Abstract
Background:
Acute kidney injury (AKI) is associated with a substantially increased risk of mortality for many hospitalized patients. It has been suggested that early initiation of renal replacement treatment has a favorable outcome in critically ill patients complicated with AKI. However, results of studies evaluating the effect of early initiation strategy of renal replacement treatment on AKI have been controversial and contradictory. The aim of this meta-analysis is to examine the effect of early initiation of renal replacement treatment on patients with AKI.
Methods:
The authors searched relevant studies in PubMed, EMBASE, and the Cochrane Library through August 2016. We searched for all eligible randomized controlled trials with regard to the role of early initiation of renal replacement treatment in mortality among patients with AKI. We extracted the following information from each study: mortality, length of stay in intensive care unit (ICU), and length of stay in hospital. Random and fixed effect models were used for pooling data.
Results:
Twelve trials including 1756 patients were included. The results of this meta-analysis showed that there was no significant difference between the mortality of early and delayed strategy for the initiation of renal replacement treatment using the random effect model (odds ratio = 0.78; 95% confidence interval [CI], 0.52–1.19; P = 0.25), with wild heterogeneity (chi2 = 33.50; I2 = 67%). Analyses from subgroup sepsis and postsurgery came to similar results. In addition, compared with delayed initiation strategy, early initiation showed no significant advantage in length of stay in ICU (mean difference = −0.80; 95% CI, −2.59 to 0.99; P = 0.56) and length of stay in hospital (mean difference = −7.69; 95% CI, −16.14 to 0.76; P = 0.07).
Conclusion:
According to the results from present meta-analysis, early initiation of renal replacement treatment showed no survival benefits in patients with AKI. To achieve optimal timing of renal replacement treatment, further large multicenter randomized trials, with widely accepted and standardized definition of early initiation, are still needed.
Keywords: acute kidney injury, renal replacement therapy, time factors
1. Introduction
Acute kidney injury (AKI) is a well recognized multidisciplinary complication of critical illness, which can lead to abrupt loss of kidney function and is associated with a substantially increased risk of mortality for many hospitalized patients.[1–4] Renal replacement therapy (RRT) is the cornerstone of the management of patients with severe kidney injury and benefits the recovery of renal function.[4] RRT in patients with AKI could prevent uremia and other adverse complications of renal failure. It has been an important part of treatment and is considered an established treatment modality for patients with AKI.[5]
Wide variations, such as timing of initiation, modalities, and dosing, may affect clinical outcomes, particularly survival, although few studies have directly examined these issues.[6] RRT initiation in critically ill patients is complex and conditional on numerous factors. Early initiation means to timely initiate RRT before condition worsened to the degree where the patients become relatively resistant to therapy.[7,8] It has been suggested that early initiation of RRT has a favorable outcome in critically ill patients complicated with AKI.[9–11] However, some other evidence suggests an absence of benefits from an early strategy compared with a delayed one for the initiation of RRT in patients with AKI.[4,12] In addition, the definitions of early initiation in these studies vary from each other. As a result, the studies concerning early initiation of RRT in patients with AKI were inconsistent, and the results remained controversial and contradictory.
Even if some evidence regarding initiation of RRT has been produced, RRT optimal timing still remains uncertain. Recently, several high-quality randomized trials involving the timing of RRT initiation in patients with AKI have been reported. In this study, we conducted a meta-analysis, which extracted results from recently published randomized controlled trials (RCTs) to investigate whether early initiation of RRT in critically ill patients with AKI improves patient survival.
2. Methods
2.1. Search strategy
The systematic review was performed in accordance with Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines.[13] Ethical approval was not required considering the nature of the study.
We searched MEDLINE, EMBASE, and the Cochrane Library through August 2016 without language restriction. The search terms were acute kidney injury, acute renal failure, renal replacement therapy, dialysis, hemodialysis, hemofiltration, time to treatment, time factors, early, earlier, time, accelerate, late, initiation, start, and randomized controlled trials. We further identified studies by reviewing the reference lists of relevant papers identified and by discussing with experts in the field to identify unpublished data.
2.2. Types of outcome measures
The primary outcome was mortality in early and delayed initiation of RRT. Intensive care unit (ICU) and hospital lengths of stay were secondary outcomes. Weighted means were calculated based on the number of patients in each study.
