Abstract
OBJECTIVE
Carotid interventions are important in helping to reduce the risk of stroke for patients with high-grade carotid artery stenosis; however, incidence of subclinical cerebral microemboli can occur during these procedures. Previously, associations have been found between incidence of microemboli and postoperative decline in memory. We therefore sought to determine whether this decline persisted long-term and to assess changes in other cognitive domains.
METHODS
Patients were prospectively recruited under an IRB-approved protocol at a single academic center. Neuropsychological testing was administered preoperatively and at 1 month and 6 month intervals postoperatively. Cognitive domains that were evaluated included verbal memory, visual memory, psychomotor speed, dexterity, and executive function. Diffusion weighted MRI (DWI) sequencing was performed preoperatively and within 48 hours postoperatively to identify procedure-related microemboli. Univariate and multivariate regression models were used to identify relationships between microembolization, demographics, and cognition.
RESULTS
80 patients were included; all were male and average age was 69 years. Forty patients underwent CAS and 40 CEA. 45% were diabetic, 50% had CAD, and 41% had prior neurologic symptoms. 45 (56%) of the patients had new postoperative microemboli. Microembolization was significantly more common in the CAS cohort (P<.005). Univariate analysis demonstrated that patients with procedure-related embolization showed decline 1 month postoperatively in verbal memory and Trail Making A measures. Multivariate analysis demonstrated that procedure-related embolization (OR: 2.8, P=.04) and pre-op symptomatic stenosis (OR: 3.2, P=.026) were independently predictors of decline for the RAVLT Short Delay measure at 1 month; however, at 6 months there was no significant relationship between emboli and decline on RAVLT Short Delay, while age (OR: 1.1, P=.005) and COPD (OR: 7.1, P=.018) were significantly associated with decline at 6 months following intervention.
CONCLUSIONS
Microembolization that is associated with carotid artery intervention predicts short-term cognitive decline. However, some of these cognitive deficits persist at 6 months following intervention, and further investigation is warranted to determine individual patient risk factors that may impact recovery.
INTRODUCTION
Cognitive impairment continues to be common in the ageing US population1. Treating patients with cognitive impairment has been shown to be significantly more costly compared to equivalent care for those without deficits2. While cognitive decline is considered part of normal ageing, events such as surgical interventions may further impact cognitive function. Several groups have found that cognitive decline is prevalent in various surgical populations3–4. Determining the cause of decline and whether there are specific risk factors involved may help decrease incidence of surgically-related cognitive impairment.
We and other groups have shown that patients with severe carotid stenosis who undergo carotid revascularization procedures are among those at risk for cognitive changes5–8. The specific cause of such changes remains unclear; however, it has been suggested that microembolization may be associated with neurocognitive changes for patients undergoing carotid interventions. Microemboli are small areas of restricted diffusion seen on diffusion weighted sequence of brain magnetic resonance imaging (DWI). Microembolization is largely considered to be subclinical, however our study and others suggest that microemboli may be associated with cognitive decline. We have previously shown that patients with postoperative microemboli exhibit decline in verbal memory at 1 month following intervention5. Other studies have resulted in variable conclusions and the long-term effects of microemboli remain unclear9–10.
In this study we analyze both immediate and long-term cognitive outcomes to determine whether decline in patients exhibiting microemboli persists after intervention. Additionally, we seek to determine whether specific cognitive domains are affected differently. While carotid interventions have been shown to be successful in reducing the risk of stroke, it is important to also minimize other potential risks of these procedures. Understanding these risks could help to better prepare patients and their caregivers for needs during the post-operative period.
