Supplemental Digital Content is available in the text
Keywords: antiretroviral therapy, HIV, HIV neurocognitive impairment, lithium, Placebo, randomized controlled clinical trial, South Africa
Abstract
Background:
HIV-associated neurocognitive disorder (HAND) remains highly prevalent despite effective anti-retroviral therapy (ART). A number of adjunctive pharmacotherapies for HAND have been studied with disappointing results, but preliminary data suggest that lithium may provide clinical benefit. In addition, the low cost of lithium would facilitate access in low- and middle-income countries which carry the greatest burden of HIV.
Methods:
Our objective was to evaluate the 24-week efficacy and safety of lithium in patients with moderate to severe HAND. Our primary efficacy endpoint was the change in Global Deficit Score (GDS) from baseline to 24 weeks, whereas our secondary endpoint was the change in proton magnetic resonance spectroscopy (1H-MRS) brain metabolite concentrations. We conducted a 24-week randomized placebo-controlled trial of lithium as adjunctive pharmacotherapy. We enrolled participants with moderate to severe HAND, on ART for at least 6 months, with suppressed viral loads and attending public sector primary care clinics in Cape Town, South Africa. We randomized 66 participants to lithium (n = 32) or placebo (n = 34). Lithium or placebo was dosed 12-hourly and titrated to achieve the maintenance target plasma concentration of 0.6 to 1.0 mmol/L. Sham lithium concentrations were generated for participants receiving placebo.
Results:
Totally 61 participants completed the study (lithium arm = 30; placebo arm = 31). Participants at enrolment had a mean age of 40 years and a median CD4+ T-cell count of 500 cells/μL. The median change in GDS between baseline and week 24 for the lithium and placebo arms were –0.57 (95% confidence interval [CI] –0.77, –0.32) and –0.56 (–0.69, –0.34) respectively, with a mean difference of –0.054 (95% CI –0.26, 0.15); P = 0.716. The improvement remained similar when analyzed according to age, severity of impairment, CD4+ count, time on ART, and ART regimen. Standard 1H-MRS metabolite concentrations were similar between the treatment arms. The study drug was well tolerated in both study arms. Six serious adverse events occurred, but none were considered related to the study drug.
Conclusion:
Adjunctive lithium pharmacotherapy in patients on ART with HAND was well tolerated but had no additional benefit on neurocognitive impairment.
1. Introduction
HIV-associated neurocognitive disorder (HAND) remains highly prevalent despite effective antiretroviral therapy (ART).[1,2] The incidence of severe HAND has decreased, but with longer life expectancy and associated risk factors for cerebrovascular disease, the overall prevalence of HAND is projected to rise.[3,4] HAND is associated with high rates of morbidity and mortality.[1,5,6] Effective neuroprotective adjunctive pharmacotherapy for HAND has not yet been identified.
A number of adjunctive pharmacotherapies for HAND have been studied with disappointing results thus far.[7] Preliminary data suggest that lithium may provide clinical benefit as adjunctive pharmacotherapy. In 2 pilot studies, adjunctive lithium in HAND improved neurocognitive impairment in 1 study, whereas neuronal integrity on imaging improved in both studies.[8,9] However, these pilot studies were limited by both the lack of a comparator arm and the short duration of lithium treatment. Lithium has also been associated with an increase in gray matter volume on neuroimaging in other patient populations.[10] In addition, lithium has been associated with an improvement in neurocognitive impairment in patients with Alzheimer's disease.[11] Lithium has complex pharmacological effects but unequivocal is the inhibition of glycogen synthase kinase-3-beta (GSK-3-β), a serine-threonine protein kinase, that mediates neuronal function, cellular substrates for learning and memory, as well as neuronal apoptosis and inflammation signaling pathways.[12–14] In addition to the potential promise of lithium as an adjuvant from preliminary work, its low cost would facilitate access in low- and middle-income countries which carries the greatest burden of HIV.
We conducted a 24-week randomized placebo-controlled trial to study lithium as an adjunctive pharmacotherapy in patients with moderate to severe HAND.
2. Methods
Our primary efficacy endpoint was the change in the Global Deficit Score (GDS) from baseline to 24 week in the placebo arm compared to the lithium arm. Baseline was the screening period up to 4 weeks prior to enrolment (–4–0 weeks). During the screening period all investigations and assessments were performed and week 1 started when the participant was enrolled and study drug dispensed. GDS summarizes the neuropsychological test results of selected cognitive domains and adjusts for age, education, gender, and ethnicity.[15] The following domains and tests were included: attention (Mental Alternation Test, Digit Span, Paced Auditory Serial Addition Test), learning and memory (the Hopkins Verbal Learning Test), motor speed (Finger Tapping Dominant Hand, Finger Tapping Non-Dominant Hand, Grooved Pegboard Test Dominant Hand, Grooved Pegboard Test Non-Dominant Hand), psychomotor speed (Trail Making Test A, Color Trails Test 1, Digit Symbol-Coding), executive function (Color Trails Test 2, Stroop Color-Word Test, Wisconsin Card-Sorting Test), visual learning and memory (Rey Complex Figure), and verbal fluency (Animals and Fruit and Vegetables). We screened for symptoms of depression using the Center for Epidemiologic Studies Depression (CES-D) scale.[16] Our secondary endpoint was the change between baseline (–4–0 weeks) and week 23 in proton magnetic resonance spectroscopy (1H-MRS, TE30, and TR2000 ms) brain metabolite concentrations of glutamate, glutamate with glutamine (Glx), myo-inositol (mI), N-acetyl-aspartate (NAA), N-acetyl-asparate with N-acetyl-aspartyl-glutamate (NAA+NAAG), choline (Cho) and creatine (Cr) in 3 brain areas (cortical: anterior cingulate cortex, white matter: left frontal white matter and deep brain structure: left thalamus). The primary safety endpoint was the severity and frequency of adverse events.
