Abstract
Active Wnt/β-catenin signaling in the dermal papilla (DP) is required for postnatal hair cycling. In addition, maintenance of the hair-inducing ability of DP cells in vitro requires external addition of Wnt molecules. However, whether DP cells are a critical source of Wnt ligands and induce both autocrine and paracrine signaling cascades to promote adult hair follicle growth and regeneration remains elusive. To address this question, we generated an animal model that allows inducible ablation of Wntless (Wls), a transmembrane Wnt exporter protein, in CD133-positive (CD133+) DP cells. CD133+ cells have been shown to be a specific subpopulation of cells in the DP, which possesses the hair-inducing capability. Here, we show that ablation of Wls expression in CD133+ DP cells results in a shortened period of postnatal hair growth. Mutant hair follicles were unable to enter full anagen (hair growth stage) and progressed toward a rapid regression. Notably, reduced size of the DP and decreased expression of anagen DP marker, versican, were observed in hair follicles when CD133+ DP cells lost Wls expression. Further analysis showed that Wls-deficient CD133+ DP cells led to reduced proliferation and differentiation in matrix keratinocytes and melanocytes that are needed for the generation of the hair follicle structure and a pigmented hair shaft. These findings clearly demonstrate that Wnt ligands produced by CD133+ DP cells play an important role in postnatal hair growth by maintaining the inductivity of DP cells and mediating the signaling cross-talk between the mesenchyme and the epithelial compartment.
Introduction
The mature hair follicle is a regenerating mini-organ that primarily comprises of epithelial and dermal compartments [1]. The cells in these two compartments interact with each other through molecular signals that collectively drive a repetitive process of hair follicle reconstitution [2], comprising cyclical periods of growth (anagen), regression (catagen) and rest (telogen) [3]. In mice, the size and shape of murine pelage hairs and their cycling properties are affected by the number of cells in the DP [4]. It has been conclusively shown that DP cells of growing hair follicles possess inductive properties that regulate the behavior of epidermal keratinocytes, resulting in the regeneration of hair follicle structure and fiber [5,6]. Unfortunately, reciprocal signaling between the epithelial compartment and the DP is complicated and still remains to be defined.
The canonical Wnt/β-catenin signaling pathway is broadly active in hair follicles and modulates the epithelial–mesenchymal interactions (EMIs) that control hair follicle regenerative cycles [7]. This pathway becomes active when the secreted Wnt ligands, which are glycoproteins, bind to Frizzled (Fzd) receptors and form a complex with Lrp5/6 coreceptors [8]. In the absence of Wnt ligands (off-state), cytoplasmic β-catenin, a dual function protein involved in both cell–cell adhesion and transcriptional regulation, is degraded upon phosphorylation by the Apc/Axin/Gsk-3β complex and subsequent ubiquitination [9]. When Wnt ligands are present (on-state), they form a complex with Fzd receptors and Lrp5/6 coreceptors, triggering inactivation of the Apc/Axin/Gsk-3β complex. Consequently, disheveled is activated to displace Gsk-3β from the Apc/Axin/Gsk-3β complex, so that cytoplasmic β-catenin can no longer be targeted for degradation. Subsequently, β-catenin accumulates in the cytoplasm and translocates to the nucleus where it forms complexes with members of the Lef1/Tcf transcription factor family. This Lef1/Tcf–β-catenin complex displaces transcriptional repressors from target gene promoters and recruits additional coactivators to activate gene expression of Wnt signaling target genes [10]. The on/off of Wnt/β-catenin signaling is not only controlled by Wnt ligands, but also by different Wnt inhibitors, including members of the Dickkopf family and Wnt inhibitory factor 1 [11]. Many genetic experiments have clearly indicated that co-ordinated Wnt/β-catenin activity in both epithelial and mesenchymal compartments of the mature hair follicle is indispensable for hair follicle regenerative cycling.
The source and nature of Wnt ligands remain intriguing questions. Epidermal Wnt ligands have been shown to be necessary for hair follicle morphogenesis and postnatal hair cycling [12–15]. Simlarly, the DP could be a source of Wnt ligands for postnatal hair cycling as well. Wnt5a, Wnt10a and Wnt11 mRNAs are expressed in the upper dermis or dermal condensate at mouse embryonic day E14.5 [16]. In one study, Wnt5a expression was identified in the DP during the adult hair growth cycle [16]. Deletion of Wnt5a in DP cells caused abnormal hair follicle differentiation [17]. Another important point to consider is that DP cells require external addition of Wnt protein to maintain their hair-inducing ability in vitro, which may also be the case in vivo [18,19]. These observations suggest an important role for DP-derived Wnt ligands in postnatal hair growth. However, despite these advances, the key question still remains on whether DP-derived Wnt ligands are required for active Wnt/β-catenin signaling in both epithelial and mesenchymal compartments in the adult hair cycle.
To address this question, we have designed a novel genetic approach to block the export of Wnt ligands from CD133+ DP cells by inducible deletion of a Wnt-exporting protein, Wntless (Wls) [20,21]. Wls, also referred to as ‘evenness interrupted’ in Drosophila and as MOM-3/MIG-14 in Caenorhabditis elegans, is a seven-pass transmembrane protein [22]. Because Wnt ligands need first to be exported from the producing cells before they can function on their target cells, the ablation of Wls will result in the accumulation of Wnt ligands inside Wnt-producing cells and consequently the absence of Wnt signaling in Wnt target cells [22–24]. Multiple reports have confirmed that Wls is indeed responsible for the surface delivery and secretion of Wnt proteins in hair follicles [12–15,25]. CD133+ DP cells are the population in the DP that has trichogenic ability and is capable of regulating DP size [26,27]. However, whether CD133+ DP cells produce Wnt proteins to maintain the hair-inducing capacaity and interact with keratiocytes to build hair follicles in vivo remains unknown.
Here, we report that ablation of Wls expression in CD133+ DP cells delays hair growth and causes premature hair follicle regression. Further analysis shows that Wls deficiency in CD133+ DP cells not only affects the proliferation and differentiation of matrix keratinocytes, but also modulates the biological properties of DP cells. Taken together, our data highlight that Wnt ligands generated by CD133+ DP cells are critical for the postnatal hair regenerative cycle by co-ordinately regulating both the epithelial compartment and the mesenchymal niche.
Methods
Mice
CD133-CreERT2 (Prom1C-L) mice and Wlsfl/fl mice were generated as previously reported [20,28]. To generate CD133-CreERT2; Wlsfl/fl mice, CD133-CreERT2 mice were crossed with Wlsfl/fl mice for several generations. TOPGAL mice were obtained from the Jackson Laboratory (stock number 004623). All mice were housed in the Laboratory Animal Services Facility of the University of Cincinnati under an artificial 12/12 light–dark cycle and were allowed free access to normal mouse feedings and water. The Institutional Animal Care and Use Committee of the University of Cincinnati approved all experimental procedures involving mice. All animals were cared daily, 7 days per week, by three full-time veterinarians, six veterinary technicians plus animal care staff.
Mice were genotyped by polymerase chain reaction (PCR) analysis of genomic DNA extracted from mouse tail biopsies. Wls alleles were detected using the following primers: P1, CTTCCCTGCTTCTTTAAGCGTC; P2, AGGCTTCGAACGTAACTGACC; P4, CTCAGAACTCCCTTCTTGAAGC. P2 and P4 primers were used for the detection of 411-bp wild-type allele and 556-bp floxed allele. P1 and P4 primers were used for 1625-bp wild-type allele and 410-bp recombined allele after successful Cre deletion. CD133 alleles were genotyped using the following primers: forward primer: CAGGCTGTTAGCTTGGGTTC; reverse primer 1: AGGCAAATTTTGGTGTACGG; reverse primer 2: TAGCGTGGTCATGAAGCAAC. Wild-type CD133 allele was genotyped using forward primer with reverse primer 2. Insertion of CreERT2 transgene in CD133 locus was genotyped using forward primer with reverse primer 1. TOPGAL transgene (LacZ) was genotyped using the following primers: forward primer: ATCCTCTGCATGGTCAGGTC and reverse primer: CGTGGCCTGATTCATTCC. The PCR protocol used was 94°C for 3 min followed by 35 cycles of 94°C for 30 s, 62°C for 30 s and 72°C for 40 s, and a final extension at 72°C for 10 min.
