Abstract
Rational:
Multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis (TB) are emerging problems in several countries. These infections require long and expensive treatment regimens. Recently, 2 new drugs, bedaquiline and delamanid, have been approved in several countries for use in adults with severe, difficult-to-treat MDR-TB, and it has been suggested that they could also be administered to children with MDR-TB and limited treatment options. However, no study has been completed on their efficacy.
Patient concerns:
This report describes a 12-year-old child with XDR-TB who was cured after a 24-month therapy regimen, which included delamanid.
Diagnoses:
The patient showed progressive clinical deterioration after 5 months of treatment with the majority of anti-TB drugs available on the market.
Interventions:
After unsuccessfull treatment with several anti-TB drugs for 5 months, he was treated with a regimen including for 24 months.
Outcomes:
Direct smear microscopy of the gastric aspirates and gastric aspirate cultures for Mycobacterium tuberculosis became negative after only 1 week and remained persistently negative. During the 24-month treatment, all blood test results remained within the normal range, no adverse events were reported, and corrected QT interval was always normal. A clinical and laboratory control was performed 3 months after discontinuation of delamanid, and the other drugs did not reveal any modification of both general conditions as well as laboratory and radiological findings. The patient was considered cured.
Lessons:
The positive outcome associated with the favorable safety and tolerability profile showed that long-term therapy with delamanid can significantly contribute to treating apparently hopeless XDR-TB cases in children.
Keywords: delamanid, extensively drug-resistant tuberculosis, multidrug resistant tuberculosis, pediatric infectious diseases, tuberculosis
1. Introduction
A recent review of the available data estimated that approximately 32,000 children worldwide suffer from multidrug-resistant (MDR) tuberculosis (TB), that is, TB caused by Mycobacterium tuberculosis strains resistant to isoniazid and rifampicin.[1] In some cases, resistance is extended to fluoroquinolones and at least 1 second-line injectable anti-TB drug. These strains are defined as extensively drug-resistant (XDR).[1] In patients with infection owing to MDR/XDR-TB, treatment with the available anti-TB drugs is challenging. These infections require long and expensive treatment regimens and are associated with severe adverse events and very low cure rates.[2] Recently, 2 new drugs, bedaquiline and delamanid, have been approved in several countries for use in adults with severe, difficult-to-treat MDR-TB, and it has been suggested that they could also be administered to children with MDR-TB and limited treatment options.[3] However, no study has been completed on bedaquiline in children to date.[4] In contrast, several clinical trials have tested the pharmacokinetics and safety of delamanid in children, but no data are available on delamanid efficacy in children.[5–7] Delamanid is a nitro-dihydro-imidazoxazole derivative with mycobacteria-specific antibacterial activity in vitro, without activity against Gram-positive or Gram-negative bacteria or intestinal flora.[8] The nitroimidazopyran derivative acts through the inhibition of the mycolic acid biosynthesis, thus preventing the formation of the mycobacterial cell envelope and secondly, facilitating better drug penetration.[8]
In a number of cases, compassionate use of delamanid was authorized by the producer after approval by an independent panel of experts after assessment of the appropriateness of the request.[9] One of these children with XDR-TB successfully completed a 24-month therapeutic regimen including delamanid during the entire duration of treatment, a period longer than any other pediatric treatment with this drug. This report describes the impact of the drug on the clinical course of the disease in this patient and the delamanid safety and tolerability during this long period of drug administration.
2. Case
2.1. Presenting concerns
The patient (male, 12 years’ old), native Italian, without comorbidities or risk factors for TB, was diagnosed in October 2013 in Milan (Italy) with both laryngeal and pulmonary TB. Clinical history was negative for relevant problems until May 2013, when he began to suffer from progressive dysphonia associated with asthenia and weakness in the absence of fever.
2.2. Clinical findings
When the pediatric infectious disease specialist visited the child, a chest radiograph was requested, and it showed extensive bronchial wall thickening with peribronchial vascular distribution in both lungs. A chest computerized tomography (CT) was requested, and it showed bilateral multiple nodular and pseudonodular infiltrates, with fibrotic and calcified areas; no excavations were recognizable, and several enlarged lymph nodes without signs of colliquation were present in the mediastinum and in both of the laterocervical regions. The aryepiglottic fold was thickened. Blood examinations were performed and they showed white blood cells (WBC) count and C-reactive protein (CRP) in normal range (WBC: 6300 cells/μL; CRP: 0.19 mg/dL). In addition, routine biochemical blood examinations were normal. However, on the basis of CT findings, laryngeal and pulmonary TB was suspected, and the patient was hospitalized.