2.1. Study design and participants
Inclusion criteria were HIV-infected adults (≥18 and ≤70 years), established on ART for at least 6 months with a suppressed viral load (HIV PCR <400 copies/mL), cognitive impairment as defined by a GDS ≥ 0.5 attending public sector ART clinics in Cape Town, South Africa. Enrolled participants were mainly recruited from Nolungile Site C clinic in Khayelitsha and were followed up at the University of Cape Town Clinical Research Centre at Groote Schuur Hospital. Eligible participants gave written informed consent; female participants were not pregnant or breastfeeding and females of child-bearing potential committed to use of contraception. We required additional written informed consent from each participant's care giver as we anticipated that participants may vary in their ability to provide consent (participants may understand the need for ART and that they have impaired memory, but may not be able to recall all aspects of the study procedures and risks). Care givers had to accompany participants to each study visit. We excluded participants who received an investigational drug within 30 days, had evidence of an active acquired immune deficiency syndrome (AIDS)-defining opportunistic infection, had a history of drug or alcohol abuse within 3 months before screening, had a positive urine drug screen for drugs of abuse (amphetamine, benzodiazepine, cannabis, cocaine, opiate), had confirmed neurosyphilis or vitamin B12 deficiency, had imaging structural abnormalities, had a significant head injury or severe mental illness. We minimized the risk of lithium exposure by excluding participants with a QTc greater than 450 ms for males and 470 ms for females, confirmed epilepsy on chronic treatment, use of any medications that may predispose the participant to lithium toxicity, clinically significant hypo- or hyperthyroidism or hypercalcaemia or hypermagnesaemia, renal impairment as defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min using the Cockroft and Gault formula and current diarrhea with dehydration.
2.2. Intervention
We dosed lithium carbonate 250 mg tablets (Camcolit, Norgine) and matching placebo (donated by Norgine). The investigational drugs were donated by Norgine who had no input into the study design, conduct, or analysis. Lithium was titrated to achieve the maintenance target plasma concentration of lithium in patients with bipolar mood disorder of between 0.6 and 1.0 mmol/L. Sham lithium concentrations were generated for participants receiving placebo (details in Section 2.4.)
2.3. Ethics and study oversight
The study was approved by the human research ethics committees of the University of Cape Town (071/2013) and Stellenbosch University (M13/07/027). The study was registered on the Pan African Clinical Trials Registry (PACTR) with the identifier number PACTR201310000635418. An independent data and safety monitoring board (DSMB) oversaw trial safety, whereas the trial steering committee mainly monitored progress of the trial.
2.4. Randomization, treatment concealment, and blinding
Participants in each cohort were randomized to placebo or the lithium carbonate prior to the start of the study using block randomization of 4, 6, or 8 which were subject to the overall constraint of adding to the total sample size. Once an enrolment number was assigned by the investigators, the study pharmacist dispensed treatment according to the randomization list. The statistician compiled the randomization list prior to study start. The randomization list was stored in a secure place with access limited to the statistician and pharmacist ensuring that the investigators and participants remained blinded throughout the study. Plasma concentrations were measured in both the lithium and placebo arms by the laboratory. The laboratory remained blinded and reported placebo concentrations as lower than level of detection. The laboratory forwarded the concentration results only to the study statistician. The study statistician generated sham lithium concentrations for the placebo patients and forwarded blinded concentrations (measured for lithium arm and simulated for placebo arm) to a coinvestigator who had no direct participant contact. This coinvestigator also received the adverse event logs, and in conjunction with the blinded concentrations, made dose-adjustment recommendations which were forwarded to the treating investigators. Only the study statistician was unblinded to arm allocation throughout this process. The sham lithium values were generated based on a random sampling from a distribution that was parameterized with the true measured lithium concentrations in the treatment arm, and with some additional rejection sampling to ensure the sham lithium values did not fall outside of feasible ranges.