Whole-mount X-gal staining
Skin biopsies of a size of 0.5 × 1 cm from the mid-dorsal region of TOPGAL mice were collected at postnatal day 25 (P25), P28, P32, P35, P40 and P45 for X-gal whole-mount staining. Briefly, skin tissues were fixed in 4% paraformaldehyde (PFA) at room temperature for 30 min, washed with phosphate-buffered saline (PBS) and immersed in X-gal staining buffer [1 mg/ml X-gal (5Primer, Gaithersburg, MD), 5 mM potassium ferricyanide (Sigma-Aldrich, St. Louis, MO), 5 mM potassium ferrocyanide (Sigma-Aldrich, St. Louis, MO) in PBS] at room temperature for 48 h. After staining, tissues were fixed in 4% PFA with 10% sucrose overnight at 4°C and then embedded in tissue embedding medium (OCT) for sectioning. Frozen sections were counterstained with nuclear red and examined under a Nikon Eclipse 80i microscope. Images were analyzed using Adobe Photoshop.
Transgene induction
To induce Cre activity and recombination of Wls floxed alleles, mice were administered with tamoxifen (TAM) (Sigma-Aldrich, St. Louis, MO) in corn oil (10 mg/ml) by intraperitoneal (IP) injection at 1 mg/g body weight starting from P21 to P27. Skin samples were collected at P28, P30, P32, P35, P40 and P45. To ablate Wls expression during mid-anagen, mice were administered with TAM at P30 by IP injection for 5 or 7 consecutive days based on the date when skin biopsies were harvested. Skin biopsies were collected at P35, P40 and P50.
Skin biopsies
Stages of normal hair cycle were determined according to the classification system published previously [1]. Mid-dorsal skin biopsies were taken from CO2 inhalation-killed mice, the standard American Veterinary Medical Association method. Death was confirmed by the lack of heartbeat. Cervical dislocation was performed to ensure mice would not recover. Two hours before sacrifice, all mice were given one IP injection of bromodeoxyuridine (BrdU) based on their body weight (50 µg/g of body weight). Hair on the back of mice was carefully shaved using an electric clipper before harvesting skin biopsies. Collected skin tissues were then fixed and processed for paraffin and frozen sectioning.
Histology and immunostaining
Histology and immunohistochemistry were performed as described previously [12]. For histology, paraffin sections were stained with hematoxylin and eosin (H&E) using standard protocols. For immunostaining, paraffin sections were deparaffinized, rehydrated and then demasked in citrate buffer (pH 6.0) using the microwave heating method. After washing with PBS, sections were blocked in 10% bovine serum albumin in PBS and subsequently incubated at 4°C overnight with each primary antibody. Slides were then washed with PBS for three times and incubated with the corresponding biotin-conjugated secondary antibodies (Vector Laboratory, Burlingame, CA) at room temperature for 1 h. For immunofluorescence staining, slides were incubated with either fluorescein or Texas red-conjugated streptavidin for 1 h. Microscopy was performed using a Nikon Eclipse 80i fluorescence microscope and images were analyzed using ImageJ (NIH).
The following primary antibodies were used: anti-Wntless (1:500, a gift from Dr Richard Lang, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH), anti-β-catenin (Invitrogen, 15B8, 1:1000), anti-BrdU (Abcam, BU1/75, 1:25), anti-Ki67 (Imgenex, 1:50), anti-Lef1 (Cell Signaling, 1:100), anti-versican (Millipore, 1:200), anti-Sox9 (Millipore, 1:100), anti-GATA3 (Sant Cruz, 1:50), anti-K15 (Vector Laboratory, 1:50), anti-AE13 (1:25, a gift from Dr Tung-Tien Sun, NYU Langone Medical Center, New York, NY), anti-AE15 (1:25, a gift from Dr Tung-Tien Sun, NYU Langone Medical Center, New York, NY) and anti-microphthalmia-associated transcription factor (MITF; Thermo Fisher, 1:200). Stained slides were examined under a Nikon Eclipse 80i fluorescence microscope.
Isolation of CD133+ DP cells
Isolation of CD133+ DP cells was performed as previously described with modification [21]. Briefly, TAM in corn oil (10 mg/ml) was administered to adult mice by IP injection at 1 mg/g body weight for 7 consecutive days from P21 to P27 to induce Cre expression and Wls ablation. Mid-dorsal back skins were harvested at P30 and floated in 0.1% dispase (Thermo Fisher, Waltham, MA) at 4°C for 16 h to separate the epidermis and dermis. The epidermis was then discarded, and the dermis was treated with 0.5% collagenase IV (Thermo Fisher, Waltham, MA). Dissociated dermal cells were filtered with cell strainer, collected by centrifugation and followed by resuspension in 100 µl of culture medium and incubation with APC-conjugated anti-CD133 antibodies (eBioscience, San Diego, CA, 1:50) for 30 min at 4°C. Cell sorting was performed using a MoFlo high-speed sorter (Dako Cytomation, Carpinteria, CA).
Western blotting
Immunoblotting for Wnt5a expression (Cell Signaling, 1:500) using isolated CD133+ DP cells was performed as described recently [29]. To verify equal loading of samples, membranes were stripped and reprobed with monoclonal anti-β-actin antibody (Invitrogen, 1:5000), followed by an HRP-conjugated goat anti-mouse IgG (Cell Signaling, 1:2000). The intensities of Wnt5a and β-actin bands were determined by scanning X-ray films and measuring using ImageJ software (NIH). The relative expression level of Wnt5a was calculated by dividing the value of Wnt5a by the net loading control of β-actin.
Hair follicle number counting
Stages of hair follicles were determined according to previously published classifications [3], which describe the key morphological features of murine hair follicles during the hair cycle comprising of hair follicle growth anagen, catagen and telogen stages. The number of hair follicles was counted manually by examining H&E-stained skin samples (×10 objective). At least 10 fields for each skin biopsy were examined for hair follicle counting. A minimum of three CD133-CreERT2; Wlsfl/fl mutant mice and three control littermates were counted for each time point.
Alkaline phosphatase staining
Alkaline phosphatase (AP) staining was performed using a VECTOR Red AP Substrate Kit (Vector Laboratory, Burlingame, CA) according to the manufacturer’s instruction. Briefly, frozen sections were washed with PBS for 5 min twice and incubated with the substrate solution for 30 min in the dark. Slides were then washed in 100 mM Tris–HCl buffer (pH 8.5) for 5 min, rinsed in water and mounted with a VECTASHIELD Mounting Medium with DAPI for observation.
DP cell number counting
DP cells in each hair follicle were counted on skin biopsy sections after AP staining and DAPI counterstaining under a Nikon Eclipse 80i fluorescence microscope using the high power 40× objective. For counting, a minimum of 10 hair follicles was randomly picked from at least two pieces of mid-dorsal skin tissues of each mouse. For each genotype, three mice were used with a total of 30 hair follicles counted.
Statistical analysis
All graphs were generated using Microsoft Excel (2016). Statistical analysis of difference was carried out by Student’s t-test using a GraphPad Prism 5.01 software package (GraphPad Software, Inc., San Diego, CA) and represented as mean ± SEM, with P < 0.05 considered statistically significant.