METHODS
Patient Selection
Patients with high-grade carotid artery stenosis, based on NACET duplex ultrasound criteria, and scheduled to undergo carotid artery interventions at the VA Palo Alto Health Care System (VAPAHCS) were recruited to participate. Patients were offered carotid artery stenting (CAS) as an alternative to carotid endarterectomy (CEA) if they exhibited a high lesion above C2 level on preoperative imaging, showed reversibility on persantine-thallium stress test, or had received prior radiation or surgery to the neck. Symptomatic patients with >60% stenosis and asymptomatic patients with >80% stenosis were treated under the standard clinical practice guidelines. A lesion represented >80% stenosis if it met two of the three velocity criteria: peak systolic velocity >330cm/s, end diastolic velocity >130cm/s, and ICA/CCA peak systolic velocity ratio >3.8. All CEA were routinely shunted and all CAS were performed with a distal embolic protection device. Inclusion criteria required the patient be 40 years of age or older; voluntarily participate in the study; be able to sign an IRB-approved informed consent and undergo MRI; and be available for follow-up neuropsychological testing. CAS patients were preferentially recruited, based on the higher incidence of microemboli in this cohort.
Demographics and clinical risk factors were collected and entered into an electronic spreadsheet for each patient. Patients were considered to have Chronic Kidney Diseases (CKD) if they had serum creatinine levels >1.5mg/dL. History of alcohol abuse and smoking were identified from the preoperative evaluation. Patients were considered to be symptomatic (prior symptoms) if they had experienced a transient ischemic attack (TIA), amaurosis fugax, or stroke within the past six months that led to their referral for carotid revascularization evaluation.
Neuropsychological Testing
Patients underwent neuropsychological testing within two weeks prior to, and at 1 month and 6 months following carotid intervention. Sessions included a battery of measures to evaluate executive function, motor speed and strength, visual and verbal memory, and language. The primary outcome measure analyzed was the Rey Auditory Verbal Learning Test (RAVLT) to evaluate verbal memory. RAVLT was chosen because it has been shown to be a sensitive measure for patients with mild cognitive impairment11. Parallel forms of RAVLT were used to minimize practice effect. The Mini Mental Status Examination (MMSE) was used as a screening measure for general cognitive function. Additional measures performed and analyzed included the Rey-Osterreith Complex Figure (RO), Trail Making Test A and B (TMTA and TMTB), Grooved Pegboard, Digit Symbol, Digit Span, and Category Fluency. These measures and their associated cognitive domains are outlined in Table I.
Table I.
Cognitive tests and associated functions.
| Test | Cognitive Function |
|---|---|
| MMSE | General function |
| RAVLT | Verbal Memory |
| Rey Osterreith | Visual Memory |
| Trails A | Psychomotor Speed |
| Trails B | Executive |
| Category Fluency | Verbal Fluency |
| Digit Symbol | Psychomotor Speed |
| Digit Span | Attention/working memory |
| Grooved Pegboard | Dexterity/fine motor control |
Magnetic Resonance Imaging (MRI)
Patients underwent MRI within one month prior to and within 24 hours following their carotid intervention. All scans were performed under a clinical protocol using a GE 750 Signa 3.0 Tesla MRI scanner. An MRA of the neck or MRI of the neck with time of flight was used to confirm degree of stenosis. For the CAS cohort, preoperative angiography of the head and neck was also used to confirm the degree of carotid stenosis. A whole brain diffusion weighted imaging (DWI) sequence was included in each scan and used to determine the incidence of procedure-related emboli. Micromeboli were identified by areas of hyperintensity on DWI with corresponding areas of hypointensity on apparent diffusion coefficient (ADC) mapping. In addition to routine clinical interpretation by the neuroradiology service, a clinical neuroradiologist carefully examined each scan.
Statistical Analysis
Data was collected and stored in an electronic spreadsheet (Microsoft Excel) and analyzed using SPSS Statistics (Version 23.0, IBM Corp., Armonk, N.Y., USA). Cognitive measures were compared using student T test with Welch correction. Demographic variables were compared using the Fisher’s exact test. Univariate and multivariate logistic regression models were used to characterize association between risk factors and microemboli as well as significant cognitive measures. A P value of <0.05 was considered statistically significance. Postoperative embolization and variables with a p<0.2 in the univariate analyses were included in the multivariate models. Individual subjects were considered to exhibit cognitive decline if they had a negative change score from post to pre-operative testing for RAVLT, Rey Osterrieth, Digit Symbol, Digit Span, and Category Fluency, or a positive change score for Grooved Pegboard and Trail Making Tests.
