Abstract
Positron emission tomography/integrated computed tomography (PET/CT) provides the most accurate imaging modality for preoperative lung cancer staging. However, the diagnostic accuracy of maximum standardized uptake value (SUVmax) for mediastinal (N2) lymph nodes (LN) is unclear. We compared SUVmax, the ratio of LN to primary tumor SUVmax (SUVn/t), and SUVn/t multiplied by maximal tumor diameter (SUVindex) in terms of their abilities to predict mediastinal LN malignancy.
We retrospectively analyzed 170 mediastinal LN stations from 73 consecutive patients who underwent systemic LN resection and PET/CT within 27 days. The SUVmax of the primary tumors was >2.0 and the SUVmax of the mediastinal LN stations ranged from 2.0 to 7.0 on PET/CT. Receiver-operating characteristic curves (ROCs) of SUVmax, SUVn/t, and SUVindex were calculated separately and the areas under the curves (AUCs) were used to assess the abilities of the parameters to predict LN malignancy. The optimal cutoff values were calculated from each ROC curve and the diagnostic abilities were also compared. The diagnostic accuracies of the 3 methods were also assessed separately in smoking and nonsmoking patients.
Twenty-eight LN stations were malignancy-positive and the remaining 142 were malignancy-negative. The AUCs for SUVindex, SUVn/t, and SUVmax were 0.709, 0.590, and 0.673, respectively, and the optimal cutoff values for SUVindex, SUVn/t, and SUVmax were 1.11, 0.34, and 3.6, respectively. The differences between SUVindex and SUVn/t were significant, but there was no significant difference between SUVindex and SUVmax. There were no significant differences between smokers and nonsmokers in the AUCs for any of the methods for predicting LN malignancy (P values >0.05).
SUVindex may be a predictor of mediastinal LN malignancy in lung cancer patients.
Keywords: diagnosis, lung neoplasms, mediastinal lymph node, neoplasm staging, positron emission tomography
1. Introduction
Lymph node (LN) staging, especially preoperative mediastinal LN assessment, is an important assessment in lung cancer patients, with implications for prognosis as well as direct impacts on patient management.[1] Positron emission tomography/integrated computed tomography (PET/CT) is considered as the best noninvasive staging modality in lung cancers,[2,3] though the sensitivity and specificity of maximal standardized uptake values (SUVmax) to predict LN malignancy have varied between studies. The sensitivity and specificity of the most widely used cutoff SUVmax of 2.5 were 40% to 97% and 60% to 96%, respectively.[2–8] The reasons for this wide range included the study of different patient populations, varying criteria for malignancy, and patient-, tumor-, and technique-specific factors.[9,10]
Three previous studies reported on the use of the ratio of LN SUVmax to primary tumor SUVmax (SUVn/t), rather than SUVmax, to predict mediastinal LN malignancy, with the aim of reducing the influence of tumor- and technique-specific factors on the diagnosis.[11–13] Although those studies determined SUVn/t to be a good predictor of mediastinal LN malignancy, the accuracies varied between studies, and tumor diameter, as an important indicator of LN metastasis, was not accounted for in the formula. Furthermore, most LNs in those studies were acquired by biopsy or mediastinoscopy, which is associated with a high potential false-negative rate. In Mattes et al's study,[13] biopsy LNs were not restricted to LNs that appeared malignant on imaging, but were sampled at the discretion of the clinician who performed the biopsy. A total of 77% (392/504) of LNs were obtained by mediastinoscopy or fine needle aspiration, suggesting that up to 77% of PET/CT-positive LNs were not matched to their pathological diagnosis. Mediastinoscopy or fine needle aspiration were used to obtain 21.3% of LNs in Iskender et al's report, but the equivalent rate is not mentioned in Cerfolio's report.
We evaluated the relative capacities of SUVn/t, SUVmax, and also SUVn/t multiplied by maximal tumor diameter (SUVindex) to predict mediastinal LN malignancy using LNs obtained by systemic lymph node resection to reduce the potential false-negative rate. We also evaluated the abilities of these parameters to predict LN malignancy among smokers and nonsmokers, respectively.
2. Methods
2.1. Ethics statement
This retrospective analysis was approved by the institutional review board of The Chinese People's Liberation Army (PLA) General Hospital. All data were extracted from the hospital medical database and were only used for academic research.
2.2. Study population
In this single-center retrospective study, we assessed 170 mediastinal LN stations from 73 patients who underwent fluorodeoxyglucose (FDG) PET/CT and thoracotomy with systemic LN resection between April 2008 and April 2016. A total of 895 consecutive patients underwent PET/CT because of suspicious lung cancer nodules at the Chinese PLA General Hospital, among whom 219 underwent surgery for highly suspected lung cancers. Patients were included if they had at least one detectable mediastinal LN on PET/CT with a LN SUVmax of 2.0 to 7.0 and an SUVmax value for suspicious primary lung cancer >2.0. The LN SUVmax was defined by LN station rather than by single LN because it was difficult to distinguish which LN within the station was responsible for the SUVmax. We also assumed that the LN with the maximal SUVmax on PET/CT in each station was most likely to be the malignant one. All PET/CT-positive LN stations underwent systemic LN dissection.