Result
Wls is expressed in DP cells during anagen stage except onset
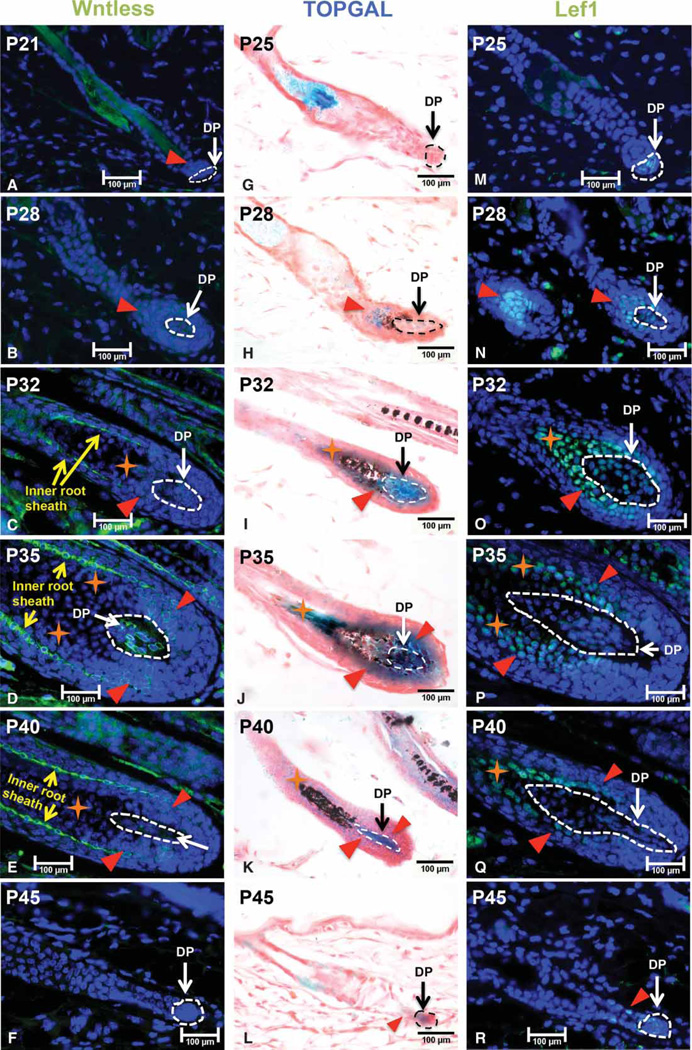
To delineate the potential contribution of the dermal papilla (DP) to the production of Wnt ligands, we evaluated the expression of Wls in murine hair follicles at successive stages of the postnatal hair growth cycle. As shown in Figure 1A, at P21, Wls expression could not be detected in DP cells when hair follicles were at the beginning of the anagen stage (surrounded by white circle). In contrast, Wls was expressed at a low level in the hair follicle epithelium (indicated by red triangle). By P28, Wls expression was significantly up-regulated in the DP while maintained at a high level in transit-amplifying keratinocytes (Figure 1B). From P32 to P40 (Figure 1C–E), high level of Wls was readily detected in the DP, matrix keratinocytes surrounding the DP (indicated by red triangle) and inner root sheath cells (indicated by yellow arrows). However, there was a lack of Wls expression in the area above the DP (denoted by orange stars) where normally hair shaft precursor cells reside. Hair shaft precursor cells are Lef1-positive but Ki67-negative as shown in Figure 1O–Q. When hair follicles enter the regression stage at P45 (Figure 1F), Wls expression could no longer be detected in the DP. These observations show that Wls is dynamically expressed in DP cells during the anagen phase, with the exception of transition stage from telogen to anagen [14].
Figure 1. Wls is expressed in the DP during hair growth anagen except onset.
Mid-dorsal skin biopsies of wild-type C57BL/6 (B6) mice were collected at P21, P25, P28, P32, P35, P40 and P45, and processed for paraffin sections. Sections were analyzed for Wls (A–F) and LEF1 (M–R) expression by immunofluorescence staining at each time point as indicated. (G–L) Mid-dorsal skin biopsies of TOPGAL mice were collected at indicated age for X-gal whole-mount staining. The DP was circled by either white or black dash line in each hair follicle. Red triangles indicate either Wls-positive or Lef1-positive cells outside the DP in their respective pictures. Orange stars denote keratinocytes that are Lef1-positive but Wls-negative. Yellow arrows indicate inner root sheath cells in the hair follicle. For every indicated mouse age, at least three mice were analyzed. Scale bar: 100 µm.
We first utilized TOPGAL Wnt reporter mice to assay for Wnt/β-catenin signaling activity in postnatal skin, in which three consensus Lef/Tcf-binding sites upstream of a minimal c-fos promoter drive the expression of lacZ transgene [30]. As shown in Figure 1G, lacZ expression was undetectable in the DP of early anagen hair follicles at P25, possibly due to the lack of sensitivity of reporter gene at this stage. At P28 (Figure 1H), there was weak blue staining in the DP, while higher lacZ expression was found in the hair matrix (indicated by red triangle). Consistent with Wls expression, TOPGAL transgene was expressed very strongly in the DP and hair matrix keratinocytes surrounding the DP (Figure 1I–K). At P45, weak lacZ expression could still be seen in the DP and the epithelial strand (Figure 1L).
Since the sensitivity of TOPGAL reporter is affected by the number and placement of Lef/Tcf sites and the integration site of the transgene, we needed additional Wnt activity indicators to confirm Wnt/β-catenin signaling in the skin. Activation of canonical Wnt signaling by Wnt ligands results in the stabilization of β-catenin, which associates with members of the Lef1/Tcf family of DNA-binding proteins to initiate target gene transcription [10]. Lef1 has been demonstrated to be a Wnt target gene; therefore, it could be used as an indicator for cells potentially responding to Wnt ligands and being able to activate Wnt/β-catenin signaling. Thus, we evaluated the expression of Lef1 in the hair follicle from anagen onset to telogen. As shown in Figure 1M, at P25, Lef1 expression was exclusively identified in the DP during early anagen. From P28 to P40 (Figure 1N–Q), expression of Lef1 was seen in both the DP and matrix and precortex keratinocytes that encircle the DP of anagen hair follicles (indicated by red triangles). At P45, when hair follicles entered the regression stage, Lef1 expression could still be detected in the DP and differentiating keratinocytes (Figure 1R). By telogen, no Lef1 expression was detected in the DP and the hair follicle epithelium (data not shown). These observations show that the expression of Wls overlaps with active Wnt/β-catenin signaling in matrix keratinocytes and DP cells during hair growth anagen.
Wls in CD133+ DP cells is required for hair follicle anagen
To determine whether CD133+ DP cells produce Wnt ligands for postnatal hair growth, we generated CD133-CreERT2; Wlsfl/fl mice, which allow for the inducible ablation of Wls in CD133+ DP cells (Figure 2A). The CD133-CreERT2 mouse line expresses a recombined CreER fusion protein consisting of Cre recombinase and a mutated ligand-binding domain of the human estrogen receptor in CD133+ cells [28]. The activation of Cre recombinase requires administration of TAM or 4-hydroxytamoxifen (4-OHT) [28,31]. It has been confirmed that Cre recombinase encoded by the CreER transgene in CD133-CreERT2 mice is uniquely expressed in CD133+ DP cells in skin [27]. The Wls allele in the Wlsfl/fl mouse is flanked by two loxP sites, which, when recombined, will result in a null allele [20]. The administration of TAM at different time points to CD133-CreERT2; Wlsfl/fl mice induces efficient ablation of Wls in CD133+ DP cells during different stages of the hair growth cycle. Control littermates were either of genotypes CD133-CreERT2; Wlsfl/+ or CD133-CreERT2; Wls+/+.
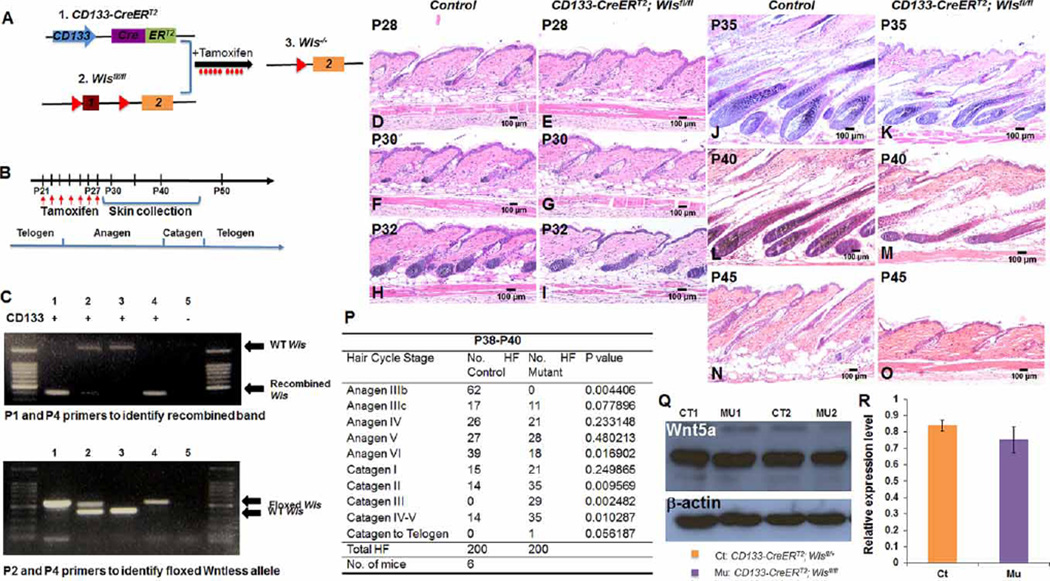
Figure 2. Ablation of Wls in CD133+ DP cells delays hair growth and induces premature hair regression.
