Supplemental Digital Content is available in the text
Keywords: East Asians, electronic health record, new-onset diabetes mellitus, statin
Abstract
Although concern regarding the increased risk for new-onset diabetes mellitus (NODM) after statin treatment has been raised, there has been a lack of evidence in real-world clinical practice, particularly in East Asians. We investigated whether statin use is associated with risk for NODM in Koreans. We conducted a retrospective cohort study using the clinical research database from electronic health records. The study cohort consisted of 8265 statin-exposed and 33,060 matched nonexposed patients between January 1996 and August 2013. Matching at a 1:4 ratio was performed using a propensity score based on age, gender, baseline glucose levels (mg/dL), and hypertension. The comparative risks for NODM with various statins (atorvastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin) were estimated by both statin exposure versus matched nonexposed and within-class comparisons. The incidence of NODM among the statin-exposed group (6.000 per 1000 patient-years [PY]) was higher than that of the nonexposed group (3.244 per 1000 PY). The hazard ratio (HR) of NODM after statin exposure was 1.872 (95% confidence interval [CI], 1.432–2.445). Male gender (HR, 1.944; 95% CI, 1.497–2.523), baseline glucose per mg/dL (HR, 1.014; 95% CI, 1.013–1.016), hypertension (HR, 2.232; 95% CI, 1.515–3.288), and thiazide use (HR, 1.337; 95% CI, 1.081–1.655) showed an increased risk for NODM, while angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker showed a decreased risk (HR, 0.774; 95% CI, 0.668–0.897). Atorvastatin-exposed patients showed a higher risk for NODM than their matched nonexposed counterparts (HR, 1.939; 95% CI, 1.278–2.943). However, the risk for NODM was not significantly different among statins in within-class comparisons. In conclusion, an increased risk for NODM was observed among statin users in a practical healthcare setting in Korea.
1. Introduction
Statins, also known as 3-hydroxy-3-methyl-glutaryl coenzyme-A reductase inhibitors, are key agents for treating dyslipidemia, a major cardiovascular disease risk factor.[1] In addition to their ability to lower cholesterol levels in serum, several beneficial pleiotropic effects such as improved endothelial function, stabilization of atherosclerotic plaques, and anti-inflammatory actions have been identified, and the effectiveness of statins for the primary and secondary prevention of cardiovascular disease has been confirmed.[2–4]
Although statins are safe and generally well tolerated by most patients, several relevant adverse effects, mostly myopathy and elevated liver enzymes, may occur.[5] Among them, one recently emerging risk is the increased incidence of new-onset diabetes mellitus (NODM) associated with statin treatment,[6–8] which prompted the United States Food and Drug Administration to add information to statin labels regarding the increased risk for NODM.[9]
However, current available evidence is mainly based on post hoc analyses of randomized controlled trials or meta-analytic results derived from predominantly Western populations; thus, further investigations on the risk for statin-induced NODM in unrestricted real-world clinical practice are necessary, particularly in East Asians. Moreover, bodies of evidence on the comparative safety and risks of NODM between various statins are conflicting.[10–13]
In contrast to clinical trial data, electronic health record (EHR) data gathered from daily practice can provide real-world evidence for diverse observational studies. Demographic information and medical records including information such as diagnoses, prescribed drugs, laboratory test results, and inpatient or outpatient visits are available in EHR data. Although using data recorded as free text (radiology reports and nursing records) is technically challenging, coded data (diagnoses, prescriptions, and laboratory test results) are precise and objective and have been widely used for observational studies.[14,15] Furthermore, EHRs contain large amount of data that can afford sufficient statistical power in many cases that clinical trials cannot, due to the limitation of sample size.
We investigated the risk for NODM with statin treatment in real-world clinical settings using a large amount of observational data from EHRs in Korea. First, we evaluated the comparative risk of NODM between statin-exposed patients versus matched nonexposed patients as the primary endpoint. Second, to evaluate the risk of NODM with various statins as a secondary endpoint, we compared patients exposed to a statin with their matched nonexposed counterparts and those exposed to other statins (within-class comparison analysis).
2. Methods
2.1. Data source
We used a clinical research database containing basic information on patient demographics, diagnoses, drug prescriptions, and laboratory test results originating from the EHRs of a tertiary teaching hospital in Korea (Ajou University Hospital) between January 1996 and August 2013. The database included 116,621,303 prescriptions and 158,122,528 laboratory test results from 1980,385 patients. This study was approved by the local Institutional Review Board, and informed consent was waived (MED-MDB-14-201).