2.3. Study selection
The inclusion criteria were as follows: definite description of factors related to timing of initiation of RRT, diagnosis of AKI, RCT/quasi-RCT, and sufficient data available to calculate a risk ratio (RR) or mean difference (MD) with 95% confidence interval (CI). The following exclusion criteria were used: studies without relevant result, study protocols, pediatric patients, and nonhuman studies.
Two investigators (HW and QC) independently reviewed all abstracts; included the full text of each trial independently; and recorded eligibility, quality, and outcomes. Disagreements between the reviewers concerning the decision to include or exclude a study were resolved through discussion. If necessary, the third reviewer (LL) would be consulted. We excluded duplicate reports, RCTs, and experimental design. Conference abstracts were also excluded, unless published as full-text reports in journals.
2.4. Quality assessment
Two reviewers (HW and QC) independently performed quality assessment. We assessed the quality of the trails according to randomization, blinding, and withdrawals and dropouts in line with the Jadad scoring system (range from 0 to 5).[14] We judged the trails as low-quality study with 2 or less points and high-quality study with 3 or more points.
2.5. Statistical analysis
Before the analysis, we converted data standardly into equivalent units. We calculated, and subsequently pooled in independent meta-analyses, RR with 95% CI for dichotomous outcomes and MD with 95% CI for continuous outcomes. Heterogeneity among pooled studies was evaluated by the Mantel–Haenszel chi-square test. We assessed the degree of interstudy variation according to the I2.[15] Homogeneity assumption was measured by P value. If a P value was less than 0.10, it suggested the evidence of statistically significant heterogeneity, and synthesis of each study was performed by the random effects model.
In this study, we evaluated publication bias by Begg test and Egger test. Sensitivity analysis was conducted by sequentially deleting a single study each time in an attempt to identify the potential influence of each study. A 2-tailed P value <0.05 was considered a criterion for statistical significance. All analyses were analyzed by Review Manager 5.3 (RevMan, The Cochrane Collaboration, Oxford, United Kingdom) and STATA 12.0 (StataCorp, College Station, TX).
3. Result
3.1. Study characteristics
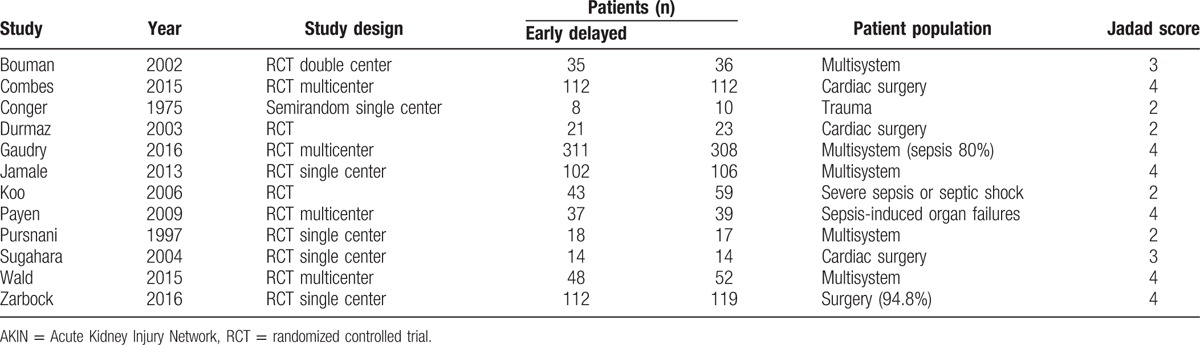
The flowchart of study selection process is presented in Fig. 1. The search strategy identified 2009 studies, and the data were from 12 RCTs comprising 1756 patients (Table 1).[4,12,16–25] One of these trails is conference abstract,[21] which was confirmed to have not been published as full-text report in a peer-reviewed journal. Jadad scores of 4 trails were less than 3.[16,17,19,21] After discussion, we regrettably excluded 2 well designed randomized controlled observational cohort studies.[26,27]
Figure 1.