(A) Illustration of the CD133-CreERT2; Wlsfl/fl transgenic mouse model, which allows specific Wls ablation in CD133+ DP cells upon TAM or 4-OHT administration. (B) Time scheme for TAM administration during early anagen stage and skin biopsy collection. CD133-CreERT2; Wlsfl/fl mice and control littermates were administered of tamoxifen daily by IP injection for 7 days starting from P21 to P27. Mid-dorsal skin biopsies were harvested for examination at P28, P30, P32, P35, P40 and P45. (C) Recombination of floxed Wls alleles in CD133+ DP cells was confirmed by PCR analysis. Upper DNA agarose gel picture: genomic DNA extracted from skin biopsies was PCR genotyped using P1/P4 primer set to identify wild-type Wls allele and recombined Wls allele. Lower DNA agarose gel picture: genomic DNA extracted from skin biopsies was PCR genotyped using P2/P4 primer set to identify wild-type Wls allele and floxed Wls allele. Lanes labeled with same number in upper and lower DNA gel pictures were genotyping results of genomic DNA extract from same mouse. Genotype of CD133-CreERT2 for each mouse is labeled above each lane of top DNA agarose gel picture. ‘+’ means mouse carried CD133-CreERT2 transgene, while ‘−’ means mouse did not carry CD133-CreERT2. (D–O) H&E-stained skin sections from CD133-CreERT2; Wlsfl/fl (D, F, H, J, L and N) and control littermates (E, G, I, K, M and O) as labeled. Scale bar: 100 µm (n = 3). (P) Comparison of hair follicle numbers that were present at different hair cycle stages between control and CD133-CreERT2; Wlsfl/fl mice from P38 to P40. A minimum of three skin biopsies from three pairs of mutant mice and control littermates were manually counted. Two-tailed paired Student’s t-test was employed. *P < 0.05. (Q) Generation of Wnt5a protein in CD133+ DP cells was confirmed by Western blot analysis. Two pairs of CD133-CreERT2; Wlsfl/fl mice (MU1, 2) and control littermates (CT1, 2) are shown. (R) Intensity of Wnt5a band was shown by scanning X-ray film and normalized to β-actin band. Three pairs of mice were analyzed.
The ablation of Wls in CD133-CreERT2; Wlsfl/fl mice and littermate controls was induced by IP injection of TAM from P21 for 7 consecutive days until P27 (Figure 2B). Mid-dorsal skin biopsies were harvest at P28, P30, P32, P35, P40 and P45 for analysis. Efficient Wls ablation was confirmed by PCR analysis of isolated genomic DNA from induced CD133-CreERT2; Wlsfl/fl mice (lanes 1 and 4 in the top panel of DNA agarose gel) showing efficient Wls recombination (Figure 2C). As reported before [32], we have observed that administration of TAM to mice led to a delay of anagen onset by 2–3 days. Therefore, timing of the hair cycle stages in our study differs slightly from those in untreated mice [3].
At P28, when hair follicles entered early anagen, hair follicles in CD133-CreERT2; Wlsfl/fl mice (Figure 2E) and controls (Figure 2D) did not show any obvious morphological difference. Starting from P30, hair follicles in control mice continued to develop normally (Figure 2F). On the contrary, the growth of hair follicles in CD133-CreERT2; Wlsfl/fl mice appeared slightly delayed (Figure 2G), suggesting that Wnt ligands from CD133+ DP cells may start to play an important role during this stage. Consistent with this observation, hair follicles in CD133-CreERT2; Wlsfl/fl (Figure 2I) grew slower than those in control (Figure 2H) at P32. As shown in Figure 2J, while hair follicles in controls entered anagen IV at P35, hair follicles in CD133-CreERT2; Wlsfl/fl skin failed to show the same advancement (Figure 2K). At P40, control hair follicles were still present in later anagen stages (Figure 2L), whereas mutant hair follicles had already regressed into catagen (Figure 2M). At P45, hair follicles in CD133-CreERT2; Wlsfl/fl skin were in telogen (Figure 2O), whereas control hair follicles just entered the catagen stage (Figure 2N). We counted the numbers of hair follicles that entered different stages of the hair cycle in P38–40 skin. As shown in Figure 2P, an average of 85% of hair follicles appeared in anagen stage in control mice, whereas an average of 60% of hair follicles had already progressed to catagen stage in CD133-CreERT2; Wlsfl/fl mice.
To determine whether Wls ablation would cause any accumulation of Wnt protein, we isolated CD133+ DP cells from P30 CD133-CreERT2; Wlsfl/fl and control skin tissues after tamoxifen induction by FACS for Wnt5a immunoblotting. Wnt5a has been repeatedly shown as a major DP signature protein in mature hair follicles [16,17]. As shown in Figure 2Q, Wnt5a was expressed in CD133+ DP cells isolated from both CD133-CreERT2; Wlsfl/fl and control hair follicles. The intensity of Wnt5a expression bands was normalized to β-actin expression and compared between CD133-CreERT2; Wlsfl/fl and control samples. As shown in Figure 2R, there was no indication of Wnt5a protein accumulation in CD133+ Wls-deficient DP cells.
Delayed hair growth and rapid regression in CD133-CreERT2; Wlsfl/fl mice are associated with inhibition of matrix cell differentiation
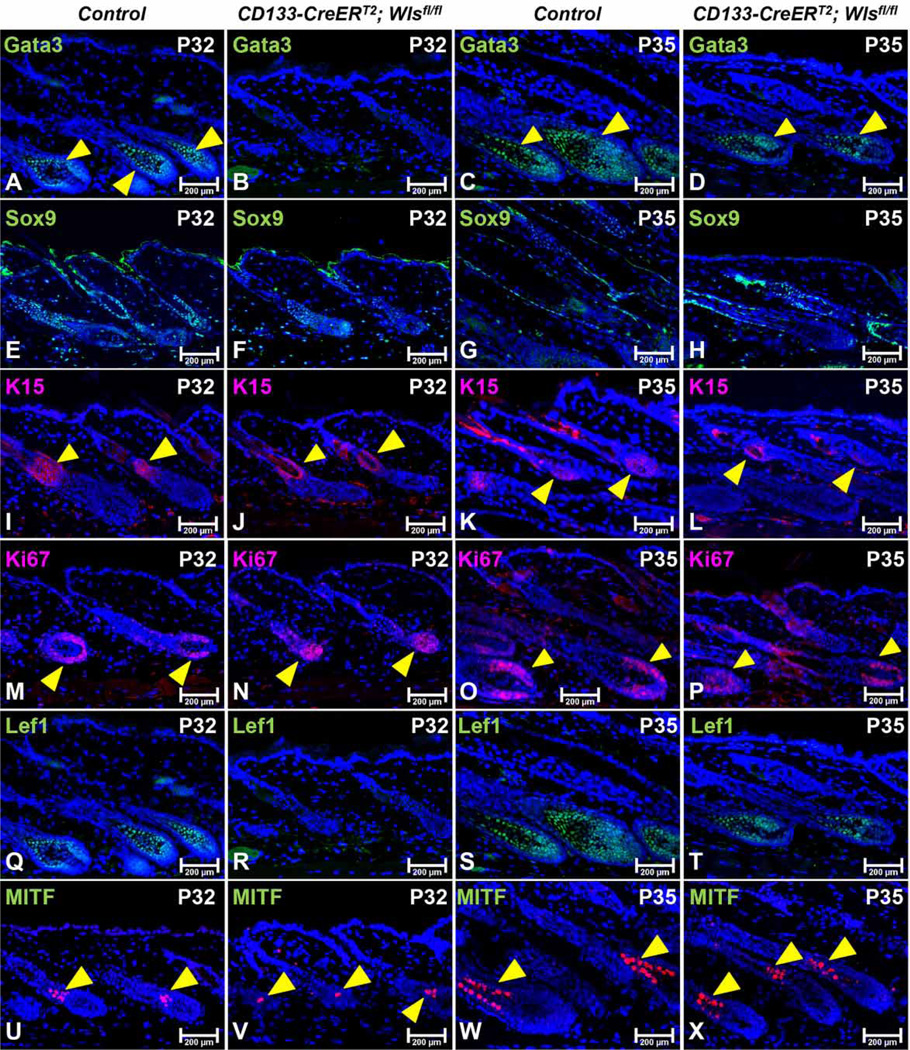
To determine whether Wls deletion in CD133+ DP cells had any effects on the hair follicle structure, the expression of Sox9 (outer root sheath) and Gata3 (inner root sheath) were analyzed by immunofluorescence staining [33]. At P32, expression levels of Gata3 (Figure 3B) and Sox9 (Figure 3F) were much lower in mutant than those of control hair follicles (Figure 3A,E). By P35, decreased Gata3 (Figure 3D) and Sox9 (Figure 3H) expression were still apparent in CD133-CreERT2; Wlsfl/fl hair follicles (Figure 3C,G). However, expression of K15, a marker for hair follicle stem cells (HFSCs) in the bulge and secondary hair germ, was readily seen in mutant hair follicles, and the level of expression was comparable between CD133-CreERT2; Wlsfl/fl and control hair follicles at P32 and P35 (Figure 3I–L), suggesting that delayed hair growth and rapid regression in CD133-CreERT2; Wlsfl/fl was not caused by major changes in the stem cell compartment.