RESULTS
A total of 80 patients who completed all study procedures were included in the analysis. All of the patients were male and had an average age of 69 years (range 55–87 years). This cohort demonstrated co-morbidities consistent with the vascular disease population, including diabetes (45%), history of smoking (84%), hypertension (94%), and coronary artery disease (CAD; 50%). 40 patients that underwent CAS and 40 underwent CEA; 41% of the subjects had prior neurologic symptoms, with similar proportions within the CAS and CEA cohorts. Six of the patients demonstrated contralateral carotid artery occlusion.
In this cohort, 45 (56%) of the subjects had microemboli evident on post-operative DWI. 17 of these patients demonstrated both ipsilateral and contralateral microemboli. Demographics for patients with and without post-operative microemboli are outlined in Table II. Microembolization was significantly more common in patients who had undergone CAS than CEA (85% vs 27.5%, P<.005). Diabetic patients were also more likely to have microemboli (P=.03). Non-significant trends of association between microembolization and co-morbidities such as contralateral occlusion (P=.17), CAD (P=.18) and CKD (P=.11) were also found. There were four patients with post-operative neurologic symptoms, including three with symptoms of TIA and one with a stroke; all had postoperative microemboli.
Table II.
Demographics of patients with and without postoperative microemboli.
| Demographic | No Microemboli (N=35) | Microemboli (N=45) | P value |
|---|---|---|---|
| Age (average) | 68.6 | 68.9 | .86 |
| CAS | 6 (17%) | 34 (76%) | <.005 |
| Contralateral carotid occlusion | 1 (3%) | 5 (11%) | .17 |
| Prior symptoms | 15 (43%) | 18 (40%) | .80 |
| Diabetes | 11 (31%) | 25 (56%) | .03 |
| Obesity | 14 (40%) | 22 (49%) | .43 |
| CAD | 14 (40%) | 26 (58%) | .18 |
| COPD | 3 (9%) | 8 (18%) | .24 |
| CKD (Chronic Kidney Disease) | 6 (17%) | 15 (33%) | .11 |
| Current smoking | 13 (37%) | 13 (29%) | .44 |
| Hx Alcohol Abuse | 16 (46%) | 17 (38%) | .48 |
| Antiplatelets | 27 (77%) | 29 (64%) | .22 |
Pre-operatively, the average MMSE score was 28 (range 22–30); there was no significant difference in MMSE between symptomatic patients compared to those without preoperative symptoms, and 2 subjects had scores <24, indicating baseline mild-cognitive impairment. Average education of this cohort was 13.5 years (range 9–18 years). Patients with procedure-related embolization showed significant decline 1 month postoperatively in RAVLT, our primary outcome measure. At 6 months, however, this trend did not persist (Figure 1). For TMTA, patients without embolization experienced improvement (shorter completion time) at 1 month following intervention, largely attributable to practice effect; however, those with embolization did not benefit from practice effect or experience the same degree of improvement. This trend persisted at 6 months (Figure 2). Interestingly, among 68 patients who completed all 3 tests of Grooved Pegboard and 65 patients who completed Category Fluency, patients with postoperative microemboli demonstrated an improvement in performance in Grooved Pegboard and Category Fluency in comparison to those patients without microemboli. No significant difference in change score was observed in Rey Osterreith, TMTB, digit symbol, or digit span measures among patients with procedure-related microemboli compared to those without.
Figure I.