2.3. Diagnostic focus and assessment
A tuberculin skin test (TST) was negative when requested, whereas an interferon-γ-release assay (IGRA; QuantiFERON GOLD, Qiagen, GmbH, Hilden, Germany) showed positive results. Direct smear microscopy of gastric aspirates for acid-fast bacilli and gastric aspirate cultures for M tuberculosis were positive. Based on early detection of rifampicin resistance by Xpert MTB/RIF (Cepheid, Sunnyvale, CA), initially (October 4, 2013) the child was treated with high-dose isoniazid, ethambutol, pyrazinamide, and moxifloxacin. However, because drug susceptibility testing (DST) revealed that the strain was resistant to all first- and second-line drugs except para-aminosalicylic acid (PAS) and linezolid, the regimen was redesigned, and PAS, linezolid, terizidone, clarithromycin, amoxicillin-clavulanate, and moxifloxacin were prescribed (October 30, 2013). After 4 weeks of treatment, both clinical and radiological improvements were achieved and laboratory examinations remained stable. Moreover, the smear microscopy of the gastric aspirate remained positive, but showed a reduced mycobacterial burden. The patient was sent home with the same drug therapy.
2.4. Therapeutic focus and assessment
Despite good treatment adherence, the patient experienced progressive clinical deterioration, with loss of 4-kg body weight (starting from a weight of 36 kg) in <2 months and evident asthenia and weakness. Hemoglobin decreased to approximately 2.5 g/dL with WBC count and CRP in normal range, and smear microscopy on direct gastric aspirate showed again positivity with high grading. He was re-admitted to the hospital. Blood transfusions were administered, and parenteral nutrition was initiated. On February 9, 2014, isoniazid, meropenem, amikacin, clofazimine, and ethionamide were added to the previous anti-TB therapy. However, 10 days later, all anti-TB drugs were stopped because the patient experienced acute pancreatic insufficiency.
On March 1, on the basis of recommendations from the TB Consilium platform and 4 experts in pediatric MDR/XDR-TB management,[10] after the approval of the Ethics Committee of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico and the signature of a written consent from both the parent and a written assent from the child, anti-TB drugs were progressively reintroduced. Amoxicillin-clavulanate (100 mg/kg/day as amoxicillin i.v.), meropenem (100 mg/kg/day i.v.), linezolid (300 mg/day i.v.), clofazimine (100 mg/day by mouth), PAS (8 g/day by mouth), and ethionamide (250 mg/day by mouth) were chosen. Moreover, delamanid, obtained from the manufacturer for compassionate use, was added at the dose of 100 mg twice daily by mouth.
This regimen was maintained for 3 months, and significant changes in the clinical and laboratory data were observed. General conditions, including weight, significantly improved, blood hemoglobin returned to normal values, and the patient never had fever. Direct smear microscopy of the gastric aspirates and gastric aspirate cultures for M tuberculosis became negative after only 1 week and remained persistently negative. All blood test results remained within the normal range, no adverse events were reported, and corrected QT interval (QTc) was always normal. Meropenem and amoxicillin-clavulanate were then discontinued before the discharge from the hospital, and the patient continued the other 5 drugs, including delamanid. A chest CT obtained before discharge documented a significant improvement in the previously described radiological findings with a reduction in the tree-in-bud signs in the right upper lobe and in the reticulonodular opacity in both upper lobes and lingula. Most of the lesions were described as fibrotic. The patient was discharged from the hospital on May 31.
2.5. Follow-up and outcomes
During the treatment period, the child completed routine monthly visits, periodic blood tests (including complete blood cell count and blood chemistry tests), and monthly electrocardiography (ECG) for QTc screening for early detection of any signs of adverse events. He persisted in good general conditions with negative results of gastric aspirate (microscopy and culture), normal blood laboratory tests, and normal QTc. Neutropenia never developed, and the liver and kidney function tests were always normal. Moreover, the QTc interval was never found to be >500 ms. Moreover, chest magnetic resonance imaging (MRI) performed after 18 months from the beginning of delamanid regimen revealed parenchymal consolidation and reticulonodular opacity predominantly in right upper lobe and lingua, with fibrotic bronchial dilatation and mediastinal lymphadenopathy with small lymph nodes. Consequently, ethionamide and clofazimine were suspended, and treatment with PAS, linezolid, and delamanid was maintained for a total of 24 months. Before discontinuation, a chest MRI confirmed that no signs of negative evolution of the previously described lesions had occurred and that no new lesions were evident.
A clinical and laboratory control performed 3 months after discontinuation of delamanid, PAS, and linezolid did not reveal any modification of both general conditions as well as laboratory and radiological findings. The patient was considered cured.