Figure 3. Wls deficiency in CD133+ DP cells inhibits matrix cells differentiation.
Paraffin sections of mid-dorsal skin biopsies of P32 or P35 old CD133-CreERT2; Wlsfl/fl mice and control littermates were analyzed by immunofluorescent staining to assess the expression of following markers: Gata3 for inner root sheath (control: A and C; mutant: B and D); Sox9 for outer root sheath (control: E and G; mutant: F and H); K15 for hair follicle stem cells and secondary hair germ cells (control: I and K; mutant: J and L); Ki67 for proliferating matrix cells (control: M and O; mutant: N and P); Lef1 for proliferating matrix cells and hair shaft precursor cells (control: Q and S; mutant: R and T); MITF for mature melanocytes (control: U and W; mutant: V and X). Sections were nuclear counterstained with DAPI (blue). Images shown are representative of at least three replicates at each indicated age. Scale bars: 200 µm.
Wnt/β-catenin signaling plays a central role in regulating hair follicle matrix cell proliferation and differentiation [34]. To determine whether the lack of hair growth in mutant mice was caused by inhibition of matrix cell proliferation and differentiation due to the blockage of Wnt export from CD133+ DP cells, we evaluated the expression of Ki67 and Lef1. Ki67 is a cell proliferation marker for matrix keratinocytes that surround the DP (transit-amplifying cells), and Lef1 is required for the differentiation of hair shaft progenitor cells [35]. At P32 and P35, there was intense labeling of matrix cells in mutant hair follicles for Ki67 (Figure 3N,P). The number of Ki67-positive cells was slightly reduced in matrix cells of CD133-CreERT2; Wlsfl/fl hair follicles and possibly due to the more advanced anagen stages in control hair follicles (Figure 3M,O). However, there was an obvious lack of Lef1+ hair shaft precursor cells in CD133-CreERT2; Wlsfl/fl hair follicles at P32 (Figure 3R) when compared with the high number of Lef1+ cells in control hair follicles (Figure 3Q). At P35, Lef1+ cells appeared in CD133-CreERT2; Wlsfl/fl hair follicles (Figure 3T), albeit at lower numbers as compared with controls (Figure 3S). These data show that Wnt ligands from CD133+ DP cells are required for hair matrix differentiation but not the maintenance of hair follicle stem cells.
The biological activities of hair follicle keratinocytes and melanocytes are co-ordinated in order to produce mature pigmented hairs. To determine whether Wls deficiency in CD133+ DP cells affected melanocytes in the hair follicles, we evaluated the expression of Mitf by immunofluorescence staining [36]. As shown in Figure 3V, X, the numbers of Mitf-positive melanocytes were significantly decreased in CD133-CreERT2; Wlsfl/fl hair follicles when compared with control hair follicles (Figure 3U,W).
Wls ablation in CD133+ DP cells leads to a reduced DP compartment and loss of versican expression
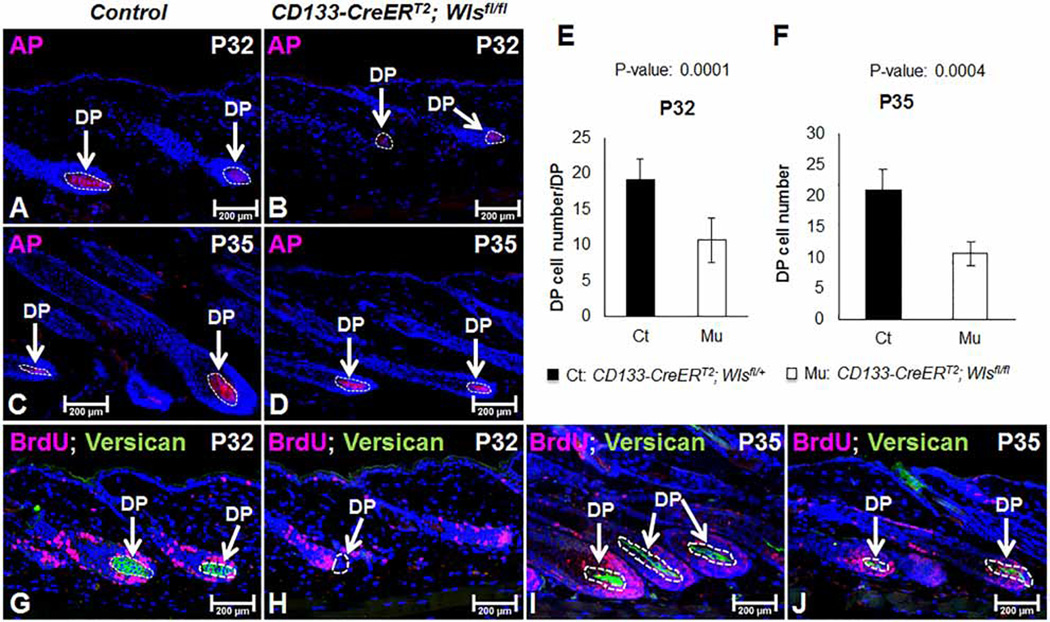
In vitro DP cell cultures require the addition of exogenous Wnt ligands to maintain hair inductivity [18,19]. To ask whether Wnt ligands from CD133+ DP cells regulate the DP in an autocrine manner, we first examined the expression of AP, which is a specific marker for the DP [37]. As shown in Figure 4B, at P32, the size of the DP in CD133-CreERT2; Wlsfl/fl mice was smaller than that in control mice (Figure 4A). Similarly, at P35, the size of mutant DP compartment (Figure 4D) was smaller than that of control DPs (Figure 4C). By manually counting, mutant DPs contained a mean of 9 DP cells at P32, whereas control DP had a mean of 19 DP cells in each hair follicle (Figure 4E). At P35, there was a mean of 11 DP cells in mutant hair follicles, compared with 21 in controls (Figure 4F; n = 30 hair follicles from control mice and HFs from mutant mice).
Figure 4. Wls deficiency in CD133+ DP cells affects DP compartments.
(A–D) Frozen sections of mid-dorsal skin biopsies of P32 or P35 old CD133-CreERT2; Wlsfl/fl mice (B and D) and control littermates (A and C) were immunostained using a VECTOR Red AP Substrate Kit to reveal the DP compartment (n = 3). (E and F) AP+ DP cells were manually counted and compared between CD133-CreERT2; Wlsfl/fl mice and control littermates at P32 (E) and P35 (F). A minimum of 30 hair follicles was counted from three mice of same genotypes (n = 3). (G–J) Expression of versican in the DP was evaluated by immunofluorescent staining (green color inside the white circle) (n = 3). Sections were co-stained with an antibody against cell proliferative marker BrdU (red) (control: G and I; mutant: H and J). Sections were nuclear counterstained with DAPI (blue). Scale bar = 200 µm.
We also examined the expression of versican by immunostaining. Versican is a marker for DP cells of anagen hair follicles [38]. At P32, expression of versican was easily detectable in control DPs (Figure 4G), but completely absent from CD133-CreERT2; Wlsfl/fl DP (Figure 4H). There was a weak versican expression in mutant DP at P35 (Figure 4J), although appearing much weaker than control DPs (Figure 4I). Thus, Wnt ligands from CD133+ DP cells contribute to the proper maintenance of DPs and the expression of anagen DP markers.