Patients with a tumor history or who underwent chemotherapy before PET/CT scan were excluded to avoid additional effects on SUVmax. Patients with ground glass opacity (GGO) lung cancer, multiple primary lung cancers, and patients whose mediastinal LNs were obtained via thoracotomy LN sampling, mediastinoscopy, and fine needle aspiration were also excluded to reduce the incidence of potential false-negatives.
Pathological results were matched to the PET/CT findings by LN station. We initially analyzed 441 mediastinal LN stations from 219 patients who had undergone both thoracotomy with systemic LN resection and PET/CT examination. Seventeen patients were excluded because their lung nodules turned out to be benign. A further 42 patients were excluded because all their mediastinal LNs were silent at PET/CT, 68 patients were excluded for having SUVmax <2.0 or >7.0, and 19 patients were excluded because of multiple primary lung cancers or GGO lung cancers.
2.3. PET/CT data collection and test methods
PET/CT scans were performed by using integrated PET/CT scanner (Siemens Biograph 64 High Definition). Patients were instructed to fast at least 6 hours before FDG administration. Whole body scans from the skull to feet (6 bed positions) were preformed 60 minutes later, after injection of 0.15 mCi/kg FDG. The CT examination performed for attenuation correction of PET images. Emission PET data were acquired for 2 minutes per bed position and iterative reconstruction with CT attenuation correction was performed. SUVmax of the primary and of each suspicious lymph node station was defined as the highest FDG uptake within a region of interest defined according to PERCIST criteria.[14] Primary tumor largest dimension, primary tumor SUVmax, and LNs SUVmax were measured at the same time. We define SUVn/t as LN SUVmax divided by primary tumor SUVmax; SUVindex was defined as SUVn/t multiply by primary tumor largest dimension.
2.4. Statistical methods
Patient, tumor, and LN characteristics were reported as descriptive statistics. SUVmax, SUVn/t, and SUVindex values were compared between pathologically positive and negative LNs using Mann–Whitney U tests. The areas under the curve (AUCs) for receiver-operating characteristic curves (ROC) were calculated for SUVmax, SUVn/t, and SUVindex to assess their abilities to predict LN malignancy, with an optimal cutoff value for each parameter for all LNs. The optimal cutoff value was defined as the point on the ROC curve with the maximum sum of sensitivity and specificity. AUCs of the ROC curves were also used to evaluate the abilities of SUVmax, SUVn/t, and SUVindex to predict malignancy in smokers and non-smokers, respectively. ROC curves were tested and compared using the Delong method.
Mann–Whitney U tests and ROC curves were tested and compared using Statistical Analysis System (SAS) version 9.4 (SAS Institute, Cary, NC). P values <0.05 were taken as significant.
3. Results
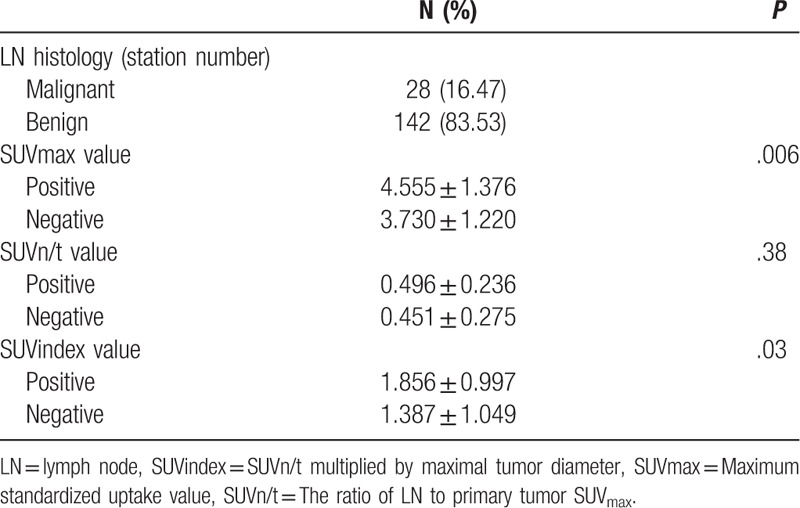
A total of 73 lung cancer patients (21 female, 52 male) with a mean age of 60.63 (median 60, range 36–81) years were included. Among all 73 patients, there were 170 PET/CT-positive mediastinal LN stations. All the LNs were confirmed pathology, with 28 malignant and 142 benign LN stations. The time interval between PET/CT and thoracotomy was 12.21 ± 5.56 (median 11, range 4–27) days. The tumor and patient characteristics are listed in Table 1 and the LN characteristics are listed in Table 2.
Table 1.
Patients’ and tumors’ characteristics.