2.5. Adverse events and safety investigations
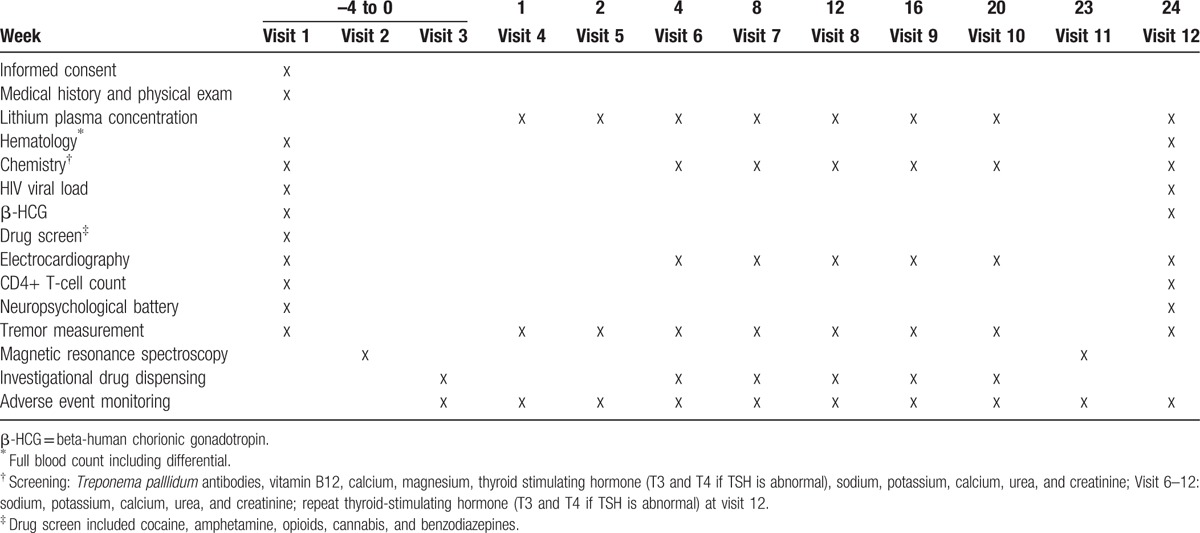
We reviewed participants weekly for adverse events for the first month followed by 4 weekly visits for adverse events and adherence. Adherence was measured using pill counts and self-report diary cards. Suspected poor adherence was flagged by the study pharmacist when a >25% discrepancy in doses taken and the pill count was noted. Participants who were noted as potentially being poorly adherent were intensively counseled by the investigators and greater emphasis was placed on evaluating adherence at subsequent visits. Participants with clinically significant adverse events were reviewed more frequently as needed. At screening (–4 to 0 weeks) and week 24 we measured full blood count and differential, Treponema palllidum antibodies (screening only), vitamin B12 levels (screening only), chemistry (calcium, magnesium, thyroid function, sodium, potassium, calcium, urea, and creatinine), viral load, CD4+ count, urine screen for amphetamines, benzodiazepine, cannabis, cocaine, and opiate abuse (screening only) and β-HCG. At other visits (week 4, 8, 12, 16, 20), we measured lithium concentrations (actual and sham) and chemistry (sodium, potassium, calcium, urea and creatinine). Other safety investigations included electrocardiogram (screening, week 4, 8, 12, 16, 20, 24) and TRG Essential Tremor Rating Assessment Scale (TETRAS) (screening, week 1, 2, 4, 8, 12, 16, 20, 24). Neuroimaging was performed at baseline and week 23 (Table 1).
Table 1.
Study procedures.
2.6. Statistical methods
We calculated our sample size to detect an absolute value change in GDS of 0.25 and required 49 participants per arm for 90% power at alpha 0.05. We aimed to enroll 54 participants in each arm to account for a 10% loss to follow-up or withdrawal. Previous research has shown that ART alone improved the GDS by a mean of 0.13 and 0.6 in patients with a GDS in the mild to moderate (>0.25 to <0.75) and severe (>0.75) ranges, respectively.[8,17] Twelve week adjunctive lithium therapy in patients stable on ART improved the GDS by 0.3 and we opted to detect a more conservative GDS difference of 0.25 with a standard deviation of 0.375, which was calculated using the range in the published studies divided by 4.[8,17] We conducted an intention-to-treat and per protocol analysis for the primary endpoint. For the intention-to-treat analysis, we carried over the last data points when the week 24 endpoints were missing, example for missing GDS at week 24 we used GDS at enrolment. For the per protocol analysis, we included only participants who completed the treatment originally allocated. We assessed the normality of the data visually and using the Shapiro–Wilk test. We compared baseline and week 24 values of continuous variables with paired t-tests or Wilcoxon sum rank depending on the distribution. Normally distributed data were described using the mean and standard deviation, whereas non-normally distributed data were described using median and interquartile ranges. We applied correction for the false discovery rate (FDR) by the method of Benjamin & Hochberg to comparisons. We report raw P values throughout and note any P values that lose or gain statistical significance after correction.