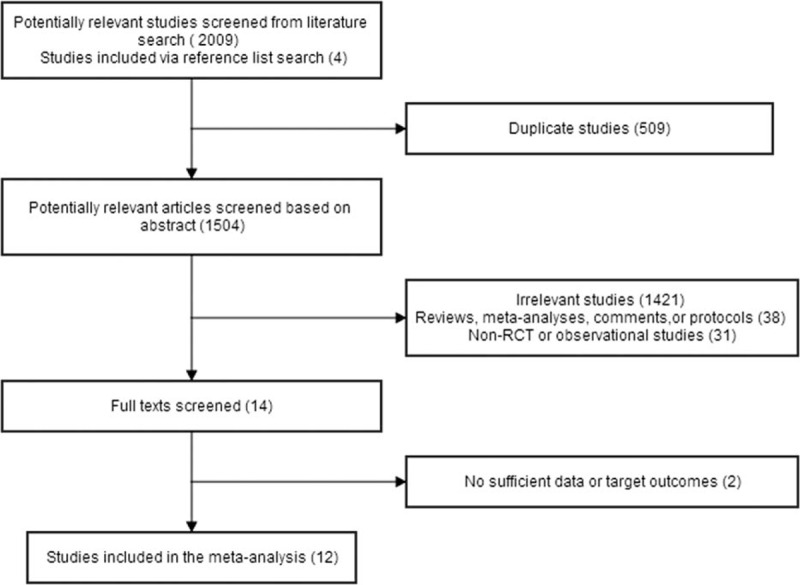
Flowchart of selection process of eligible studies. RCT = randomized controlled trial.
Table 1.
Characteristics of studies included in meta-analysis.
3.2. Primary outcome
A total of 12 RCTs including 1756 patients were included, and the overall mortality in patients with AKI was about 41.57% (344/861 in the early group and 386/895 in the delayed group). There was no significant difference between overall mortality of early and delayed strategy for the initiation of RRT using the random effect model (odds ratio [OR] = 0.78; 95% CI, 0.52–1.19; P = 0.25), with wild heterogeneity (chi2 = 33.50, I2 = 67%) (Fig. 2). Sensitivity analysis sequentially deleting a single study each time revealed that most individual study was consistent. No significant publication bias was detected, with P = 0.193 in Begg test and P = 0.155 in Egger test.
Figure 2.
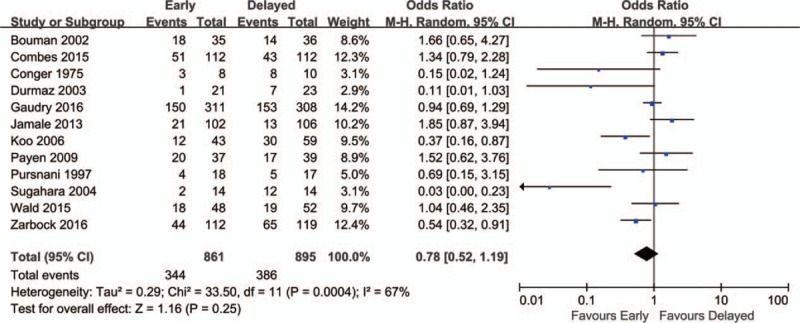
Forest plot for overall mortality.
Subgroup analyses were conducted according to the etiology. Early initiation did not reduce the mortality in subgroup of sepsis (OR = 0.83; 95% CI, 0.43–1.58; P = 0.56) (Fig. 3A). Subgroup analysis from patients after surgery also found that early initiation did not lower mortality compared with the delayed strategy for the initiation of RRT (OR = 0.72; 95% CI, 0.31–1.70; P = 0.46) (Fig. 3B).
Figure 3.