2.6. Timeline
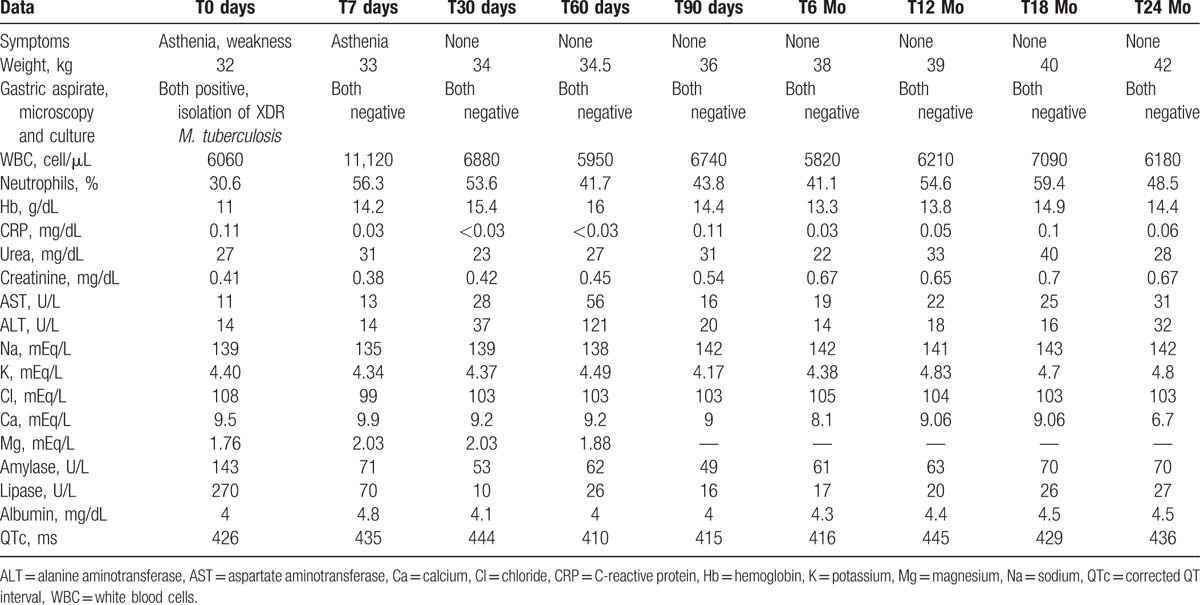
Table 1 summarizes the clinical and laboratory data of the child.
Table 1.
Clinical and laboratory data of the 12-year-old child with extensively drug-resistant (XDR) tuberculosis during the 24-month regimen including delamanid.
3. Discussion
Clinical trials with delamanid carried out in adults with MDR/XDR-TB receiving 100 or 200 mg twice a day of this drug added to a standardized background therapy have shown that it is effective, safe, and well tolerated.[11] The results indicate that delamanid reduced the rates of positive cultures after only 2 months of administration. However, better results were achieved when delamanid was administered for 6 months or 8 months with a break in the middle. With the latter regimen, there was a higher reduction in positive cultures and a relevant impact on mortality.[12] The safety data were reassuring because the most common adverse events following delamanid administration were nausea, vomiting, and dizziness, which were observed in approximately one-third of treated adults, but they were usually mild and did not lead to treatment discontinuation. The only troubling side effect of this drug was QT prolongation that was observed in approximately 10% of the treated subjects and was apparently related to the dose.[12] However, data regarding the use of delamanid for periods >6 months have not been reported. Moreover, data collected in children, which are limited in number and were mainly obtained in adolescents receiving 100 mg 2 times daily, do not allow for definitive conclusions regarding efficacy.
This is the first case showing that delamanid can significantly contribute to treating for several months apparently hopeless XDR-TB cases with a favorable safety and tolerability profile. Long-term prescription of anti-TB drugs is common in patients with MDR/XDR-TB.[13] However, the long duration of treatment, the toxicity of certain drugs, and the high cost discourage many patients and pose a major challenge to the health system. This explains why recently the World Health Organization (WHO) has recommended a standardized treatment regimen for MDR-TB lasting no >9 months: kanamycin, moxifloxacin, prothionamide, clofazimine, high-dose isoniazid, pyrazinamide, and ethambutol given together in an initial phase of 4 months, followed by 5 months of treatment with 4 of the drugs (e.g., moxifloxacin, clofazimine, pyrazinamide, and ethambutol).[13] Although this regimen has resulted in high rates of relapse-free cures in selected MDR-TB patients with an acceptable safety profile, it is not indicated in very complicated cases, such as the one reported in this study. The presence of a strain resistant to one of the drugs included in the WHO regimen together with an extrapulmonary localization of the infection are 2 of the conditions that contraindicate the WHO suggested regimen.[13] Consequently, particularly in subjects with XDR-TB, the use of long-term treatment with effective drugs remains the major recommended treatment. The case here described shows that delamanid was effective and well tolerated even when provided for 24 months.