3. Results
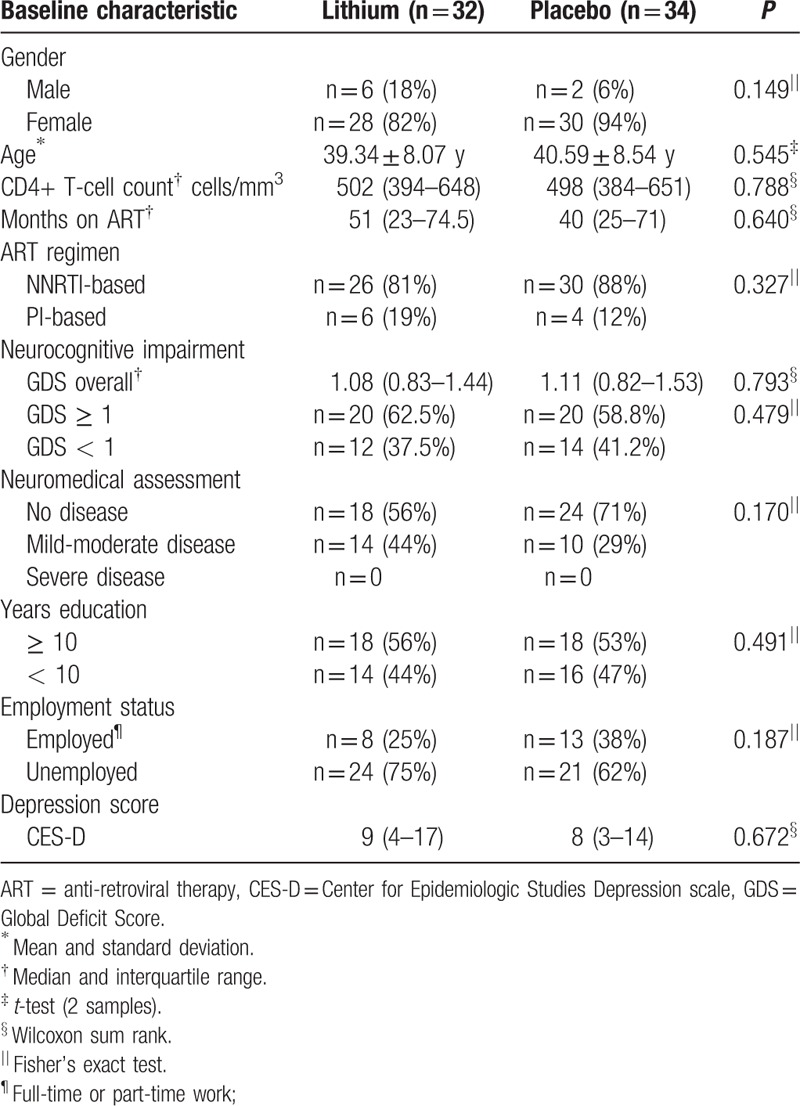
We enrolled our first participant in December 2013 and had our last study visit in June 2015. Due to slow accrual we were unable to enroll our original calculated sample size and randomized 66 participants to lithium (n = 34) or placebo (n = 32), whereas 61 participants completed the study (lithium arm = 30; placebo arm = 31) (diagram 1). All participants were black Africans, first language Xhosa. Baseline characteristics were similar between the 2 groups with the majority of participants presenting with severe neurocognitive impairment with GDS of ≥ 1 (Table 2).
Figure 1.
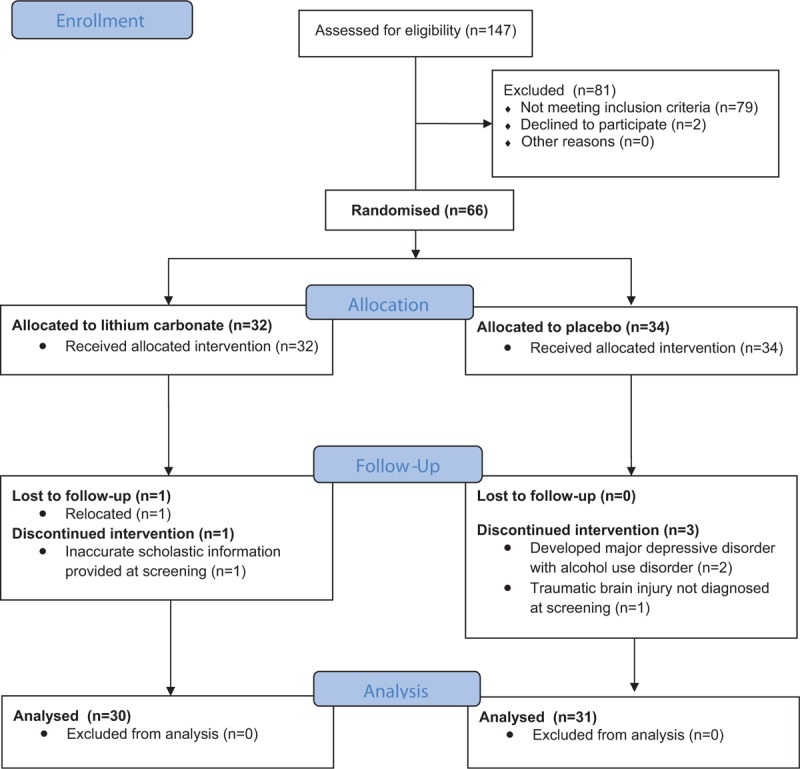
Diagram 1. Trial profile: eligibility, randomization, follow-up, and analysis.