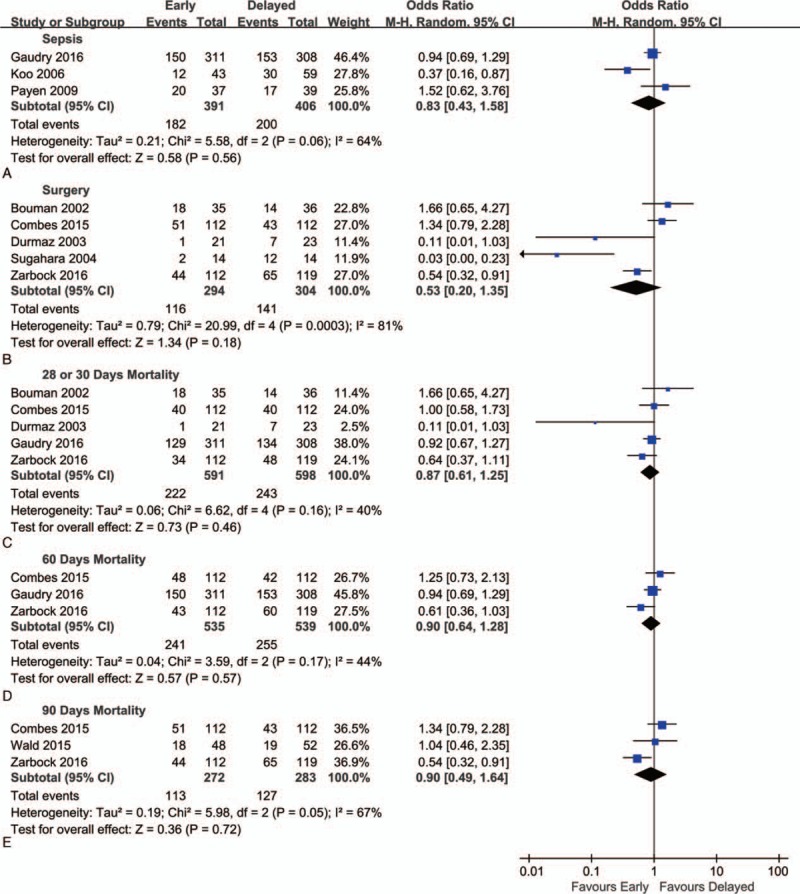
Forest plot for subgroup analyses of mortality. (A) Subgroup analysis for patients with sepsis. (B) Subgroup analysis for patients after surgery. (C–E) Subgroup analysis for mortality at days 30, 60, and 90, respectively.
We analyzed mortalities at 3 different time points (day 28 or 30, day 60, and day 90). But early initiation of RRT failed to show any advantage at each time point (Fig. 3C–E).
3.3. Secondary outcomes
3.3.1. Effect of early initiation of RRT on length of ICU stay
Five of included studies were analyzed to assess effect of early initiation of RRT on length of ICU stay. There was no statistically significant difference in the overall mortality between 2 groups (mean difference, −0.80; 95% CI, −2.59 to 0.99; P = 0.38) with no heterogeneity (chi2 = 2.00; I2 = 0%) (Fig. 4).
Figure 4.

Forest plot for length of stay in intensive care unit.
3.3.2. Effect of early initiation of RRT on length of hospital stay
Available information on the length of hospital stay was analyzed. No statistically significant difference was observed between early and delayed initiation of RRT (mean difference, −7.69; 95% CI, −16.14 to 0.76; P = 0.07) (Fig. 5).
Figure 5.
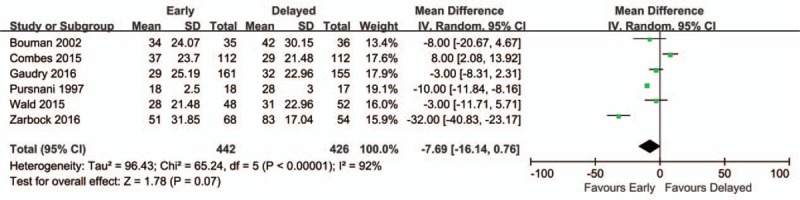
Forest plot for length of stay in hospital.
4. Discussion
The meta-analysis reported detailed analyses of 12 trails comparing early with delayed initiation of RRT on AKI. The results of this meta-analysis showed no significant difference of mortality between an early and a delayed strategy for the initiation of RRT. No significant differences were found in length of stay in ICU and that in hospital.
AKI is a common disease or complication in critically ill patients in ICU. Among patients with AKI requiring RRT, in-hospital mortality rates ranged from 20% to 60.3% when accompanied with nonrenal organ system failure.[28,29] Several studies showed high survival rates and kidney recovery among patients who received early RRT.[30–32] Recently, a single-center trial,[25] comparing early RRT with delayed RRT in patients with AKI trial, reported that early initiation resulted in a 15.4% reduction in 90-day mortality compared with delayed RRT (39.3% vs 53.6%; P = 0.03). For the early group, RRT was initiated within 8 hours of diagnosis of stage 2 AKI using the Kidney Disease: Improving Global Outcomes (KDIGO) classification, while delayed RRT was initiated within 12 hours of stage 3 AKI. Early RRT also showed shorter hospital stay and reduction in selected plasma proinflammatory mediators. There were no differences in organ dysfunction scores or dialysis dependence beyond 90 days. However, in this research, the vast majority (94.8%) of patients were from surgical ICU.
