difference was statistically significant (MD = 7.86, 95%CI [4.44, 11.29], P < 0.00001).GFR of 48 hours after the operation: Comparing with the control group, the GFR of 48 hours in the experimental group was significantly increased, the difference was statistically significant (MD = 18.23, 95%CI [13.76, 22.69], P < 0.00001). GFG of 72 hours after the operation: Comparing with the control group, the GFRof 48 hours in the experimental group was significantly increased, the difference was statistically significant (MD = 12.81, 95%CI [8.51, 17.11], P < 0.00001) as shown in Fig. 6.
Figure 6.
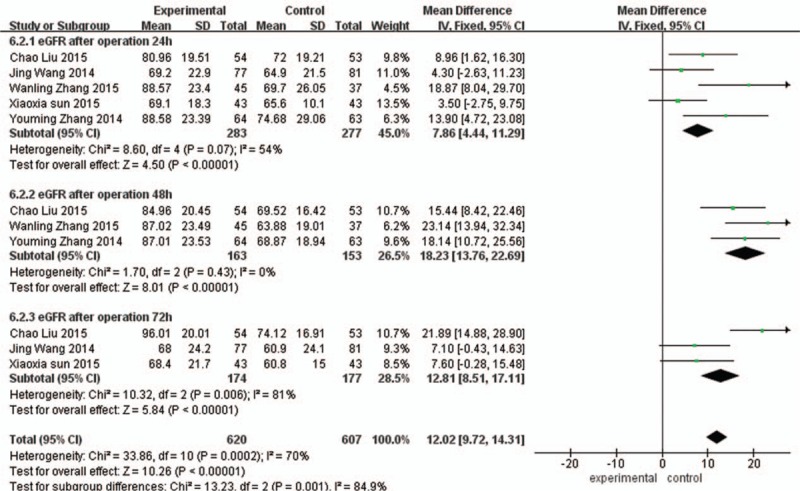
Comparison of 24 h, 48 h, and 72 h glomerular filtration rate between the experimental group and the control group.
3.8. Serum creatinine
Scr of 24 hours after operation: Comparing with the control group, Scr of 24 hours in the experimental group was significantly decreased, the difference was statistically significant (MD = –9.09, 95%CI [−12.67, –5.51], P < 0.00001). Scr of 48 hours after operation: Comparing with the control group, Scr of 48 hours in the experimental group was significantly decreased, the difference was statistically significant (MD = –19.14, 95%CI [−23.61, –14.66], P < 0.00001).
Scr of 72 hours after operation: Comparing with the control group, Scr of 72 hours in the experimental group was significantly decreased, the difference was statistically significant (MD = –6.50, 95%CI (−8.29, –4.71), P < 0.00001), as shown in Fig. 7.
Figure 7.
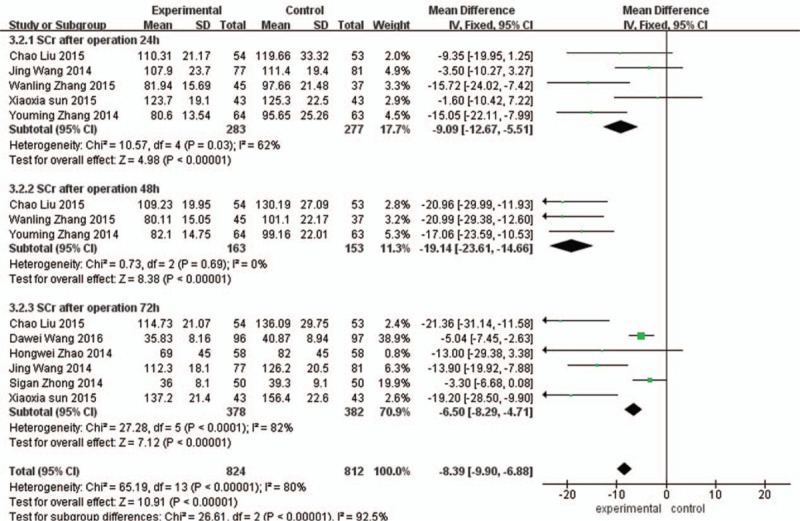
Comparison of 24 h, 48 h, and 72 h serum creatinine between the experimental group and the control group.