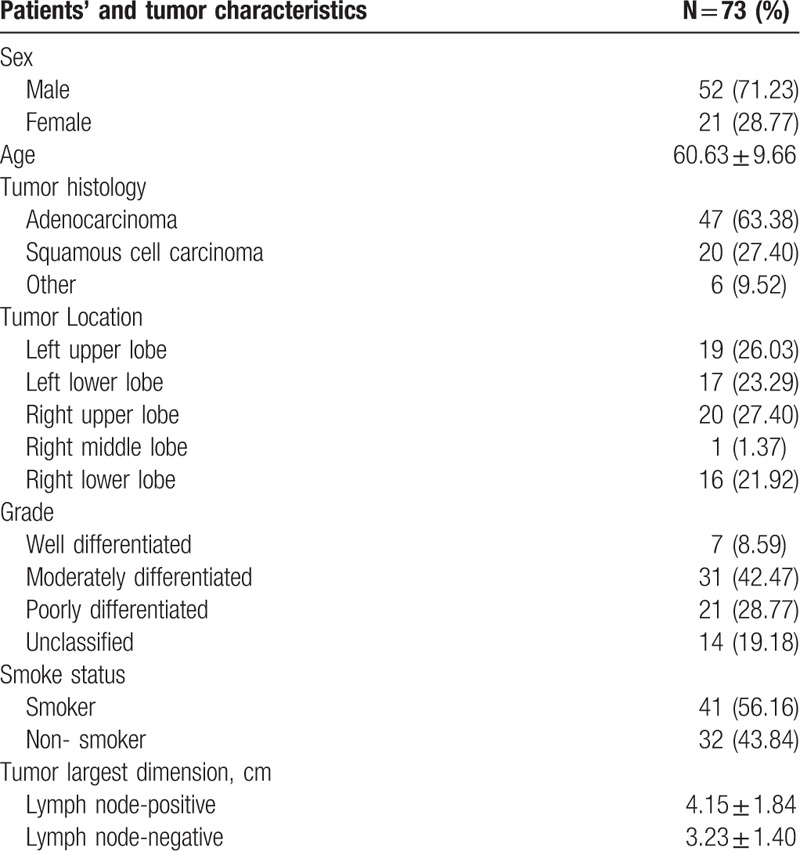
Table 2.
Lymph node characteristics.
According to the ROC curves, SUVindex (AUC = 0.71, P < 0.001) was more accurate in predicting LN malignancy than SUVmax (AUC = 0.67, P < 0.001) and SUVn/t (AUC = 0.59, P = 0.09) among all LNs (Fig. 1). The difference between SUVindex and SUVn/t was statistically significant (P = 0.0245), but that between SUVindex and SUVmax was not significant (P = 0.60).
Figure 1.
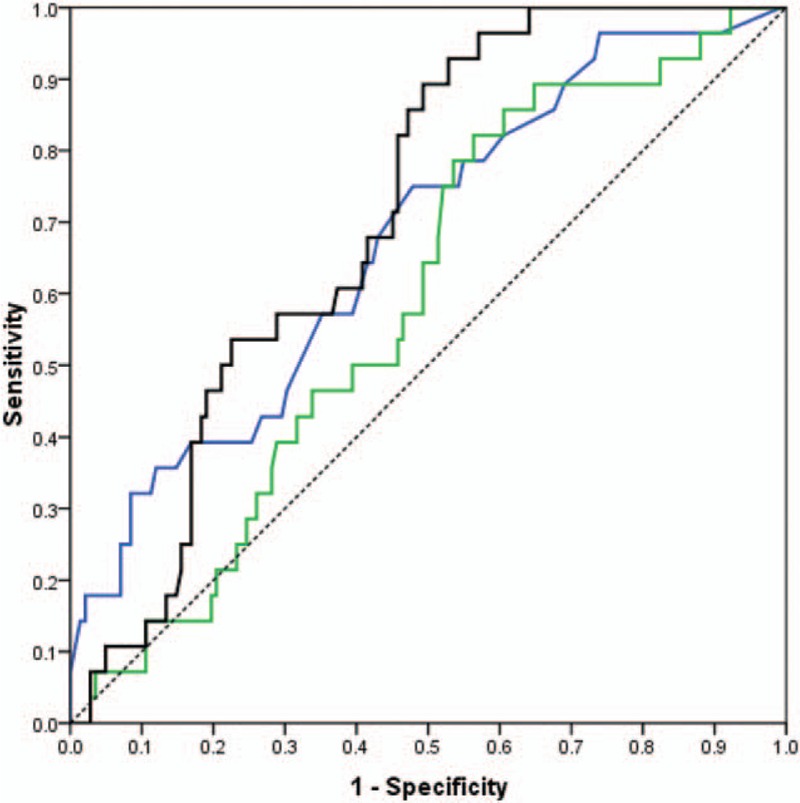
Receiver-operating characteristic (ROC) Curves for all patients who had both primary tumor SUVmax >2.0 and lymph nodes SUVmax 2.0 to 7.0, for SUVmax (blue), SUVn/t (green), and SUVindex (black) with diagonal reference line (dashed).
We also evaluated the diagnostic utilities of SUVmax, SUVn/t, and SUVindex as predictors of LN malignancy among nonsmokers and smokers separately. SUVindex (AUC = 0.72, P < 0.001) was more accurate in predicting malignancy than SUVmax (AUC = 0.69, P < 0.001) and SUVn/t (AUC = 0.62, P = 0.05) among non-smokers (LN stations = 75, N = 32). SUVmax (AUC = 0.71, P = 0.02) was more accurate than SUVindex (AUC = 0.69, P = 0.003) and SUVn/t (AUC = 0.53, P = 0.84) in predicting LN malignancy among smokers (LN stations = 95, patients = 41). However, the differences between smokers and nonsmokers for all tests were not significant (P > 0.05).