Ablation of Wls in CD133+ DP cells during mid-anagen leads to shortened hair follicle growth stage
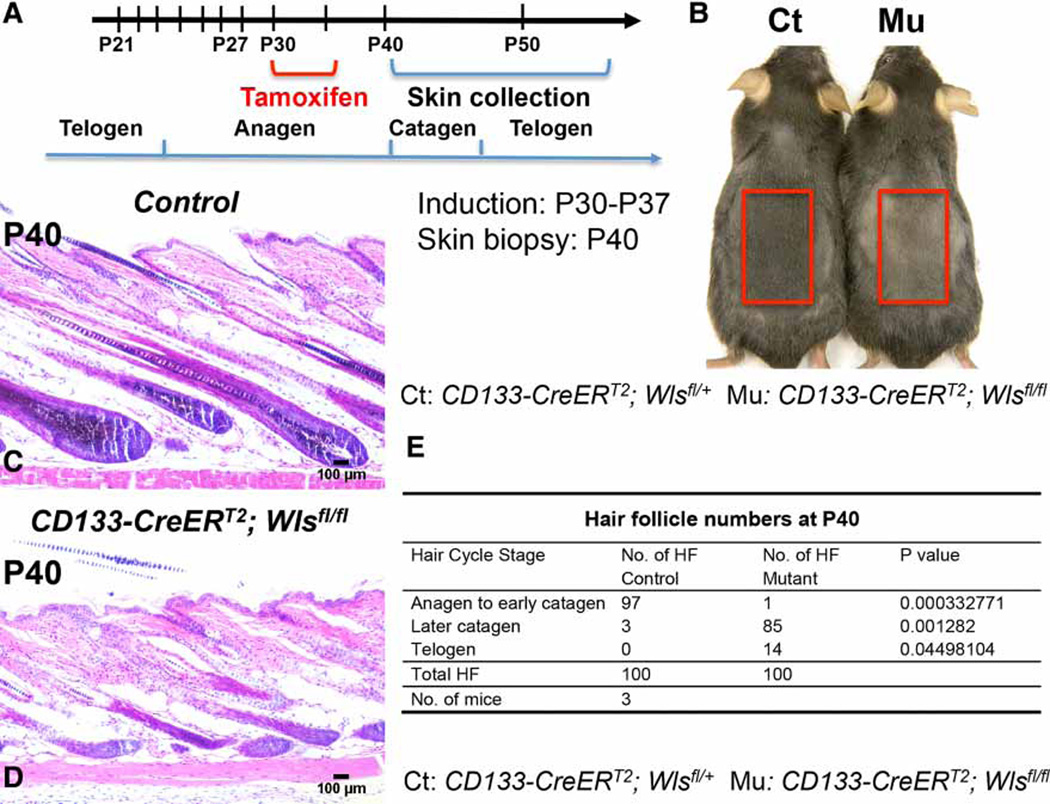
To exclude the possibility that delayed growth and accelerated regression in CD133-CreERT2; Wlsfl/fl hair follicles were due to the loss of Wls at anagen onset, we designed an alternative strategy to induce Wls ablation in CD133+ DP cells from P30 after anagen onset (Figure 5A). The Cre activity induction lasted for a maximum of 7 days depending on when skin biopsies were collected. There was no significant difference in hair follicle histology between CD133-CreERT2; Wlsfl/fl and control littermates at P35 (data now shown). However, at P40, 10 days after initial induction of Wls loss, control mice showed darker skin color because of hair growth (Figure 5B). Skin of CD133-CreERT2; Wlsfl/fl mice appeared lighter, indicating that the growth of hair follicles was blocked. Histological analysis confirmed this observation. As shown in Figure 5C, hair follicles in control littermates had entered full anagen, whereas a majority of hair follicles in mutant skin either were arrested at early anagen stages or regressed prematurely and entered catagen (Figure 5D). Overall, no hair follicles in CD133-CreERT2; Wlsfl/fl mice appeared in full anagen, whereas a majority of hair follicles in control littermates were in anagen to early catagen stages (Figure 5E).
Figure 5. Ablation of Wls in CD133+ DP cells during mid-anagen induces premature hair regression.
(A) Time scheme for TAM administration during mid-anagen and sample collection. Briefly, CD133-CreERT2; Wlsfl/fl mice and control littermates were administered with TAM by IP injection at P30 for 5 or 7 days based on the date of skin biopsy collection. (B) Representative pictures of skin color of CD133-CreERT2; Wlsfl/fl mouse and control littermate at P40 after shaving (n = 3). (C and D) H&E-stained skin biopsies of CD133-CreERT2; Wlsfl/fl mouse (D) and control littermate (C) at P40 (n = 3). Scale bar: 100 µm. (E) Comparison of hair follicle numbers that were present at different hair cycle stages between CD133-CreERT2; Wlsfl/fl mice and control mice at P40 (n = 3). A minimum of three skin biopsies from three pairs of mutant mice and control littermates were manually counted. Two-tailed paired Student’s t-test was employed.
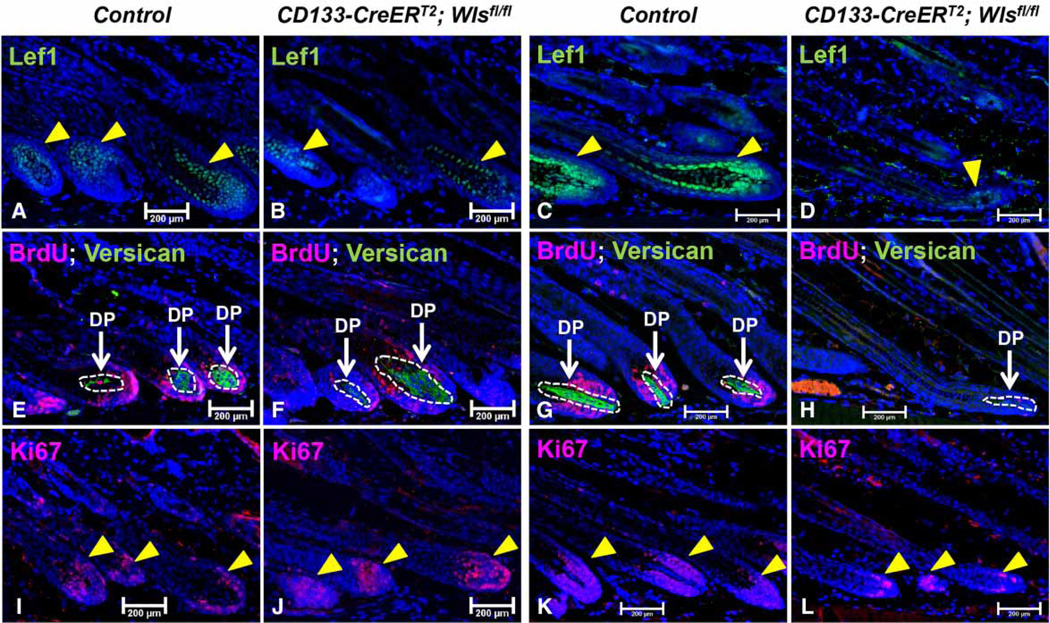
Similar to our previous set of experiments where we induced Wls ablation starting in early anagen, Lef1 expression was also remarkably reduced in CD133-CreERT2; Wlsfl/fl hair follicles at P35 (Figure 6B) and P40 (Figure 6D). Interestingly, while the expression of versican was maintained in mutant hair follicles at P35 (Figure 6F), it was clearly lost at P40 (Figure 6H). This was also the case for Ki67 expression. While mutant hair follicles exhibited strong Ki67 expression at P35 (Figure 6J), the level of Ki67 expression was significantly decreased at P40 (Figure 6L) when compared with control hair follicles (Figure 6K). The findings show that Wnt ligands from CD133+ DP cells are important for postnatal hair growth by regulating events in both the epithelial and mesenchymal compartments.
Figure 6. Wls deficiency in CD133+ DP cells during mid-anagen affects both epithelial compartment and the DP.
Paraffin sections of skin biopsies from P35 and P40 CD133-CreERT2; Wlsfl/fl mice and control littermates (n = 3) after Wls ablation in CD133+ DP cells during mid-anagen stage (starting from P30) were analyzed for the expression of following markers: Lef1 (control: A and C; mutant B and D); Ki67 (control: E and G; mutant: F and H) and versican/BrdU (control: I and K; mutant: J and L). Sections were nuclear counterstained with DAPI (blue). Scale bar = 200 µm.