2.2. Patient selection and cohort definition
The study cohort consisted of statin-exposed patients and nonexposed patients who were 18 years of age or older in the subject hospital (Fig. 1). Statin-exposed patients were patients exposed to statins for more than 90 consecutive days. Continuous exposure was defined as follows: if repeated prescription of the drug was followed within a 30-day window, 2 prescriptions were considered continuous administration. For the selection of nonexposed patients, those followed up for more than 90 days and not exposed to any statins were selected. The exposure patients were divided into 6 subgroups according to the type of statin: atorvastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin. Each subgroup included patients who took each statin continuously for more than 90 days. To include only incident users, when a patient had more than 2 continuous administration periods, only the first one was included in the study. Patients with a proportion of days covering <60% were excluded. Patients who had a psychiatric disorder or received organ transplant(s) were excluded due to the established high risk for NODM in these groups.
Figure 1.

Overview of the study design. To evaluate the risk for new-onset diabetes mellitus (NODM) after exposure to statins, statin-exposed patients were compared to matched nonexposed patients (comparison 1). To evaluate the risks associated with 6 different statins (atorvastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin), comparison 2 (comparison between patients exposed to each statin and the matched nonexposed patients) and comparison 3 (within-class analysis) were conducted.
Observation for statin exposure began at day 91 from the first exposure to evaluate the long-term effects of statins on NODM. At the observation start point, patients who already had diabetes mellitus (DM) were excluded (Fig. 1). To exclude DM patients, we only included patients who visited the subject hospital more than once, regardless of outpatient visits or hospitalization, and patients who had more than 1 fasting glucose measurement before the start of observation. We also excluded patients with abnormal random glucose levels (≥200 mg/dL), abnormal fasting glucose levels (≥126 mg/dL), abnormal hemoglobin A1c (HbA1c) results (≥6.5%), those with International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) diagnosis codes related to diabetes (E10–E14), and those who had received a prescription for diabetes medication(s) (acarbose, gemigliptin, glibenclamide, gliclazide, glimepiride, linagliptin, metformin, mitiglinide, nateglinide, pioglitazone, repaglinide, saxagliptin, sitagliptin, vildagliptin, and voglibose) including insulin before the start of observations.
The statin nonexposed group included patients who were never exposed to a statin in the subject hospital and were followed up for more than 90 days (Fig. 1). Observations for the nonexposed group were started at the earliest point meeting the following conditions: the patient visited the subject hospital more than twice, regardless of outpatient visits or hospitalization, and had more than 1 fasting and random glucose measurement, but before having abnormal random glucose levels (≥200 mg/dL), abnormal fasting glucose levels (≥126 mg/dL), abnormal HbA1c results, ICD-10 codes related to diabetes (E10–E14), or prescriptions for diabetes medication(s) as described above. Patients who had no observation period defined in this study were excluded.
Observation was ended when the endpoint, NODM, occurred at the subject hospital. NODM was detected using an existing NODM-detection algorithm.[16] The original version used ICD-9-CM codes, but we modified it to use ICD-10 codes to apply to our data (Supplementary figure S1). First, the algorithm excludes patients who have type 1 DM diagnosis codes (ICD-10 E10). If patients have type 2 DM (T2DM) diagnosis codes (ICD-10 E11), the algorithm checks whether their medication history met the T2DM treatment standard. In cases without T2DM diagnosis codes, patients who received medication(s) for T2DM and had abnormal glucose or HbA1c results were identified as T2DM patients. The earliest time at which patients met the algorithm was considered the time the event occurred.
2.3. Observational variables
We obtained information on age, gender, baseline glucose, the Charlson comorbidity index (CCI), and all concomitant drugs, including thiazide-type diuretics, beta-blockers, angiotensin-converting enzyme inhibitor (ACEi), and angiotensin II receptor blocker (ARB). The age-adjusted CCI is an index of comorbidity. It is calculated using age and the presence of diverse medical conditions. Many studies have used the CCI to select subject groups, minimize group variability, and relay the risk of morbidity. Patients exposed to a fixed-dose combination of ACEi or ARB and thiazide were considered exposed to both ACEi/ARB and thiazide-type diuretics. For each observed drug, the level of exposure was categorized into 3 levels: 0 for nonexposed, 1 for exposure to the drug but the prescribed drug count was less than the median value among the patients exposed to the drug, and 2 for the prescribed drug count equal to or greater than the median value.
