Abstract
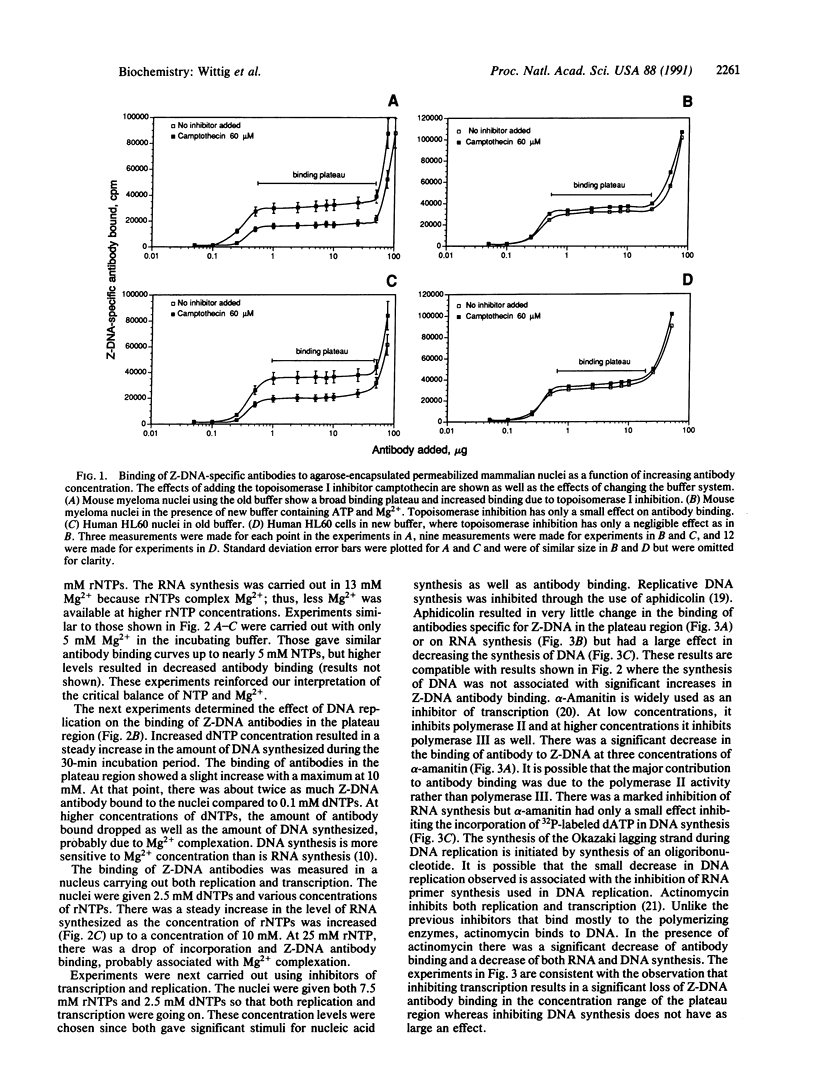
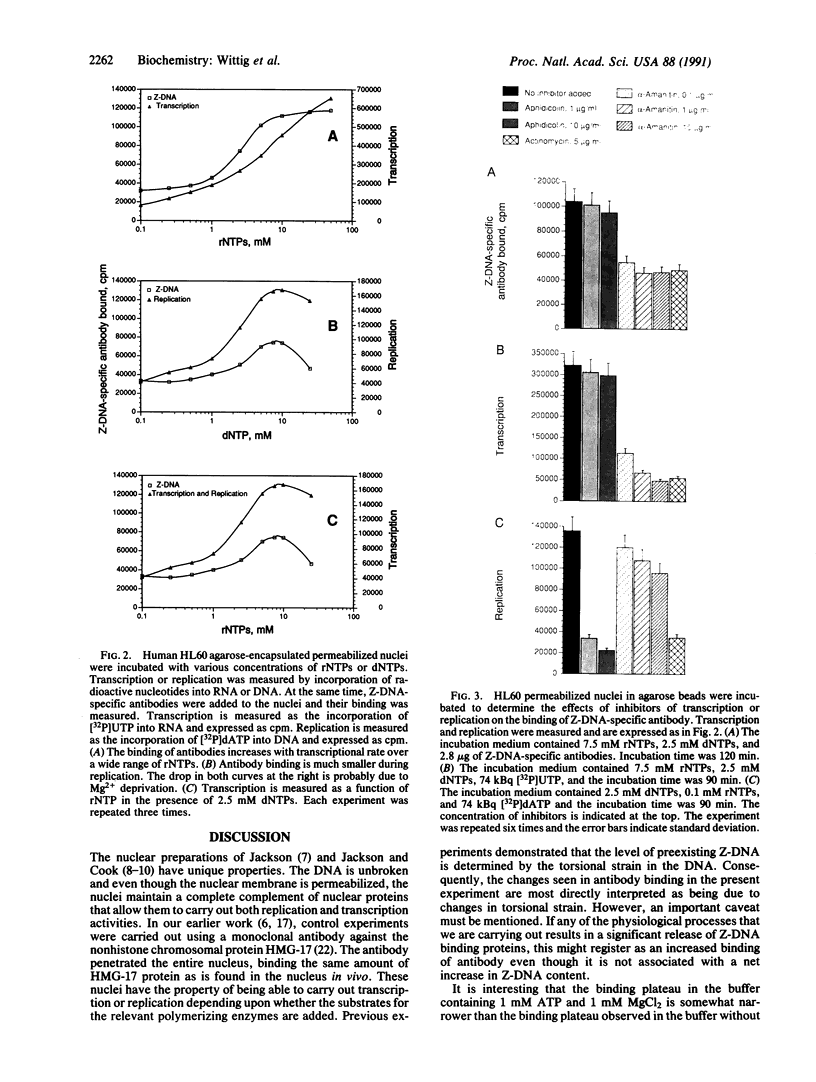
Mammalian cells have been encapsulated in agarose microbeads, and from these cells metabolically active permeabilized nuclei were prepared. Previously, we showed that biotin-labeled monoclonal antibodies against Z-DNA can be diffused into the nuclei and, over a specific concentration range, they will bind to Z-DNA within the nucleus in a concentration-independent manner. By using radiolabeled streptavidin, we showed that the amount of Z-DNA antibody bound is related to the torsional strain of the DNA in the nucleus. Relaxation of the DNA results in a decrease of Z-DNA formation, whereas increasing torsional strain through inhibiting topoisomerase I results in increased Z-DNA formation. Here we measure the influence of RNA transcription and DNA replication. Transcription is associated with a substantial increase in the binding of anti-Z-DNA antibodies, paralleling the increased level of RNA synthesized as the level of ribonucleoside triphosphate in the medium is increased. DNA replication yields smaller increases in the binding of Z-DNA antibodies. Stopping RNA transcription with inhibitors results in a large loss of Z-DNA antibody binding, whereas only a small decrease is associated with inhibition of DNA replication.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dorbic T., Wittig B. Chromatin from transcribed genes contains HMG17 only downstream from the starting point of transcription. EMBO J. 1987 Aug;6(8):2393–2399. doi: 10.1002/j.1460-2075.1987.tb02517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Franco R. J. Inhibitors of DNA topoisomerases. Biochemistry. 1988 Apr 5;27(7):2253–2259. doi: 10.1021/bi00407a001. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Cook P. R. A cell-cycle-dependent DNA polymerase activity that replicates intact DNA in chromatin. J Mol Biol. 1986 Nov 5;192(1):65–76. doi: 10.1016/0022-2836(86)90464-x. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Cook P. R. A general method for preparing chromatin containing intact DNA. EMBO J. 1985 Apr;4(4):913–918. doi: 10.1002/j.1460-2075.1985.tb03718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. A., Cook P. R. Replication occurs at a nucleoskeleton. EMBO J. 1986 Jun;5(6):1403–1410. doi: 10.1002/j.1460-2075.1986.tb04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. A., Cook P. R. Transcription occurs at a nucleoskeleton. EMBO J. 1985 Apr;4(4):919–925. doi: 10.1002/j.1460-2075.1985.tb03719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. A., Cook P. R. Visualization of a filamentous nucleoskeleton with a 23 nm axial repeat. EMBO J. 1988 Dec 1;7(12):3667–3677. doi: 10.1002/j.1460-2075.1988.tb03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. A., Yuan J., Cook P. R. A gentle method for preparing cyto- and nucleo-skeletons and associated chromatin. J Cell Sci. 1988 Jul;90(Pt 3):365–378. doi: 10.1242/jcs.90.3.365. [DOI] [PubMed] [Google Scholar]