3.9. Serum beta 2-microspheres
β2-MG of 24 hours after operation: Comparing with the control group, β2-MG of 24 hours in the experimental group was significantly decreased, the difference was statistically significant (MD = –0.12, 95%CI (−0.27, 0.03), P = 0.12]. β2-MG of 48 hours after operation: Comparing with the control group, the β2-MG of 48 hours in the experimental group was significantly decreased, the difference was statistically significant (MD = –0.55, 95%CI (−0.71, –0.39), P < 0.00001). β2-MG of 72 hours after operation: Comparing with the control group, the β2-MG of 72 hours in the experimental group was significantly decreased, the difference was statistically significant (MD = –0.50, 95%CI [−0.60, –0.39], P < 0.00001], as shown in Fig. 8.
Figure 8.
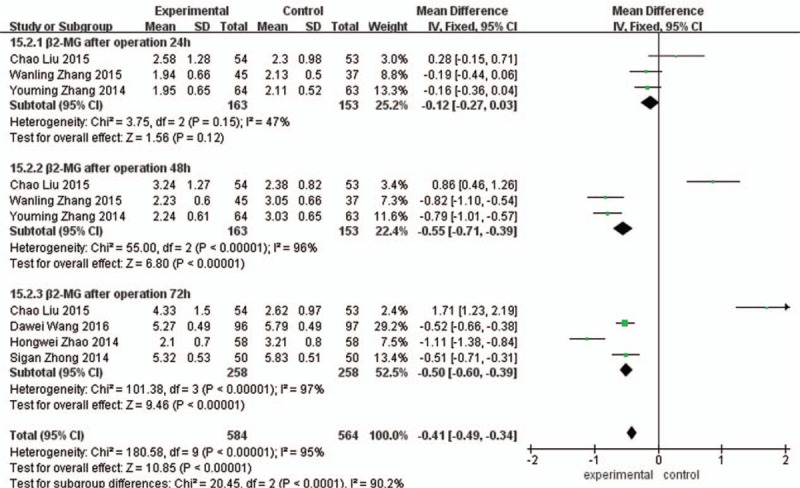
Comparison of 24 h, 48 h, and 72 h serum beta 2- microspheres between the experimental group and the control group.
3.10. Publication bias
Funnel plot indicated that there is a certain bias between all articles included in our meta- analysis, but the bias is small, which is basically about symmetry, as shown in Fig. 9.
Figure 9.
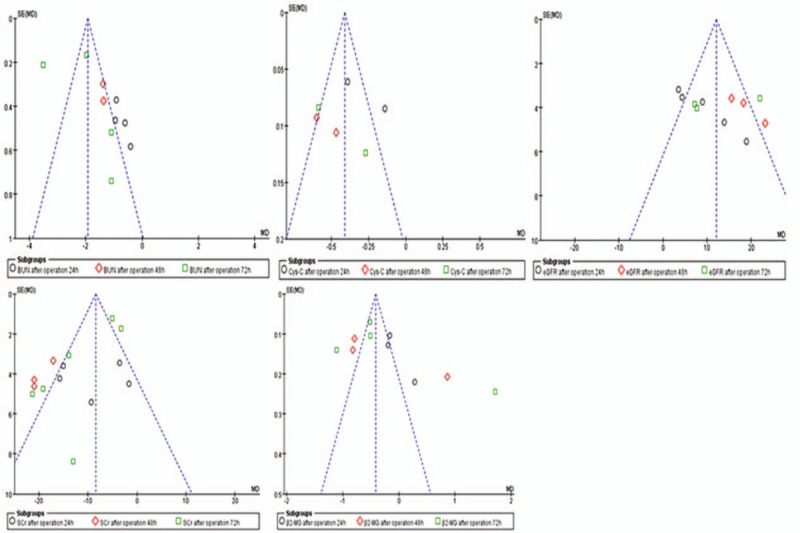
Publication bias of the included literature.