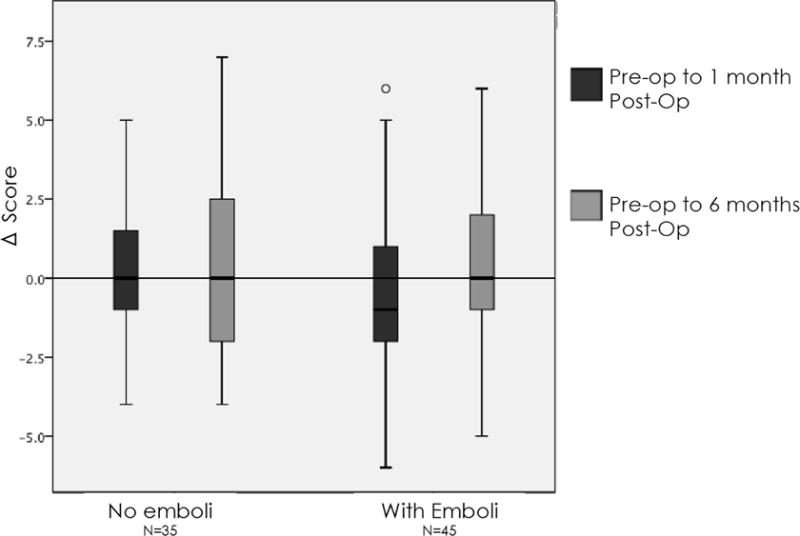
Patients with microemboli demonstrated a postoperative decline in verbal memory (RAVLT) performance at one month.
Figure II.
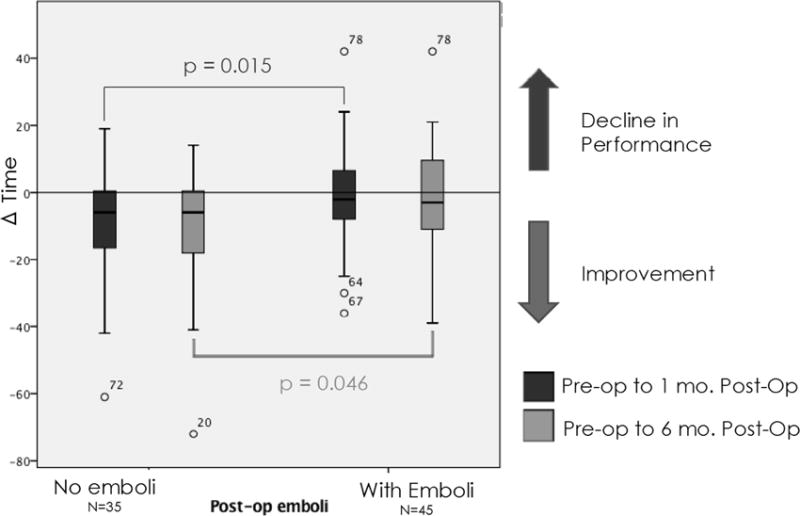
Patients with microemboli did not demonstrate improvement in psychomotor speed performance (Trail Making Test A)
After adjusting for demographics, comorbidities, procedure type, and medications (Table II), univariate and multivariate analysis demonstrated that procedure-related embolization (OR: 2.8, P=.04) and pre-op symptoms (OR: 3.2, P=.026) were independent predictors of decline for the RAVLT short delay measure at 1 month (Table IIIa). Additionally, age (OR: 1.1, P=.005) and COPD (OR: 7.1, P=.018) were independent predictors for long-term RAVLT decline (Table IIIa). For TMTA, a test of psychomotor speed, no significant predictor was identified (Table IIIb). For Grooved Pegboard, a measure of dexterity, only diabetes showed a trend toward significance for protection against decline at 1 month post-procedure (OR: 0.3, P=.056) (Table IIIc). At baseline, patients with diabetes had a significantly longer completion time for Grooved Pegboard than patients without diabetes (124 seconds vs 100 seconds, P=.017). For Category Fluency, a measure of verbal fluency, alcohol abuse was a strong predictor of postoperative decline at 1 month (OR: 3.5, P=.036), while preoperative symptomatic status appeared to be protective (OR: 0.29, P=.049) against decline at 1 month following interventions (Table IIId). Post-operative embolization was not a significant predictor for changes in performance on the TMTA, Grooved Pegboard, and Category Fluency measures in their respective multivariate regression models for neither the 1 month or 6 month time points (Tables IIIb–d).