- Jaworski A., Hsieh W. T., Blaho J. A., Larson J. E., Wells R. D. Left-handed DNA in vivo. Science. 1987 Nov 6;238(4828):773–777. doi: 10.1126/science.3313728. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Nordheim A., Rich A., Wang J. C. Flipping of cloned d(pCpG)n.d(pCpG)n DNA sequences from right- to left-handed helical structure by salt, Co(III), or negative supercoiling. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4560–4564. doi: 10.1073/pnas.79.15.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Runkel L., Nordheim A. Chemical footprinting of the interaction between left-handed Z-DNA and anti-Z-DNA antibodies by diethylpyrocarbonate carbethoxylation. J Mol Biol. 1986 Jun 5;189(3):487–501. doi: 10.1016/0022-2836(86)90319-0. [DOI] [PubMed] [Google Scholar]
- Schröder H. C., Trölltsch D., Friese U., Bachmann M., Müller W. E. Mature mRNA is selectively released from the nuclear matrix by an ATP/dATP-dependent mechanism sensitive to topoisomerase inhibitors. J Biol Chem. 1987 Jun 25;262(18):8917–8925. [PubMed] [Google Scholar]
- Seki S., Oda T., Ohashi M. Differential effects of aphidicolin on replicative DNA synthesis and unscheduled DNA synthesis in permeable mouse sarcoma cells. Biochim Biophys Acta. 1980 Dec 11;610(2):413–420. doi: 10.1016/0005-2787(80)90022-2. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Klysik J., Stirdivant S. M., Wells R. D. Left-handed Z-DNA is induced by supercoiling in physiological ionic conditions. Nature. 1982 Sep 23;299(5881):312–316. doi: 10.1038/299312a0. [DOI] [PubMed] [Google Scholar]
- Stollar B. D. Antibodies to DNA. CRC Crit Rev Biochem. 1986;20(1):1–36. doi: 10.3109/10409238609115899. [DOI] [PubMed] [Google Scholar]
- Wieland T., Faulstich H. Amatoxins, phallotoxins, phallolysin, and antamanide: the biologically active components of poisonous Amanita mushrooms. CRC Crit Rev Biochem. 1978 Dec;5(3):185–260. doi: 10.3109/10409237809149870. [DOI] [PubMed] [Google Scholar]
- Wittig B., Dorbic T., Rich A. The level of Z-DNA in metabolically active, permeabilized mammalian cell nuclei is regulated by torsional strain. J Cell Biol. 1989 Mar;108(3):755–764. doi: 10.1083/jcb.108.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Wang J. C., Liu L. F. Involvement of DNA topoisomerase I in transcription of human ribosomal RNA genes. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1060–1064. doi: 10.1073/pnas.85.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]