3.11. Sensitivity analysis
To determine sensitivity, we excluded 1 study at a time. In the comparison between the incidence of CIN, the incidence of adverse reactions, BUN, Cys C, GFR, SCR, and β2-MG,and the RRs (MD) and 95% CIs did not change substantially after removing 1 study at a time. This result was consistent with the meta-analysis results.
3.12. Subgroup analysis
Because all of the studies were from the same country and nation, this study was not conducted in a subgroup analysis by the nation and country.
4. Discussion
At present, the application of coronary artery interventional therapy (such as coronary angiography, stent implantation, etc.) is more and more extensive. The common features of this kind of surgery need to use the contrast agent. Meanwhile, old age, diabetes mellitus, renal insufficiency and hyperlipidemia are the risk factors of contrast induced nephropathy.[30] At the same time, the acute renal failure caused by the contrast was 57.2% of the patients with renal failure, 23.8% of irreversible renal damage occurred, and 24% of patients developed end-stage renal failure.
Results have shown that patients with CIN risk factors should be treated with 6–24 hours Hydration of ISO osmotic fluid 12 hours before angiography. At present, it is generally believed that the occurrence of CIN is the common result of many factors, such as the direct toxic effect of contrast agent on renal tubule, the imbalance of renal medulla, oxygen free radical damage, inflammatory reaction, renal tubular blockage, cell apoptosis and so on. However, the main component of alprostadil is prostaglandin E1, which has potent vasodilator effect, through the regulation of adenylate cycles and phosphodiesterase activity promotes adenosine concentration increased. Activation of a series of protein kinases, which rely on cyclic AMP, enables the expansion of blood vessels, reducing glomerular pressure, high filtration, and high perfusion. At the same time, alprostadil can inhibit the release of IL-1, TNF-αand other inflammatory mediators, reduce the formation of antigen-antibody reaction, inhibit platelet aggregation, reduce blood viscosity, and prevent the formation of thrombosis and atherosclerotic plaque. Through the above role, improving renal ischemia, hypoxia, prevent the occurrence of CIN.[31–34]
Our study included 969 patients, which was divided into hydration combined with alprostadil group (n = 433) and pure water group (n = 429),the results from Meta-analysis showed that the incidence of CIN in the experimental group was less than that of the control group (11.5% [50/433] vs 26.6% [114/429]), the difference was statistically significant (OR = 0.28, 95%CI [0.18, 0.42],P < 0.00001), the incidence of adverse reactions in the experimental group was less than that of the control group (12.6% [18/142] vs 24.3% [34/140]). And 3 days after surgery, Scr, urea nitrogen of the experimental group were lower than the control group, glomerular filtration rate of the experimental group was significantly higher than the control group, and the difference was statistically significant (P < 0.05). The results suggest that alprostadil can reduces the incidence of CIN through various metabolic pathways, and protect renal function in patients with diabetic patients after PCI.
There are several limitations to this study: (1) a total of 8 randomized controlled studies were included in this study, but most of the studies have some limitations. The inclusion of the study was more concentrated in the same region and country (Asia), and the lack of randomized clinical trials of Western and African race; (2) in the study, there is no unified standard for the using dose, method, and time of alprostadil before and after surgery; (3) although the studies were randomized controlled trials, but the study of the distribution of hidden, the specific random method is not a complete description, there is no evidence to rule out the possibility of patient selection bias. (4) In the study, short-term changes of BUN and Scr were included in the study. But Scr, BUN is not suitable for long-term follow-up and act as a main observing targe, it still need to extend the time of follow-up and selection of new indicators to determine the final outcome of patients. (5) The sample size of the study included in this study was small. And the crowd mainly comes from China, not for the state or nation for further subgroup analysis and sensitivity analysis, so we did not carry out subgroup analysis according to country or nation. Therefore, this meta-analysis also has certain enlightenment to the future randomized controlled trial: (1) Detailed description of the specific methods of test groups; clear intervention time, record the short-term efficacy and long-term efficacy; (2) Uniform drug administration time and dosage; (3) The articles included in the study should come from different countries and regions, in order to clarify the clinical effect of different countries and nationalities, so as to draw the correct conclusion.