All the AUC values and 95% confidence intervals (CI), optimal cutoff values, sensitivities and specificities, and P values are listed in Table 3. ROC curves for smokers and nonsmokers are shown in Figures 2 and 3, and LN PET/CT images for nonsmokers and smokers are shown in Figures 4 and 5.
Table 3.
Receiver=operating characteristic curves parameters for different LN groups.
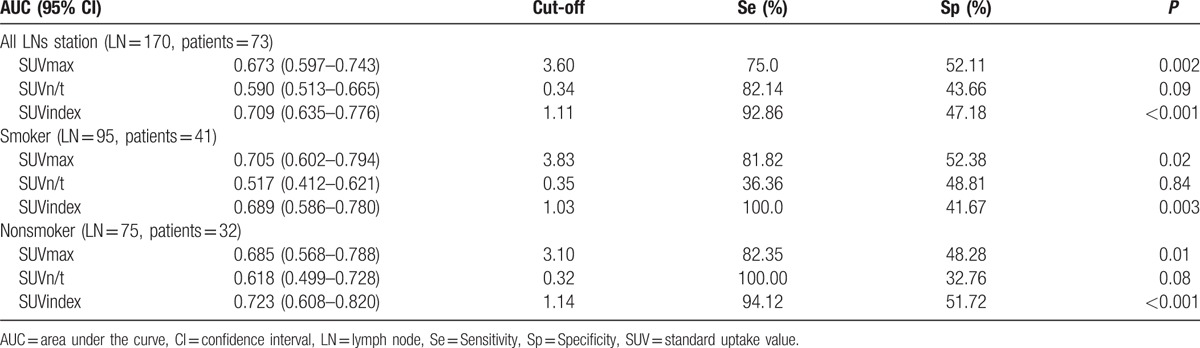
Figure 2.
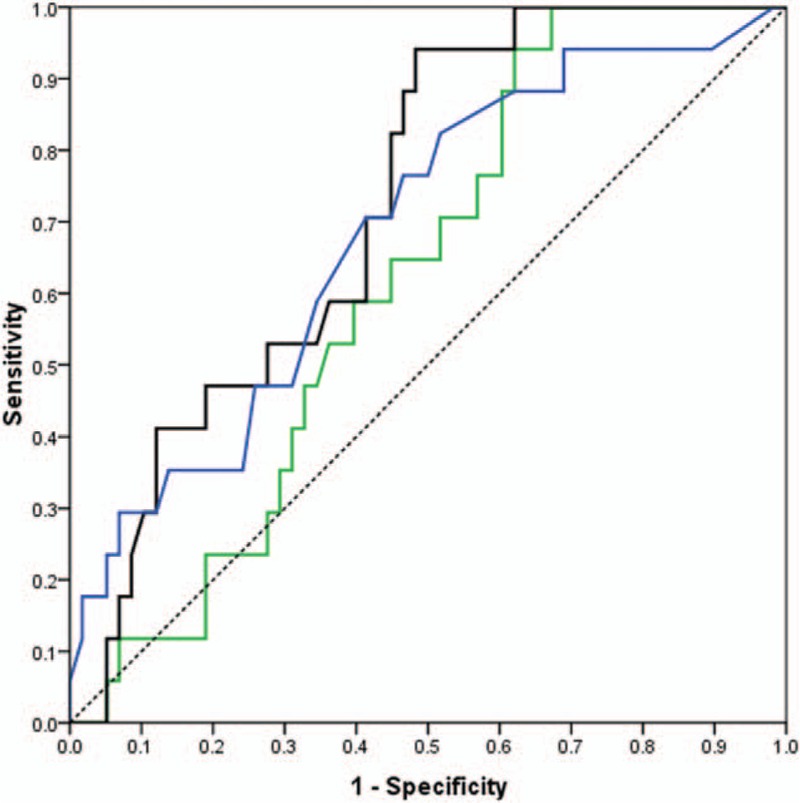
Receiver-operating characteristic (ROC) curves for non-smoker patients who had both primary tumor SUVmax >2.0 and lymph nodes SUVmax 2.0 to 7.0, for SUVmax (blue), SUVn/t (green), and SUVindex (black) With diagonal reference line (dashed).
Figure 3.