2.4. Risk evaluation of NODM after statin exposure
Three models of comparison were used to evaluate the risk for NODM after statin exposure and exposure to individual statins (Fig. 1). In the first model, we compared the risk for NODM between statin-exposed patients and their matched nonexposed counterparts using propensity score matching (1:4 ratio), including the observation period, age, gender, baseline glucose levels, CCI, and hypertension.
In comparisons 2 and 3, we evaluated the risk for NODM after exposure to atorvastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin. In comparison 2, the risk for NODM was compared between patients exposed to a statin and matched nonexposed counterparts. The matching method and variables used for matching were the same as in comparison 1. In comparison 3, a within-class comparison was performed to compare the risk for NODM in patients exposed to 1 of the 6 statins with patients exposed to other statins.
2.5. Statistical analysis
For descriptive analyses, we report means with standard deviations for continuous variables and numbers with percentages for categorical variables. Differences in the characteristics between the exposure and comparison groups were compared using chi-square tests and t tests. To determine the incidence of NODM, we used events per 1000 patient-years (PY) during the observation period. The risk for NODM was compared using Kaplan–Meier analysis with the log-rank test. The adjusted hazard ratios (HRs) of statin exposure were estimated using Cox proportional hazards regression analysis after adjusting for age, gender, baseline glucose levels (per mg/dL); CCI at the start of observation; whether hypertension was present at the start of observation; and level of exposure to ACEi, ARB, beta-blockers, and thiazide-type diuretics during the observation period. Based on the results of the JUPITER trial,[6] we adopted the predicted diabetes incidence rate as 3.0% among statin-exposed patients and 2.4% among controls for the statistical power analysis. The estimated numbers of statin-exposed patients and 1:4 matched controls to be included in the study were 6967 and 27,868, respectively, with 80% power and a 5% 2-sided significance level. The numbers consider the planned sampling process used in our study. We used MS-SQL 2012 (Microsoft, Redmond, WA) as the database-management system. The R package (R Development Core Team, Vienna, Austria) was used for statistical analyses. A P value <0.05 was considered to indicate statistical significance.
3. Results
3.1. Study group
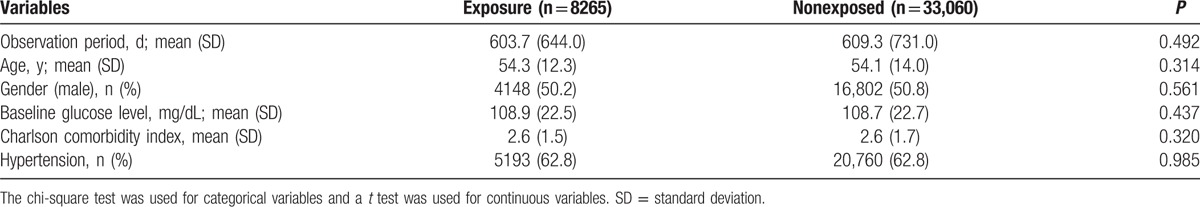
We identified 14,607 patients as the statin-exposed group and 70,474 patients as their matched nonexposed counterparts (Fig. 1). During the observation period, 4328 patients were exposed to atorvastatin, 359 to fluvastatin, 403 to pitavastatin, 1357 to pravastatin, 1429 to rosuvastatin, 1148 to simvastatin, and 5583 to 2 or more types of statins. Based on propensity score matching, 8265 and 33,060 patients were assigned to the exposed and nonexposed groups, respectively. The matched baseline characteristics are presented in Table 1. Major risk factors for the occurrence of DM were well balanced between the exposed and nonexposed groups. In the exposed group, beta-blockers, ACEi/ARB, and thiazide-type diuretics were more frequently used and total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglyceride levels were higher compared to the nonexposed group.
Table 1.
Baseline characteristics of the statin-exposed and matched nonexposed groups.