Table 2.
Baseline characteristics.
Suspected poor adherence was similar in the placebo and lithium arms. We recorded 47 poor adherence episodes of which 23 episodes occurred in 16 lithium arm participants and 24 episodes occurred in 17 placebo arm participants. In the 16 lithium arm participants: 10 participants had 1 poor adherence episode, 5 participants had 2 poor adherence episodes, and 1 participant had 3 poor adherence episodes. In the 17 placebo arm participants: 12 participants had 1 poor adherence episode, 3 participants had 2 poor adherence episodes, and 2 participants had 3 poor adherence episodes. The majority of poor adherence episodes occurred within the first 8 weeks of the study (57%). Week 24 viral loads were not predictive of poor adherence as the 2 participants with slightly raised viral loads at the end of the study (highest value 585 copies per mL) were not identified with poor adherence. Both participants were allocated to the lithium arms.
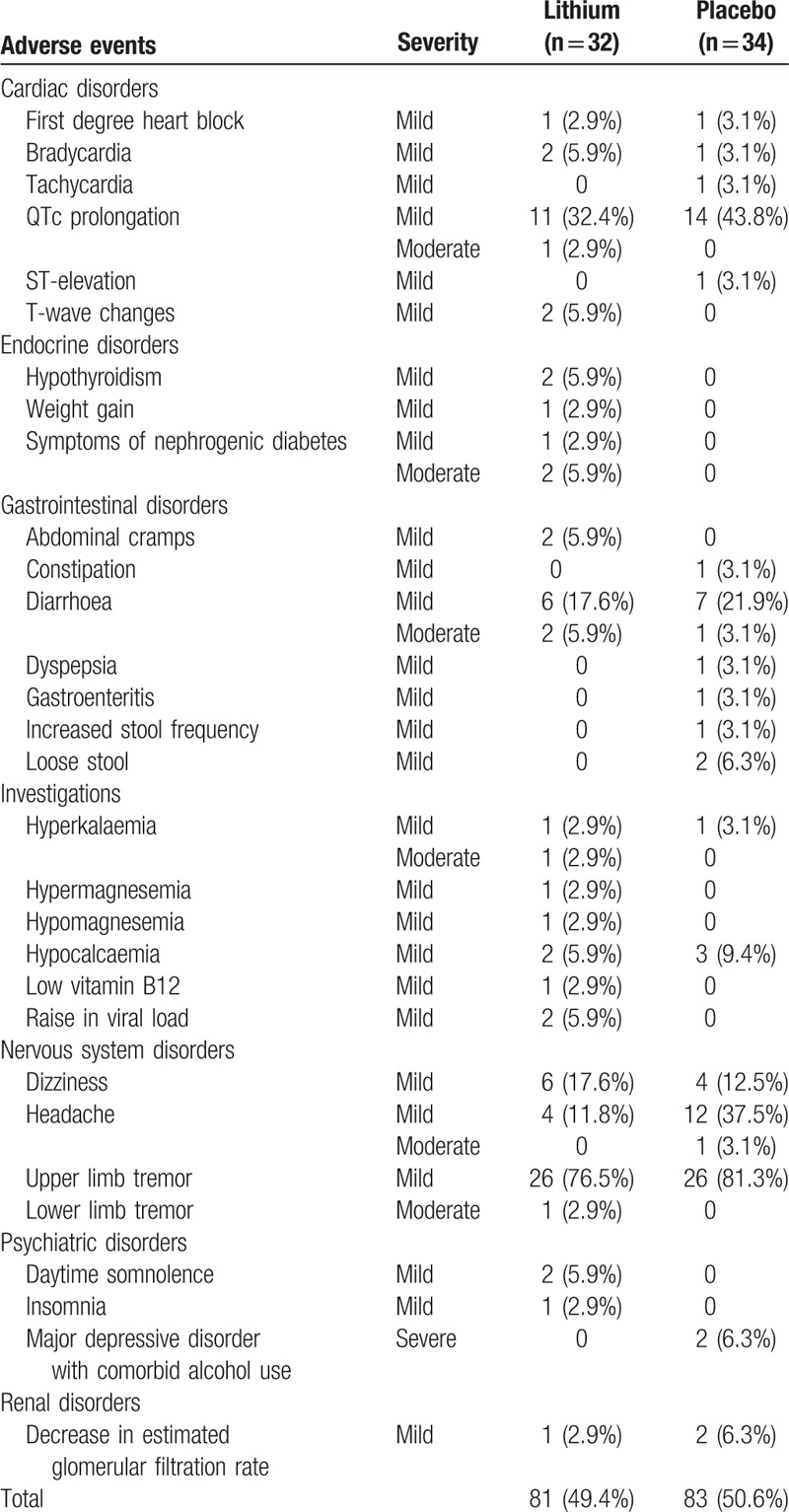
The improvement in GDS was not different between the treatment arms in both the intent-to-treat and the per protocol analysis (Table 3, supplemental file table 1, diagram 2 (A) (B)). The median change in GDS scores between baseline and week 24 for the lithium and placebo arms were –0.57 (95% CI –0.77, –0.32) and –0.56 (–0.69, –0.34) respectively, with a mean difference of –0.054 (–0.26, 0.15); P = 0.716. The improvement remained similar when analysed according to age, severity of impairment, CD4+ count, time on ART, and ART regimen. 1H-MRS metabolite concentrations (supplemental file table 2) were also not different between the treatment arms. However, the 1H-MRS metabolite concentrations could not be measured for all participants due to intermittent periods of technical downtime of the MRI scanner. The study drug was well tolerated with no statistically significant difference (P = 0.413) in total adverse events between the 2 study arms (Table 4). Six serious adverse events occurred but none were considered related to the study drug (supplemental file table 3).