Table IIIa.
Multivariate analysis for postoperative decline in verbal learning memory.
| RAVLT Short Delay Pre – 1 month post |
OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.067 | 0.998 – 1.140 | .056 |
| COPD | 3.216 | 0.724 – 14.276 | .125 |
| Symptomatic | 3.214 | 1.148 – 8.996 | .026 |
| Postop microemboli | 2.864 | 1.048 – 7.824 | .040 |
| RAVLT Short Delay Pre – 6 month post |
OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.110 | 1.033 – 1.193 | .005 |
| COPD | 7.118 | 1.392 – 36.391 | .018 |
| Obesity | 0.480 | 0.167 – 1.386 | .175 |
| Postop microemboli | 0.863 | 0.301 – 2.473 | .783 |
Table IIIb.
Multivariate analysis for postoperative decline in psychomotor speed.
| Trails A Pre – 1 month post |
OR | 95% CI | P value |
|---|---|---|---|
| CKD | 2.556 | 0.795 – 8.219 | .115 |
| CAS | 1.257 | 0.328 – 4.809 | .739 |
| Hypertension | 0.288 | 0.043 – 1.914 | .198 |
| Reversibility of P-thallium | 0.413 | 0.093 – 1.835 | .245 |
| Symptomatic | 0.686 | 0.236 – 1.989 | .488 |
| Postop microemboli | 1.485 | 0.419 – 5.262 | .540 |
| Trails A Pre – 6 month post |
OR | 95% CI | P value |
|---|---|---|---|
| CAS | 1.564 | 0.410 – 5.964 | .513 |
| Age | 1.019 | 0.948 – 1.095 | .604 |
| Diabetes | 1.891 | 0.640 – 5.586 | .249 |
| Current Smoking | 0.386 | 0.098 – 1.523 | .174 |
| CAD | 2.567 | 0.804 – 8.201 | .112 |
| Postop microemboli | 1.020 | 0.288 – 3.616 | .975 |
Table IIIc.
Multivariate analysis for postoperative decline in dexterity.
| Grooved Pegboard Pre – 1 month post |
OR | 95% CI | P value |
|---|---|---|---|
| Diabetes | 0.302 | 0.088 – 1.032 | .056 |
| CRI | 0.836 | 0.203 – 3.445 | .804 |
| Antiplatelets | 2.177 | 0.619 – 7.655 | .225 |
| Postop microemboli | 0.684 | 0.224 – 2.093 | .506 |
| Grooved Pegboard Pre – 6 month post |
OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.072 | 0.996 – 1.153 | .062 |
| Postop microemboli | 0.572 | 0.194 – 1.685 | .311 |
Table IIId.
Multivariate analysis for postoperative decline in verbal fluency
| Category Fluency Pre – 1 month post |
OR | 95% CI | P value |
|---|---|---|---|
| Severe Alcohol Abuse | 3.513 | 1.086 – 11.362 | .036 |
| CKD | 0.265 | 0.064 – 1.091 | .066 |
| Symptomatic | 0.290 | 0.085 – .996 | .049 |
| Postop microemboli | 0.474 | 0.156 – 1.440 | .188 |
| Category Fluency Pre – 6 month post |
OR | 95% CI | P value |
|---|---|---|---|
| BMI | 1.091 | 0.984 – 1.209 | .097 |
| Contralateral Carotid Stenosis/Occlusion | 0.200 | 0.022 – 1.796 | .151 |
| Postop microemboli | 0.405 | 0.146 – 1.118 | .081 |
DISCUSSION
In this study we found that cerebral microembolization was associated with cognitive deficits one month postoperatively in patients undergoing carotid interventions. We have previously identified decline in verbal learning memory at 1 month following carotid interventions for patients with microemboli5. In this larger dataset, we have found that patients with microemboli experienced significant decline in verbal memory at 1 month, although these deficits largely disappeared at 6 months following intervention. A deficit in psychomotor speed, however, persisted long-term.