5. Conclusion
Our research shows that alprostadil has preventive effects on CIN, which can effectively reduce the incidence of CIN in coronary heart disease patients complicated with diabetes. For patients with diabetes undergoing PCI, the use of alprostadil before operation can reduce the incidence of CIN. Due to the limitations of the quality and quantity of the articles, this conclusion still needs further research to confirm. In the future, it still needs to explore appropriate scheme of alprostadil, including the dosage, usage, and use time. It also needs more and more large-scale design rigorous RCT and long-term follow-up, to obtain a wealth of clinical data and draw a more credible conclusion, so as to guide clinical drug use.
Acknowledgments
The authors thank the First Affiliated Hospital of Guangxi Medical University, for her helpful statistical advice.
Footnotes
Abbreviations: β2-MG = Serum beta 2-microspheres, BUN = blood urea nitrogen, CIN = contrast-induced nephropathy, Cys-C = Cystatin C, GFR = glomerular filtration rate, MD = mean difference, OR = odds ratio, PCI = percutaneous coronary intervention, Scr = serum creatinine.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533. [DOI] [PubMed] [Google Scholar]
- 2.Steinhubl SR, Berger PB, Mann JT 3rd, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420. [DOI] [PubMed] [Google Scholar]
- 3.Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 2004; 44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 4.Smith SC, James TD, Alice KJ, et al. ACC/AHA guidelines for percutaneous coronary intervention (revision of the 1993 PTCA guidelines) 333: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty) endorsed by the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol 2001; 37:2239–12239. [DOI] [PubMed] [Google Scholar]
- 5.Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013; 381:629–638. [DOI] [PubMed] [Google Scholar]
- 6.Morice M-C, Serruys PW, Kappetein AP, et al. Five-year outcomes in patients with left main disease treated with either percutaneous coronary intervention or coronary artery bypass grafting in the synergy between percutaneous coronary intervention with taxus and cardiac surgery trial. Circulation 2014; 129:2388–2394. [DOI] [PubMed] [Google Scholar]
- 7.Kappetein AP, Head SJ, Morice MC, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardio-Thorac Surg 2013; 43:1006–1013. [DOI] [PubMed] [Google Scholar]
- 8.Farooq V, van Klaveren D, Steyerberg EW, et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet 2013; 381:639–650. [DOI] [PubMed] [Google Scholar]
- 9.Bolognese L, Falsini G, Schewenke C, et al. Impact of iso-osmolar versus low-osmolar contrast agents on contrast-induced nephropathy and tissue reperfusion in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention (from the Contrast Media and Nephrotoxicity Following Primary Angioplasty for Acute Myocardial Infarction [CONTRAST-AMI] Trial). Am J Cardiol 2012; 109:67–74. [DOI] [PubMed] [Google Scholar]
- 10.Mohammed Nazar MA, Mahfoz Ahmed, Achkar Katafan, et al. Contrast-induced nephropathy. Heart Views 2013; 14:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon RJ, Mehran R, Natarajan MK, et al. Contrast-induced nephropathy and long-term adverse events: cause and effect? Clin J Am Soc Nephrol 2009; 4:1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tereza Pucelikova, Dangas George, Mehran Roxana. Contrast-induced nephropathy. Cathet Cardiovasc Intervent 2008; 71:62–72. [DOI] [PubMed] [Google Scholar]
- 13.Walsh SR, Tang T, Gaunt ME, et al. Contrast-induced nephropathy. J Endovasc Ther 2007; 14:92–100. [DOI] [PubMed] [Google Scholar]
- 14.Solomon R, Dauerman HL. Contrast-induced acute kidney injury. Circulation 2010; 122:2451–2455. [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg I, Michel C, Victor G. Reversible acute kidney injury following contrast exposure and the risk of long-term mortality. Am J Nephrol 2008; 29:136–144. [DOI] [PubMed] [Google Scholar]