Other studies showed no significant survival or renal function benefit compared with early RRT.[27,33] A multicenter high-quality RCT on this issue involved 620 patients with AKI of KDIGO stage 3.[4] The early strategy started RRT within 6 hours of fulfilling KDIGO stage 3 AKI, while the delayed treatment strategy initiated upon fulfilling clinical criteria related to worsening AKI or complications. The primary outcome, mortality at 60 days, did not differ differently in the 2 groups: 48.5% (95% CI, 42.6–53.8) in the early-strategy group and 49.7% (95% CI, 43.8–55.0) in the delayed strategy group (P = 0.79). There was no difference in secondary endpoints including ventilator and vasoactive-free days through day 28, ICU stay, hospital stay, and 60-day dialysis. However, it is worth noting that only 61% of the patients in the delayed group received dialysis. A 12-center open-label pilot trial by Wald et al[12] compared early (12 hours or less from eligibility) and standard RRT initiation in critically ill adults with volume replete severe AKI. Clinical outcomes were similar, all patients at 90 days following enrollment, with mortality 38% in the accelerated and 37% in the standard group. In another prospective randomized trial,[23] earlier start of dialysis therapy before the onset of significant hyperkalemia, hypervolemia, or uremia did not result in improved survival (relative risk, 1.67; 95% CI, 0.88–3.17; P = 0.2) in patients with community-acquired AKI. They even reported that very early RRT delayed the recovery of kidney function in patients with sepsis. It was showed that RRT was associated with a higher mortality, a longer ICU, and hospital stay in comparison with conservative approach (volume, electrolyte, acid–base homeostasis, and specific drug management without dialysis) in patients with AKI.
However, these should be interpreted cautiously. There are already widely accepted indications for RRT in patients with AKI, which generally include refractory fluid overload; hyperkalemia (plasma potassium concentration >6.5 mEq/L) or rapidly rising potassium levels and/or ECG abnormalities; signs of uremia, such as pericarditis, bleeding, or encephalopathy; severe metabolic acidosis (pH < 7.15); certain alcohol and drug intoxications; and urine output less than 200 mL/12 h or anuria.[6] The definition of early initiation is different from each other, which could explain the heterogeneity in these studies.[34] By early initiation of RRT, clinicians may get better control of fluid and electrolyte status, removal of uremic toxins, and prevention of overt complications attributable to AKI before the patients becoming relatively resistant to therapy.[7,12] But it may also expose them to the potential harms (e.g., hemodynamic instability, hemorrhage, thrombosis, and bacteremia). On the contrary, a delayed strategy may allow for spontaneous recovery of kidney function.[4,12]
4.1. Limitations
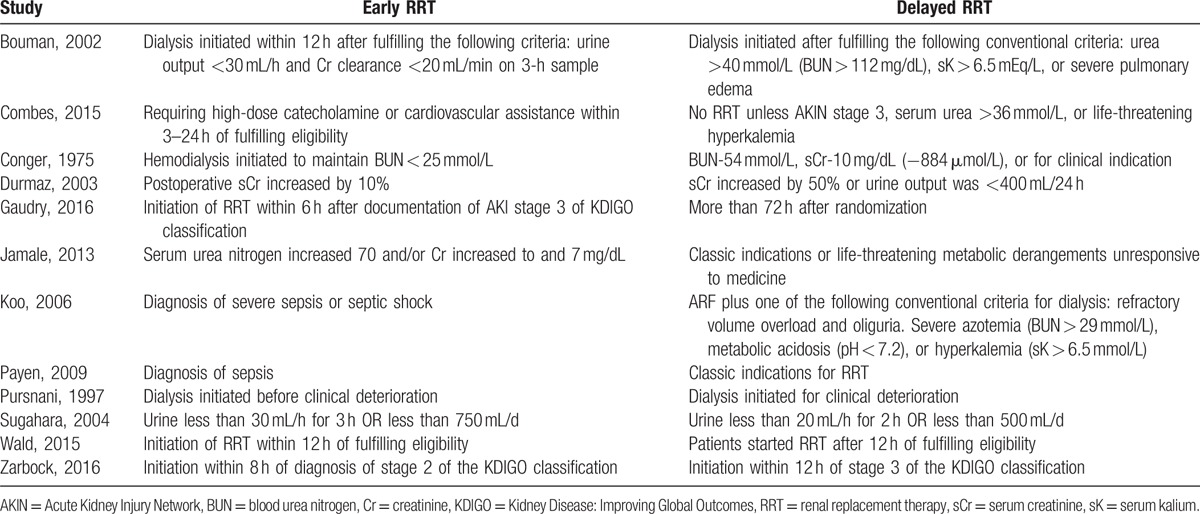
Our meta-analysis has several limitations. First, most studies were not comparable because definition of early was somewhat arbitrary and varies in the literatures (Table 2). A survey, distributed broadly to Canadian nephrologists and intensivists, showed that there was little agreement on what constitutes a trigger for initiation.[35] In addition, the mortality was calculated according to the data of the researches with different follow-up time, which included 14, 30, 60, and 90 days. Second, the studies included were not conducted blindly. It was difficult to perform a double- or triple-blind method study. Third, there were differences in dose, mode, and intensity among these studies. However, there were balances between the 2 groups in each study. Fourth, the morbidity of each trail ranges differently. AKI is a multidisciplinary complication, and about 50% of AKI was caused by sepsis or septic shock. By subgroup analysis, we found similar results in postsurgery and septic patients.