The impact of microembolization on patients undergoing carotid interventions has been unclear due to discrepant findings. It is difficult to compare studies, as they differ in how microemboli are characterized, the patient inclusion criteria, and specific neuropsychological measures used to measure cognition. While a number of studies have been conducted that analyze cognition in patients undergoing carotid interventions12, few have looked specifically at the impact of microembolization on cognition in this population since our own initial analysis in 2012. Many similar studies seek to compare differences in outcomes between patients undergoing CAS and CEA. Due to differences in patient selection and procedure-related hemodynamics, we believe that patients who undergo CEA and CAS are not comparable. CAS was reserved for patients at high surgical or medical risks.
CAS patients have been shown to have a higher incidence of microemboli13, however microemboli have also been observed following CEA. We therefore included both intervention types in our study and controlled for procedure in our analyses. Notably, we identify a higher rate of microembolization in both CEA and CAS populations than what is found in other published datasets and our previous report14–16. As patient selection criteria and operators have not changed since our previous publication in 2012, we believe that this may be due to our use of a 3.0T MRI for our postoperative MRI studies, which is more sensitive than a 1.5T magnet, and that each scan was carefully reviewed by a neuroradiologist included in the research protocol.
Investigations by other groups suggest that microemboli do not have a long-term impact on cognition9–10. This present study found a decline in verbal learning memory at 1 month following intervention in patients with microemboli. Despite this, there were no differences in visual memory change between those with and without microemboli. This may have been due to the fact that we used parallel forms for the verbal memory measure but not for the visual memory measure, and therefore a learning effect may have impacted the visual memory results. At the 6 month time point, verbal memory performance of the patients with procedure-related embolization had largely returned to baseline levels, suggesting that these initial deficits are transient. These findings reflect the remarkable plasticity of human brain.
We also found deficits in psychomotor speed in patients with microemboli. Patients without microemboli showed significant improvement on the TMTA task at one month following intervention, while those with microemboli demonstrated minimal to nonexistent gains. Since this task is unchanged across testing sessions, we expected patients to show improvement on this measure due to practice effects. At 6 months following intervention, patients with microemboli still demonstrated a lack of improvement on this measure in comparison to those without microemboli, suggesting a longer-term impact on this cognitive domain. Nevertheless, in multivariate models we did not identify an independent association between emboli and psychomotor speed.
Interestingly, we found an improvement in performance on the Grooved Pegboard and Category Fluency measures for patients with post-operative microemboli at both 1 month and 6 months following intervention. The Grooved Pegboard task measures dexterity and fine motor control, and the Category Fluency is a measure of verbal fluency and semantic memory. While these findings are contrary to our observations in the other cognitive domains, other groups have previously reported improvement in motor skill tasks and executive function following carotid interventions17. Our cohort showed an overall average improvement on the Grooved Pegboard measure postoperatively, which might be expected due to a practice effect and improved cerebral perfusion; however, it is unclear why there was significantly more improvement for patients with microemboli. Given that microembolization is not an independent predictor for improvement or decline, it is likely that other risk factors play a more significant role in changes in these cognitive domains. In fact, a history of severe alcohol abuse was an independent predictor for decline in verbal fluency decline 1 month following interventions (Table IIId).
It is also unclear why diabetes showed a trend of protection against decline on Grooved Pegboard at 1 month and preoperative symptoms appeared protective against decline in Category fluency at 1 month following procedures. One possible explanation is that diabetics in this cohort performed significantly worse at the pre-operative assessment, and thus may have created a floor effect for subsequent postoperative decline. Recent studies in mice have also shown that diabetes is associated with cognitive deficits18. There was no difference in pre-operative performance between symptomatic and asymptomatic patients for any of the cognitive measures analyzed, so the same rationale cannot be used to explain why symptoms were protective against decline. Symptomatic patients with a lesser degree of stenosis (60% or more) were treated compared to asymptomatic patients (>80% stenosis) in our clinical practice; therefore, we suspect that symptomatic patients overall might have less stenosis in our cohort, and perhaps had better brain reserve. Patients with a lesser degree of stenosis and greater blood flow may have better brain integrity, thus allowing them to recover from ischemic insult more effectively than those with low blood flow at baseline. However, this speculation will need to be further investigated.