- 16.Aguirre A, Carlos F, Gasca R, et al. Comparison of overall costs between alprostadil and limb amputation in patients affected by peripheral arterial disease stages III and IV in Mexico. Value Health 2015; 18:A392–A392. [Google Scholar]
- 17.Montorsi F, Guazzoni G, Strambi LF, et al. Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. J Urol 1997; 158:1408–1410. [PubMed] [Google Scholar]
- 18.Linet OI, Ogrinc FG. Efficacy and safety of intracavernosal alprostadil in men with erectile dysfunction. N Engl J Med 1996; 334:873–877. [DOI] [PubMed] [Google Scholar]
- 19.Der Simonian R, Nan L. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 20.Higgins Julian PT, Green Sally. Wiley-Blackwell, Cochrane Handbook for Systematic Reviews of Interventions. Vol. 5. Chichester:2008. [Google Scholar]
- 21.Higgins J.P.G.S., Sally Green. “Cochrane handbook for systematic reviews of interventions 4.2. 5 [updated May 2005].” The Cochrane Library 3 (2005). [Google Scholar]
- 22.Zhangyouming. Alprostadil preventing diabetes in patients undergoing coronary intervention on postoperative contrast induced nephropathy. MS thesis. of Kunming Medical University, 2014. [Google Scholar]
- 23.Zhang Wan Ling. Alprostadil PCI in patients of coronary heart disease with diabetes prevention of contrast induced nephropathy effect observed. Shandong Med 2015; 55:29–30. [Google Scholar]
- 24.Liu Chao. Alprostadil combined with hydration treatment protective effects on renal function of diabetic nephropathy patients with PCI. MS thesis. Hebei Medical University of, by 2015. [Google Scholar]
- 25.Sigan Z, Fei Y, Aiwen C, et al. Alprostadil on diabetic patients with coronary heart disease in patients with interventional treatment of contrast induced nephropathy prevention effect. China General Med 2014; 31:3720–3723. [Google Scholar]
- 26.Dawei W, Dan L, Zhang Z, et al. Alprostadil on diabetic patients with coronary heart disease patients with intervention treatment of contrast induced nephropathy prevention effect [J]. Diabetes New World 2016; 11:30–31. [Google Scholar]
- 27.Xiaoxia Sun, Ali Wang, Hong Zuo, et al. Effect of alprostadil on coronary heart disease combined with interventional therapy in prevention of contrast induced nephropathy in patients with diabetes mellitus. Chin J Evidence Based Med, 2015(5):647–649. [Google Scholar]
- 28.Peng Wang Jing Yong Ping, Jianbin Gong, Cai Xiao Min, et al. Alprostadil on coronary heart disease with diabetes patients after treatment of contrast induced nephropathy. Southeast National Defense Med Intervent 2014; 1:28–30. [Google Scholar]
- 29.Wei ZH. Water vein of combined with alprostadil on coronary heart disease complicated with type 2 diabetes intervention therapy on early contrast induced nephropathy clinical study. Chin Med Innovation 2014; 25:33–35. [Google Scholar]
- 30.Feng W, Li J, Huang B, et al. Clinical survey on contrast-induced nephropathy after coronary angiography. Ren Fail 2013; 35:1255–1259. [DOI] [PubMed] [Google Scholar]
- 31.Heyman SN, Rosen S, Khamaisi M, et al. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol 2010; 45:188–195. [DOI] [PubMed] [Google Scholar]
- 32.Wen-Hua Li, Li DY, Qian WH, et al. Prevention of contrast-induced nephropathy with prostaglandin E1 in high-risk patients undergoing percutaneous coronary intervention. Int Urol Nephrol 2014; 46:781–786. [DOI] [PubMed] [Google Scholar]
- 33.Fan Y, Wei Q, Cai J, et al. Preventive effect of oral nicorandil on contrast-induced nephropathy in patients with renal insufficiency undergoing elective cardiac catheterization. Heart Vessels 2016; 31:1–7. [DOI] [PubMed] [Google Scholar]
- 34.Zhao SJ, Zhong ZS, Qi GX, et al. The efficacy of N-acetylcysteine plus sodium bicarbonate in the prevention of contrast-induced nephropathy after cardiac catheterization and percutaneous coronary intervention: A meta-analysis of randomized controlled trials. Int J Cardiol 2016; 221:251–259. [DOI] [PubMed] [Google Scholar]