Table 2.
Definitions of early strategy and late strategy initiation of RRT.
5. Conclusion
In present systematic meta-analysis, early initiation of RRT showed no survival benefits in patients with AKI. In this difficult debate, we suggest that the decision to initiate RRT be made according to the specific condition of each patient with AKI. To achieve optimal timing of RRT, large multicenter randomized trials, with widely accepted and standardized definition of early initiation, are still needed.
Footnotes
Abbreviations: AKI = acute kidney injury, CI = confidence interval, ICU = intensive care unit, KDIGO = Kidney Disease: Improving Global Outcomes, OR = odds ratio, RCT = randomized controlled trial, RR = risk ratio, RRT = renal replacement therapy.
Final approval of manuscript: all authors.
Funding/support: The research was carried out with the support from the Fund of Department of Anesthesiology, The First affiliated Hospital of Zhengzhou University.
The authors have no conflicts of interest to disclose.
References
- 1.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015; 41:1411–1423. [DOI] [PubMed] [Google Scholar]
- 2.Hsu CN, Lee CT, Su CH, et al. Incidence, outcomes, and risk factors of community-acquired and hospital-acquired acute kidney injury: a retrospective cohort study. Medicine 2016; 95:e3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta RL, Cerda J, Burdmann EA, et al. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet 2015; 385:2616–2643. [DOI] [PubMed] [Google Scholar]
- 4.Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 2016; 375:122–133. [DOI] [PubMed] [Google Scholar]
- 5.Lins RL, Elseviers MM, Van der Niepen P, et al. Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol Dial Transplant 2009; 24:512–518. [DOI] [PubMed] [Google Scholar]
- 6.Ricci Z, Ronco C. Timing, dose and mode of dialysis in acute kidney injury. Curr Opin Crit Care 2011; 17:556–561. [DOI] [PubMed] [Google Scholar]
- 7.Wierstra BT, Kadri S, Alomar S, et al. The impact of “early” versus “late” initiation of renal replacement therapy in critical care patients with acute kidney injury: a systematic review and evidence synthesis. Crit Care 2016; 20:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 2009; 302:1179–1185. [DOI] [PubMed] [Google Scholar]
- 9.Boussekey N, Capron B, Delannoy PY, et al. Survival in critically ill patients with acute kidney injury treated with early hemodiafiltration. Int J Artif Organs 2012; 35:1039–1046. [DOI] [PubMed] [Google Scholar]
- 10.Carl DE, Grossman C, Behnke M, et al. Effect of timing of dialysis on mortality in critically ill, septic patients with acute renal failure. Hemodial Int 2010; 14:11–17. [DOI] [PubMed] [Google Scholar]
- 11.Shiao CC, Ko WJ, Wu VC, et al. U-curve association between timing of renal replacement therapy initiation and in-hospital mortality in postoperative acute kidney injury. PLoS One 2012; 7:e42952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wald R, Adhikari NKJ, Smith OM, et al. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int 2015; 88:897–904. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001; 135:982–989. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conger JD. A controlled evaluation of prophylactic dialysis in post-traumatic acute renal failure. J Trauma 1975; 15:1056–1063. [DOI] [PubMed] [Google Scholar]
- 17.Pursnani ML, Hazra DK, Singh B, et al. Early haemodialysis in acute tubular necrosis. J Assoc Physicians India 1997; 45:850–852. [PubMed] [Google Scholar]