3.2. Incidence of NODM
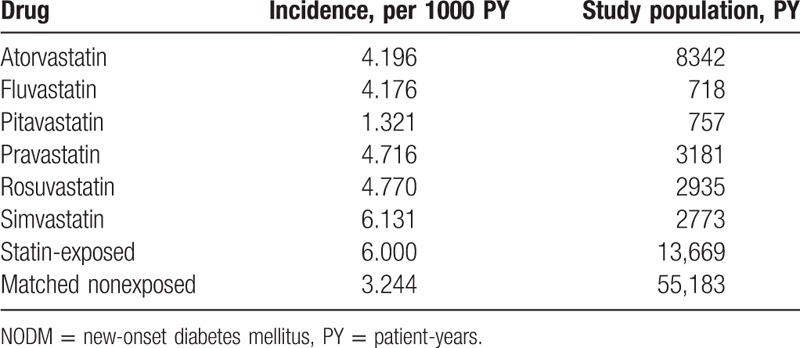
The incidence of NODM in the exposed group was 6.000 per 1000 PY and 3.244 in the matched nonexposed group (Table 2). The incidence rates according to the type of statin were as follows: 4.196 for atorvastatin, 4.176 for fluvastatin, 1.321 for pitavastatin, 4.716 for pravastatin, 4.770 for rosuvastatin, and 6.131 for simvastatin per 1000 PY.
Table 2.
Incidence of NODM according to statin exposure.
3.3. Risk for NODM due to statins
NODM-free survival curves of each group are shown in Fig. 2. Kaplan–Meier survival curves showed a significantly higher occurrence rate of the primary endpoint NODM in the exposed group (P < 0.001, log-rank test). A significant relationship between statin exposure and NODM was consistently shown even after adjusting for age, gender, baseline glucose levels, CCI, hypertension, ACEi/ARB, beta-blockers, and thiazide when using Cox proportional hazard regression analysis (Table 3). The HR of statin exposure was 1.872 (1.432–2.445). Older age, being male, having higher levels of baseline glucose, hypertension, and exposure to thiazide were the factors that significantly increased the risk for NODM, whereas having taken ACEi or ARB significantly decreased the risk.
Figure 2.
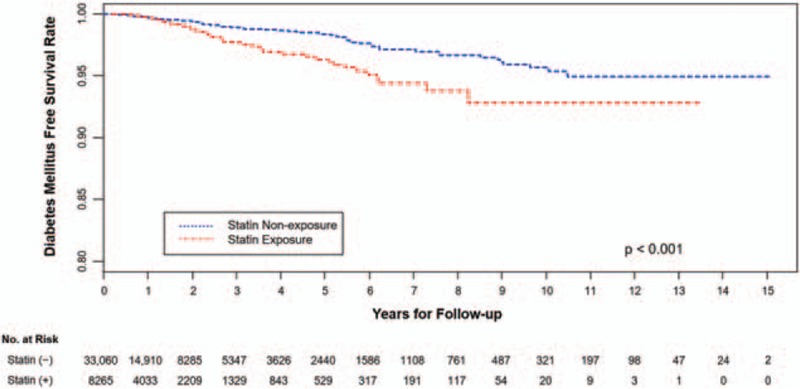
Kaplan–Meier plot for new-onset diabetes mellitus (NODM)-free survival in the statin-exposed group and matched nonexposed group. Kaplan–Meier survival curves showed a significantly higher occurrence rate of the primary endpoint NODM in the statin-exposed group compared with that in the matched nonexposed group (P < 0.001, log-rank test).
Table 3.
HR of statins and observed variables in NODM.