Discussion
Identification of the intricacies of EMIs during the hair follicle growth cycle is a prerequisite to improve our abilities to artificially generate hair follicles for clinical applications. Wnt signaling is a critical player in EMIs [39]. To better understand how Wnt signals regulate cell–cell interactions between epithelial and mesenchymal compartments during the hair growth cycle, more detailed studies on the source and nature of Wnt ligands that are responsible for triggering Wnt/β-catenin signaling are required. Although the requirements of Wnt ligands from the hair follicle epithelium have been well reported [12–14,25], there is a surprising lack of progress in the understanding of the role of Wnt ligands secreted from the DP.
Here, we have started to explore the role of Wnt ligands expressed in the DP and how they influence postnatal hair growth. Our study clearly demonstrates that Wnt ligands generated by CD133+ DP cells are important for postnatal hair regenerative cycling by exerting influences over both the hair follicle epithelium and the DP.
For the purpose of the study, we have introduced the Wls conditional knockout mouse (Wlsfl/fl) as our genetic tool since Wls is essential for Wnt secretion from Wnt-producing cells [24]. Although Wls might also have other functions, it has been clearly demonstrated in several reports that ablation of Wls blocks the release of Wnt proteins and results in the blockade of the activation of Wnt/β-catenin signaling in Wnt target cells [22,23]. We and several other groups have recently demonstrated that Wls ablation in the hair follicle epithelium blocks postnatal hair cycling [12–14,25]. Owing to the pivotal importance of the DP in hair follicle biology, key components of the Wnt signaling pathway have been investigated in the DP. However, the focus so far has generally been on β-catenin with the notable exception of Wnt5a [17]. Therefore, the genetic approach to ablate Wls expression in DP cells may offer an opportunity for us not only to further explore Wnt-mediated dynamic interactions between the epithelial compartment and the DP, but also to identify the nature and identity of Wnt ligands produced in the DP during the postnatal hair cycle.
An unique CD133+ cell population exists in the DP during the anagen stage [21]. It has been convincingly demonstrated that CD133+ DP cells are a subpopulation in the DP that has the ability to induce hair follicles when mixed with keratinocytes [26]. This observation clearly suggests that CD133+ DP cells may possess the ability to produce and secret Wnt ligands required for adult hair follicle growth. Our model, CD133-CreERT2; Wlsfl/fl, is the first to actually show that Wnt ligands originating from CD133+ DP cells are essential for adult hair follicle growth in vivo. Because CD133+ DP cells are actually not present at the anagen onset, we focused our attention on the role of Wnt ligands in regulating hair growth and how they affect the activity of the DP and adjacent keratinocytes, mainly matrix and hair shaft precursor cell populations. It is poorly understood how these epithelial cell populations are actually regulated by the DP and how Wnts originating in the DP are used to regulate proliferation and differentiation of matrix cell populations. Surprisingly, we found that Wls ablation in CD133+ DP cells inhibits the proliferation and differentiation of matrix cells and progeny cells derived from them. At P32, there was a lack of Lef1 expression in matrix cells and hair shaft precursor cells that eventually differentiate into inner root sheath and hair shaft cells. Furthermore, the number of mature melanocytes in the hair matrix was also reduced upon Wls ablation in CD133+ DP cells. This phenotype can be best explained taking into consideration that the behavior of keratinocytes, melanocytes and DP cells is co-ordinated and synchronized during hair growth [40]. Consequently, changes in one cell population should at some level result in changes in the other cell population during anagen progression. Although it is unclear whether these interactions between melanocytes, keratinocytes and fibroblasts solely and directly rely on Wnt ligands from the DP or are indirectly influenced by the lack of unknown molecules from the DP that are affected by Wls deficiency in CD133+ DP cells, it is important to note that Wnt/β-catenin signaling is highest in the bulb region of the hair follicle. Based on the canonical model of Wnt signaling, this activity is induced by actual Wnt ligands. Our data strongly suggest that one possible source of these Wnt ligands at this stage is the DP, although there clearly exists the possibility of Wnt redundancy between the epithelium and the DP.
Several Wnt ligands have, at least in in vitro systems, exhibited the ability to maintain DP cell function and DP inductive capacity [19]. In vivo, Wnt ligands from DP cells may function as autocrine factors that induce nuclear localization of β-catenin within DP cells, which can be detected in many DP cells during anagen. The Corin+ DP cell population requires β-catenin to maintain proliferation of matrix keratinocytes at the base of the follicle, and loss of β-catenin in these Corin+ DP cells also results in catagen induction and prevents subsequent anagen induction [32]. However, these results only indicate that DP cells have to receive Wnt signals to initiate the canonical Wnt/β-catenin signaling pathway. The origin, timing and exact nature of this Wnt signal has not been fully addressed in detail. In the present study, we have shown that CD133+ DP cells produce Wnt5a, a major DP Wnt, during early anagen. However, it remains to be seen whether this DP subpopulation generates other Wnt signals as well. Regardless, it will be important to identify Wnt ligands that are produced in the DP for hair follicle growth and regeneration in order to improve approaches to treat hair growth disorders such as alopecia.
Data from our study suggest that Wnt ligands generated by CD133+ DP cells function within the DP potentially in an autocrine fashion. Support for this interpretation stems from a lack of versican expression, a Wnt signaling target gene [41], in the DPs of P32 hair follicles upon Wls ablation in CD133+ DP cells. Versican is a specific marker for anagen DP [42], and its reduction indicates that DP cells have lost biological properties that are needed to maintain hair inductivity. Deficiencies in the DP functionality may also contribute to the abnormal hair phenotypes in CD133-CreERT2; Wlsfl/fl mice. These results suggest that Wnt ligands generated by DP cells during anagen stages possibly contribute to active Wnt/β-catenin signaling in both epithelial and mesenchymal compartments of hair follicles to promote hair growth.
Functional redundancy of Wnt ligands generated by hair follicle keratinocytes and DP cells needs always to be taken into consideration. This could well be the case for CD133-CreERT2; Wlsfl/fl hair follicle as we saw the weak appearance of Lef1 expression in hair matrix and precursor cells from P35 onwards. Wnt ligands made by hair follicle matrix cells and inner root sheath cells could eventually substitute for the missing DP-derived Wnts. On the other hand, since Wnt ligands, as activators, could travel for a short distance in the hair follicle, it is possible that Wnt ligands from the hair follicle epithelium could eventually diffuse from the epithelium to the DP and subsequently activate Wnt/β-catenin signaling in DP cells. This compensatory mechanism could be one explanation as to why the DP maintained weak versican expression at P35. In addition, there remains the possibility that there are other DP populations that have the ability to generate Wnt ligands when hair follicles enter different stages of the hair growth cycle and substitute the functional activity of CD133+ DP cells. To address this issue, we may need to develop and use additional genetic tools to target CD133− DP cell populations.
The lack of changes in anagen onset in CD133-CreERT2; Wlsfl/fl hair follicles is not a surprise as endogenous CD133 expression and the resulting Cre activity is absent at anagen onset but initiated shortly after. Therefore, this particular cell population is not available to interact with HFSCs during anagen onset. This limitation of our CD133-CreERT2-based model means that the role of DP-derived Wnts at anagen onset before the CD133+ cell population emerges cannot be studied in the model we present here. Further development of alternative models in combination with our data will help to refine our understanding of the role of Wnts in DP biology.
Regardless, it is also unlikely that the bulge stem cell population would be fundamentally affected by the lack of Wnt ligands from the DP after anagen onset. This is supported by our observation that HFSCs and secondary hair germ were not disturbed to an obvious degree in CD133-CreERT2; Wlsfl/fl mutant hair follicles, although we cannot exclude the possibility that intrinsic properties of HFSCs were actually affected contributing to later phenotypes. Therefore, in future studies, we will address the long-term effects of Wls ablation in CD133+ DP cells during the first hair cycle and observe whether the second hair cycle or induced hair growth by depilation is affected, which will definitely answer the question as to whether the activation of HFSCs requires Wnt ligands from CD133+ DP cells.
In summary, our study demonstrates that CD133+ DP cells generate Wnt ligands that are important for postnatal hair regenerative cycling. Without Wls, and therefore without Wnts, from CD133+ DP cells, hair follicles are not able to sustain a normal anagen. This finding carries important implications as it provides a new insight into the biology of EMIs and DP cell biology that may lead to the discovery and development of new targets for treating hair growth disorders.