There are several limitations to our study. While we have a larger number of patients than in our previous analyses, we are still limited by the size of our cohort. These patients were recruited from a single academic center; a larger multi-center study would provide data for a more robust statistical analysis. Secondly, we are also limited in our ability to determine causality for cognitive decline. While we found associations between incidence of microemboli and changes in cognition, it is possible that cognitive decline occurred due to other reasons. This patient population has a number of co-morbidities that may have also contributed to poor cognitive outcomes following surgery, including CAD, contralateral carotid occlusion, and diabetes. Our multivariate models were designed to help correct for such factors. While DWI is a sensitive and specific tool to identify procedure-related microembolization, it does not reflect real-time information. Transcranial Doppler (TCD), on the other hand, can provide real-time information that help clinicians to optimize stenting techniques. However, TCD detects both gaseous and solid lesions that may or may not lead to neurocognitive changes15, 19, 20. Finally, we also did not account for variability in volume or location of microemboli; we plan to investigate this in the future.
CONCLUSION
Although carotid revascularization has an overall positive impact on cognitive function, microembolization in patients undergoing carotid interventions can result in transient cognitive decline. While some deficits, particularly in psychomotor speed measured by TMTA, persist at 6 months following intervention, most cognitive function returns to baseline. Further investigation is warranted to determine individual patient risk factors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 2016 Society for Clinical Vascular Surgery Meeting in Las Vegas, Nevada. March 12–16, 2016.
References
- 1.Abbott A. Dementia: a problem for our age. Nature. 2011;475(7355):S2–4. doi: 10.1038/475S2a. [DOI] [PubMed] [Google Scholar]
- 2.Kelley A, McGarry K, Gorges R, Skinner J. The Burden of Health Care Costs for Patients With Dementia in the Last 5 Years of Life. Ann Intern Med. 2015;163(10):729–736. doi: 10.7326/M15-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho PM, Arciniegas DB, Grigsby J, McCarthy M, Jr, McDonald GO, Moritz TE, et al. Predictors of cognitive decline following coronary artery bypass graft surgery. Ann Thorac Surg. 2004;77(2):597–603. doi: 10.1016/S0003-4975(03)01358-4. [DOI] [PubMed] [Google Scholar]
- 4.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 5.Zhou W, Hitchner E, Gillis K, Sun L, Floyd R, Lane B, et al. Prospective neurocognitive evaluation of patients undergoing carotid interventions. J Vasc Surg. 2012;56(6):1571–8. doi: 10.1016/j.jvs.2012.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mocco J, Wilson DA, Komotar RJ, Zurica J, Mack WJ, Halazun HJ, et al. Predictors of neurocognitive decline after carotid endarterectomy. Neurosurgery. 2006;58(5):844–50. doi: 10.1227/01.NEU.0000209638.62401.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lal BK, Younes M, Cruz G, Kapadia I, Jamil Z, Pappas PJ. Cognitive changes after surgery vs stenting for carotid artery stenosis. J Vasc Surg. 2011;54(3):691–8. doi: 10.1016/j.jvs.2011.03.253. [DOI] [PubMed] [Google Scholar]