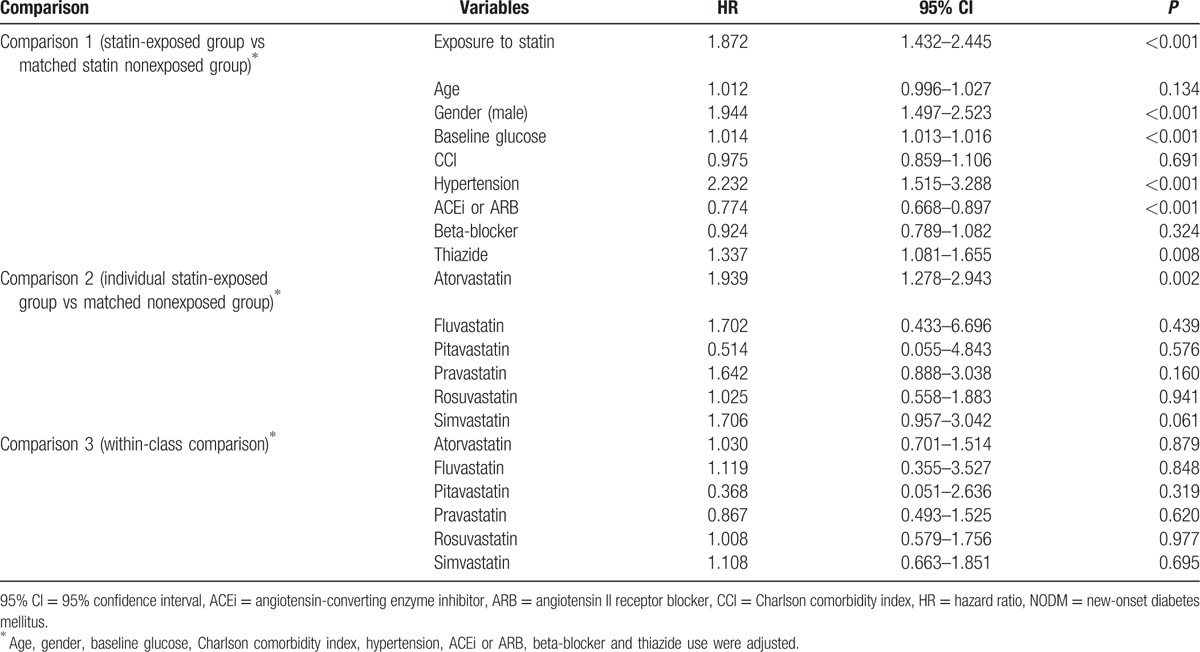
In comparison 2, among the various statins, only the atorvastatin-exposed group had a significantly higher risk for NODM than their matched nonexposed counterparts. In comparisons 2 and 3, pitavastatin tended to have the lowest HR among the 6 statins but without statistical significance.
In addition, the body mass index (BMI), which was suggested to be a risk factor for statin-induced NODM, was available in 3392 statin-exposed patients with their 13,568 matched nonexposed counterparts. In this subgroup of patients, a significant association between statin exposure and NODM was observed after adjusting for age, gender, baseline glucose levels, hypertension, and BMI when using Cox proportional hazards regression analysis (Supplementary table S1). The HR of statin exposure was 1.641 (1.095–2.458). Higher levels of baseline glucose, hypertension, and BMI ≥ 25 were the factors that significantly increased the risk for NODM. Among the various statins, atorvastatin and simvastatin had a significantly higher risk for NODM than their matched nonexposed counterparts, while pitavastatin tended to have the lowest HR among the 6 individual statins but without statistical significance in comparisons 2 and 3 (refer to Supplementary table S1, Supplemental Content, which illustrates HR of statins and observed variables including BMI for NODM).
4. Discussion
We observed that statin treatment increases the risk for NODM in Korean patients. By providing evidence from real-world practice using a large-scale clinical database through an EHR processing algorithm, the results of the present study support and extend previous reports on the increased risk for incidental diabetes with statin treatment.[6–8,10]
Statins are an important medication for the primary and secondary prevention of atherosclerotic cardiovascular diseases and are the most widely used drug prescription due to their well proven benefits and level of safety.[1,2,4,17] However, after a concern was raised in 2009 that statins increase the risk for NODM, various follow-up studies have been performed, urging treatment guidelines to include labeling on the risk for NODM associated with statins.[6–9,18]
Statins not only have therapeutic value for hyperlipidemia but also various pleiotropic effects and are considered essential medications for the prevention and treatment of cardiovascular diseases.[2,4,17] However, because the risk for NODM is a serious adverse effect, reassessing the risks and benefits of statins is important to have balanced insight for treatment.
Most results from previous studies, however, have been based on post hoc analyses from large clinical studies or meta-analyses,[6,7,10,19,20] and additional evidence based on real-world clinical settings is needed, particularly in East Asians, due to the lack of studies in those populations. Although a prospective, large-scale clinical trial would be warranted to resolve these remaining issues, due to the amount of time, cost, and workforce that it would require, such a study will probably not be conducted in the near future. Given this context, the usefulness of the present study is enhanced. We identified occurrences of NODM using an EHR-based algorithm (Supplementary figure S1), and this was deemed a reliable method for the surveillance of NODM.[16] This algorithm provided stricter and more accurate criteria for detecting occurrences of DM than those used in previous studies that defined NODM mainly based on blood glucose, HbA1c levels, and the use of antidiabetic medications. In addition, by using EHR data, including a large number of patients, we were able to minimize selection bias and reflect real-world clinical settings properly despite the retrospective nature of our research.