In conclusion, delamanid is active against XDR M tuberculosis and can be considered for selected children with very difficult-to-treat diseases. Future studies investigating the long-term use of delamanid in children are needed. They may help solve any potential problems of effectiveness and safety of this drug in the pediatric population of any age and permit delamanid inclusion in the armamentarium of drugs useful for controlling severe pediatric cases of XDR-TB.
3.1. Patient's parents’ perspective
We were very worried when we were informed of the XDR-TB in our child. In particular, we were worried after the second hospitalization when his clinical conditions were rapidly worsening. We were confident in the pediatricians who followed our child, and we were very happy to discover that delamanid was able to negativize the gastric aspirate. The treatment was long, but we are really happy considering the results!
3.2. Informed consent
The patient's parents provided their written informed consent for the publication of this study.
Acknowledgments
We would like to thank Raffaella Pinzani, Luca Castellazzi, Lia D’Ambrosio, and Rosella Centis for their support in following this case.
Footnotes
Abbreviations: CRP = C-reactive protein, CT = chest computerized tomography, DST = drug susceptibility testing, ECG = electrocardiography, IGRA = interferon-γ-release assay, MDR = multidrug-resistant, PAS = para-aminosalicylic acid, QTc = corrected QT interval, TB = tuberculosis, TST = tuberculin skin test, WBC = white blood cells, XDR = extensively drug-resistant tuberculosis.
The study was supported by a grant from the Italian Ministry of Health (Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Ricerca Corrente Grant 2014 850/01).
The authors have no conflict of interest to declare.
References
- 1.Jenkins HE, Tolman AW, Yuen CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet 2014; 383:1572–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Migliori GB, Sotgiu G, Gandhi NR, et al. Drug resistance beyond extensively drug-resistant tuberculosis: individual patient data meta-analysis. Eur Respir J 2013; 42:169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zumla A, Gillespie S, Hoelscher M, et al. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect Dis 2014; 14:327–340. [DOI] [PubMed] [Google Scholar]
- 4.Resist-TB. Drug-resistant tuberculosis, clinical trials progress report. Available at: http://www.resisttb.org/wp-content/uploads/2016/04/RESIST-TB-Clinical-Trials-Progress-eport_20Apr2016.pdf Accessed on May 18, 2016. [Google Scholar]
- 5.Hafkin J, Frias M, Hesseling A, et al. Pharmacokinetics and safety of delamanid in children aged 6–17 years with MDR-TB. Proceedings of The International Congress of Antimicrobial Agents and Chemotherapy. San Diego (CA), USA, September 17–21, 2014. Abstract A-960. [Google Scholar]
- 6.Hafkin J, Gler M, Frias M, et al. Long-term safety, tolerability, and pharmacokinetics of delamanid in children aged 12-17. The 46th Union Worls Conference on Lung Health, Cape Town, SA 2015; Poster EP-115-04. [Google Scholar]
- 7.Clinical Trials.gov. A 6-month safety, efficacy, and PK trial of delamanid in pediatric patients with multidrug resistant tuberculosis. Available at: https://clinicaltrials.gov/ct2/show/NCT01859923 Accessed on May 18, 2016. [Google Scholar]
- 8.Esposito S, Bianchini S, Blasi F. Bedaquiline and delamanid in tuberculosis. Expert Opin Pharmacother 2015; 16:2319–2330. [DOI] [PubMed] [Google Scholar]
- 9.Tadolini M, Garcia-Prats AJ, D’Ambrosio L, et al. Compassionate use of new drugs in children with multidrug-resistant and extensively-drug resistant tuberculosis: early experiences and challenges. Eur Resp J 2016; 48:938–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esposito S, D’Ambrosio L, Tadolini M, et al. ERS/WHO Tuberculosis Consilium assistance with extensively drug-resistant tuberculosis management in a child: case study of compassionate delamanid use. Eur Respir J 2014; 44:811–815. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Liu Y, Tang S, et al. Clinical benefit of delamanid (OPC-67683) in the treatment of multidrug-resistant tuberculosis patients in China. Cell Biochem Biophys 2013; 67:957–963. [DOI] [PubMed] [Google Scholar]
- 12.Skripconoka V, Danilovits M, Pehme L, et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J 2013; 41:1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Orgnization WHO treatment guidelines for drug-resistant tuberculosis - 2016 update (WHO/HTM/TB/2016.04). Geneva:World Health Organization; 2016. [PubMed] [Google Scholar]