Acknowledgments
We thank Dr Richard J. Gilbertson and the St Jude Children’s Research Hospital for CD133-CreERT2 (Prom1C-L) mice.
Funding
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) R03 AR062788 (Y.Z.).
Abbreviations
- 4-OHT
4-hydroxytamoxifen
- AP
alkaline phosphatase
- APC
Adenomatous polyposis coli
- Axin
axis inhibition protein
- BrdU
bromodeoxyuridine
- CD133+
CD133-positive
- DP
dermal papilla
- EMIs
epithelial–mesenchymal interactions
- Fzd
Frizzled
- GSK-3β
Glycogen synthase kinase 3 beta
- H&E
hematoxylin and eosin
- HFSCs
hair follicle stem cells
- IP
intraperitoneal
- Mitf
microphthalmia-associated transcription factor
- P25
postnatal day 25
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- TAM
tamoxifen
- Wls
Wntless
Footnotes
Author Contribution
L.Z. and K.Y. performed the experiments. A.C. and R.L. generated and provided the animal model. Y.Z. and T.A. contributed to the analysis and interpretation of the data. Y.Z. supervised the study and wrote the manuscript with feedback from all authors.
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Alonso L, Fuchs E. The hair cycle. J. Cell Sci. 2006;119:391–393. doi: 10.1242/jcs.02793. [DOI] [PubMed] [Google Scholar]
- 2.Botchkarev VA, Kishimoto J. Molecular control of epithelial–mesenchymal interactions during hair follicle cycling. J. Invest. Dermatol. Symp. Proc. 2003;8:46–55. doi: 10.1046/j.1523-1747.2003.12171.x. [DOI] [PubMed] [Google Scholar]
- 3.Müller-Röver S, Kerstin F, Paus R, Handjiski B, van der Veen C, Eichmüller S, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 4.Chi W, Wu E, Morgan BA. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development. 2013;140:1676–1683. doi: 10.1242/dev.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen J. The transplantation of individual rat and guinea-pig whisker papillae. J. Embryol. Exp. Morphol. 1961;9:117–127. PMID: 13694386. [PubMed] [Google Scholar]
- 6.Jahoda CA, Reynolds AJ, Oliver RF. Induction of hair growth in ear wounds by cultured dermal papilla cells. J. Invest. Dermatol. 1993;101:584–590. doi: 10.1111/1523-1747.ep12366039. [DOI] [PubMed] [Google Scholar]
- 7.Millar SE. Molecular mechanisms regulating hair follicle development. J. Invest. Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 8.Millar SE. WNTs: multiple genes, multiple functions. J. Invest. Dermatol. 2003;120:7–8. doi: 10.1046/j.1523-1747.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- 9.Willert K, Nusse R. β-catenin: a key mediator of Wnt signaling. Curr. Opin. Genet. Dev. 1998;8:95–102. doi: 10.1016/s0959-437x(98)80068-3. [DOI] [PubMed] [Google Scholar]
- 10.Cadigan KM, Waterman ML. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb. Perspect. Biol. 2012;4:a007906. doi: 10.1101/cshperspect.a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruciat C-M, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb. Perspect. Biol. 2013;5:a015081. doi: 10.1101/cshperspect.a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi YS, Zhang Y, Xu M, Yang Y, Ito M, et al. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell. 2013;13:720–733. doi: 10.1016/j.stem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu J, Hsu W. Epidermal Wnt controls hair follicle induction by orchestrating dynamic signaling crosstalk between the epidermis and dermis. J. Invest. Dermatol. 2013;133:890–898. doi: 10.1038/jid.2012.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myung PS, Takeo M, Ito M, Atit RP. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J. Invest. Dermatol. 2013;133:31–41. doi: 10.1038/jid.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen D, Jarrell A, Guo C, Lang R, Atit R. Dermal β-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development. 2012;139:1522–1533. doi: 10.1242/dev.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 17.Hu B, Lefort K, Qiu W, Nguyen B-C, Rajaram RD, Castillo E, et al. Control of hair follicle cell fate by underlying mesenchyme through a CSL–Wnt5a–FoxN1 regulatory axis. Genes Dev. 2010;24:1519–1532. doi: 10.1101/gad.1886910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–1185. PMCID: PMC316619. [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu H, Morgan BA. Wnt signaling through the β-catenin pathway is sufficient to maintain, but not restore, anagen-phase characteristics of dermal papilla cells. J. Invest. Dermatol. 2004;122:239–245. doi: 10.1046/j.0022-202X.2004.22224.x. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter AC, Rao S, Wells JM, Campbell K, Lang RA. Generation of mice with a conditional null allele for Wntless. Genesis. 2010;48:554–558. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito Y, Hamazaki TS, Ohnuma K, Tamaki K, Asashima M, Okochi H. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J. Invest. Dermatol. 2007;127:1052–1060. doi: 10.1038/sj.jid.5700665. [DOI] [PubMed] [Google Scholar]
- 22.Bänziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 23.Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Ching W, Nusse R. A dedicated Wnt secretion factor. Cell. 2006;125:432–433. doi: 10.1016/j.cell.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Huang S, Zhu X, Liu Y, Tao Y, Feng G, He L, et al. Wls is expressed in the epidermis and regulates embryonic hair follicle induction in mice. PLoS ONE. 2012;7:e45904. doi: 10.1371/journal.pone.0045904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Driskell RR, Juneja VR, Connelly JT, Kretzschmar K, Tan DW-M, Watt FM. Clonal growth of dermal papilla cells in hydrogels reveals intrinsic differences between Sox2-positive and -negative cells in vitro and in vivo. J. Invest. Dermatol. 2012;132:1084–1093. doi: 10.1038/jid.2011.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaushal GS, Rognoni E, Lichtenberger BM, Driskell RR, Kretzschmar K, Hoste E, et al. Fate of prominin-1 expressing dermal papilla cells during homeostasis, wound healing and Wnt activation. J. Invest. Dermatol. 2015;135:2926–2934. doi: 10.1038/jid.2015.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou L, Yang K, Randall Wickett R, Zhang Y. Dermal fibroblasts induce cell cycle arrest and block epithelial-mesenchymal transition to inhibit the early stage melanoma development. Cancer Med. 2016;5:1566–1579. doi: 10.1002/cam4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. PMID: 10498690. [DOI] [PubMed] [Google Scholar]
- 31.Metzger D, Clifford J, Chiba H, Chambon P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc. Natl Acad. Sci. USA. 1995;92:6991–6995. doi: 10.1073/pnas.92.15.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch MH, O’Guin WM, Hardy C, Mak L, Sun TT. Acidic and basic hair/nail (“hard”) keratins: their colocalization in upper cortical and cuticle cells of the human hair follicle and their relationship to "soft” keratins. J. Cell Biol. 1986;103:2593–2606. doi: 10.1083/jcb.103.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rishikaysh P, Dev K, Diaz D, Qureshi WM, Filip S, Mokry J. Signaling involved in hair follicle morphogenesis and development. Int. J. Mol. Sci. 2014;15:1647–1670. doi: 10.3390/ijms15011647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006;12:406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Iida M, Ihara S, Matsuzaki T. Hair cycle-dependent changes of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Dev. Growth Differ. 2007;49:185–195. doi: 10.1111/j.1440-169X.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 38.Kishimoto J, Ehama R, Wu L, Jiang S, Jiang N, Burgeson RE. Selective activation of the versican promoter by epithelial-mesenchymal interactions during hair follicle development. Proc. Natl Acad. Sci. USA. 1999;96:7336–7341. doi: 10.1073/pnas.96.13.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, et al. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- 40.Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L, et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941–955. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahmani M, Read JT, Carthy JM, McDonald PC, Wong BW, Esfandiarei M, et al. Regulation of the versican promoter by the β-catenin-T-cell factor complex in vascular smooth muscle cells. J. Biol. Chem. 2005;280:13019–13028. doi: 10.1074/jbc.M411766200. [DOI] [PubMed] [Google Scholar]
- 42.du Cros DL, LeBaron RG, Couchman JR. Association of versican with dermal matrices and its potential role in hair follicle development and cycling. J. Invest. Dermatol. 1995;105:426–431. doi: 10.1111/1523-1747.ep12321131. [DOI] [PubMed] [Google Scholar]