The HR of 1.87 obtained in this study is a slightly higher value than the results from previous studies. However, this result parallels recent research conducted on Korean patients, in which the relative risk for NODM after statin treatment was high at 1.99, compared to a control group, even though the study included patients who used low-dose atorvastatin.[21] Accordingly, our results suggest that the risk for NODM related to statins might be higher in East Asians, including Koreans, than in Westerners and that, under real-world conditions, the risk for NODM occurrence might be higher than that in a well controlled prospective clinical trial.
One plausible explanation about the relatively higher HR for statin-induced NODM is the possibility that not only patients with simple dyslipidemia but patients with comorbid cardiovascular diseases might be included relatively more in the present study because it was conducted with patients treated at a tertiary hospital. However, although our results may not be generalizable to every patient in need of statin treatment, considering that patients with comorbid cardiovascular diseases (in whom strict secondary prevention is essential[22]) need more active statin treatment, our results may hold important clinical implications.
The methodology that we used allowed us to evaluate the comparative risk for NODM according to the different types of statins. The results suggested that atorvastatin, one of the most lipophilic statins, significantly increased the risk for NODM. Although the mechanisms associated with an increased risk for DM and exposure to statins have not yet been fully revealed, lipophilic statins might be more diabetogenic via several harmful effects, they may have on glucose intolerance, because they penetrate the cell membrane easier and thus are likely to have more extrahepatic effects than hydrophilic statins. Lipophilic statins such as atorvastatin and simvastatin can reduce insulin secretion by inhibiting L-type Ca2+ channels and exocytosis in pancreatic beta-cells.[23] In addition, they decrease the expression of glucose transporter 4 in adipocytes and aggravate insulin sensitivity.[24,25] However, these differences between lipophilic and hydrophilic statins need to be clarified further.[26]
On the other hand, although not statistically significant, pitavastatin tended to lower the risk for NODM in the present study. Similarly, in recent Japanese studies, it had a positive impact on glucose metabolism.[9,27,28] A decrease in coenzyme Q10 due to statins lowers the inflow of Ca2+, which is associated with insulin secretion, leading to abnormal glucose metabolism.[29] However, pitavastatin has been suggested to have minimal effects on coenzyme Q10 via a unique pharmacological mechanism.[30,31] In addition, it has been argued that, by increasing adiponectin and high-density lipoprotein cholesterol, pitavastatin favorably affects glucose metabolism.[30,32]
Meanwhile, when comparing HRs between statins, pitavastatin and pravastatin tended to produce a lower risk for NODM. However, because statistical significance was not observed, caution should be applied when interpreting these results, and further study is needed to verify this finding.
In addition, the use of ACEi or ARB reduced the occurrence of DM, and thiazide-type diuretics increased it. These results are consistent with previous reports[20,33] and should be considered by patients at high risk for abnormal glucose metabolism.
The present study does not discredit the proven benefits of statin treatment or the current guidelines recommending the active use of statins for patients at high risk for cardiovascular diseases, including diabetes.[18,34,35] However, our major results suggest that, for low-risk patients (i.e., those with simple dyslipidemia and/or subclinical atherosclerosis) or patients at high risk for developing diabetes, the balance of risks and benefits should be considered more carefully when determining statin treatment, including the type and dose. Particularly in East Asians, there might be a need to be more cautious regarding the intensity of statin use because relatively less-intensive treatments could bring sufficient lipid-lowering and even plaque regression in those populations compared to Western populations.[36,37]
Importantly, the actions that should be taken if diabetes occurs after using a statin remain unanswered. Additional investigations on whether reducing or stopping statin treatment or changing the type of statin being used would restore glucose metabolism and diabetes are needed.
The present study had several limitations. First, the intensity of statin was not considered. Despite a report on a higher risk for NODM after intensive statin treatment,[35] whether there is a definite correlation between dosage and risk for NODM, as suggested by a recent paper showing an increased risk for NODM even with a low dose of statins in Asian populations,[21] remains controversial. Previous studies have reported that the mere use of a statin is important for NODM development itself, regardless of the dosage.[8,19,21] Second, the comparative risk for NODM between various statins was analyzed as a secondary endpoint; therefore, it was underpowered, and further studies are needed. Third, BMI was not available for all of the patients and was not included in the main analysis, and BMI ≥ 25 was used in propensity matching and Cox regression analysis in the subgroup of patients. However, considering that the association between BMI and statin-induced diabetes remains controversial[7,21] and that BMI may exhibit ethnic differences (only 4.7% of the Korean population has a BMI > 30, the level at which it is considered a risk factor for DM[8,38]), a different criteria regarding BMI might be appropriate to apply to East Asians, and these findings need to be prospectively verified in further studies.