Table 3.
Intent to treat analysis of neuropsychological changes.
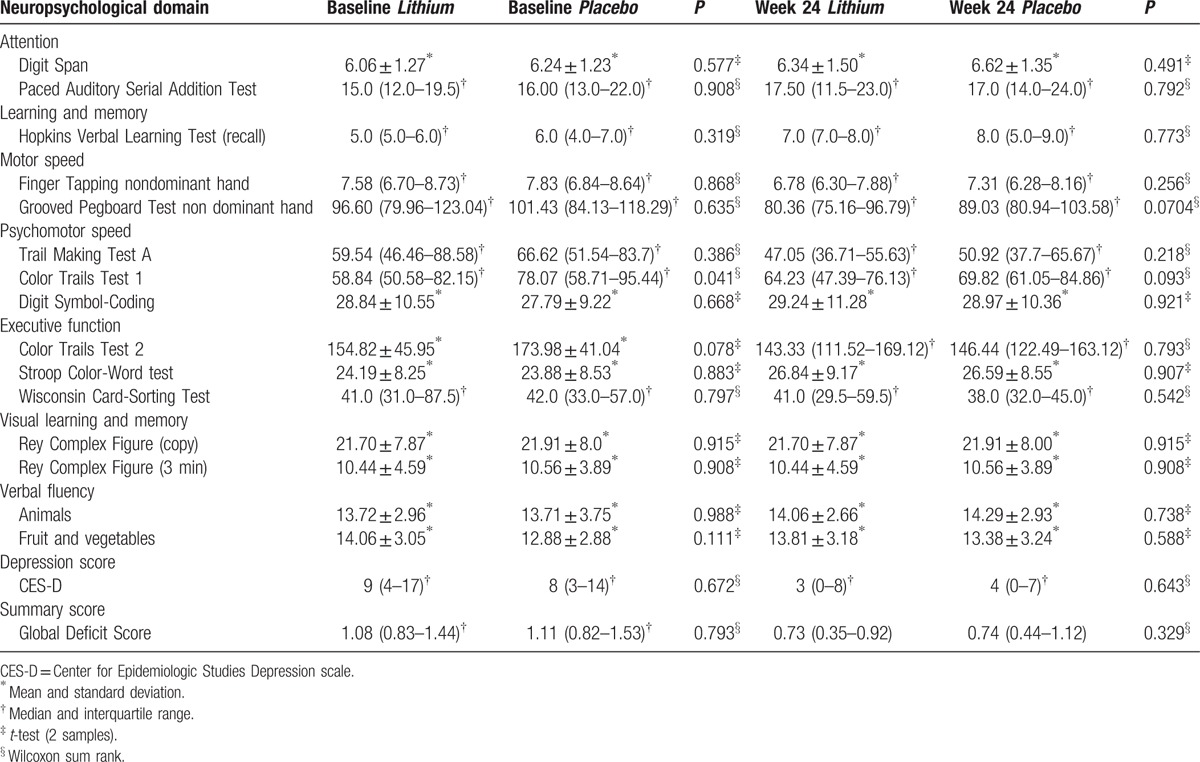
Figure 2.

Diagram 2. Box-and-whisker plots of (GDS) at week 1 and week 24 analyzed (A) per protocol analysis and (B) intention to treat analysis. GDS = Global Deficit Score.
Table 4.
Selected adverse events considered relevant to lithium therapy.
4. Discussion
Our study is the first to test adjunctive lithium therapy in patients with HAND in a randomized double blind controlled trial for a period of 6 months. We found that adjunctive lithium in patients with HAND was well tolerated but had no benefit on neurocognitive impairment compared with placebo when assessing neuropsychological test performance and 1H-MRS metabolite concentrations. Neurocognitive impairment improved similarly in both the lithium and placebo arm.