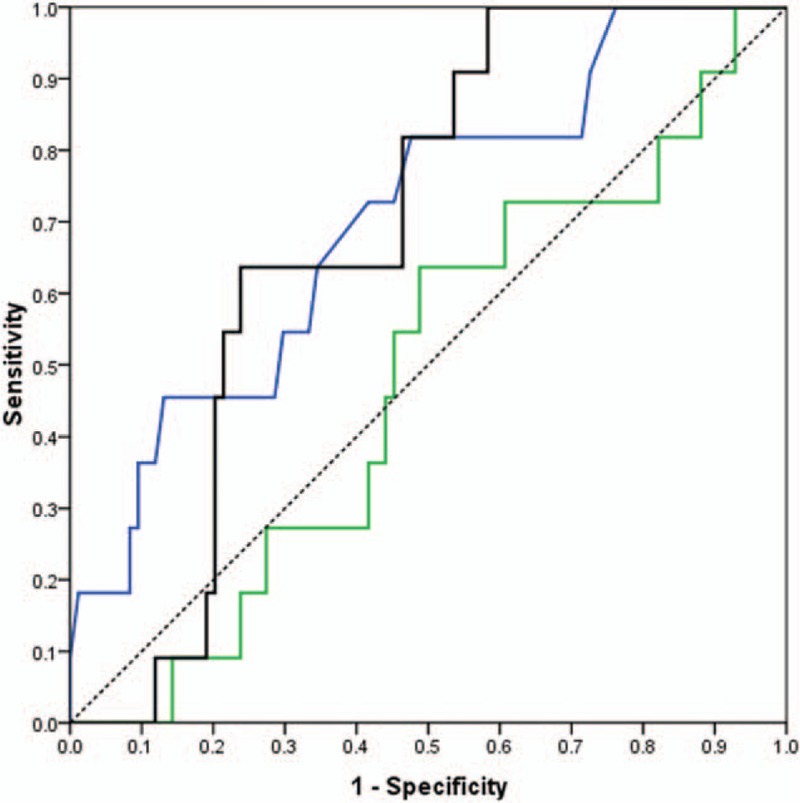
Receiver-operating characteristic (ROC) curves for smoker patients who had both primary tumor SUVmax >2.0 and lymph nodes SUVmax 2.0 to 7.0, for SUVmax (blue), SUVn/t (green), and SUVindex (black) with diagonal reference line (dashed).
Figure 4.
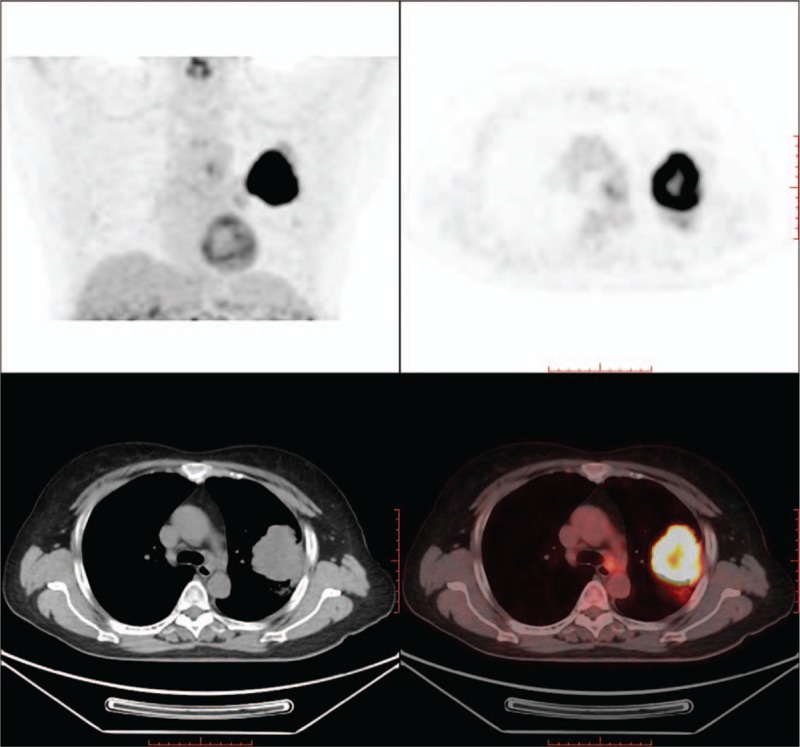
Nonsmoker lung cancer patients positron emission tomography/integrated computed tomography figure. Mediastinal lymph nodes SUVmax value was 2.2 and tumor SUVmax value was 23.9. This positron emission tomography/integrated computed tomography positive mediastinal lymph nodes turns out to be pathological benign.
Figure 5.
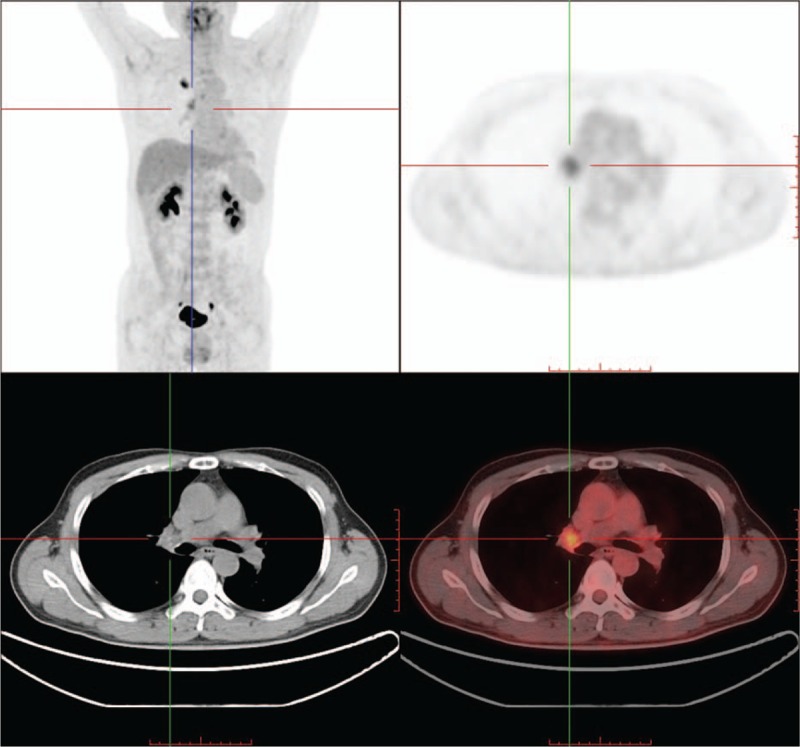
Smoker lung cancer patients positron emission tomography/integrated computed tomography figure. Mediastinal lymph nodes SUVmax value was 2.4 and tumor SUVmax value was 7.9. This positron emission tomography/integrated computed tomography positive mediastinal lymph nodes turns out to be pathological benign.