- 18.Bouman CSC, Oudemans-van Straaten HM, Tijssen JGP, et al. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med 2002; 30:2205–2211. [DOI] [PubMed] [Google Scholar]
- 19.Durmaz I, Yagdi T, Calkavur T, et al. Prophylactic dialysis in patients with renal dysfunction undergoing on-pump coronary artery bypass surgery. Ann Thorac Surg 2003; 75:859–864. [DOI] [PubMed] [Google Scholar]
- 20.Sugahara S, Suzuki H. Early start on continuous hemodialysis therapy improves survival rate in patients with acute renal failure following coronary bypass surgery. Hemodial Int 2004; 8:320–325. [DOI] [PubMed] [Google Scholar]
- 21.Koo J, Yoon J, Oh J. Prospective evaluation of early continuous venovenous hemofiltration (CVVH) on the outcome in patients with severe sepsis or septic shock. J Am Soc Nephrol 2006; 17:50A. [Google Scholar]
- 22.Payen D, Mateo J, Cavaillon JM, et al. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Crit Care Med 2009; 37:803–810. [DOI] [PubMed] [Google Scholar]
- 23.Jamale TE, Hase NK, Kulkarni M, et al. Earlier-start versus usual-start dialysis in patients with community-acquired acute kidney injury: a randomized controlled trial. Am J Kidney Dis 2013; 62:1116–1121. [DOI] [PubMed] [Google Scholar]
- 24.Combes A, Brechot N, Amour J, et al. Early high-volume hemofiltration versus standard care for post-cardiac surgery shock the HEROICS study. Am J Respir Crit Care Med 2015; 192:1179–1190. [DOI] [PubMed] [Google Scholar]
- 25.Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA 2016; 315:2190–2199. [DOI] [PubMed] [Google Scholar]
- 26.Iyem H, Tavli M, Akcicek F, et al. Importance of early dialysis for acute renal failure after an open-heart surgery. Hemodial Int 2009; 13:55–61. [DOI] [PubMed] [Google Scholar]
- 27.Jun M, Bellomo R, Cass A, et al. Timing of renal replacement therapy and patient outcomes in the randomized evaluation of normal versus augmented level of replacement therapy study. Crit Care Med 2014; 42:1756–1765. [DOI] [PubMed] [Google Scholar]
- 28.Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of veterans health administration data. Am J Kidney Dis 2016; 67:872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005; 294:813–818. [DOI] [PubMed] [Google Scholar]
- 30.Lim CC, Tan CS, Kaushik M, et al. Initiating acute dialysis at earlier Acute Kidney Injury Network stage in critically ill patients without traditional indications does not improve outcome: a prospective cohort study. Nephrology (Carlton) 2015; 20:148–154. [DOI] [PubMed] [Google Scholar]
- 31.Shiao CC, Wu VC, Li WY, et al. Late initiation of renal replacement therapy is associated with worse outcomes in acute kidney injury after major abdominal surgery. Crit Care 2009; 13:R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Fernandez N, Perez-Valdivieso JR, Bes-Rastrollo M, et al. Timing of renal replacement therapy after cardiac surgery: a retrospective multicenter Spanish cohort study. Blood Purif 2011; 32:104–111. [DOI] [PubMed] [Google Scholar]
- 33.Chou YH, Huang TM, Wu VC, et al. Impact of timing of renal replacement therapy initiation on outcome of septic acute kidney injury. Crit Care 2011; 15:R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bagshaw SM, Lamontagne F, Joannidis M, et al. When to start renal replacement therapy in critically ill patients with acute kidney injury: comment on AKIKI and ELAIN. Crit Care 2016; 20:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark E, Wald R, Walsh M, et al. Timing of initiation of renal replacement therapy for acute kidney injury: a survey of nephrologists and intensivists in Canada. Nephrol Dial Transplant 2012; 27:2761–2767. [DOI] [PubMed] [Google Scholar]