Lithium has demonstrated neuroprotection with an increase in gray matter volume in various patient populations.[10,13] However, controlled clinical data demonstrating neuroprotection with clinical endpoints were lacking. The improvement in GDS we observed in the lithium arm is similar to the improvement noted by Letendre et al[8] (median improvement 0.29 while we found a median improvement of 0.47) in an open-label 12-week lithium study in patients with HAND. The similar improvement we observed in the placebo arm highlights the importance of a comparator arm. There are a number of potential explanations for our findings that lithium was no better than placebo. First, the placebo effect is a well-described response accompanied by psychobiological changes in the brain.[18] Clinicians are held in high regard and could have biased our participants’ expectations and response.[19] Second, participants may have become more familiar with the neuropsychological assessments leading to a practice effect. We deliberately scheduled the neuropsychological assessments 6 months apart to limit a potential practice effect, but cannot completely exclude some practice effect. In addition, no participant underwent a neuropsychological assessment prior to enrolment into this study. Third, we assessed endpoints only twice 6 months apart which prevents a longitudinal description of natural disease progression, placebo response, and lithium effect. The trajectory of natural disease, placebo, and lithium would be best described in longer term studies where quantitative modeling is applied.[20] The possibility exists that the placebo response may be temporary. Fourth, cognitive assessment is influenced by HIV infection, physical -, psychiatric -, and social comorbidity.[21] We monitored HIV -, physical- and psychiatric comorbidities and did not detect an improvement, but it is plausible that we missed social comorbidity improvement explained by trial participation. Lastly, it is possible that only patients with certain covariates or characteristics (such as depression comorbidity) may respond significantly better to lithium compared with placebo. Recently a genome-wide association between lithium response and common genetic variants on chromosome 21 has been identified in patients with bipolar disorder.[22]
Our study has a number of differences when compared with the open-label pilot studies of adjunctive lithium in HAND: longer study duration, randomized double-blind placebo-controlled design, lithium, and placebo dose adjusted using therapeutic drug monitoring with a target range used in the treatment of bipolar mood disorder and mostly African female participants.[8,9] The Letendre et al[8] study found that lithium improved the GDS from impaired to normal after 12 weeks in 8 participants, whereas Schiffitto et al found no neurocognitive improvement after 10 weeks in 13 participants, but found a decrease in glutamate with glutamine (Glx) metabolites in the frontal gray matter.[8,9] However, both studies were uncontrolled.
Our study has a number of limitations. First, our findings are limited by the fact that we were unable to enroll our original calculated sample due to slow accrual. However, an increase in sample size is unlikely to change our findings as an interim review by the DSMB determined that a sample size of 65 using the same assumptions as the original calculation have a power of 70% to 90% for the standard deviation ranging from 0.3 to 0.5. Our GDS standard deviation was 0.53 and 0.39 in the placebo and lithium arms, respectively. To the contrary, the between-group difference of GDS may be smaller than the assumed 0.25 and an even larger sample size than originally calculated may have been required to detect a significant difference. Second, 6 month trial duration could not exclude a beneficial effect of lithium on long-term functional worsening. Third, we cannot exclude selection bias as the majority of our participants were unemployed females with significant neurocognitive impairment. Fourth, all our participants were black Xhosa speaking Africans which limits the generalizability of our results.
In summary, we found no additional benefit of adjunctive lithium to placebo in African patients with HAND after 6 months of treatment. Future adjunctive lithium studies should follow-up patients for a longer duration to determine whether lithium has a beneficial effect on HAND progression.
Supplementary Material
Acknowledgments
The acknowledge the study participants: The rest of the study team: Kareema Poggenpoel (administration support), Laura Comrie (clinical support), Nicky Kramer (study pharmacist), Nozipho Mawisa (study nurse), Pam Jordan (data capturer), Queen Maswana (recruiter), Rasmita Ori (clinical support), Shireen Surtie (study coordinator), Teboho Linda (neuropsychology technician) and Wynand Smythe (study pharmacist).
Members of the DSMB and the trial steering committee: Giovanni Schifitto, Ned Sacktor, Carl Lombard, Rhoderick Machekano, Haylene Nell and Bernd Rosenkranz.
Norgine Pty (Ltd) who unconditionally donated lithium carbonate and identical placebo.
Footnotes
Abbreviations: 1H-MRS = proton magnetic resonance spectroscopy, AIDS = acquired immune deficiency syndrome, ART = anti-retroviral therapy, CES-D = Center for Epidemiologic Studies Depression, Cho = choline, CI = confidence interval, Cr = creatine, DSMB = Data and safety monitoring board, eGFR = estimated glomerular filtration rate, FDR = false discovery rate, GDS = Global Deficit Score, Glx = glutamate with glutamine, GSK-3-β = glycogen synthase kinase-3-beta, HAND = HIV-associated neurocognitive disorder, mI = Myo-inositol, NAA = N-acetyl-aspartate, NAA+NAAG = N-acetyl-asparate with N-acetyl-aspartyl-glutamate, PACTR = Pan African Clinical Trials Registry, TETRAS = TRG Essential Tremor Rating Assessment Scale.