5. Conclusion
We confirmed an increased risk for incident diabetes after statin treatment based on data from real-world clinical practice in Korea. Among various statins, atorvastatin might be more prone to increase the risk for DM than their matched nonexposed counterparts. We need more careful consideration regarding the balance between the risks and benefits when determining statin treatment.
Supplementary Material
Footnotes
Abbreviations: ACEi = angiotensin-converting enzyme inhibitor, ARB = angiotensin II receptor blocker, BMI = body mass index, CCI = Charlson comorbidity index, CI = confidence interval, DM = diabetes mellitus, EHR = electronic health record, HR = hazard ratio, ICD-10 = the International Classification of Diseases 10th Revision, NODM = new-onset diabetes mellitus.
DY and SSS have contributed equally to the article.
Funding/support: The research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C3201). We also received support from the Bio and Medical Technology Development Program of the National Research Foundation funded by the Ministry of Science, Information and Communications Technology, and Future Planning, Republic of Korea (no. 2013M3A9B5075838), and the Ajou University School of Medicine (M-2015-C0460-00109).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
The English in this document has been checked by at least 2 professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/QfVXGe.
References
- 1.Mendis S. The contribution of the Framingham Heart Study to the prevention of cardiovascular disease: a global perspective. Prog Cardiovasc Dis 2010; 53:10–14. [DOI] [PubMed] [Google Scholar]
- 2.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 3.Mihos CG, Pineda AM, Santana O. Cardiovascular effects of statins, beyond lipid-lowering properties. Pharmacol Res 2014; 88:12–19. [DOI] [PubMed] [Google Scholar]
- 4.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013; 31:CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation 2004; 109:III50–III57. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 7.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010; 375:735–742. [DOI] [PubMed] [Google Scholar]
- 8.Waters DD, Ho JE, DeMicco DA, et al. Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J Am Coll Cardiol 2011; 57:1535–1545. [DOI] [PubMed] [Google Scholar]
- 9.Daido H, Horikawa Y, Takeda J. The effects of pitavastatin on glucose metabolism in patients with type 2 diabetes with hypercholesterolemia. Diabetes Res Clin Pract 2014; 106:531–537. [DOI] [PubMed] [Google Scholar]
- 10.Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246,955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes 2013; 6:390–399. [DOI] [PubMed] [Google Scholar]
- 11.Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ 2013; 346:f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaharan NL, Williams D, Bennett K. Statins and risk of treated incident diabetes in a primary care population. Br J Clin Pharmacol 2013; 75:1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho Y, Choe E, Lee YH, et al. Risk of diabetes in patients treated with HMG-CoA reductase inhibitors. Metabolism 2015; 64:482–488. [DOI] [PubMed] [Google Scholar]
- 14.Park I, Sheen SS, Lim HS, et al. Comparison of hyperkalemic risk in hospitalized patients treated with different angiotensin receptor blockers: a retrospective cohort study using a Korean clinical research database. Am J Cardiovasc Drugs 2012; 12:255–262. [DOI] [PubMed] [Google Scholar]
- 15.Yoon D, Park MY, Choi NK, et al. Detection of adverse drug reaction signals using an electronic health records database: Comparison of the Laboratory Extreme Abnormality Ratio (CLEAR) algorithm. Clin Pharmacol Ther 2012; 91:467–474. [DOI] [PubMed] [Google Scholar]
- 16.Kho AN, Hayes MG, Rasmussen-Torvik L, et al. Use of diverse electronic medical record systems to identify genetic risk for type 2 diabetes within a genome-wide association study. J Am Med Inform Assoc 2012; 19:212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mills EJ, Wu P, Chong G, et al. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170,255 patients from 76 randomized trials. QJM 2011; 104:109–124. [DOI] [PubMed] [Google Scholar]
- 18.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S1–45. [DOI] [PubMed] [Google Scholar]