- 8.Maggio P, Altamura C, Landi D, Migliore S, Lupoi D, Moffa F, et al. Diffusion-weighted lesions after carotid artery stenting are associated with cognitive impairment. J Neurol Sci. 2013 May 15;328(1–2):58–63. doi: 10.1016/j.jns.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Wasser K, Pilgram-Pastor SM, Schnaudigel S, Stojanovic T, Schmidt H, Knauf J, et al. New brain lesions after carotid revascularization are not associated with cognitive performance. J Vasc Surg. 2011 Jan;53(1):61–70. doi: 10.1016/j.jvs.2010.07.061. [DOI] [PubMed] [Google Scholar]
- 10.Akkaya E, Vuruskan E, Gul ZB, Yildirim A, Pusuroglu H, Surgit O, et al. Cerebral microemboli and neurocognitive change after carotid artery stenting with different embolic protection devices. Int J Cardiol. 2014 Sep 20;176(2):478–83. doi: 10.1016/j.ijcard.2014.07.241. [DOI] [PubMed] [Google Scholar]
- 11.Estévez-González A, Kulisevsky J, Boltes A, Otermín P, García-Sánchez C. Rey verbal learning test is a useful tool for differential diagnosis in the preclinical phase of Alzheimer’s disease: comparison with mild cognitive impairment and normal aging. Int J Geriatr Psychiatry. 2003 Nov;18(11):1021–8. doi: 10.1002/gps.1010. [DOI] [PubMed] [Google Scholar]
- 12.Kougias P, Collins R, Pastorek N, Sharath S, Barshes NR, McCulloch K, et al. Comparison of domain-specific cognitive function after carotid endarterectomy and stenting. J Vasc Surg. 2015 Aug;62(2):355–61. doi: 10.1016/j.jvs.2015.02.057. [DOI] [PubMed] [Google Scholar]
- 13.Tedesco MM, Lee JT, Dalman RL, Lane B, Loh C, Haukoos JS, et al. Postprocedural microembolic events following carotid surgery and carotid angioplasty and stenting. J Vasc Surg. 2007;46(2):244–50. doi: 10.1016/j.jvs.2007.04.049. [DOI] [PubMed] [Google Scholar]
- 14.Hammer FD, Lacroix V, Duprez T, Grandin C, Verhelst R, Peeters A, et al. Cerebral microembolization after protected carotid artery stenting in surgical high-risk patients: results of a 2-year prospective study. J Vasc Surg. 2005 Nov;42(5):847–53. doi: 10.1016/j.jvs.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 15.Gossetti B, Gattuso R, Irace L, Faccenna F, Venosi S, Bozzao L, et al. Embolism to the brain during carotid stenting and surgery. Acta Chir Belg. 2007 Mar-Apr;107(2):151–4. [PubMed] [Google Scholar]
- 16.Poppert H, Wolf O, Resch M, Theiss W, Schmidt-Thieme T, Graefin von Einsiedel H, et al. Differences in number, size and location of intracranial microembolic lesions after surgical versus endovascular treatment without protection device of carotid artery stenosis. J Neurol. 2004 Oct;251(10):1198–203. doi: 10.1007/s00415-004-0502-4. [DOI] [PubMed] [Google Scholar]
- 17.Heyer EJ, Adams DC, Solomon RA, Todd GJ. Neuropsychometric changes in patients after carotid endarterectomy. Stroke. 1998 doi: 10.1161/01.str.29.6.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuloaga KL, Johnson LA, Roese NE, Marzulla T, Zhang W, Nie X, et al. High fat diet-induced diabetes in mice exacerbates cognitive deficit due to chronic hypoperfusion. J Cereb Blood Flow Metab. 2015 Nov 11; doi: 10.1177/0271678X15616400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skjelland M, Krohg-Sørensen K, Tennøe B, Bakke SJ, Brucher R, Russell D. Cerebral microemboli and brain injury during carotid artery endarterectomy and stenting. Stroke. 2009 Jan;40(1):230–4. doi: 10.1161/STROKEAHA.107.513341. [DOI] [PubMed] [Google Scholar]
- 20.Garami ZF1, Bismuth J, Charlton-Ouw KM, Davies MG, Peden EK, Lumsden AB. Feasibility of simultaneous pre- and postfilter transcranial Doppler monitoring during carotid artery stenting. J Vasc Surg. 2009 Feb;49(2):340–4, 345. doi: 10.1016/j.jvs.2008.08.102. [DOI] [PubMed] [Google Scholar]