Statistical analysis: Dr Maia Lesosky, Division of Epidemiology and Biostatistics, School of Public Health and Family Medicine, University of Cape Town, South Africa.
Search terms: South Africa; Randomized controlled clinical trial; HIV neurocognitive impairment; HIV; Lithium; Placebo; Antiretroviral therapy
Authorship: ED—study concept and design, analysis and interpretation of data, drafting and revising the manuscript for content, acquisition of data, study supervision, and obtaining funds; CF—revising the manuscript for content, interpretation of data, acquisition of data, study supervision and coordination; FH—analysis and interpretation of data; MC-C—analysis and interpretation of data, revising the manuscript for content, acquisition of data and study coordination; ML—analysis and interpretation of data, statistical analysis; EK—study concept and design, revising the manuscript for content, and obtaining funds; SL—study concept and design, revising the manuscript for content and obtaining funds; GM—study concept and design, revising the manuscript for content, and obtaining funds; JJ—study concept and design, revising the manuscript for content, study supervision, and obtaining funds.
Disclosure: Norgine Pty (Ltd) unconditionally donated lithium carbonate and identical placebo, and had no input in any aspect of the study.
Funding: This study was funded by the European and Developing Countries Clinical Trials Partnership (EDCTP Grant number SP.2011.41304.065/BMBF 01KA1306).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacktor N, Skolasky RL, Seaberg E, et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 2016; 86:334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabbiani M, Ciccarelli N, Tana M, et al. Cardiovascular risk factors and carotid intima-media thickness are associated with lower cognitive performance in HIV-infected patients. HIV Med 2013; 14:136–144. [DOI] [PubMed] [Google Scholar]
- 5.Cysique LA, Vaida F, Letendre S, et al. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology [Internet] 2009; 73:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cysique LA, Bain MP, Brew BJ, et al. The burden of HIV-associated neurocognitive impairment in Australia and its estimates for the future. Sex Health 2011; 8:541–550. [DOI] [PubMed] [Google Scholar]
- 7.McGuire JL, Barrett JS, Vezina HE, et al. Adjuvant therapies for HIV-associated neurocognitive disorders. Ann Clin Transl Neurol 2014; 1:938–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letendre SL, Woods SP, Ellis RJ, et al. Lithium improves HIV-associated neurocognitive impairment. AIDS 2006; 20:1885–1888. [DOI] [PubMed] [Google Scholar]
- 9.Schifitto G, Zhong J, Gill D, et al. Lithium therapy for human immunodeficiency virus type 1-associated neurocognitive impairment. J Neurovirol [Internet] 2009; 15:176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajek T, Weiner MW. Neuroprotective effects of lithium in human brain? Food for thought. Curr Alzheimer Res 2016; 13:862–872. [DOI] [PubMed] [Google Scholar]
- 11.Forlenza OV, Aprahamian I, de Paula VJ, Hajek T, et al. Lithium, a therapy for AD: current evidence from clinical trials of neurodegenerative disorders. Curr Alzheimer Res 2016; 13:879–886. [DOI] [PubMed] [Google Scholar]
- 12.Crowder RJ, Freeman RS. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci 1998; 18:2933–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazzara CA, Kim YH. Potential application of lithium in Parkinson's and other neurodegenerative diseases. Front Neurosci 2015; 9:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peineau S, Bradley C, Taghibiglou C, et al. The role of GSK-3 in synaptic plasticity. Br J Pharmacol 2008; 153 (suppl):S428–S437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 2004; 26:307–319. [DOI] [PubMed] [Google Scholar]
- 16.Myer L, Smit J, Roux LL, et al. Common mental disorders among HIV-infected individuals in South Africa: prevalence, predictors, and validation of brief psychiatric rating scales. AIDS Patient Care STDS 2008; 22:147–158. [DOI] [PubMed] [Google Scholar]
- 17.Joska JA, Westgarth-Taylor J, Hoare J, et al. Neuropsychological outcomes in adults commencing highly active anti-retroviral treatment in South Africa: a prospective study. BMC Infect Dis 2012; 12:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benedetti F, Carlino E, Pollo A. How placebos change the patient's brain. Neuropsychopharmacology 2011; 36:339–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray D, Stoessl AJ. Mechanisms and therapeutic implications of the placebo effect in neurological and psychiatric conditions. Pharmacol Ther 2013; 140:306–318. [DOI] [PubMed] [Google Scholar]
- 20.Holford N. Clinical pharmacology = disease progression + drug action. Br J Clin Pharmacol 2015; 79:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tedaldi EM, Minniti NL, Fischer T. HIV-associated neurocognitive disorders: The relationship of Hiv infection with physical and social comorbidities. BioMed Res Int 2015; 2015:641913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou L, Heilbronner U, Degenhardt F, et al. Genetic variants associated with response to lithium treatment in bipolar disorder: a genome-wide association study. Lancet 2016; 387:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