- 19.Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol 2013; 111:1123–1130. [DOI] [PubMed] [Google Scholar]
- 20.Shen L, Shah BR, Reyes EM, et al. Role of diuretics, beta blockers, and statins in increasing the risk of diabetes in patients with impaired glucose tolerance: reanalysis of data from the NAVIGATOR study. BMJ 2013; 347:f6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JY, Rha SW, Choi B, et al. Impact of low dose atorvastatin on development of new-onset diabetes mellitus in Asian population: three-year clinical outcomes. Int J Cardiol 2015; 184:502–506. [DOI] [PubMed] [Google Scholar]
- 22.Miura M, Yamasaki M, Uemura Y, et al. Effect of statin treatment and low-density lipoprotein-cholesterol on short-term mortality in acute myocardial infarction patients undergoing primary percutaneous coronary intervention – Multicenter Registry From Tokyo CCU Network Database. Circ J 2016; 80:461–468. [DOI] [PubMed] [Google Scholar]
- 23.Yada T, Nakata M, Shiraishi T, et al. Inhibition by simvastatin, but not pravastatin, of glucose-induced cytosolic Ca2+ signalling and insulin secretion due to blockade of L-type Ca2+ channels in rat islet beta-cells. Br J Pharmacol 1999; 126:1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganesan S, Ito MK. Coenzyme Q10 ameliorates the reduction in GLUT4 transporter expression induced by simvastatin in 3T3-L1 adipocytes. Metab Syndr Relat Disord 2013; 11:251–255. [DOI] [PubMed] [Google Scholar]
- 25.Nakata M, Nagasaka S, Kusaka I, et al. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia 2006; 49:1881–1892. [DOI] [PubMed] [Google Scholar]
- 26.Izawa A, Kashima Y, Miura T, et al. Assessment of lipophilic vs. hydrophilic statin therapy in acute myocardial infarction – ALPS-AMI study. Circ J 2015; 79:161–168. [DOI] [PubMed] [Google Scholar]
- 27.Mita T, Nakayama S, Abe H, et al. Comparison of effects of pitavastatin and atorvastatin on glucose metabolism in type 2 diabetic patients with hypercholesterolemia. J Diabetes Investig 2013; 4:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu PY, Lin LY, Lin HJ, et al. Pitavastatin and atorvastatin double-blind randomized comparative study among high-risk patients, including those with type 2 diabetes mellitus, in Taiwan (PAPAGO-T Study). PLoS One 2013; 8:e76298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mabuchi H, Higashikata T, Kawashiri M, et al. Reduction of serum ubiquinol-10 and ubiquinone-10 levels by atorvastatin in hypercholesterolemic patients. J Atheroscler Thromb 2005; 12:111–119. [DOI] [PubMed] [Google Scholar]
- 30.Kawashiri MA, Nohara A, Tada H, et al. Comparison of effects of pitavastatin and atorvastatin on plasma coenzyme Q10 in heterozygous familial hypercholesterolemia: results from a crossover study. Clin Pharmacol Ther 2008; 83:731–739. [DOI] [PubMed] [Google Scholar]
- 31.Brault M, Ray J, Gomez YH, et al. Statin treatment and new-onset diabetes: a review of proposed mechanisms. Metabolism 2014; 63:735–745. [DOI] [PubMed] [Google Scholar]
- 32.Kurogi K, Sugiyama S, Sakamoto K, et al. Comparison of pitavastatin with atorvastatin in increasing HDL-cholesterol and adiponectin in patients with dyslipidemia and coronary artery disease: the COMPACT-CAD study. J Cardiol 2013; 62:87–94. [DOI] [PubMed] [Google Scholar]
- 33.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet 2007; 369:201–207. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Pradhan A, MacFadyen JG, et al. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012; 380:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011; 305:2556–2564. [DOI] [PubMed] [Google Scholar]
- 36.Lee CW, Kang SJ, Ahn JM, et al. Comparison of effects of atorvastatin (20 mg) versus rosuvastatin (10 mg) therapy on mild coronary atherosclerotic plaques (from the ARTMAP trial). Am J Cardiol 2012; 109:1700–1704. [DOI] [PubMed] [Google Scholar]
- 37.Takayama T, Hiro T, Yamagishi M, et al. Effect of rosuvastatin on coronary atheroma in stable coronary artery disease: multicenter coronary atherosclerosis study measuring effects of rosuvastatin using intravascular ultrasound in Japanese subjects (COSMOS). Circ J 2009; 73:2110–2117. [DOI] [PubMed] [Google Scholar]
- 38.Korea Health Statistics 2013: Korea National Health and Nutrition Examination Survey (KNAHNES VI-1), 2013; 652, table 3-37. https://knhanes.cdc.go.kr/knhanes/index.do. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