4. Discussion
SUVmax derived from PET/CT is the most widely used predictor of mediastinal LN malignancy in patients with lung cancer. However, the sensitivity and specificity using a cutoff value of 2.5 have varied considerably among studies. Differences may be caused by several factors, such as differences in patient populations, FDG blood levels, and tumor- and technique-specific factors, all of which may affect the abilities of SUVmax to diagnose LN malignancy accurately. We assessed the ability of SUVn/t, defined as lymph node SUVmax divided by tumor SUVmax, to predict LN malignancy, to eliminate the impacts of some of these factors and thus standardize SUVmax.
Three previous studies focused on the diagnostic accuracy of SUVn/t.[11–13] Cerfolio and Bryant[11] first confirmed SUVn/t as a potential predictor of mediastinal LN malignancy in 2007. They assessed SUVn/t in 335 FDG-avid mediastinal LNs in 239 patients, with an optimal cut-off for predicting malignancy of 0.56 (sensitivity 94%, specificity 72%) and AUC value of 0.79 (95% CI 0.66–0.88, P < 0.001). However, they did not consider the diagnostic utility of SUVmax or compare it with SUVn/t. Iskender's study in 2011 produced similar results. The AUC of SUVn/t (referred to as PET predictive ratio) was 0.69 with an optimal cut-off of 0.49 for predicting malignancy (sensitivity 70%, specificity 65%).[12] However, the diagnostic utility of LN SUVmax was assessed, with an optimal cutoff value 2.75 for predicting malignancy (sensitivity 84%, specificity 87%), whereas the AUC of SUVmax was not mentioned or compared with the PET predictive ratio. LN SUVmax was shown to be more accurate than SUVn/t for predicting LN malignancy. The diagnostic utility of SUVn/t in these 2 previous studies was similar to that found in our study, and SUVmax appeared to be more accurate than SUVn/t for predicting mediastinal LN malignancy.
Mattes recently focused on the diagnostic utility of SUVn/t,[13] using a similar study design to our present study. They also selected patients with both LN SUVmax values from 2.0 to 7.0 and primary tumor SUVmax values >2 because it was relatively difficult to distinguish if these LNs were malignant or benign based on SUVmax value alone. Mattes assessed and compared the diagnostic utilities of SUVmax and SUVn/t and found that SUVn/t (AUC 0.85, 95% CI 0.78–0.92) was significantly more accurate than SUVmax (AUC 0.65, 95% CI 0.55–0.76) for predicting LN malignancy. However, these results differed from those of the present study, which demonstrated that the accuracy of SUVn/t (AUC 0.59, 95% CI 0.51–0.67) was worse than that of SUVmax (AUC 0.67, 95% CI 0.64–0.78).
Differences in the methods used to obtain the LNs may be the most important factor contributing to the differences in results between the present and Mattes's study. All LNs in the current study were obtained by systemic LN resection, whereas most of the LNs in previous studies were obtained by mediastinoscopic biopsy or thoracotomy LN sampling. LN biopsy via mediastinoscopy or sampling may lead to potential false-negative results because the biopsied LNs may not be the positive LNs displayed in the PET/CT image. This potential false-negative rate was mentioned as a major limitation in Mattes's study.[13] Furthermore, most metastatic mediastinal LNs contain only microscopic tumor deposits, and it was therefore hard to obtain and confirm mediastinal LN tumor cells via mediastinoscopic biopsy or LN sampling. The false-negative rates of mediastinoscopic biopsy and LN sampling ranged from 4.4% to 8.2% according to previous studies.[15–17] Differences between studies may also have arisen because we evaluated PET/CT-positive LNs by station, not by single positive LNs, as in all the previous studies. Furthermore, systemic LN resection guaranteed the resection of all PET/CT-positive LNs.
A third possible reason for the apparent discrepancies between study results may be the more-exclusive patient selection criteria employed in the present study. We excluded patients with GGO lung cancer because of its locally invasive characteristics and rare metastasis to LNs.[18–21] We also excluded patients with multiple primary lung cancers because it was hard to confirm which tumors had caused the LN metastasis. However, unlike the previous studies, we did not exclude patients with small-cell lung cancer because we focused on the preoperative diagnostic accuracy of PET/CT, which was not limited to nonsmall cell lung cancers. It is not possible to perform preoperative lung nodule biopsies for all patients, and small-cell lung cancer patients may thus undergo surgery without pathologic confirmation, making it meaningless to limit the use of this diagnostic method to non-small cell lung cancer. Because the AUCs for SUVn/t and SUVmax were not particularly informative, we hypothesized that tumor diameter may also influence the diagnostic accuracy because bigger or higher-stage tumors would have a greater possibility of LN metastasis.[22–24] We therefore assessed the diagnostic efficiency of SUVindex, defined as SUVn/t multiplied by maximal tumor diameter on PET/CT. According to the results, SUVindex (AUC 0.71, 95% CI 0.64–0.78) provided a better predictive index of mediastinal LN malignancy in lung cancer patients than SUVmax or SUVn/t. The optimal SUVindex cutoff value was 1.11, with 92.86% sensitivity and 47.18% specificity. The SUVindex AUC was significantly higher than that for SUVn/t, though there was no significant difference in AUCs between SUVmax (AUC = 0.67, P = 0.002) and SUVindex. However, it is possible that the lack of significance was because of the limited sample size in this study.
We also evaluated the AUCs for SUVmax, SUVn/t, and SUVindex for predicting LN malignancy among non-smokers and smokers, respectively. Among smokers (LN station = 95, N = 41), SUVmax (AUC = 0.71) was more accurate for predicting LN malignancy than and SUVn/t (AUC = 0.52) and SUVindex (AUC = 0.69), whereas SUVindex (AUC = 0.72) showed better diagnostic capabilities than SUVn/t (AUC = 0.69) and SUVmax (AUC = 0.61) in nonsmokers. However, there were no significant differences among the 3 AUCs in either smokers or nonsmokers. It was interesting to note that the diagnostic abilities of SUVmax and SUVindex differed between smokers and nonsmokers, even though the differences were not statistically significant. The SUVmax and SUVindex ROC cutoff values in smokers were higher than in nonsmokers, indicating that malignant LNs had higher SUVmax values in smokers. This may be explained by an increase in noncarcinogenic SUVmax as a result of an inflammatory reaction in intrapulmonary LNs in smokers. These results suggest that it would be difficult to distinguish between inflammatory and malignant LNs on the basis of SUVmax value alone.[25–27] In contrast, increases in LN SUVmax in nonsmokers are less likely to be caused by an inflammatory reaction, resulting in lower SUVmax values compared with smokers. However, given that there were no significant differences in AUCs for SUVmax, SUVn/t, and SUVindex between smokers and nonsmokers, we were unable to conclude that SUVindex would be more accurate for predicting LN malignancy among nonsmokers than smokers.
All the patients in the current study underwent PET/CT and surgery in the same hospital. A lack of data from other hospitals was thus a major limitation of the present study, and we are unable to draw any conclusions about the abilities of SUVindex to predict LN malignancy in other centers, or using different PET/CT scanners. Our study was also limited by the relatively small sample size, as a result of excluding many patients to avoid potential false-positive LNs obtained by mediastinoscopic biopsy or LN sampling.
Further studies conducted in more centers and with larger sample sizes are needed to confirm the accuracy of SUVindex for predicting LN malignancy. Furthermore, the value of SUVmax for predicting malignancy has been reported to differ between LN stations, and further studies are needed to assess the predictive accuracy for each station.[11]
5. Conclusion
In conclusion, SUVindex may provide a more accurate preoperative prediction of mediastinal LN malignancy in patients with lung cancer patients compared with either SUVmax or SUVn/t.
Footnotes
Abbreviations: AUC = area under the curve, GGO = ground glass opacity, N2 LN =mediastinal lymph nodes, PET/CT = positron emission tomography integrated computed tomography, ROC = receiver-operating characteristic, SUVindex = SUVn/t multiplied by maximal tumor diameter, SUVmax = maximum standardized uptake value, SUVn/t = The ratio of LN to primary tumor SUVmax.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997; 111:1718–1723. [DOI] [PubMed] [Google Scholar]
- 2.Truong MT, Viswanathan C, Erasmus JJ. Positron emission tomography/computed tomography in lung cancer staging, prognosis, and assessment of therapeutic response. J Thorac Imag 2011; 26:132–146. [DOI] [PubMed] [Google Scholar]
- 3.Broderick SR, Patterson GA. Performance of integrated positron emission tomography/computed tomography for mediastinal nodal staging in non-small cell lung carcinoma. Thorac Surg Clin 2013; 23:193–198. [DOI] [PubMed] [Google Scholar]
- 4.Lv YL, Yuan DM, Wang K, et al. Diagnostic performance of integrated positron emission tomography/computed tomography for mediastinal lymph node staging in non-small cell lung cancer: a bivariate systematic review and meta-analysis. J Thorac Oncol 2011; 6:1350–1358. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Welch K, Wang L, et al. Negative predictive value of positron emission tomography and computed tomography for stage T1-2N0 non-small-cell lung cancer: a meta-analysis. Clin Lung Cancer 2012; 13:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S, Zheng Q, Ma Y, et al. Implications of false negative and false positive diagnosis in lymph node staging of NSCLC by means of (1)(8)F-FDG PET/CT. PloS One 2013; 8:e78552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harders SW, Madsen HH, Hjorthaug K, et al. Mediastinal staging in non-small-cell lung carcinoma: computed tomography versus F-18-fluorodeoxyglucose positron-emission tomography and computed tomography. Cancer Imag 2014; 14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt-Hansen M, Baldwin DR, Hasler E, et al. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst Rev 2014; 11:CD009519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westerterp M, Pruim J, Oyen W, et al. Quantification of FDG PET studies using standardised uptake values in multi-centre trials: effects of image reconstruction, resolution and ROI definition parameters. Eur J Nucl Med Mol Imag 2007; 34:392–404. [DOI] [PubMed] [Google Scholar]
- 10.Allen TL, Kendi AT, Mitiek MO, et al. Combined contrast-enhanced computed tomography and 18-fluoro-2-deoxy-D-glucose-positron emission tomography in the diagnosis and staging of non-small cell lung cancer. Semin Thorac Cardiovasc Surg 2011; 23:43–50. [DOI] [PubMed] [Google Scholar]
- 11.Cerfolio RJ, Bryant AS. Ratio of the maximum standardized uptake value on FDG-PET of the mediastinal (N2) lymph nodes to the primary tumor may be a universal predictor of nodal malignancy in patients with nonsmall-cell lung cancer. Ann Thorac Surg 2007; 83:1826–1829. [DOI] [PubMed] [Google Scholar]
- 12.Iskender I, Kadioglu SZ, Kosar A, et al. Is there any maximum standardized uptake value variation among positron emission tomography scanners for mediastinal staging in non-small cell lung cancer? Interact Cardiovasc Thorac Surg 2011; 12:965–969. [DOI] [PubMed] [Google Scholar]
- 13.Mattes MD, Moshchinsky AB, Ahsanuddin S, et al. Ratio of lymph node to primary tumor SUV on PET/CT accurately predicts nodal malignancy in non-small-cell lung cancer. Clin Lung Cancer 2015; 16:e253–e258. [DOI] [PubMed] [Google Scholar]
- 14.Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 2009; 50 (suppl 1):122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei B, Bryant AS, Minnich DJ, et al. The safety and efficacy of mediastinoscopy when performed by general thoracic surgeons. Ann Thorac Surg 2014; 97:1878–1883. [DOI] [PubMed] [Google Scholar]
- 16.Citak N, Buyukkale S, Kok A, et al. Does video-assisted mediastinoscopy offer lower false-negative rates for subcarinal lymph nodes compared with standard cervical mediastinoscopy? Thorac Cardiovasc Surg 2014; 62:624–630. [DOI] [PubMed] [Google Scholar]
- 17.Ibeas P, Cantos B, Gasent JM, et al. PET-CT in the staging and treatment of non-small-cell lung cancer. Clin Translational Oncol 2011; 13:368–377. [DOI] [PubMed] [Google Scholar]
- 18.Tsutani Y, Miyata Y, Nakayama H, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest 2014; 145:66–71. [DOI] [PubMed] [Google Scholar]
- 19.Lee HY, Lee KS. Ground-glass opacity nodules: histopathology, imaging evaluation, and clinical implications. J Thorac Imag 2011; 26:106–118. [DOI] [PubMed] [Google Scholar]
- 20.Ye B, Cheng M, Li W, et al. Predictive factors for lymph node metastasis in clinical stage IA lung adenocarcinoma. Ann Thorac Surg 2014; 98:217–223. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura S, Fukui T, Kawaguchi K, et al. Does ground glass opacity-dominant feature have a prognostic significance even in clinical T2aN0M0 lung adenocarcinoma? Lung Cancer 2015; 89:38–42. [DOI] [PubMed] [Google Scholar]
- 22.Gulack BC, Yang CF, Speicher PJ, et al. The impact of tumor size on the association of the extent of lymph node resection and survival in clinical stage I non-small cell lung cancer. Lung Cancer 2015; 90:554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada E, Ishii G, Aramaki N, et al. Tumor-size-based morphological features of metastatic lymph node tumors from primary lung adenocarcinoma. Pathol Int 2014; 64:591–600. [DOI] [PubMed] [Google Scholar]
- 24.Xu ZQ, Xie LJ, Fan W, et al. Risk factors for mediastinal lymph node metastasis in non-small-cell lung cancer by PET/CT. Nucl Med Commun 2014; 35:466–471. [DOI] [PubMed] [Google Scholar]
- 25.Lin WY, Hsu WH, Lin KH, et al. Role of preoperative PET-CT in assessing mediastinal and hilar lymph node status in early stage lung cancer. J Chinese Med Assoc: JCMA 2012; 75:203–208. [DOI] [PubMed] [Google Scholar]
- 26.Cerfolio RJ, Bryant AS. The role of integrated positron emission tomography-computerized tomography in evaluating and staging patients with non-small cell lung cancer. Semin Thorac Cardiovasc Surg 2007; 19:192–200. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Zhao B, Wang X, et al. Overestimated value of (18)F-FDG PET/CT to diagnose pulmonary nodules: Analysis of 298 patients. Clin Radiol 2014; 69:e352–e357. [DOI] [PubMed] [Google Scholar]


